Abstract
Alterations at the molecular level are a hallmark of cancer. Prostate cancer is associated with the overexpression of prostate-specific membrane antigen (PSMA) in a majority of cases, predominantly in advanced tumors, increasing with the grade or Gleason’s score. PSMA can be selectively targeted using radiolabeled PSMA ligands. These small molecules binding the PSMA can be radiolabeled with γ-emitters like 99mTc and 111In or positron emitters like 68Ga and 18F for diagnosis as well as with their theranostic pairs such as 177Lu (β-emitter) or 225Ac (α-emitter) for therapy. This review summarizes the theranostic role of PSMA ligands for molecular imaging and targeted molecular radiotherapy, moving towards precision oncology.
Introduction
Theranostics in the context of nuclear medicine aims to identify the appropriate molecular targets in neoplasms, so that the optimal ligands and radionuclides with favorable labelling chemistry can be selected for personalized management of disease, taking into consideration the specific patient.1, 2 Personalized medicine improves tailoring and timing of preventive and therapeutic measures by utilizing biological information and biomarkers at the level of molecular disease pathways, genetics, proteomics, and metabolomics.3 Theranostics using PET/CT (or PET/MRI) as an in vivo companion diagnostic for decision-making and monitoring of therapy with radiolabeled ligands, is part and parcel of personalized medicine.2, 4 The successful application of 68Ga for diagnosis, and 177Lu and 90Y for radionuclide therapy using the same peptide for targeting somatostatin receptors in neuroendocrine neoplasms, has paved the way to other indications of theranostics.1 The recently published results of the randomized controlled NETTER-1 trial revealed a fivefold improvement in response with 177Lu-DOTATATE (Lutathera™) compared with conventional treatment of gastroenteropancreatic neuroendocrine tumors.4
Using a ligand targeting, the prostate-specific membrane antigen (PSMA), which is overexpressed in a majority of prostate cancer (PCa) cells, enables effective molecular imaging and targeted radioligand therapy of PCa, with acceptable toxicity. The PSMA ligand labeled with a positron emitter like 68Ga/18F helps not only to select patients who are likely to benefit from the PSMA-radioligand therapy (PRLT) using later on the same ligand labeled with a βbeta emitter like 177Lu or an α-emitter like 225Ac, but also enables detection of recurrent and metastatic disease (staging), assessment of molecular response to therapy, and long-term follow-up after the initial diagnosis. In addition, pre- and/or post-therapeutic dosimetry ensures the optimum balance between risk and therapeutic benefit, and helps to predict toxicity.1
PCa is the second most common cancer in males worldwide and causes an estimated 90,000 deaths per year in Europe.5 The primary treatment for localized PCa is radical prostatectomy, following which salvage radiotherapy and lymphadenectomy are the options with a curative approach in patients with residual or recurrent PCa.6 However, 20–40% of the clinically localized PCa patients will present with rising prostatic-specific antigen (PSA) after surgery, which is referred to as biochemical recurrence.7–9 In fact, about 60% of patients with stage pT3 PCa have biochemical recurrence within 5 years of surgery, indicative of local tumor progression and/or metastatic disease. The appropriate time point to initiate a multimodal therapy is vital, since the course of biochemical recurrence after surgery varies, and does not necessarily correlate with clinical recurrence.10, 11
The conventional imaging modalities like CT and MRI have a limited role, especially when the PSA levels are low.12 Indeed, the current guidelines do not recommend additional imaging for staging low-risk PCa due to the poor accuracy of conventional imaging, e.g. in detecting small lymph nodes.13 Pelvic multiparametric MRI and abdominopelvic cross-sectional imaging are recommended in the staging of high-risk localized or locally advanced PCa. Multiparametric MRI has a high accuracy in PCa with higher Gleason score and a larger volume of disease, and is also used to guide biopsy.14, 15 However, its performance is limited in the identification of extraprostatic extension of disease. The guidelines recommend bone scan for the screening of metastases.13 But this is constrained, especially, by a lack of specificity and the inherent inability to detect extraosseous disease.
Androgen deprivation therapy is an established treatment option after salvage surgery or radiotherapy. However, at some point of time, the disease continues to progress with rise of PSA under hormone therapy, indicating castration resistance. Metastatic castration-resistant prostate cancer (mCRPC) has a poor prognosis and is responsible for nearly all PCa-specific deaths.16 Abiraterone acetate and enzalutamide, targeting the androgen receptor (AR) signaling, have demonstrated encouraging results in mCRPC.17–19 Randomized controlled clinical trials in mCRPC have demonstrated a small benefit in the overall survival (OS) with taxane-based chemotherapy and the therapy of skeletal metastases with the α-emitter 223Radium.20–23
Molecular imaging
The unmet need in PCa has been to identify local recurrence, lymph node, bone and visceral metastases with high sensitivity and specificity in patients with biochemical relapse after initial curative therapy. Molecular imaging with PET/CT or PET/MRI has a great potential to counter the drawbacks of conventional imaging and consequently improve the overall diagnostic accuracy.
18F-FDG PET/CT has limitations in the evaluation of PCa.24 However, a recent study concluded that assessment of glycolytic activity in addition to the AR expression, had prognostic implications in mCRPC.25 Most of the mCRPC lesions express ARs, consistent with initial benefit of androgen receptor-signaling inhibitors. On a patient basis, 49% had at least one FDG-positive lesion, the imaging phenotype with the most negative effect on survival, possibly due to androgen receptor-signaling inhibitors resistance.25 11C-choline PET/CT has demonstrated a potential in the therapy response assessment after chemotherapy in mCRPC.26 A meta-analysis revealed a pooled detection rate of 62% for biochemical recurrence in PCa, although the detectability was poor when the PSA levels were lower (<2 ng ml−1).27 Primary staging with choline PET/CT is limited by the non-specific uptake in benign prostatic hyperplasia.28
PSMA is a Type II transmembrane glycoprotein with an intracellular, transmembrane, and an extensive extracellular domain, which is overexpressed in PCa, especially in poorly differentiated mCRPC.29–31 Radiolabeled monoclonal PSMA antibodies such as J591 have been demonstrated to have a role in PCa.32 However, their long half-life and poor tumor penetration represent a significant limitation of monoclonal antibodies in imaging and therapy. On the other hand, the 68Ga labeled urea-based PSMA inhibitors have nearly ideal pharmacokientics.30, 33 The major uses of PET/CT using PSMA ligands in PCa are: detection of biochemical recurrence, primary staging, radioguided surgery, selection of patients and monitoring the response/follow up after PRLT. Most of the studies reported so far have been using 68Ga-PSMA PET/CT.
The pooled detection rates of biochemical recurrence for 68Ga-PSMA-11 PET/CT in a meta-analysis were found to be 58 and 76% for PSA levels of 0.2–1 and 1–2 ng ml−1, respectively.34 Afshar-Oromieh et al reported a detection rate of 88.1% on a patient basis in a retrospective study of 319 patients, with a sensitivity of 76.6% and a specificity of 100%.35 In a head-to-head comparison with 11C-/18F-choline, 68Ga-PSMA-11 demonstrated a superiority for the PET/CT detection of biochemical recurrence.36–38 Many studies have revealed a higher detection rate for 68Ga-PSMA-11 PET/CT than any other imaging modality for PSA levels less than 0.5 ng ml−1.39 This enables an early and effective salvage treatment modality, e.g. lymphadenectomy or radiotherapy.
18F has lower mean positron energy than 68Ga, resulting in a higher intrinsic spatial resolution. In first-in-human studies by the group of Pomper, the two 18F-labeled tracers DCFBC and DCFPyL demonstrated a favorable dosimetry and biodistribution, as well as a superior efficiency for the detection of PCa.40–43 18F-DCFPyL PET/CT revealed additional lesions in 3 of 14 patients (21.4%), who had either negative or inconclusive findings on 68Ga-PSMA-11 PET/CT.44 More recently, Giesel et al published their findings using a 18F-labeled PSMA ligand PSMA-1007, demonstrating a lesion detectability as good as with 68Ga-PSMA PET/CT.45
PSMA PET/CT plays an important role in the primary staging, especially the detection of lymph node and distant metastases. 68Ga-PSMA-11 PET was found to be significantly better than cross-sectional imaging for lymph node staging in 130 patients with primary intermediate- to high-risk PCa.46 The specificity was greater than 95%, which was also confirmed by another study in patients who underwent salvage lymphadenectomy.47 The intraprostatic tumor could be localized by 68Ga-PSMA-11 PET/CT, and the findings also correlated with histopathology.48–50 The positive segments demonstrated a significantly higher uptake of 68Ga-PSMA-11 than the negative segments.48, 49 Combination of 68Ga-PSMA-11 PET and mpMRI in 53 intermediate-/high-risk patients revealed a significantly better performance than mpMRI or 68Ga-PSMA-11 PET alone, in terms of sensitivity and specificity for localization of tumor.51 Therefore, hybrid PET/mpMRI may enable an accurate image-guided biopsy of the most relevant area within the prostate.
68Ga-PSMA PET/CT could have significant impact on the therapy planning, e.g. standard or extended lymph node dissection and change in radiotherapy planning and systemic treatment (Figure 1).52–54 Radioguided surgery is feasible using pre-operative labeling of lymph node metastases with a γ-emitting PSMA-ligand (e.g. 111In-PSMA I&T), allowing detection and resection of very small metastatic lesions.55, 56
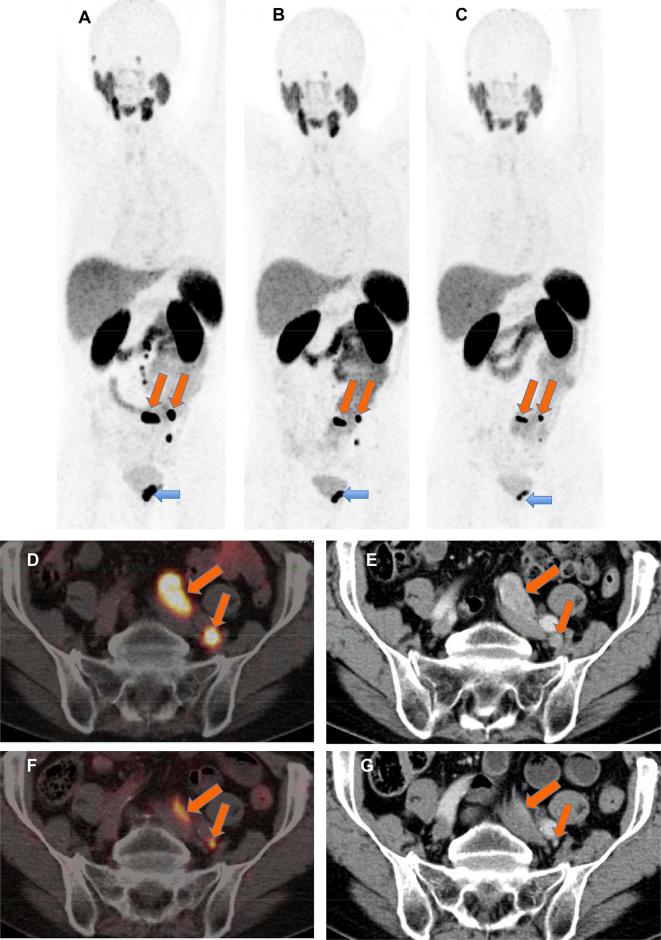
Figure 1. .
A 76-year-old patient with progressive mCRPC s.p. prostatectomy, pelvic lymphadenectomy, ADT as well as enzalutamide and 16 cycles of docetaxel chemotherapy. After 3 cycles of 177Lu-PRLT (cumulative administered activity 21.8 GBq), there was PR of the LNM (oblique arrows) as well as of the primary tumor (horizontal arrow). (A) 68Ga-PSMA MIP image before PRLT; (B) after two cycles and (C), after three cycles; (D,F) axial PET/CT images; (E,G) contrast-enhanced CT images. There was response to PRLT (PR) according to RECIST 1.1 as well as EORTC criteria (reduction of uptake) of the left iliac LNM (D,E, before PRLT; F, G, after three PRLT cycles) and of the primary tumor. ADT, androgen deprivation therapy; LNM, lymph node metastases; mCRPC, metastatic castration-resistant prostate cancer; PR, partial remission; PRLT, PSMA radioligand therapy; PSMA, prostate-specific membrane antigen.
Precision molecular radiotherapy using PSMA ligands
PRLT involves selective binding of a radioligand to PSMA, which is overexpressed in mCRPC, in order to increase tumor dose and to spare the normal tissue.57 Internalization and retention within the tumor cell are essential mechanisms for the cell-killing effect of this molecular radiotherapy (also called endoradiotherapy), which has the advantage of selectively targeting multiple metastases.58 PRLT is based on the principle of theranostics. The overexpression of PSMA in tumors can be confirmed by pre-therapeutic molecular imaging using 68Ga-PSMA PET/CT (Figure 2). Therefore, we treat what we see. 177Lu, being a γ-emitter, permits post-therapy imaging for the assessment of biodistribution, intensity of uptake as well as dosimetry. Hence, we can see what we treat. 68Ga-PSMA PET/CT can be used as a sensitive and specific imaging modality for patient selection, response assessment and follow up after PRLT.
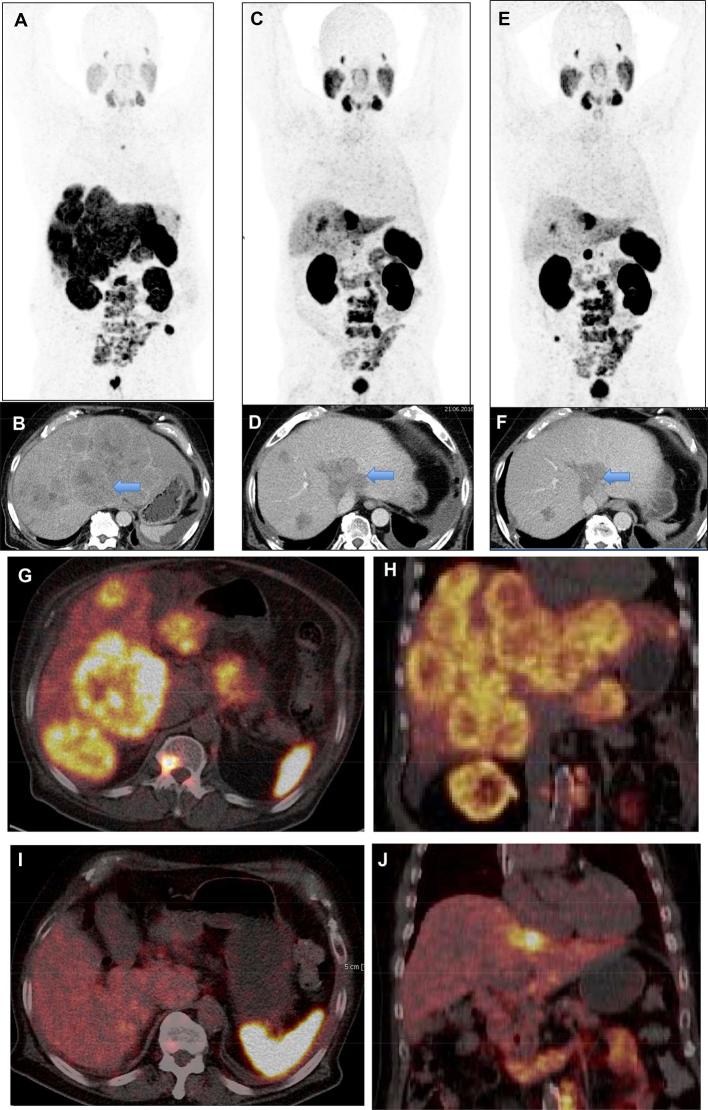
Figure 2. .
A 75-year-old patient with progressive mCRPC, s.p. prostatectomy, ADT and enzalutamide as well as docetaxel chemotherapy (wheel-chair bound with pain and low Karnofsky performance status) with disseminated osseous as well as extensive liver metastases (hepatomegaly) exhibiting very high PSMA expression. After 3 cycles of 177Lu-PSMA-radioligand therapy (cumulative administered activity 22.7 GBq), excellent response of the multiple liver metastases (PR according to RECIST 1.1 and EORTC criteria) with significant decrease in size of the lesions as well as of the whole liver occurred. The general condition of the patient improved remarkably (coming for follow-up studies driving his own car) and he lived for another 2 years after the last PRLT cycle. (A) 68Ga-PSMA MIP image before PRLT; (C), after two cycles and (E), after three cycles; (B,D,F) contrast-enhanced CT images. (G,I) axial PET/CT images; (H,J) coronal PET/CT images; (G,H) before PRLT; (I,J) after 3 PRLT cycles. ADT, androgen deprivation therapy; LNM, lymph node metastases; mCRPC, metastatic castration-resistant prostate cancer; MIP, maximum intensity protection; PR, partial remission; PRLT, PSMA radioligand therapy.
The patients currently receive PRLT under compassionate basis after treatment failure following chemotherapy and newer antihormonal agents, but also possibly after exhaustion of monoclonal antibody therapy or 223Ra-chloride therapy. Distant metastases with high PSMA expression confirmed on pre-therapy 68Ga-PSMA PET/CT, and progressive disease despite extensive previous treatments, are currently the essential inclusion criteria, as stated in the consensus recommendations of the German society of nuclear medicine, which were published in 2016.59
Dosimetry
Individualized dosimetry is imperative for precision molecular radiotherapy using 177Lu-PSMA. The kinetics of a certain ligand varies in patients and depends on a number of factors like renal function and tumor load, to name a few. There is additionally a significant intrapatient variability due to tumor responses and varying tumor loads between different therapy cycles.60 Therefore, for the direct comparison of the different PSMA ligands, patient-specific factors need to be considered. A differential analysis for PSMA I&T and PSMA-617 revealed comparable results for both ligands. In a dosimetry study of 18 patients receiving 1–4 PRLT cycles using 177Lu-PSMA-I&T, Okamoto et al demonstrated organ- and tumor-absorbed doses comparable to 177Lu-PSMA-617.61 However, they found relatively constant doses among the four different treatment cycles, quite contrary to the results by our group.61, 62 Kabasakal et al also stressed the need for individual dosimetry based on the large inter-individual variation in a study using 177Lu-PSMA-617.63
The organs at risk are the salivary glands, lacrimal glands and the kidneys. The highest dose was demonstrated for the lacrimal glands (1–3.8 Gy GBq-1) followed by the salivary glands (0.5–1.4 Gy GBq-1) and then the kidneys (0.53–0.88 Gy GBq-1).57,61–67 In fact, the dosimetry with the red marrow was the most favorable (0.01–0.04 Gy GBq-1). Therefore, the threshold absorbed dose of 2 Gy to the red marrow for severe hematotoxicity, implies a maximal tolerated cumulative activity of at least 45 GBq.67, 68 Considering the threshold for renal toxicity, a cumulative activity of 40 GBq would be safe.69, 70 Whereas, a maximal dose limit of 45 Gy for salivary dysfunction would allow the administration of a cumulative activity of around 50 GBq of 177Lu-PSMA.71 However, it must be stressed that no universal dose limits have yet been defined for a molecular radiotherapy, and the ones mentioned above are only extrapolated from the external radiation therapy.
Adverse effects
The potential adverse effects to be kept in mind are hematological, renal and salivary gland toxicities. Overall, 177Lu-PRLT is tolerated well by all the patients with no severe acute or long-term adverse events. Short-lasting mild fatigue was the most common immediate side effect.60 Long-term side effects seem to be relatively mild with transient xerostomia, being a non-hematological side effect in about 5–10% of the patients, and grade 3/4 hematological toxicity being reported in a few cases.60,72–76
Docetaxel, the most commonly used chemotherapeutic agent in mCRPC, is associated with different adverse effects impairing also the quality of life.77 One or more serious adverse events were observed in 26% of the patients receiving docetaxel every 3 weeks including two (0.3 %) treatment-related deaths.78The most common severe (grade ≥3) side effects of the second-line chemotherapy with cabazitaxel were neutropenia, leukopenia, anemia, and thrombocytopenia, with neutropenia being the most common, in 82% of the patients. On the other hand, diarrhea was the most common non-hematological adverse event, seen in 47% of the patients. 18 patients (5%) died due to the side-effects.79 The most common adverse events with abiraterone include fluid retention/ edema, hypokalemia, hypertension, cardiac disorders, atrial fibrillation, and an increase in liver enzymes.80 On the other hand, fatigue, diarrhea, hot flashes, musculoskeletal pain, headache, cardiac disorder, seizure (<1%), and myocardial infarction (<1%) were associated with enzalutamide.81
In a systematic review of third-line treatment and 177Lu-PRLT, G3-4 hematological toxicities were reported in about 2% of the patients undergoing PRLT.82 On the other hand, the first study of 177Lu-PSMA I&T reported only G1-2 anemia/pancytopenia and no G3-4 hematological toxicity.57 Indeed, renal insufficiency may also increase the risk due to a higher circulation time and hence dose to the bone marrow. The risk of development of hematotoxicity increases with extensive bone marrow involvement and previous chemotherapy or 223Ra-treatment.60, 65 Long-term low-dose concept, i.e. fractionation, may be beneficial to avoid/minimize bone marrow toxicity.
Since 2013, we have not seen any clinically significant nephrotoxicity in long-term follow-up of over 200 patients treated with up to 12 cycles of PRLT, even in the more than 15 patients with a single functioning kidney.83 Yordanova et al found elevated cystatin C in 32/55 patients (58%); however, 14 of who already had elevation of cystatin C before treatment. The renal function significantly correlated with age, hypertension and prior renal disease.84 The renal specific PSMA-binding can be blocked by PMPA (2- (phosphonomethyl)pentanedioic acid), a PSMA-inhibitor, which has been validated in pre-clinical studies.85 But due to its lack of availability and also the possibility of concurrent blockade within the tumor, this compound is not in routine clinical use.
Reversible xerostomia has been reported in about 5–10% of patients treated with 177Lu-PRLT, and is likely to be caused by high PSMA-specific binding of the tracer in the salivary glands.60,72–76 This was objectively assessed by Scarpa et al, who found a significant reduction in the SUVmax on 68Ga-PSMA PET/CT as well as a decrease in the volume of the salivary glands after PRLT.67 The SUVmax on 68Ga-PSMA PET/CT decreased on cooling of the glands.86 A possible significant breakthrough for salivary gland protection, especially crucial in the context of targeted α therapy using 225Ac-PSMA, was the demonstration of reduced ligand uptake 45 days after injection of botulinum toxin into the salivary gland unilaterally.87 The SUVmean on Ga-68 PSMA PET/CT in the injected parotid gland showed a highly significant decrease of up to 64% compared with the other side. The vascularization of the salivary glands on Doppler was demonstrated to remained unchanged after botulinum use by Coskun et al.88 This implies that additional mechanisms might be playing part in the action of Botulinim toxin, probably a post-denervation atrophy, causing a decrease in PSMA expression.89
Efficacy
The excellent tumor response is attributable to the high doses delivered to metastases based on the specific 177Lu-PSMA tumor uptake. High uptake can be demonstrated pre-therapy on 68Ga- PSMA PET/CT, which is an important pre-requisite for PRLT. PSMA PET/CT, therefore, plays an important role in the selection of patients for PRLT. Tumor doses exceeding 50 Gy and ranging up to 500 Gy have been reported.60, 67 Significant PSA decline (by ≥50%) was observed in 30–60% of the patients.57,60,72–76 von Eyben et al noted that177Lu-PSMA RLT caused a best decline of PSA ≥50% twice as often as the third-line treatment with a higher frequency of objective remission as well as fewer side effects than third-line treatment.82 Patients undergoing PRLT tended to live longer than patients given third-line treatment (median of 14 months v s 11 months), but the difference was not statistically significant. Third-line treatment was stopped more often due to adverse effects.
68Ga-PSMA PET/CT is a very sensitive and specific modality for the early assessment of response in comparison with the morphological imaging like CT, since molecular response precedes morphological changes (Figure 3). Lymph node metastases of mCRPC responded better to PRLT than bone metastases.60 This may be explained by a higher and more uniform absorbed radiation dose by lymph node metastases, which—in general—exhibit a higher uptake (SUV) on 68Ga-PSMA PET/CT as compared to bone lesions. In addition, the biological differences in radiation sensitivity might be an influencing factor. 68Ga-PSMA PET/CT is also superior in response assessment of skeletal metastases compared to CT alone, in which the actual size of the osteoblastic metastases is difficult to measure and change in size is difficult to appreciate.
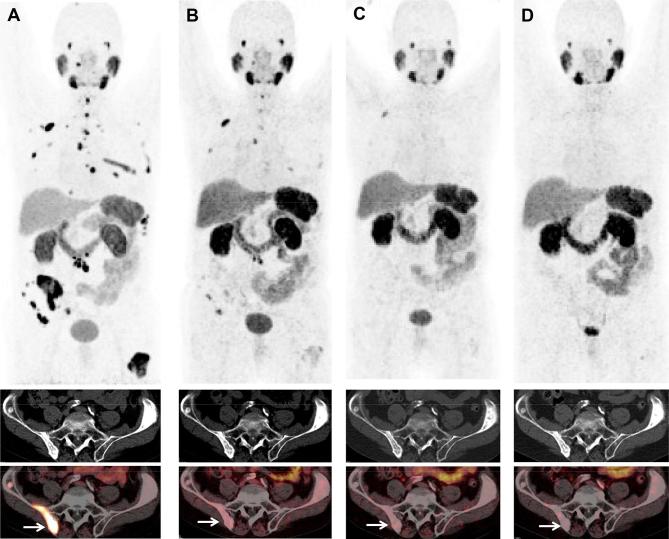
Figure 3.
A 77-year-old patient (first diagnosis in 1998) with progressive mCRPC and initial osseous metastases, s.p. orchiectomy, ADT and abiraterone, repeated external beam radiotherapy and chemotherapy with docetaxel and cabazitaxel. After 3 cycles of 177Lu-PSMA PRLT (cumulative administered radioactivity 12.9 GBq) between October 2015 and March 2016, the patient experienced nearly complete remission (with no toxicity), persisting for 2 years after the last cycle. This patient presented with chronic renal insufficiency and G2 anemia before PRLT, which did improve (!) after PRLT. 68Ga-PSMA PET/CT images: (A), October 2015; (B) March 2016; (C), July 2016 and (D) December 2017; upper panel, MIP images; middle panel, CT images revealing no significant change over time in the osteosclerotic iliac bone lesions; lower panel, fused 68Ga-PSMA PET/CT images after PRLT exhibiting a significant decrease in PSMA expression of the metastases (arrow showing metastasis in the right iliac bone), related to treatment response according to molecular imaging criteria. ADT, androgen deprivation therapy; mCRPC, metastatic castration-resistant prostate cancer; MIP, maximum intensity protection; PRLT, prostate-specific membrane antigen; PSMA, prostate-specific membrane antigen.
Only a few studies have reported response as assessed according to morphological (RECIST) or molecular imaging criteria.57, 60,67,74,90,91 Often, there is a discordance between the PSA levels and the PET/CT imaging findings implying that PSA alone is definitely not a reliable parameter for the assessment of therapy response.57, 60,65,67 Yadav et al demonstrated according to molecular imaging criteria, a complete remission (CR) in 2/6 patients, PR in 3/6 patients and stable disease (SD) in 1/6 patients.90 An overall assessment of bone and soft tissue metastases by Heck et al revealed a CR in 5% of patients, SD in 63% and PD in 32%.91 On the other hand, Fendler et al used RECIST to define response and found PR in 4/15, SD in 6/15, and PD 5/15 patients after two PRLT-cycles with177Lu- PSMA-617.74 Scarpa et. al showed an objective molecular and radiological response in half of the patients (5/10), wherein in addition to PR and SD, they also defined a mixed response as patients responding remarkably to PRLT at one metastatic lesion site, but developing new lesions at another site.67
In an analysis of 224 patients with metastatic PCa treated at our center since April 2013, we observed any PSA reduction in 157/224 (70 %) patients; 121/224 patients (54%) demonstrated a PSA decline by >50% and the best response was CR with undetectable PSA. The response according to RECIST was as follows: CR in 9 patients (4%), PR in 53 patients (23.7%), SD in 91 patients (40.6%), and PD in 71 patients (31.6%). According to the molecular imaging criteria, CR was noted in 10 patients (4.5%), PR in 78 (34.8 %), SD in 61 (27.2 %) and PD in 75 patients (33.5%). The median OS in all patients was 27 months and the median progression-free survival (PFS) was 11.5 months. First-line PRLT (with no previous hormone therapy) was associated with the longest OS (median not reached at 55 months, all 18 patients are alive). Chemotherapy-pre-treated patients lived significantly shorter (median OS 19 months) as compared to chemotherapy naive patients (38 months, p < 0.05). OS was also shorter in patients with previous 223Ra treatment (17 months). Addition of abiraterone or enzalutamide provided a significant prolongation of survival (40 months, p < 0.05). On the other hand, prior surgical or radiation treatment of primary tumor had no significant effect on the OS (30 months, p > 0.05). In patients demonstrating a PSA decline of >50% after at least two PRLT cycles, the OS was significantly longer (38 months). The significantly shorter OS reported by other groups might be due to use of PRLT as last line after exhaustion of other therapy options (newer antiandrogen agents, chemotherapy).72, 76 On the other hand, patients treated at an earlier stage of the disease had a favorable outcome in our study, leading to a significantly longer median OS.
Prognostic factors influencing the outcome of PRLT have been studied. In the retrospective multicenter German study, negative predictors were elevated alkaline phosphatase and the presence of visceral metastases, whereas the total number of therapy cycles were associated with a favorable outcome.75 Ahmadzadehfar et al found that patients with any PSA decline had a significantly longer OS than patients without PSA decline (68 vs 33 weeks).72 The median OS is significantly longer in patients without hepatic involvement, with high levels of albumin and Hb and low levels of aspartate aminotransferase and a decline in PSA levels of more than 14% was the most important response parameter with regard to OS.92 Bräuer et al noted that PSA decline after the first therapy cycle was associated with a longer OS and only alkaline phosphatase <220 U l−1 lended a longer PFS (median PSA-PFS 18 weeks).73 On the other hand, a lack of PSA response after the first therapy cycle should not preclude further treatments, since these patients did respond after the second or third therapy cycle.93
α-emitter labeled PSMA ligands
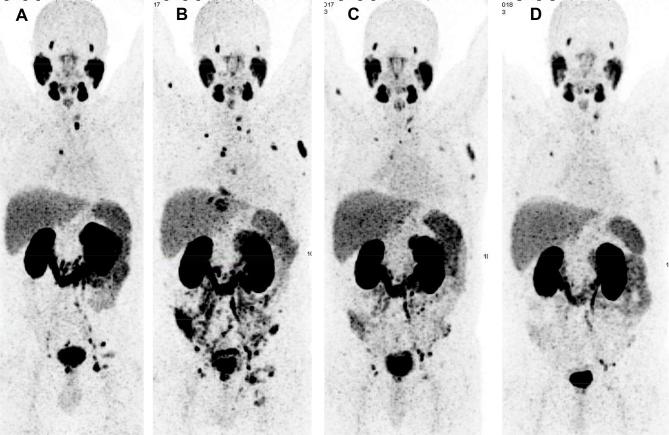
Despite the high doses delivered to tumors, approximately a fourth to a third of the patients are refractory to treatment with 177Lu-PSMA, presenting with primary progression under PRLT (Figure 4). Hematological toxicity tends to be frequent after 177Lu-PSMA in patients having disseminated bone and bone marrow involvement. The application of α-emitters with a short range and high linear energy transfer is a very promising option to overcome this limitation, as has been demonstrated by an excellent therapy response using 225Ac-PSMA in the above-mentioned two scenarios.94, 95
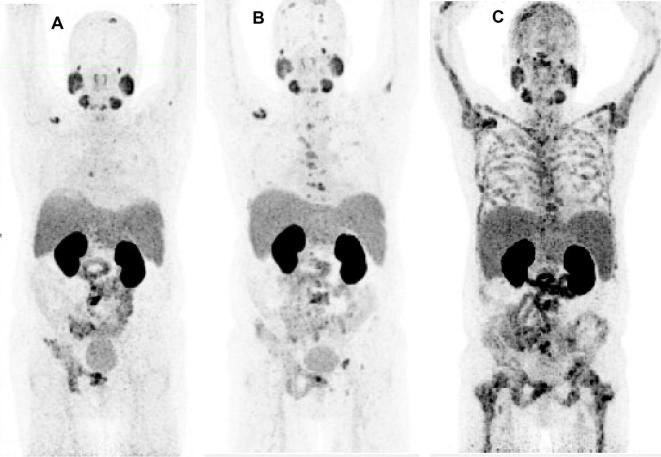
Figure 4. .
A 56-year-old patient with mCRPC, s.p. orchiectomy and 20 cycles of docetaxel/cabazitaxel chemotherapy (stopped due to severe anemia, neutropenia and fatigue). The patient underwent 177Lu-PSMA RLT as a last line therapy option, however, experiencing progression under PRLT, with disseminated bone and bone marrow involvement (which could be an indication for therapy with the α-emitter 225Ac-PSMA). (A)68Ga-PSMA PET/CT MIP image before PRLT; (B) after two cycles and (C) after four cycles of PRLT. mCRPC,metastatic castration-resistant prostate cancer; MIP, maximum intensity protection; PRLT, PSMA radioligand; PSMA, prostate-specific membrane antigen.
A first-in-human study reported by Sathekge et al demonstrated a marked response in one patient after two cycles of 213Bi-PSMA-617 using a cumulative activity of 592 MBq.96 In our experience using 213Bi-PSMA with the administered radioactivities per cycle [median 390 MBq (155–623 MBq)], no significant acute/subacute toxicity was noted and minor responses could be demonstrated. However, higher activities or more frequent cycles of Bi-213 PSMA might be required due to very short half-life of 213Bi (46 min) to achieve the desired response to therapy, which was constrained in our study due to the limited available activity and very high cost of the generator. 225Ac must be considered the first-choice isotope for PSMA-TAT in the setting of PCa.97
The efficacy and toxicity of salvage therapy using four different administered radioactivities of 225Ac- PSMA-617 was compared, namely 50 kBq kg-1 (n = 4), 100 kBqkg-1 (n = 4), 150 kBq kg-1 (n = 2), 200 kBq kg-1 (n = 4). Severe xerostomia was the dose-limiting toxicity for activities exceeding 100 kBq kg-1 per cycle. Therefore, an administered activity of 100 kBqkg-1 225Ac-PSMA-617 per cycle every 8 weeks was concluded to be a reasonable trade-off between toxicity and biochemical response.94
Kratochwil et al further retrospectively analyzed the remarkable antitumor activity of 225Ac-PSMA-617 therapy in 40 patients, demonstrating a promising duration of tumor control (median 9 months).95 A significant PSA response (>50%) was noted in 24/38 (63 %) of the patients. Five patients presented with enduring responses of >2 years. Xerostomia was the main reason to discontinue therapy (in 4/38 patients) as in the case of non-responders (in 5/38 patients). Hence, they concluded that further modifications of the treatment regimen regarding the adverse events were necessary to yield maximal response.
Personalized PRLT—on the way to precision medicine
A growing literature supports the use of PRLT in advanced PCa with potential benefit in OS and acceptable side-effects, when compared with the competing modalities. In clinical practice, we frequently observe good responses despite progression under extensive pre-treatments like newer antihormonal agents, 223Ra and chemotherapy, and poor performance status. The currently unmet need in metastatic PCa is to determine the optimal choice and sequencing of therapy. An ideal patient for PRLT could possibly be one receiving PRLT before chemotherapy with good baseline bone marrow function and a good baseline performance status.60
Patients can be effectively selected and the likely response to therapy predicted as well as assessed with molecular imaging (PET/CT or PET/MRI), making use of the same PSMA ligand. In contrast to this theranostic approach, a conventional chemotherapy regimen, e.g. is standardized not personalized and pre-defined by a previous randomized controlled clinical trial in a typical patient cohort. A personalized approach using the theranostic concept can be tailored towards an individual patient rather than the concept of “one size fits all”.
Various factors like adjusting the administered activity, number of cycles and interval between the cycles are important for obtaining favorable therapeutic responses, e.g. a large volume of disease necessitates administering higher radioactivities and vice versa. We define herewith, the imaging phenotypes α or β for choosing the isotope. An α imaging phenotype would be extensive bone and bone marrow involvement or superscan and/or status post-chemotherapy, where a PSMA-targeted α radioligand therapy may be more suitable in terms of efficacy and toxicity, than the β phenotype with strongly PSMA-positive, relatively limited disease amenable to PRLT using βbeta emitters like 177Lu.
At our center, restaging is performed using 68Ga-PSMA PET/CT 3–4 months after PRLT. In case of a stable disease or remission (complete or partial), the patient is restaged with PET/CT every 6 months until disease progression is evident on imaging. PRLT can be resumed after detection of progression after a therapy interruption, what we refer to as the next phase of PRLT (Figure 5). Additionally, laboratory parameters (erythrocytes, hemoglobin, platelets, leucocytes, creatinine, BUN, SGOT, SGPT, bilirubin, SAP, TSH, γ-GT and PSA) are evaluated prior to each cycle and at restaging. Renal function is monitored by tubular extraction rate using 99mTc-MAG3 renography. We also assess the function of the parotid and submandibular salivary glands by dynamic salivary gland scintigraphy, which is a very useful and easy to perform imaging modality for the objective evaluation of xerostomia following 177Lu-PRLT.98
Figure 5. .
A 60-year-old patient with mCRPC, s.p. brachytherapy, ADT and enzalutamide, experiencing partial remission of the extensive lymph node and bone metastases after 3 cycles (21.2 GBq) of PRLT (A, 68Ga-PSMA PET/CT MIP image after the third cycle). The disease progressed 6 months later (B) and he underwent a second phase (fourth and fifth PRLT) of treatment (salvage PRLT) with 7.9 and 8 GBq, respectively and concurrent treatment with abiraterone. The combination therapy had an effect/PR (C, 68Ga-PSMA PET/CT MIP image after the fourth cycle and D, after the fifth cycle) without any toxicity after a cumulative administered activity of 37.1 GBq. G1 anemia (present before PRLT) improved over time. ADT, androgen deprivation therapy; mCRPC, metastatic castration-resistant prostate cancer; MIP, maximum intensity protection; PRLT, PSMA radioligand therapy; PSMA, prostate-specific membrane antigen.
It must be emphasized that PRLT of metastatic PCa involves a multidisciplinary management with close collaboration with the referring urologists/oncologists as well as palliative medicine physicians. Treatment as a last line option implies that many patients present in a relatively poor general condition, which necessitates also the treatment of accompanying symptoms, commonly pain and anemia (administration of packed red blood cells).
Future perspectives
Early initiation of 177Lu-PRLT may be effective in metastatic PCa and may offer a significant survival benefit. Randomized controlled studies are required to best determine the place of this agent (e.g. before chemotherapy) in the management of mPC. Administration of higher activities or hyperfractionation may be considered for a better efficacy. A total activity of 30 GBq given 6–10 weeks apart was proved to be safe, considering dose limit to the kidney and bone marrow.67 Adjusting the amount of 177Lu administered during each cycle is important in contrast to a standardized approach of a fixed activity for each cycle.99 We analyzed the intrapatient variability in the absorbed doses during different therapy cycles and noted that the mean absorbed tumor dose demonstrated a significant reduction during subsequent cycles. Hence, applying a higher radioactivity in the first cycle seems to be logical in order to obtain the maximal antitumor effect.99
Future clinical studies should address the enhancement of the efficacy of PRLT by the combination with radiosensitizers, PARP inhibitors, immune-checkpoint inhibitors etc. Targeted multimodality options like combination with external beam radiation therapy (a concept which we refer to as COMBIERT, combined internal–external radiation therapy) or with bone-targeting agents like 177Lu labeled bisphosphonates (in case of a discordance between PSMA expression and the osteoblastic activity) must be considered for the maximal therapeutic effect. Treatment with newer agents like abiraterone or enzalutamide, which inhibit the AR signaling, leads to the upregulation of PSMA (Figure 5).100 Therefore, 177Lu-PSMA-RLT may produce a synergistic effect in combination with these agents.101
The preliminary results with 225Ac-PSMA-PRLT are definitely highly encouraging. Further studies to overcome the potential side effects, predominantly xerostomia, are urgently warranted. The demonstration of a proof-of-principle by the intraparencymal injection of botulinum toxin is a step forward in this direction.87
Newer radionuclides with favorable kinetics have been studied, permitting pre-therapeutic dosimetry.102–105 44Sc has a half-life of 4 h and can be made available using a cyclotron production route in substantial quantities as a highly pure product.102 It can be effectively labeled with PSMA ligands with in vitro and in vivo characteristics similar to 177Lu-PSMA-617. This permits delayed imaging after 24 h or later.103, 104 Another attractive radionuclide for PET/CT imaging with its 17.5 h half-life is 152Tb, particularly for predictive dosimetry before PRLT.105
Contributor Information
Harshad R Kulkarni, Email: harshad.kulkarni@zentralklinik.de.
Aviral Singh, Email: aviral.singh@zentralklinik.de.
Thomas Langbein, Email: thomas.langbein@zentralklinik.de.
Christiane Schuchardt, Email: christiane.schuchardt@zentralklinik.de.
Dirk Mueller, Email: dirk.mueller@zentralklinik.de.
Jingjing Zhang, Email: jingjing.zhang@zentralklinik.de.
Coline Lehmann, Email: coline.lehmann@zentralklinik.de.
Richard P Baum, Email: richard.baum@zentralklinik.de.
REFERENCES
- 1. Baum RP, Kulkarni HR, Carreras C. Peptides and receptors in image-guided therapy: theranostics for neuroendocrine neoplasms. Semin Nucl Med 2012; 42: 190–207. doi: 10.1053/j.semnuclmed.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 2. Baum RP, Kulkarni HR. Theranostics: from molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy— the Bad Berka experience. Theranostics 2012; 2: 437–47. doi: 10.7150/thno.3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Puranik AD, Kulkarni HR, Baum RP. Companion diagnostics. Cancer J 2015; 21: 213–7. [DOI] [PubMed] [Google Scholar]
- 4. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B. NETTER-1 trial investigators. Phase 3 trial of (177)Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med 2017; 376: 125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 6. Pfister D, Bolla M, Briganti A, Carroll P, Cozzarini C, Joniau S, et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol 2014; 65: 1034–43. doi: 10.1016/j.eururo.2013.08.013 [DOI] [PubMed] [Google Scholar]
- 7. Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ Jr, Dotan ZA, Fearn PA. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98:715-7. Erratum in. J Natl Cancer Inst 2012; 104: 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol 2002; 167: 528–34. doi: 10.1016/S0022-5347(01)69079-7 [DOI] [PubMed] [Google Scholar]
- 9. Kuriyama M, Wang MC, Lee CI, Papsidero LD, Killian CS, Inaji H, et al. Use of human prostate-specific antigen in monitoring prostate cancer. Cancer Res 1981; 41: 3874–6. [PubMed] [Google Scholar]
- 10. van den Bergh RC, Albertsen PC, Bangma CH, Freedland SJ, Graefen M, Vickers A, et al. Timing of curative treatment for prostate cancer: a systematic review. Eur Urol 2013; 64: 204–15. doi: 10.1016/j.eururo.2013.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moschini M, Sharma V, Zattoni F, Quevedo JF, Davis BJ, Kwon E, et al. Natural history of clinical recurrence patterns of lymph node-positive prostate cancer after radical prostatectomy. Eur Urol 2016; 69: 135–42. doi: 10.1016/j.eururo.2015.03.036 [DOI] [PubMed] [Google Scholar]
- 12. Rouvière O, Vitry T, Lyonnet D. Imaging of prostate cancer local recurrences: why and how? Eur Radiol 2010; 20: 1254–66. doi: 10.1007/s00330-009-1647-4 [DOI] [PubMed] [Google Scholar]
- 13. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on PC. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71: 618–29. doi: 10.1016/j.eururo.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 14. Turkbey B, Brown AM, Sankineni S, Wood BJ, Pinto PA, Choyke PL. Multiparametric prostate magnetic resonance imaging in the evaluation of prostate cancer. CA Cancer J Clin 2016; 66: 326–36. doi: 10.3322/caac.21333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Visschere PJ, Briganti A, Fütterer JJ, Ghadjar P, Isbarn H, Massard C, et al. Role of multiparametric magnetic resonance imaging in early detection of prostate cancer. Insights Imaging 2016; 7: 205–14. doi: 10.1007/s13244-016-0466-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Halabi S, Vogelzang NJ, Kornblith AB, Ou SS, Kantoff PW, Dawson NA, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J Clin Oncol 2008; 26: 2544–9. doi: 10.1200/JCO.2007.15.0367 [DOI] [PubMed] [Google Scholar]
- 17. de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al . Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. doi: 10.1056/NEJMoa1014618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. COU-AA-301 Investigators Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13: 983–92. doi: 10.1016/S1470-2045(12)70379-0 [DOI] [PubMed] [Google Scholar]
- 19. Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al . Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet 2010; 375: 1437–46. doi: 10.1016/S0140-6736(10)60172-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seruga B, Tannock IF. Chemotherapy-based treatment for castration-resistant prostate cancer. J Clin Oncol 2011; 29: 3686–94. doi: 10.1200/JCO.2010.34.3996 [DOI] [PubMed] [Google Scholar]
- 21. de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al . Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–54. doi: 10.1016/S0140-6736(10)61389-X [DOI] [PubMed] [Google Scholar]
- 22. Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al . Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–23. doi: 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 23. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al . Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–97. doi: 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 24. Jadvar H. Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: utility and limitations. Eur J Nucl Med Mol Imaging 2013; 40(suppl 1): 5–10. doi: 10.1007/s00259-013-2361-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fox JJ, Gavane SC, Blanc-Autran E, Nehmeh S, Gönen M, Beattie B, et al. Positron emission tomography/computed tomography-based assessments of androgen receptor expression and glycolytic activity as a prognostic biomarker for metastatic castration-resistant prostate cancer. JAMA Oncol 2018; 4: 217–24. doi: 10.1001/jamaoncol.2017.3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwarzenböck SM, Eiber M, Kundt G, Retz M, Sakretz M, Kurth J, et al. Prospective evaluation of [11C]Choline PET/CT in therapy response assessment of standardized docetaxel first-line chemotherapy in patients with advanced castration refractory prostate cancer. Eur J Nucl Med Mol Imaging 2016; 43: 2105–13. doi: 10.1007/s00259-016-3439-9 [DOI] [PubMed] [Google Scholar]
- 27. Fanti S, Minozzi S, Castellucci P, Balduzzi S, Herrmann K, Krause BJ, et al. PET/CT with 11C-choline for evaluation of prostate cancer patients with biochemical recurrence: meta-analysis and critical review of available data. Eur J Nucl Med Mol Imaging 2016; 43: 55–69. doi: 10.1007/s00259-015-3202-7 [DOI] [PubMed] [Google Scholar]
- 28. Souvatzoglou M, Weirich G, Schwarzenboeck S, Maurer T, Schuster T, Bundschuh RA, et al. The sensitivity of [11C]choline PET/CT to localize prostate cancer depends on the tumor configuration. Clin Cancer Res 2011; 17: 3751–9. doi: 10.1158/1078-0432.CCR-10-2093 [DOI] [PubMed] [Google Scholar]
- 29. Eder M, Eisenhut M, Babich J, Haberkorn U. PSMA as a target for radiolabelled small molecules. Eur J Nucl Med Mol Imaging 2013; 40: 819–23. doi: 10.1007/s00259-013-2374-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem 2004; 91: 528–39. doi: 10.1002/jcb.10661 [DOI] [PubMed] [Google Scholar]
- 31. Hellwig D, Moosbauer J, Eilles C. Ga-68-PSMA PET/CT for prostate cancer. Aktuelle Urol 2014; 45: 457–63. doi: 10.1055/s-0034-1395529 [DOI] [PubMed] [Google Scholar]
- 32. Bander NH, Trabulsi EJ, Kostakoglu L, Yao D, Vallabhajosula S, Smith-Jones P, et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urol 2003; 170: 1717–21. doi: 10.1097/01.ju.0000091655.77601.0c [DOI] [PubMed] [Google Scholar]
- 33. Banerjee SR, Pullambhatla M, Byun Y, Nimmagadda S, Green G, Fox JJ, et al. 68Ga-labeled inhibitors of prostate-specific membrane antigen (PSMA) for imaging prostate cancer. J Med Chem 2010; 53: 5333–41. doi: 10.1021/jm100623e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomogra- phy in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol 2016; 70: 926–37. [DOI] [PubMed] [Google Scholar]
- 35. Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015; 42: 197–209. doi: 10.1007/s00259-014-2949-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective comparison of 18F- fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med 2015; 56: 1185–90. doi: 10.2967/jnumed.115.160382 [DOI] [PubMed] [Google Scholar]
- 37. Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging 2017; 44: 92–101. doi: 10.1007/s00259-016-3490-6 [DOI] [PubMed] [Google Scholar]
- 38. Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2014; 41: 11–20. doi: 10.1007/s00259-013-2525-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical pros- tatectomy. J Nucl Med 2015; 56: 668–74. doi: 10.2967/jnumed.115.154153 [DOI] [PubMed] [Google Scholar]
- 40. Cho SY, Gage KL, Mease RC, Senthamizhchelvan S, Holt DP, Jeffrey-Kwanisai A, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med 2012; 53: 1883–91. doi: 10.2967/jnumed.112.104661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rowe SP, Macura KJ, Ciarallo A, Mena E, Blackford A, Nadal R, et al. Comparison of prostate-specific mem- brane antigen-based 18F-DCFBC PET/CT to conventional imaging modalities for detection of hormone-naive and castration-resistant metastatic prostate can- cer. J Nucl Med 2016; 57: 46–53. doi: 10.2967/jnumed.115.163782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol 2015; 17: 565–74. doi: 10.1007/s11307-015-0850-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rowe SP, Macura KJ, Mena E, Blackford AL, Nadal R, Antonarakis ES, et al. PSMA-based [18F]DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol 2016; 18: 411–9. doi: 10.1007/s11307-016-0957-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dietlein M, Kobe C, Kuhnert G, Stockter S, Fischer T, Schomäcker K, et al. Comparison of [18F]DCFPyL and [68Ga] Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol 2015; 17: 575–84. doi: 10.1007/s11307-015-0866-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging 2017; 44: 678–88. doi: 10.1007/s00259-016-3573-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol 2016; 195: 1436–43. doi: 10.1016/j.juro.2015.12.025 [DOI] [PubMed] [Google Scholar]
- 47. Rauscher I, Maurer T, Beer AJ. Value of 68Ga‐PSMA HBED‐CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med 2016: 1713–9. [DOI] [PubMed] [Google Scholar]
- 48. Fendler WP, Schmidt DF, Wenter V. 68Ga‐PSMA PET/CT detects the location and extent of primary prostate cancer. J Nucl Med 2016: 1720–5. [DOI] [PubMed] [Google Scholar]
- 49. Rahbar K, Weckesser M, Huss S, Semjonow A, Breyholz HJ, Schrader AJ, et al. Correlation of intraprostatic tumor extent with ⁶⁸Ga-PSMA distribution in patients with prostate cancer. J Nucl Med 2016; 57: 563–7. doi: 10.2967/jnumed.115.169243 [DOI] [PubMed] [Google Scholar]
- 50. Zamboglou C, Drendel V, Jilg CA, Rischke HC, Beck TI, Schultze-Seemann W, et al. Comparison of 68Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics 2017; 7: 228–37. doi: 10.7150/thno.16638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol 2016; 70: 829–36. doi: 10.1016/j.eururo.2015.12.053 [DOI] [PubMed] [Google Scholar]
- 52. Albisinni S, Artigas C, Aoun F, Biaou I, Grosman J, Gil T, et al. Clinical impact of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach. BJU Int 2017; 120: 197–203. doi: 10.1111/bju.13739 [DOI] [PubMed] [Google Scholar]
- 53. Bluemel C, Linke F, Herrmann K, Simunovic I, Eiber M, Kestler C, et al. Impact of 68Ga-PSMA PET/CT on salvage radiotherapy planning in patients with prostate cancer and persisting PSA values or biochemical relapse after prostatectomy. EJNMMI Res 2016; 6: 78. doi: 10.1186/s13550-016-0233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dewes S, Schiller K, Sauter K, Eiber M, Maurer T, Schwaiger M, et al. Integration of (68)Ga-PSMA-PET imaging in planning of primary definitive radiotherapy in prostate cancer: a retrospective study. Radiat Oncol 2016; 11: 73. doi: 10.1186/s13014-016-0646-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maurer T, Weirich G, Schottelius M, Weineisen M, Frisch B, Okur A, et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur Urol 2015; 68: 530–4. doi: 10.1016/j.eururo.2015.04.034 [DOI] [PubMed] [Google Scholar]
- 56. Rauscher I, Düwel C, Wirtz M, Schottelius M, Wester HJ, Schwamborn K, et al. Value of 111 In-prostate-specific membrane antigen (PSMA)-radioguided surgery for salvage lymphadenectomy in recurrent prostate cancer: correlation with histopathology and clinical follow-up. BJU Int 2017; 120: 40-47. doi: 10.1111/bju.13713 [DOI] [PubMed] [Google Scholar]
- 57. Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant pros- tate cancer: safety and efficacy. J Nucl Med 2016; 57: 1006–13. [DOI] [PubMed] [Google Scholar]
- 58. Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med 2015; 56: 1169–76. doi: 10.2967/jnumed.115.158550 [DOI] [PubMed] [Google Scholar]
- 59. Fendler WP, Kratochwil C, Ahmadzadehfar H, Rahbar K, Baum RP, Schmidt M, et al. 177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer. Nuklearmedizin 2016; 55: 123–8. [PubMed] [Google Scholar]
- 60. Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y, et al. PSMA-based radioligand therapy for metastatic castrationresistant prostate cancer: the Bad Berka experience since 2013. J Nucl Med 2016; 57(Suppl 3): 97S–104. doi: 10.2967/jnumed.115.170167 [DOI] [PubMed] [Google Scholar]
- 61. Okamoto S, Thieme A, Allmann J, D’Alessandria C, Maurer T, Retz M. Radiation dosimetry for 177Lu-PSMA-I&T in mCRPC: absorbed activity in normal organs and tumour lesions. J Nucl Med 2017; 58: 445–50. [DOI] [PubMed] [Google Scholar]
- 62. Kulkarni HR, Schuchardt C, Singh S, Baum RP. Serial dosimetry during Lu-177 PSMA radioligand therapy in the same patient. J Nucl Med 2017; 58: 58316. [Google Scholar]
- 63. Kabasakal L, AbuQbeitah M, Aygün A, Yeyin N, Ocak M, Demirci E, et al. Pre-therapeutic dosimetry of normal organs and tissues of (177)Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2015; 42: 1976–83. doi: 10.1007/s00259-015-3125-3 [DOI] [PubMed] [Google Scholar]
- 64. Delker A, Fendler WP, Kratochwil C, Brunegraf A, Gosewisch A, Gildehaus FJ, et al. Dosimetry for (177)Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging 2016; 43: 42–51. doi: 10.1007/s00259-015-3174-7 [DOI] [PubMed] [Google Scholar]
- 65. Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med 2016; 57: 1170–6. doi: 10.2967/jnumed.115.171397 [DOI] [PubMed] [Google Scholar]
- 66. Hohberg M, Eschner W, Schmidt M, Dietlein M, Kobe C, Fischer T, et al. Lacrimal glands may represent organs at risk for radionuclide therapy of prostate cancer with [177Lu]DKFZ-PSMA-617. Mol Imaging Biol 2016; 18: 437–45. doi: 10.1007/s11307-016-0942-0 [DOI] [PubMed] [Google Scholar]
- 67. Scarpa L, Buxbaum S, Kendler D, Fink K, Bektic J, Gruber L. The 68Ga/177Lu theragnostic concept in PSMA targeting of CRPC: correlation of SUVmax values and absorbed activity esti- mates. Eur J Nucl Med Mol Imaging 2017; 44: 788–800. [DOI] [PubMed] [Google Scholar]
- 68. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991; 21: 109–22. doi: 10.1016/0360-3016(91)90171-Y [DOI] [PubMed] [Google Scholar]
- 69. Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J Nucl Med 2005; 46 Suppl 1: 83S–91. [PubMed] [Google Scholar]
- 70. Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging 2008; 35: 1847–56. doi: 10.1007/s00259-008-0778-1 [DOI] [PubMed] [Google Scholar]
- 71. Li Y, Taylor JM, Ten Haken RK, Eisbruch A. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys 2007; 67: 660–9. doi: 10.1016/j.ijrobp.2006.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ahmadzadehfar H, Wegen S, Yordanova A, Fimmers R, Kürpig S, Eppard E, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging 2017; 44: 1448–54. doi: 10.1007/s00259-017-3716-2 [DOI] [PubMed] [Google Scholar]
- 73. Bräuer A, Grubert LS, Roll W, Schrader AJ, Schäfers M, Bögemann M, et al. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2017; 44: 1663–70. doi: 10.1007/s00259-017-3751-z [DOI] [PubMed] [Google Scholar]
- 74. Fendler WP, Reinhardt S, Ilhan H, Delker A, Böning G, Gildehaus FJ, et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget 2017; 8: 3581–90. doi: 10.18632/oncotarget.12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. Germanmulticenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med 2017; 58: 85–90. doi: 10.2967/jnumed.116.183194 [DOI] [PubMed] [Google Scholar]
- 76. Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schäfers M, Essler M, et al. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging 2018; 45: 12–19. doi: 10.1007/s00259-017-3848-4 [DOI] [PubMed] [Google Scholar]
- 77. Al-Batran SE, Hozaeel W, Tauchert FK, Hofheinz RD, Hinke A, Windemuth-Kieselbach C, et al. The impact of docetaxel-related toxicities on health-related quality of life in patients with metastatic cancer (QoliTax. Ann Oncol 2015; 26: 1244–8. doi: 10.1093/annonc/mdv129 [DOI] [PubMed] [Google Scholar]
- 78. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–12. doi: 10.1056/NEJMoa040720 [DOI] [PubMed] [Google Scholar]
- 79. de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–54. doi: 10.1016/S0140-6736(10)61389-X [DOI] [PubMed] [Google Scholar]
- 80. Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16: 152–60. doi: 10.1016/S1470-2045(14)71205-7 [DOI] [PubMed] [Google Scholar]
- 81. Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al . Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–97. doi: 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 82. von Eyben FE, Roviello G, Kiljunen T, Uprimny C, Virgolini I, Kairemo K, et al. Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: a systematic review. Eur J Nucl Med Mol Imaging 2018; 45: 496–508. doi: 10.1007/s00259-017-3895-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Singh A, Schuchardt C, Kulkarni H, Baum RP. Is Lu-177 PSMA radioligand therapy of prostate cancer safe for patients with single functioning kidney? Eur J Nucl Med Mol Imaging 2016; 43(Suppl 1): S1–S734. [Google Scholar]
- 84. Yordanova A, Becker A, Eppard E, Kürpig S, Fisang C, Feldmann G, et al. The impact of repeated cycles of radioligand therapy using [177Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur J Nucl Med Mol Imaging 2017; 44: 1473–9. doi: 10.1007/s00259-017-3681-9 [DOI] [PubMed] [Google Scholar]
- 85. Kratochwil C, Giesel FL, Leotta K, Eder M, Hoppe-Tich T, Youssoufian H, et al. PMPA for nephroprotection in PSMA-targeted radionuclide therapy of prostate cancer. J Nucl Med 2015; 56: 293–8. doi: 10.2967/jnumed.114.147181 [DOI] [PubMed] [Google Scholar]
- 86. Bohn KP, Kletting P, Solbach C, Beer AJ, Krohn T, v EderK. Speicheldrüsen bei der Therapie mit PSMA- Radioliganden. Nuklearmedizin 2017; 56: A2–A91. [Google Scholar]
- 87. Baum RP, Langbein T, Singh A, Shahinfar M, Schuchardt C, Volk GF, et al. Injection of botulinum toxin for preventing salivary gland toxicity after PSMA radioligand therapy: an empirical proof of a promising concept. Nucl Med Mol Imaging 2018; 52: 80–1. doi: 10.1007/s13139-017-0508-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Coskun BU, Savk H, Cicek ED, Basak T, Basak M, Dadas B. Histopathological and radiological investigations of the influence of botulinum toxin on the submandibular gland of the rat. Eur Arch Otorhinolaryngol 2007; 264: 783–7. doi: 10.1007/s00405-007-0254-8 [DOI] [PubMed] [Google Scholar]
- 89. Teymoortash A, Sommer F, Mandic R, Schulz S, Bette M, Aumüller G, et al. Intraglandular application of botulinum toxin leads to structural and functional changes in rat acinar cells. Br J Pharmacol 2007; 152: 161–7. doi: 10.1038/sj.bjp.0707375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A. 177Lu-DKFZ-PSMA-617 therapy in mCRPC: safety, effica- cy, and quality of life assessment. Eur J Nucl Med Mol Imaging 2017; 44: 81–91. [DOI] [PubMed] [Google Scholar]
- 91. Heck MM, Retz M, D’Alessandria C, Rauscher I, Scheidhauer K, Maurer T. Systemic radioligand therapy with 177Lu-labeled prostate-specific membrane antigen-ligand for imaging and thera- py in patients with mCRPC. J Urol 2016; 196: 382–91. [DOI] [PubMed] [Google Scholar]
- 92. Ahmadzadehfar H, Schlolaut S, Fimmers R, Yordanova A, Hirzebruch S, Schlenkhoff C, et al. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving [177Lu]Lu-PSMA-617 radioligand therapy. Oncotarget 2017; 8: 103108–16. doi: 10.18632/oncotarget.21600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rahbar K, Bögeman M, Yordanova A, Eveslage M, Schäfers M, Essler M, et al. Delayed response after repeated 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur J Nucl Med Mol Imaging 2018; 45: 243–6. doi: 10.1007/s00259-017-3877-z [DOI] [PubMed] [Google Scholar]
- 94. Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med 2017; 58: 1624–31. doi: 10.2967/jnumed.117.191395 [DOI] [PubMed] [Google Scholar]
- 95. Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U. Targeted Alpha Therapy of mCRPC with (225)Actinium-PSMA-617: Swimmer-Plot analysis suggests efficacy regarding duration of tumor-control. J Nucl Med 2018; 59: 795–802. [DOI] [PubMed] [Google Scholar]
- 96. Sathekge M, Knoesen O, Meckel M, Modiselle M, Vorster M, Marx S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in mCRPC. Eur J Nucl Med Mol Imaging 2017; 44: 1099–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kratochwil C, Schmidt K, Afshar-Oromieh A, Bruchertseifer F, Rathke H, Morgenstern A, et al. Targeted alpha therapy of mCRPC: dosimetry estimate of 213Bismuth-PSMA-617. Eur J Nucl Med Mol Imaging 2018; 45: 31–7. doi: 10.1007/s00259-017-3817-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Langbein T, Kulkarni HR, Singh A, Baum RP. Functional imaging of the salivary glands for evaluation of radiation-induced sialadenitis before and after Lu-177 PSMA radioligand therapy. Eur J Nucl Med Mol Imaging 2017; 44(Suppl 2): S328. [Google Scholar]
- 99. Weber W. SNMMI highlights lecture: oncology. J Nucl Med 2017; 2018: 7N–13. [PubMed] [Google Scholar]
- 100. Evans MJ, Smith-Jones PM, Wongvipat J, Navarro V, Kim S, Bander NH, et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci U S A 2011; 108: 9578–82. doi: 10.1073/pnas.1106383108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. DiPippo VA, Nguyen HM, Brown LG, Olson WC, Vessella RL, Corey E. Addition of PSMA ADC to enzalutamide therapy significantly improves survival in in vivo model of castration resistant prostate cancer. Prostate 2016; 76: 325–34. doi: 10.1002/pros.23124 [DOI] [PubMed] [Google Scholar]
- 102. Singh A, van der Meulen NP, Müller C, Klette I, Kulkarni HR, Türler A, et al. First-in-human PET/CT imaging of metastatic neuroendocrine neoplasms with cyclotron-produced 44Sc-DOTATOC: a proof-of-concept study. Cancer Biother Radiopharm 2017; 32: 124–32. doi: 10.1089/cbr.2016.2173 [DOI] [PubMed] [Google Scholar]
- 103. Khawar A, Eppard E, Sinnes JP, Roesch F, Ahmadzadehfar H, Kürpig S, et al. 44Sc]Sc-PSMA-617 biodistribution and dosimetry in patients with metastatic castration-resistant prostate carcinoma. Clin Nucl Med 2018; 43: 1. doi: 10.1097/RLU.0000000000002003 [DOI] [PubMed] [Google Scholar]
- 104. Umbricht CA, Benešová M, Schmid RM, Türler A, Schibli R, van der Meulen NP, et al. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617-preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res 2017; 7: 9. doi: 10.1186/s13550-017-0257-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Baum RP, Singh A, Benešová M, Vermeulen C, Gnesin S, Köster U, et al. Clinical evaluation of the radiolanthanide terbium-152: first-in-human PET/CT with 152Tb-DOTATOC. Dalton Trans 2017; 46: 14638–46. doi: 10.1039/C7DT01936J [DOI] [PubMed] [Google Scholar]