Abstract
The brain is inherently multiscalar in both space and time. We argue that this multiscalar nature is reflected in the blood oxygenation level dependent (BOLD) fluctuations used to map functional connectivity. We present evidence that global fluctuations in activity, quasiperiodic spatiotemporal patterns, and aperiodic time-varying activity coexist within the BOLD signal. These processes can be separated using careful analysis and appear to reflect electrical activity on similar scales, suggesting that the BOLD signal fluctuations can provide novel insight into the functional architecture of the brain.
I. Introduction
To interpret functional magnetic resonance imaging (fMRI) experiments, researchers rely on a model where increased neural activity raises the demand for metabolic substrates, which in turn leads to a localized rise in cerebral blood flow that increases the blood oxygenation level dependent (BOLD) signal [1]. This model performs reasonably well for task-based fMRI, where background activity or ongoing fluctuations are suppressed by averaging. For resting state fMRI (rs- fMRI), the situation is more complex. Because functional connectivity maps are based on statistical relationships between areas, any process that changes the MRI signal in a spatially structured manner and on the appropriate time scale will contribute to the measured functional connectivity. Head motion, respiration, and cardiac pulsation all create structured patterns of correlation, and numerous strategies have been devised to mitigate their effects [2]. Much less is known about the relative contributions of various neurophysiological processes that contribute to the BOLD signal. Neural and glial activity is coordinated across multiple scales, ranging from the firing of a single neuron to widespread modulations of cortical excitability. The complex multiscale architecture of the elements that participate in normal brain function is one of the greatest challenges to our understanding of how the brain works. We propose that the BOLD signal should be modeled as a combination of processes that occur on different spatial and temporal scales, and provide evidence that different processes reflect underlying activity in the brain.
II. Contributors to the bold signal
In healthy, wakeful subjects, rs-fMRI analysis is typically performed assuming that the BOLD signal correlations reflect coordinated but time-varying interactions between areas that are key to normal information processing and cognitive functioning. However, not all BOLD fluctuations arise from this type of activity. Network structures are largely preserved during sleep, anesthesia and coma [3, 4], suggesting that a persistent, spatially-structured background activity coexists with time-varying information processing. Here we describe three potential contributors to the BOLD signal that exhibit different spatial and temporal scales.
A. Global BOLD Signal
Regression of the BOLD signal averaged across the whole brain (the global signal, GS) prior to rs-fMRI analysis was originally touted as a method for removing noise unrelated to the time-varying localized interactions ideally detected with rs-fMRI. Regression of the GS improves specificity and reproducibility of functional networks [5]. However, it can be shown mathematically that the removal of the GS forces some correlations within the brain to become negative, making interpretation problematic [6]. Recent studies have found that the GS has ties to neural activity and therefore is not merely an aggregate of noise sources. The most direct evidence of a link comes from a study in monkeys, which found that gamma power recorded from a single site in the brain exhibited positive correlations with the rs-fMRI signal from most of the brain [7]. Studies in humans have shown that the BOLD GS is also linked to changes detectable by EEG. Caffeine affects both neural activity and GS amplitude [8], and GS amplitude is related to changes in vigilance measured by EEG [9].
B. Quasiperiodic Patterns (QPPs)
Quasiperiodic spatiotemporal patterns (QPPs) of BOLD signal changes were first observed as bilateral waves of signal propagating from lateral to medial areas in rats that repeated many times over the course of a single rs-fMRI scan [10]. The implementation of a pattern-finding algorithm allowed the detection of similar patterns in human subjects that involves alternation between a default mode-like network (DMN) and a task positive-like network (TPN), with propagation along the cortex between network nodes [11]. To examine QPPs on a group basis, the images are first normalized to a common space and then concatenated in time (similar to group ICA; details in [11]). A spatiotemporal block of data is randomly selected and correlated with the entire time course in a sliding fashion. Time points where correlation is high (indicating a similar spatiotemporal pattern) are averaged together to form a new template and the process is repeated until the template converges. The results of the pattern finding algorithm are the spatiotemporal template of the QPP and a plot showing the strength of the QPP over the course of the scan. The patterns are consistent in spatial localization and timing across subjects and contribute ongoing background patterns of activity to the BOLD fluctuations.
We have shown in the rat that QPPs are similar in spatial extent and timing to the correlation between infraslow activity and the BOLD signal [12,13], suggesting that they reflect infraslow electrical activity. These findings are consistent with other observations of quasiperiodicity in brain function, all of which fall into the infraslow regime. Of particular note, Ko et al. reported a quasiperiodic pattern of DMN activity using electrophysiology [14] that is consistent with both our work and a recent study in animals, where Li et al. found that correlation between sites was mostly confined to a narrow band of frequencies superimposed on a scale-free distribution [15]. The studies also show that quasiperiodic activity persists across a number of states, including sleep, wakefulness, and anesthesia. The relatively long time scale and repetitive nature of the patterns provides additional evidence that they are not directly tied to cognitive processing.
C. Aperiodic Activity
A number of studies have shown that brain activity in general follows a P~1/fβ distribution [16], where P is power at frequency f and β is the power law exponent. Additive peaks correspond to particular oscillations (such as alpha activity). Because cognitively-relevant activity is unlikely to be periodic, our working hypothesis is that the 1/fβ portion contains the time-varying interactions that are of primary interest in rs-fMRI, while the additive bandlimited peaks are tied to large-scale modulatory processes such as the QPPs. These aperiodic time-varying processes are of primary interest in dynamic functional connectivity studies and have been investigated with a wide range of techniques including sliding window correlation, clustering, independent component analysis, and Markovian modeling. Dynamic analysis of rs-fMRI clearly contains information about the underlying neural activity, as the network dynamics have been linked to electrophysiology, behavior, and brain dysfunction. However, standard analysis methods ignore contributions from other neurophysiological processes ongoing in the brain, potentially reducing sensitivity to cognitively-relevant changes.
III. PROPOSED MODEL
The model that we propose considers the BOLD signal as a linear combination of the global signal, the quasiperiodic spatiotemporal patterns, and the aperiodic activity.
| (1) |
where xi(t) is the measured time series for the ith voxel, QPPi(t) is the spatiotemporal template derived from the pattern finding algorithm for that voxel, Tstr(t) is the strength of the QPP template over time, and Aperi(t) is the desired aperiodic time-varying fluctuations related to neural activity. The weighting coefficients a and b indicate the relative contribution of GS and aperiodic activity in each voxel (this information is implicit in the QPP term). The linear nature of the model implies that the components of the BOLD signal can be separated into at least three different signals representing different processes. In the next section, we give evidence that each of these signals can be isolated from the rest and provides unique information about brain function.
IV. FEASIBILITY AND DESIRABILITY OF SEPARATING COMPONENTS
A. Global BOLD Signal
All animal experiments were approved by the Emory IACUC committee; all human studies, by the Emory IRB committee.
The global signal is by definition not related to the time-varying interactions that would ideally define functional networks. When we examined simultaneous rs-fMRI and electrophysiological data from the rat using sliding window correlation to capture dynamic interactions, we found that sensitivity to time-varying correlation in neural activity was enhanced after GS regression [17]. This is in line with previous studies showing that GS regression improves specificity of networks and suggests that if the BOLD GS does have a neural origin, it arises from a type of nonlocalized activity that differs from the coordination of functional networks and may provide distinct information about brain function.
Further evidence that the global BOLD signal contains information of interest comes from recent clinical studies that have demonstrated that the GS can distinguish patients with schizophrenia from those with bipolar disorder [18] or from healthy controls [19]. Because GS regression can distort differences between groups, other differences in patient populations that have been observed could have been due to differences in GS rather than localized neural activity [20].
To look for a neural basis of the GS, we measured local field potentials (LFPs) from six widely spread electrodes implanted across DMN and sensorimotor regions of three anesthetized rats. All pairs of electrodes exhibited strong coherence in the low frequencies. Average pairwise coherence from one rat is shown in Fig. 1 and exhibits the highest values in frequencies below 10 Hz. This coherence is a plausible source for the BOLD global signal.
Fig. 1.
Average coherence as a function of frequency for 6 widely distributed electrodes.
B. Quasiperiodic Patterns
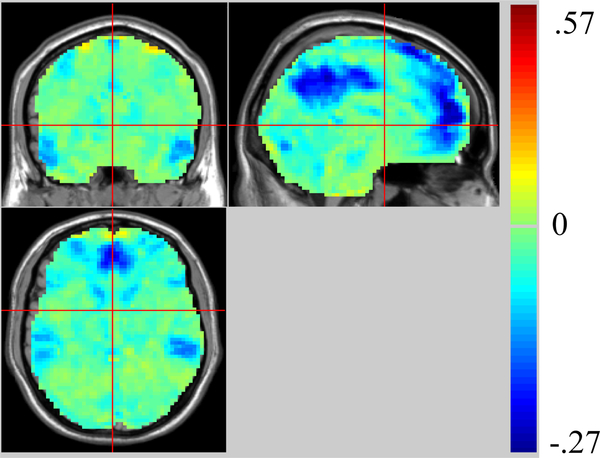
The quasiperiodic patterns have a period of approximately 20–30 s in humans, falling well within the typical low frequency range used to map functional connectivity (0.01–0.08 or 0.01–0.1 Hz). As a preliminary examination of the contribution of the QPPs to functional connectivity, we analyzed data acquired for another study (16 healthy subjects; 3T; TE/TR 30ms/2000ms; 30 4 mm slices; 7 min scan). Standard preprocessing procedures were followed, including slice timing, realignment, coregistration, nuisance covariates regression, normalization and smoothing with a 4mm Gaussian kernel. The resultant data was detrended and filtered (0.01–0.08 Hz). The QPP template and plot of template strength were calculated for the concatenated data. We then convolved the template with the normalized timecourse of template strength and regressed the result from the original time course. By comparison with the original data, we mapped the reduction in variance that resulted from the regression of QPPs (Fig. 2). As can be seen, the QPP accounted for up to a quarter of the variance in the BOLD signal, with the largest changes in areas of the DMN. Pearson correlation was calculated between the time course of the posterior cingulate cortex (PCC) and the rest of the brain using the original BOLD signal and the QPP- corrected signal. A t-test was performed voxel by voxel to highlight the differences in connectivity. The results, corrected for multiple comparisons (AlphaSim, p< 0.05), indicate that the QPPs account for a significant amount of the functional connectivity in this network (Fig. 3).
Fig. 2.
Change in BOLD signal variance after QPP regression across the brain.
Fig. 3.
Areas of significant reduction in functional connectivity to a seed in the posterior cingulate cortex after QPP regression.
C. Aperiodic Activity
Research from our lab and others has shown that aperiodic activity and quasiperiodic patterns coexist and contribute to the BOLD signal. In different studies, the aperiodic BOLD fluctuations have been linked to neural activity in gamma, beta, delta, and theta bands, infraslow activity,and slow variations in firing rates [12,17, 21–25]. Interestingly, the frequencies that are most closely related to the local BOLD fluctuations are not the same as the frequencies that mediate correlation between areas [21–23]. The diverse results suggest that the BOLD signal reflects a variety of neural and potentially non-neural processes, and the relative contribution of these processes could differ from network to network. Some of these differences may be due to the anesthesia used in animal models. We compared sliding window BOLD correlation from left and right somatosensory cortex to correlation between simultaneously –acquired band-limited power from electrodes in the same area (Fig. 4). The pattern of bandlimited power correlation with BOLD is similar for low and high dose isoflurane, but differs for dexmedetomidine.
Fig. 4.
Correlation between BOLD sliding window time courses and bandlimited power sliding window time courses under isoflurane and dexmedetomidine.
To examine whether QPPs were somehow linked to the network dynamics observed with sliding window techniques and presumed to arise from aperiodic contributions, we first looked at phase amplitude coupling between infraslow activity and higher frequencies, and found little evidence of a relationship [26]. Further work showed that infraslow electrical activity correlated with QPPs but higher frequency activity correlated more closely to sliding window BOLD correlation [27]. This is strong evidence that the QPPs and remaining BOLD have differing sensitivities to neural activity and do not reflect the same underlying process. If we consider the QPPs as a sort of periodic noise, their removal by regression should increase sensitivity to fluctuations that occur on shorter time scales, potentially increasing the sensitivity of rs-fMRI to cognitive processing.
V. DISCUSSION
A. Limitations of the Linear Model
While our work so far has found that aperiodic activity and QPPs contribute separately to the BOLD signal, it is possible that a weak relationship exists between the two processes. We did not observe phase-amplitude coupling, but many more sophisticated measures of interaction remain to be examined. For example, mutual information measures might prove useful, or an examination of phase locking. Similarly, the global signal may weakly modulate the other contributors, something that has not been examined in detail at this point in time. Nevertheless, to a first approximation, our data show that the three signal sources are separable, with different spatial and temporal signatures.
B. Other Potential Contributors
The three contributors to the BOLD signal identified in this paper are by no means the only processes that can contribute to functional connectivity measurements. A prominent omission is vasomotion, the spontaneous fluctuation of small vessels that can exist independently of neural activity. Because the BOLD signal is influenced by changes in blood flow, these oscillations can cause coordinated fluctuations that then contribute to functional connectivity between areas. Other possible sources include glial cells, particularly the astrocytes, which are known to participate in coordinated waves of activation. These changes again may or may not be coupled to the neural activity that is of primary interest. Future multimodal experiments in the animal model can begin to unravel the complicated sources of the coordinated BOLD fluctuations.
C. Application in Basic and Clinical Science
The feasibility of separating contributions from brain processes that occur on different spatial and temporal scales opens the door to new studies that explore the functional architecture of the brain in ways that could not be examined previously. While much more work is needed to understand how the BOLD signal reflects multiple spatial and temporal scales, the experiments that address these issues will improve our understanding of the systems-level function of the brain, and may also provide insight into the alterations that occur in clinical disorders. For example, we speculate that quasiperiodic patterns may prove most sensitive to the changes that occur in one type of disorder, while aperiodic activity is more sensitive to another.
ACKNOWLEDGMENT
The authors thank Hyun Koo Chung, Dieter Jaeger, and Eric Schumacher for scientific discussions.
Research supported by NIH and NSF.
Contributor Information
Shella D. Keilholz, Emory University/Georgia Institute of Technology, Atlanta, GA 30322 USA (phone: 404-727-2433; fax: 404-727-9873; shella.keilholz@bme.gatech.edu)..
Jacob C.W. Billings, Emory University/Georgia Institute of Technology (billings.jacob@gmail.com)
Kai Wang, Tsinghua University, Beijing, China (wangkai12@mails.tsinghua.edu.cn)..
Anzar Abbas, Emory University/Georgia Institute of Technology, anzar.abbas@emory.edu.
Claudia Hafeneger, Max Planck Institute, Cologne, Germany (Claudia.hafeneger@sf.mpg.de)..
Wen-Ju Pan, Emory University/Georgia Institute of Technology, wpan5@emory.edu.
Sadia Shakil, Emory University/Georgia Institute of Technology, sadia_shakil@gatech.edu.
Maysam Nezafati, Emory University/Georgia Institute of Technology, maysam.nezafati@emory.edu.
REFERENCES
- [1].Bandettini PA et al. , “Time course EPI of human brain function during task activation.” Magn Reson Med 25, 1992, p. 390–7. [DOI] [PubMed] [Google Scholar]
- [2].Murphy K et al. , “Resting state fMRI confounds and cleanups.” NeuroImage 80, 2013, p. 349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Boly M et al. , “Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function?” Ann N Y Acad Sci 1129, 2008, p. 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fukunaga M et al. ,”Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages.” Magn Reson Imaging 24, 2006, p. 979–992. [DOI] [PubMed] [Google Scholar]
- [5].Fox MD et al. , “ The global signal and observed anticorrelated resting state brain networks. “ J Neurophysiol 101, 2009, p. 3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Murphy K et al. , “The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced?” Neuroimage 44, 2009, p. 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Scholvinck ML et al. “Neural basis of global resting-state fMRI activity.” Proc Natl Acad Sci U S A 107, 2010, p. 10238–10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wong CW et al. , “ Anti-correlated networks, global signal regression, and the effects of caffeine in resting-state functional MRI.” Neuroimage 63,2012, p. 356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wong CW, et al. , “ The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures.” Neuroimage 83, 2013, p. 983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Majeed W et al. , “ Spatiotemporal Dynamics of Low Frequency Fluctuations in BOLD fMRI of the Rat.” J Magn Reson Imag 30, 2009, p. 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Majeed W et al. , “Spatiotemporal dynamics of low frequency BOLD fluctuations in rats and humans.” Neuroimage 54, 2011, p. 1140–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pan W-J et al. , “Infraslow LFP correlates to resting-state fMRI BOLD signals.” Neuroimage 74, 2013, p. 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thompson GJ et al. , “Quasi-periodic patterns (QPP): Large-scale dynamics in resting state fMRI that correlate with local infraslow electrical activity.” Neuroimage 2014, 84, 1018–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ko AL et al. , “Quasi-periodic fluctuations in default mode network electrophysiology.” J Neurosci 31, 2011, p. 11728–11732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li JM et al. , “Functional connectivity arises from a slow rhythmic mechanism.” Proc. Natl. Acad. Sci. U. S. A 112,2015, p. E2527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].He BJ, “Scale-free brain activity: past, present, and future.” Trends Cogn. Sci 2014, p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thompson GJ et al. , “Neural correlates of time-varying functional connectivity in the rat.” Neuroimage 83, 2013, p.826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yang GJ et al. , “Altered global brain signal in schizophrenia.” Proc. Natl. Acad. Sci. U. S. A 111, 2014, p. 7438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hahamy A et al. , “Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging.” Brain Connect. 4, 2014, p. 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Saad ZS et al. , “Trouble at rest: how correlation patterns and group differences become distorted after global signal regression.” Brain Connect. 2, 2012, p.25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lu H et al. , “Synchronized delta oscillations correlate with the resting-state functional MRI signal.” Proc Natl Acad Sci U S A 104, 2007, p.18265–18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shmuel A & Leopold DA, “ Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest.” Hum Brain Mapp 29, 2008, p.751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pan W-J et al. ,”Broadband Local Field Potentials Correlate with Spontaneous Fluctuations in Functional Magnetic Resonance Imaging Signals in the Rat Somatosensory Cortex Under Isoflurane Anesthesia.” Brain Connect. 1, 2011, p. 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nir Y et al. , “Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex.” Nat Neurosci 11, 2008, p. 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].He BJ et al. , “Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture.” Proc Natl Acad Sci U S A 105, 2008, p. 16039–16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Thompson GJ et al. , “Phase-amplitude coupling and infraslow (<1 Hz) frequencies in the rat brain: relationship to resting state fMRI. “ Front Integr Neurosci 8, 2014, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thompson GJ, Pan W-J & Keilholz SD, “Different dynamic resting state fMRI patterns are linked to different frequencies of neural activity.” J. Neurophysiol 114, 2015, p.114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]