Abstract
Objective:
To evaluate the efficacy and toxicity of a repeat peptide receptor radionuclide therapy (PRRT) course in neuroendocrine tumour patients who have progressed following previous PRRT and to identify factors contributing to retreatment outcomes.
Methods:
This was a retrospective analysis of 47 consecutive patients who had been treated with PRRT (PRRT1) and following disease progression were retreated with a second course of PRRT (PRRT2). We reviewed patient, tumour and treatment characteristics, time to progression after PRRT1 and PRRT2, overall survival and toxicity. We evaluated Kaplan–Meier survival plots, multiple regression analysis on factors predictive of time to progression and toxicity.
Results:
PRRT1: 45/47 patients were initially were treated with 90Y-DOTATATE, with two patients treated with 177Lu-DOTATATE. The median progression free survival (PFS) following PRRT1 was 30 months [95% confidence interval (CI) (26.9–36.6 months)]. Two patients developed Grade 1 renal toxicity. 3/47 patients had bone marrow toxicity, with 1 of these patients having Grade 3 toxicity. PRRT2: At the second course of treatment, 29 patients were treated with 90Y-DOTATATE and 18 patients with 177Lu-DOTATATE. Of the 44 patients with evaluable survival data, 41 patients developed disease progression. The median PFS after PRRT2 was 17.5 months [95% CI (11–23.8 months)]. There was no statistically significant difference in median PFS dependent on the choice of radiopharmaceutical: median PFS for 177Lu-DOTATATE = 17.2 months, median PFS for 90Y-DOTATATE = 17.3 months. Male sex and high burden of liver metastases were associated with shorter PFS following a PRRT retreatment course. 17/41 (41%) patients had bone marrow toxicity (2/17 had Grade 3 toxicity; no Grade 4 toxicity was seen). One patient developed myelodysplastic syndrome. 6/41 (14.6%) developed Grade 1 renal toxicity and 1/41 (2.4%) had Grade 4 renal toxicity. The median overall survival from commencement of first PRRT cycle was 71 months.
Conclusion:
PRRT retreatment is safe and offers patients, who had progressed following initial PRRT course, a reasonably good PFS. Extra consideration is needed in patients with multiple comorbidities, as they may be at greater risk of renal and haematological toxicity. Male sex and high burden of liver metastases seem to be associated with shorter PFS following PRRT retreatment.
Advances in knowledge:
The majority of studies on PRRT have shown that it is effective as an initial treatment. This study with long-term follow-up demonstrates that PRRT is safe and effective retreatment option in patients that have progressed following initial PRRT course.
Introduction
Neuroendocrine tumours (NETs) are heterogeneous in nature, varying in differentiation and proliferation. They can produce a variety of bioactive substances, e.g. gastrin, insulin or can be non-functioning.1 In patients with metastatic disease, the only realistic curative option is surgery; however, this is feasible in less than 10% of patients. Several licensed and unlicensed systemic treatment options for metastatic NETs are currently available that have been shown to provide symptomatic benefit and prolong survival. These include long-acting somatostatin analogues (SSAs), chemotherapy, molecular targeted treatments (everolimus and sunitinib), alpha-interferon and peptide receptor radionuclide therapy (PRRT).2
Of these therapies, PRRT appears to have the greatest benefit in terms of progression-free survival (PFS), with the recent NETTER-1 trial demonstrating a PFS of approximately 40 months in patients with progressive metastatic midgut NETs.3 PRRT first became prevalent in practice in the late 1990s and the two most predominant radionuclides used are 90Yttrium (90Y) and 177Lutetium (177Lu). The somatostatin receptor (SSR) compounds these are most commonly labelled with are DOTATATE and DOTATOC. 90Y-PRRT was used clinically from the early 2000s, whilst 177Lu-PRRT became prevalent in the mid/late-2000s. Both 90Y-PRRT and 177Lu-PRRT have been shown to be effective treatments, with approximately 80% of patients having partial response (PR)/disease stabilisation.4, 5 The average time to progression ranges from 13 to 40 months.3–8 90Y has a more energetic beta particle and higher amounts of bone marrow/renal toxicity have been described with this agent.9–11
It is standard practice to fractionate treatments at between two and four cycles to reduce toxicity with commonly used regimes being four cycles of 7.4 GBq of 177Lu-DOTATATE or three cycles of 3.7 GBq m–2 90Y-DOTATATE/DOTATOC. Although PRRT is an effective treatment, patients with metastatic disease will invariably progress over time. Its use as a single course (four cycles 177Lu-PRRT or three cycles 90Y-PRRT) has been much explored but there is limited outcome data for its use in the retreatment setting. We aim to review the experiences in a national referral centre for retreatment with 177Lu-DOTATATE and 90Y-DOTATATE.
Aim
To evaluate the efficacy and toxicity of a repeat PRRT course in patients who have progressed following previous PRRT and identify factors contributing to retreatment outcomes.
Methods and materials
This was a retrospective analysis of consecutive patients who had been treated with PRRT (PRRT1) and following disease progression were retreated with a second course of PRRT (PRRT2). Ethics approval was not required as this was a retrospective analysis of patient data acquired during routine clinical care and no patient identifiable data were released or transferred from any standard hospital database. The data have not been previously analysed for this purpose.
Patient selection
All patients who initiated treatment with PRRT from December 2000 to December 2012 were eligible for inclusion.
Patient suitability for PRRT was decided upon and documented at a dedicated NET tumour board meeting. Histologically confirmed well-differentiated gastro-entero-pancreatic NET, thoracic NET, medullary thyroid cancer (MTC), paragangliomas and NET of unknown primary was included.
Patients required sufficient uptake on their SSR imaging (111Indium-octreotide or 68Gallium-DOTATATE) by demonstration of tumour uptake greater than that of background liver at >90% sites of disease. In addition, patients required radiological progressive disease (PD) according to response evaluation in solid tumours within the past 6 months, despite maximum dose SSAs for those with mid- and hindgut NET or despite maximum dose SSAs and other systemic therapies for those with pancreatic (p)NET or foregut NET. Patients who had clinical disease progression, despite other treatments were also suitable. An adequate functional status (Performance score of ECOG 0–2) was required. In borderline cases, a ward assessment was performed to determine self-caring capabilities of the patient. Patients required an adequate renal function (eGFR >65 ml per min per 1.73 m2; if EGFR <65, a radionuclide GFR of >50 ml per min per 1.73 m2) and bone marrow reserve as per European Neuroendocrine Tumour Society guidelines.10
In addition, patients being retreated with PRRT would have to have previously demonstrated a reasonable response to treatment defined as a time to progression at least 1 year after completion of the last cycle of PRRT1.
Treatment protocol
Each cycle of 177Lu-DOTATATE consisted of approximately 7.4 GBq, which was either labelled in-house (2011–2013) or acquired from Imaging Equipment Limited. Each cycle of 90Y-DOTATATE was labelled in-house. The usual administered activity was 3.2 GBq. If patients had liver only/predominant disease, one of the three cycles would include an intra-arterial administration of 2.2 GBq of 90Y-DOTATATE through the common/branch hepatic artery.
Administration of the radiopharmaceutical was performed over approximately 30 min. Patients stopped their long acting SSAs at least 4 weeks, and their short acting SSAs at least 24 h, prior to treatment. An intravenous infusion containing 25 g each of lysine and arginine in saline for renal protection was administered over 4–6 h, commencing 30 min before administration of radiopeptide. Patients with borderline reduced renal function received additional renal protection with gelofusine 500 ml over 4 h. The patient is retreated after 10–12 weeks if no intervening contraindications have developed, up to a maximum of four cycles (three cycles for 90Y-DOTATATE). Patients are restaged after two cycles of treatment. If the disease is stable or has responded according to response evaluation in solid tumours, the patient continued with further cycles of PRRT, whilst if there has been disease progression PRRT is discontinued and different treatments considered. Renal impairment, thrombocytopaenia and clinical deterioration are other reasons not to proceed with further cycles.
Following the completion of PRRT, patients are followed up with cross-sectional imaging at 3–4 month intervals. If there is suggestion of disease progression, rediscussion at the multidisciplinary team meeting occurred including consideration of retreatment with PRRT (along with up to date SSR imaging).
PRRT2 was administered in patients who had progressed on PRRT1. Most patients were retreated with two cycles of 90Y-DOTATATE or 177Lu-DOTATATE.
Data collection and response assessment
Patient and disease data were collected from hospital electronic records and imaging, data were not complete for all variables due to patients being partially managed at other hospitals.
Hepatic tumour load was determined by assessment of the most recent imaging (either CT or MRI) preceding treatment and classified as 0, <25, 25–50 and >50% of the volume of the liver taken up with metastases. PFS was calculated from date of start of PRRT to date of progression defined as either clinical (worsening symptoms or general deterioration), radiological (either at mid or end of treatment restaging) or death (where there was no documentation of prior deterioration). For patients who had not progressed, the date of most recent imaging showing stable disease (SD) was recorded and used in the statistical analysis.
Full blood count and urea and electrolytes pre- and post-treatment were used to identify treatment related toxicity. This was defined according to the Common Toxicity Criteria Adverse Events v. 4 (CTCAE). In addition, we determined that a drop of >15% from baseline was also necessary in order to ensure any change in Common Toxicity Criteria Adverse Events toxicity classification was due to the therapy rather than non-significant fluctuations in blood result parameters (e.g. eGFR dropping from 63 to 59 ml min–1 m– 2). Clinic letters and blood results were reviewed to determine whether the long-term haematological complications or renal toxicity occurred.
Data analysis
Kaplan–Meier (K–M) survival plots of time to progression were performed for the initial course of treatment and for the retreatment group. K–M overall survival (OS) plots from the time of initiating PRRT were also performed.
Multivariate cox regression analysis was performed on pre-defined variables to determine if they were predictive of PFS following the salvage PRRT treatment, and to determine if there were any factors associated with bone marrow toxicity.
Results
Patient and tumour characteristics are listed in Table 1. The presence and extent of liver and bone metastases that were present at the imaging prior to commencing PRRT2 is documented.
Table 1.
Patient and tumour characteristics
| Variable | Number | |
| Gender | Male | 22 (47%) |
| Female | 25 (53%) | |
| Age | Range (median) | 23–80 (54) |
| <50 | 17 (36%) | |
| 50–59 | 14 (30%) | |
| 60–69 | 12 (26%) | |
| >69 | 4 (9%) | |
| Location of primary | Midgut | 21 (45%) |
| Pancreas | 15 (32%) | |
| Hindgut | 2 (4%) | |
| Lung | 3 (6%) | |
| Unknown | 2 (4%) | |
| Other (neuroectodermal and MTC) | 4 (9%) | |
| Tumour Grade | Grade 1 | 14 (30%) |
| Grade 2 | 17 (36%) | |
| Grade 3 | 4 (9%) | |
| Unavailable | 12 (26%) | |
| Hepatic tumour load (% of liver with metastatic involvement) | 0% | 5 (11%) |
| 0–25% | 13 (29%) | |
| 25–50% | 18 (40%) | |
| >50% | 9 (20%) | |
| Presence of bone metastases | Yes | 19 (42%) |
| No | 26 (58%) | |
| Previous treatments | SSAs | 40 (85%) |
| Resection of primary | 22 (47%) | |
| Chemotherapy | 19 (40%) | |
| Radiotherapy | 8 (17%) | |
| Liver targeted treatments | 15 (32% | |
| Interferon/sunitinib/everolimus | 1 (2%) |
MTC, medullary thyroid cancer; SSAs, somatostatin analogues.
PRRT1
45/47 of the patients were initially treated with 90Y-DOTATATE. Of these 45, 41 had three cycles of 90Y-DOTATATE, 1 patient had four cycles and 3 patients two cycles. The median cumulative activity was 7661 MBq (Range 2227–9429 MBq). Two patients initially treated with 177Lu-DOTATATE (four cycles). The median cumulative activity for patients initially treated with 177Lu-DOTATATE was 30,299 MBq.
On radiological response assessment, 37/47 patients had SD and 10/47 patients had PR to PRRT. All 47 patients progressed after initial PRRT1. Figure 1 shows the K–M estimate for PFS together with the 95% CIs for the whole cohort. The median PFS following PRRT1 was 30 months [95% CI (26.9–36.6 months)].
Figure 1. .
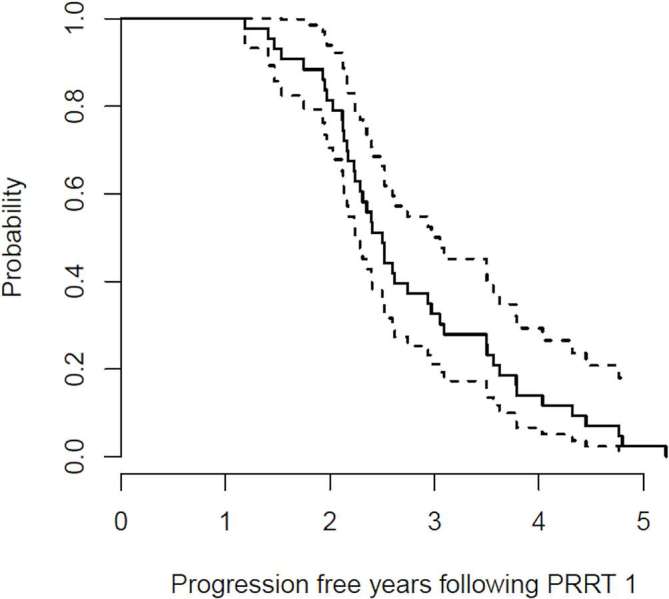
Kaplan-Meier estimate for PFS together with 95% confidence intervals following PRRT1; Median PFS = 30 months.
Toxicity data were present in 46/47 patients. Two patients developed Grade 1 renal toxicity. 3/47 patients had bone marrow toxicity, with 1 of these patients having Grade 3 toxicity. No Grade 4 toxicity was observed. No prolonged bone marrow suppression was recorded.
PRRT2
The average time to commencement of PRRT2 after completion of the last cycle of the PRRT1 was 2.6 years (range 1.5–7.1 years).
At PRRT2, 29 patients were treated with 90Y-DOTATATE and 18 patients with 177Lu-DOTATATE. The median number of cycles administered was 2 (range 1–4, mean 2.3). The median cumulative active activity for 90Y-DOTATATE was 6018 MBq and for 177Lu-DOTATATE was 14,884 MBq.
44/47 patients had evaluable response data. At the 3 month post PRRT radiological response assessment, 7 patients had PR, 26 patients had SD, 10 patients had radiological PD and 1 patient had clinical PD. The disease control rate was 33/44 (75%).
At the time of analysis, 41/44 had progressed. The median PFS after PRRT2 was 17.5 months [95% CI (11–23.8 months)] (Figure 2). There was no statistically significant difference in the median PFS dependent on the choice of radiopharmaceutical: median PFS for 177Lu-DOTATATE = 17.2 months, median PFS for 90Y-DOTATATE = 17.3 months.
Figure 2. .
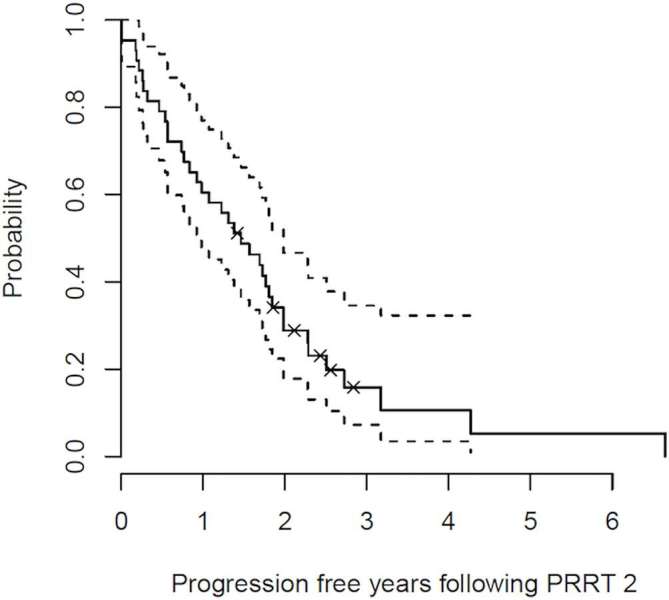
Kaplan-Meier estimate for PFS together with 95% confidence intervals following PRRT2; Median PFS = 18 months.
A multivariate analysis was performed to determine if there were any factors impacting on treatment response (Table 2). Variables studies included sex, age, length of PFS following the first course of PRRT, grade of tumour, site of tumour primary, and extent of liver metastases.
Table 2.
Multivariate analysis of factors associated with PFS at PRRT2
| Variables | Categories | No. of patients | No. of patients progressed | Median PFS (months) | Median PFS 95% CI | p-value |
| Sex | F | 22 | 19 | 19.7 | (11.8–32.7) | |
| M | 21 | 18 | 14.7 | 6.4-not reached | 0.012 | |
| Grade of tumour | G1 | 13 | 11 | 20.7 | 11.1-not reached | |
| G2 | 16 | 14 | 13.2 | 8.9- not reached | 0.053 | |
| G3 | 4 | 3 | 15.3 | 3.8- not reached | 0.841 | |
| Site of primary NET | Midgut | 20 | 19 | 17.2 | (6.4–27.5) | |
| Pancreas | 13 | 11 | 20.3 | 14.7-not reached | 0.154 | |
| Hindgut | 2 | 1 | 6.7 | 6.7-not reached | 0.355 | |
| Other | 8 | 6 | 10.8 | 6.8-not reached | 0.625 | |
| Percentage liver lesions | No metastases | 5 | 3 | 12.8 | 8.9-not reached | |
| 0–25% metastases | 12 | 10 | 21.4 | 14.7-not reached | 0.031 | |
| 25–50% metastases | 18 | 17 | 17.2 | (5.5–32.7) | 0.041 | |
| >50% metastases | 6 | 6 | 12.1 | 2.1-not reached | 0.007 | |
| PFS post-PRRT1 | PFS < 30 months | 21 | 19 | 12.8 | (6.8–21.2) | |
| PFS > 30 months | 20 | 17 | 22.9 | (18.7–32.7) | 0.073 | |
| Age | <55 | 23 | 20 | 17.5 | (11.8–23.8) | |
| 55+ | 20 | 17 | 18.4 | 6.7-not reached | 0.123 |
CI, confidence interval; NET, neuroendocrine tumour; PFS, progression-free survival; PRRT, peptide receptor radionuclide therapy.
The variables that were found to be significant on multivariate analysis were sex and extent of liver metastases. Males were associated with a worse survival (p = 0.012). The extent of liver metastases was also associated with worse survival, with patients with >50% metastases in particular associated with worst survival (p = 0.007).
Patients with a longer PFS after the first course of treatment appeared to have a longer PFS after PRRT2 (median PFS 22.9 vs 12.8 months). However, this was not statistically significant. Grade 2 tumours appeared to have worse PFS than Grade 1 (median PFS 13.2 vs 20.7 months), although not reaching statistical significance.
Toxicity PRRT2
At PRRT2, 41 patients had evaluable toxicity data. There were 7/41 (17.2%) patients who developed renal toxicity. Of these, 6 (14.6%) patients had Grade 1 renal toxicity and 1 (2.4%) patient had Grade 4 renal toxicity. The patient with Grade 4 toxicity had a pancreatic NET, was hypertensive and diabetic and had an initial three cycles of 90Y-DOTATATE (cumulative activity 3.3 GBq). No immediate renal toxicity occurred and the patient had a PR to treatment. 3 years later, he progressed and was treated with six cycles of streptozocin/5-fluorouracil/carboplatin and developed grade renal toxicity whilst on treatment. He subsequently developed Grade 3 renal impairment. It was decided to proceed with further cycles of 90Y-DOTATATE (cumulative activity 8.8 GBq) following further progression. He responded well with a PR. However, his renal impairment deteriorated, resulting in him being dialysis dependent. His renal failure was probably a result of the combination of treatments on a background of hypertension and diabetes.
There were 17/41 (41%) patients who developed bone marrow toxicity. Of these, 11 patients had Grade 1 toxicity, 4 patients had Grade 2 toxicity (1 prolonged bone marrow suppression) and 2 patients (4.8%) had Grade 3 toxicity (1 of these patients had a previous Grade 3 toxicity at PRRT1). No Grade 4 toxicity was seen. One patient developed myelodysplastic syndrome (MDS). This patient had a metastatic pancreatic NET and in total was treated with five cycles of 90Y-DOTATATE with a cumulative activity of 15 GBq. He had also been treated with chemotherapy (2 × 3 cycles of 5-fluorouracil/cisplatin/streptozocin) and α-interferon. He developed MDS 8 months after completion of the last cycle of 90Y-DOTATATE.
Greater bone marrow toxicity was seen with 90Y used at PRRT2 (41% vs 28% with 177Lu) and in patients with existing bone metastases (47% vs 31% with no bone metastases). However, there were no factors associated with increased risk of bone marrow toxicity on multivariate analysis (Table 3).
Table 3.
Multivariate analysis of factors associated with any bone marrow toxicity at PRRT2
| Variable | No. of patients | BM toxicity | p-value | |
| Chemotherapy | Yes | 19 | 7 (36.8%) | 0.949 |
| No | 28 | 10 (35.7%) | ||
| Hypertension | Yes | 15 | 6 (40%) | 0.767 |
| No | 32 | 11 (34.4%) | ||
| Bone metastases pre-PRRT 2 | Yes | 19 | 9 (47.4%) | 0.371 |
| No | 26 | 8 (30.8%) | ||
| Type of PRRT2 | 90Y | 29 | 12 (41.4%) | 0.451 |
| 177Lu | 18 | 5 (27.8%) |
PRRT, peptide receptor radionuclide therapy.
Overall survival data
39/47 patients had OS data, with 8 patients lost to follow up (several of these were patients from abroad). 29/39 patients have died at the time of analysis. The median OS from the time of first starting PRRT was 71 months [95% CI (57–89 months)] (Figure 3).
Figure 3. .
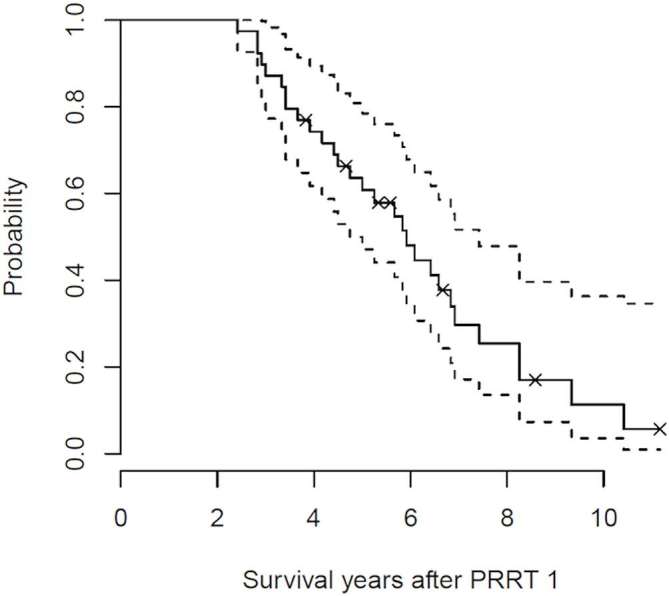
Kaplan-Meier estimate for OS together with 95% confidence intervals from the time of commencement of first cycle of PRRT; Median OS = 71 months.
Discussion
PRRT is an established therapeutic option and has increasingly been utilised in patients with non-resectable NETs. However, there is less published research into its use in the retreatment setting. This retrospective study of 47 patients who underwent retreatment with PRRT has shown that this is an effective treatment with reasonable PFS. It has also demonstrated that possible side effects of nephrotoxicity and haematotoxicity are largely mild and self-limiting.
We demonstrated a median PFS after PRRT1 of 30 months. Following PRRT 2, there was a median PFS of 18 months which is in line with other published data on retreatment outcomes. In other PRRT retreatment studies, Severi et al12 demonstrated a PFS of 22 months, Sabet et al13 demonstrated a PFS of 13 months and Van Essen et al14 demonstrated a PFS of 17 months.
Van Essen et al highlighted the possible positive impact of PRRT retreatment but did highlight that antitumour effects appear to be less than initial PRRT treatment.14 This is similar to our findings. There are several possible reasons for the lower PFS at PRRT2. This may be a degree of tumour dedifferentiation over time with reduced expression of SSR (however, all patients had a positive SSR scan prior to PRRT2). It is possible that tumours have acquired radioresistance in patients that have been previously treated with chemotherapy/radiotherapy.15 Radiation-induced vascular damage increases tumour hypoxia, hypoxic tumours are less likely to respond to radiotherapy. It is also probable that patients would have suboptimal absorbed dose. Invariably patients would have more extensive disease at PRRT2. This coupled with the reduced amount of administered cycles, may result in reduced tumour absorbed dose. Despite the reduction in PFS following PRRT2 compared to PRRT1, this represents valuable time for patients with metastatic NETs many of whom have limited other treatment options.
Our multivariate analysis has highlighted statistically significant factors associated with reduced PFS post-treatment. Two variables, male gender and burden of liver metastases have been shown to reduce PFS in PRRT retreatment. Our work has demonstrated that the higher the proportion of hepatic metastases the worse the retreatment response.
Patients with hepatic metastases of over 50% on imaging were most strongly statistically significant for short PFS. This impact of hepatic disease is in line with previous work which has shown tumour burden and number of liver metastases to be important prognostic factors.12 Hepatic NET metastases are the most common cause of death for patients with gastro-entero-pancreatic-NETs16 and controlling hepatic disease is one of the most important aspects in the management of metastatic disease. Patients with larger volume liver metastases may have reduced absorbed dose due to larger volume of disease may thus have been undertreated.
Male sex was also strongly statistically significant in poor response to retreatment. A large epidemiological study of over 35,000 patients from the USA also found that being male was associated with worse OS (114 vs 145 months for females).1
Previous work has shown duration of PFS after PRRT1 to be statistically significant factor in duration of PFS after PRRT2 (Sabet). In our study, although there was a difference in median PFS after PRRT2 in patients with a prolonged PFS on PRRT1 vs those without (23 vs 13 months), this was not a statistically significant variable on multivariate analysis.
All patients had PD prior to being treated with PRRT2. Disease control rates achieved at PRRT2 was 75% which is similar to previous prospective work.12 For the 39 patients where follow-up data were available, OS from the time of commencement of PRRT1 was 71 months. This OS result is favourable compared with other published data.5, 17
Previous prospective research showed significantly increase in creatinine and drop in haemoglobin during PRRT retreatment in a small sample size of patients.18 In our cohort, only a small number of patients had lasting side effects and those patients were found to have multiple comorbidities prior to treatment. One patient suffered Grade 4 renal toxicity following PRRT2. This patient was a diabetic, hypertensive patient whose renal function deteriorated following chemotherapy and further PRRT2 such that he became dialysis dependent. Our experiences during retreatment with PRRT have once again highlighted the importance of patient selection for PRRT retreatment and renoprotection during treatment.
A greater number of our sample suffered bone marrow toxicity at PRRT2. However, only 5% of patients developed Grade 3 toxicities and no patient developed Grade 4 toxicity. One patient developed MDS. It is thought that this patient’s concurrent chemotherapy and αinterferon were contributing factors to this significant side effect. More patients developed bone marrow toxicity with 90Y PRRT2 (41 vs 28% with 177Lu) and with existing bone metastases (47 vs 31% with no bone metastases). Multivariate analysis showed that no one factor alone proved a statistically significant risk factor for haematological toxicity. This work has highlighted the need for close post-treatment monitoring of blood results in order that patients have potential side effects managed accordingly.
Limitations to our work include incomplete follow up of three patients who either returned to their home country from abroad for ongoing follow up or returned to their local centre for post-treatment monitoring. Despite efforts to obtain this data, treating physicians were unable to obtain up to date medical records.
Conclusion
These data suggest that PRRT retreatment is safe and offers patients, who progressed following initial PRRT course, a prolonged PFS. PRRT retreatment appears to be an effective therapeutic option for metastatic progressive NET patients many of whom may not have many other effective treatment options available. Extra consideration is needed in patients with multiple comorbidities, as they may be at greater risk of renal and haematological toxicity. Male sex and high burden of liver metastases seem to be associated with shorter PFS following a PRRT retreatment course.
Contributor Information
Emily Vaughan, Email: emily.vaughan@nhs.net.
Joseph Machta, Email: josephmachta@gmail.com.
Martin Walker, Email: m.walker06@imperial.ac.uk.
Christos Toumpanakis, Email: c.toumpanakis@ucl.ac.uk.
Martyn Caplin, Email: m.caplin@ucl.ac.uk.
Shaunak Navalkissoor, Email: s.navalkissoor@nhs.net.
REFERENCES
- 1. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26: 3063–72. doi: 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 2. Modlin IM, Moss SF, Oberg K, Padbury R, Hicks RJ, Gustafsson BI, et al. Gastrointestinal neuroendocrine (carcinoid) tumours: current diagnosis and management. Med J Aust 2010; 193: 46–52. [DOI] [PubMed] [Google Scholar]
- 3. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med 2017; 376: 125–35. doi: 10.1056/NEJMoa1607427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011; 29: 2416–23. doi: 10.1200/JCO.2010.33.7873 [DOI] [PubMed] [Google Scholar]
- 5. Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008; 26: 2124–30. doi: 10.1200/JCO.2007.15.2553 [DOI] [PubMed] [Google Scholar]
- 6. Bushnell DL, O'Dorisio TM, O’Dorisio MS, Menda Y, Hicks RJ, Van Cutsem E, et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol 2010; 28: 1652–9. doi: 10.1200/JCO.2009.22.8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cwikla JB, Sankowski A, Seklecka N, Buscombe JR, Nasierowska-Guttmejer A, Jeziorski KG, et al. Efficacy of radionuclide treatment DOTATATE Y-90 in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs): a phase II study. Ann Oncol 2010; 21: 787–94. doi: 10.1093/annonc/mdp372 [DOI] [PubMed] [Google Scholar]
- 8. Pencharz D, Walker M, Yalchin M, Quigley AM, Caplin M, Toumpanakis C, et al. Early efficacy of and toxicity from lutetium-177-DOTATATE treatment in patients with progressive metastatic NET. Nucl Med Commun 2017; 38: 593–600. doi: 10.1097/MNM.0000000000000685 [DOI] [PubMed] [Google Scholar]
- 9. Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging 2015; 42: 5–19. doi: 10.1007/s00259-014-2893-5 [DOI] [PubMed] [Google Scholar]
- 10. Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O’Dorisio MS, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2013; 40: 800–16. doi: 10.1007/s00259-012-2330-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabet A, Ezziddin K, Pape UF, Ahmadzadehfar H, Mayer K, Pöppel T, et al. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med 2013; 54: 1857–61. doi: 10.2967/jnumed.112.119347 [DOI] [PubMed] [Google Scholar]
- 12. Severi S, Sansovini M, Ianniello A, Bodei L, Nicolini S, Ibrahim T, et al. Feasibility and utility of re-treatment with 177Lu-DOTATATE in GEP-NENs relapsed after treatment with 90Y-DOTATOC. Eur J Nucl Med Mol Imaging 2015; 42: 1955–63. doi: 10.1007/s00259-015-3105-7 [DOI] [PubMed] [Google Scholar]
- 13. Sabet A, Haslerud T, Pape UF, Sabet A, Ahmadzadehfar H, Grünwald F, et al. Outcome and toxicity of salvage therapy with 177Lu-octreotate in patients with metastatic gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2014; 41: 205–10. doi: 10.1007/s00259-013-2547-z [DOI] [PubMed] [Google Scholar]
- 14. van Essen M, Krenning EP, Kam BL, de Herder WW, Feelders RA, Kwekkeboom DJ. Salvage therapy with 177Lu-octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumors. J Nucl Med 2010; 51: 383–90. doi: 10.2967/jnumed.109.068957 [DOI] [PubMed] [Google Scholar]
- 15. Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer 2015; 15: 409–25. doi: 10.1038/nrc3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salvia AL, Partelli S, Tampellini M, Tamburrino D, Falconi M, Scagliotti GV, et al. Management of hepatic metastases of well/moderately differentiated neuroendocrine tumors of the digestive tract. J Cancer Metastasis Treat 2016; 2: 294–303. doi: 10.20517/2394-4722.2016.37 [DOI] [Google Scholar]
- 17. Villard L, Romer A, Marincek N, Brunner P, Koller MT, Schindler C, et al. Cohort study of somatostatin-based radiopeptide therapy with [90Y-DOTA]-TOC versus [90Y-DOTA]-TOC plus [177Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol 2012; 30: 1100–6. doi: 10.1200/JCO.2011.37.2151 [DOI] [PubMed] [Google Scholar]
- 18. Forrer F, Uusijärvi H, Storch D, Maecke HR, Mueller-Brand J. Treatment with 177Lu-DOTATOC of patients with relapse of neuroendocrine tumors after treatment with 90Y-DOTATOC. J Nucl Med 2005; 46: 1310–6. [PubMed] [Google Scholar]


