Abstract
Objective:
To determine the diagnostic performance of CT in the assessment of mild hepatic steatosis by comparison with MR mDIXON-Quant as a reference standard, and to explore their clinical applications.
Methods:
In this prospective study 169 volunteers were included. Each subject underwent CT and MR mDIXON-Quant examinations. Hepatic steatosis evaluations were performed via liver attenuation alone (CT L), liver to spleen attenuation ratio (CT L/S), difference between liver and spleen attenuation (CT L-S), and MR mDIXON-Quant imaging. The effectiveness of CT L, CT L/S, and CT L-S in diagnosing hepatic steatosis severity of ≥5%, ≥10%, and ≥15% was compared, using mDIXON-Quant results as standard.
Results:
65 subjects exhibited mild hepatic steatosis. Hepatic steatosis measurement with mDIXON-Quant was strongly correlated with the three CT methods. Using cutoff value, the sensitivity and specificity of diagnosing hepatic steatosis ≥5, ≥10, and ≥15% were 64.6, 91.3, 100%, and 90.4, 89.7, 93.0% for CT L; 50.8, 87.0, 100%, and 96.2, 98.6, 97.5% for CT L/S; and 67.7, 87.0, 100%, and 81.7, 98.6, 97.5% for CT L-S, respectively. ROC analysis indicated that 58.9, 56.5, and 52.8 HU for CT L; 1.06, 0.98, and 0.90 HU for CT L/S; and 6.21,–1.04, and −4.93 HU for CT L-S were cutoff values for diagnosing hepatic steatosis ≥5%,≥10%, and ≥15%, respectively.
Conclusions:
The three CT methods exhibit better agreements with mDIXON-Quant imaging for diagnosing hepatic steatosis ≥10%. Hence, CT and mDIXON-Quant could serve as suitable tools for the accurate quantification of mild hepatic steatosis.
Significant finds of the study:
The close agreement between the three different CT methods (based on our cutoff values) and mDIXON-Quant imaging suggests that CT could accurately diagnose hepatic steatosis ≥10%. Thus, CT and mDIXON-Quant imaging can accurately measure mild hepatic steatosis.
What this study adds:
Only few studies have compared hepatic steatosis quantification between CT and mDIXON-Quant. We are the first to determine the diagnostic performance of unenhanced CT for quantitatively assessing mild hepatic steatosis, in reference to magnetic resonance mDIXON-Quant imaging.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease worlwide,1 and presents a significant health burden along with serious public and clinical health concerns.2 The prevalence of NAFLD is as high as 30% in the adult Western population, and is approximately 15% (range, 6.3–27.0%) in China,3, 4 with a maximum prevalence of 53% among obese children.5
NAFLD is characterized by the fatty infiltration of the liver parenchyma that exceeds 5% on histological examination.6 Increasing evidence has suggested an association between NAFLD and metabolic syndrome, insulin resistance, Type 2 diabetes mellitus (DM), cardiovascular diseases and ultimately mortality.7 Hepatic steatosis is traditionally graded as mild (<30% infiltration), moderate (30–60% infiltration), and severe (>60% infiltration) based on histologic examination findings, and patients with NAFLD frequently exhibit a mild grade of steatosis8–10 Nevertheless, mild steatosis is not always quiescent, and simple steatosis may develop non-alcoholic steatohepatitis (NASH) and fibrosis progression.11 A previous study indicated that patients with simple steatosis developed NASH within 3 years, wherein 58% of the patients with histological NAFLD activity score <3 exhibited increased disease activity after 3 years and 28% of the patients exhibited fibrosis progression.8 According to recent data, even the mildest degree of steatosis increases the incidence of primary non-function and decreases patient survival after liver transplantation, and thus has an significant impact on mortality and patient outcome.12
Liver biopsy continues to be the gold standard for the quantification of hepatic steatosis.13 However, it is an invasive procedure, and is associated with a substantial degree of sampling error and complications such as bleeding.14 The noninvasive methods for quantification of hepatic steatosis include ultrasound, CT, and MRI. Ultrasound is a subjective assessment that has a small field of view; the findings are often dependent on the operator and equipment.15 Moreover, although ultrasound has a high specificity, it underestimates the prevalence of hepatic steatosis in cases with liver fat <20%.16 Both CT and MRI can supply a more objective assessment that is both reproducible and well correlated. CT offers a semi-quantitative approach for the evaluation of hepatic steatosis, and has a high specificity for diagnosing moderate and severe hepatic steatosis17 However, the accuracy of CT in the detection of a mild degree of hepatic steatosis is low.15 Unlike ultrasound and CT, MRI can gauge the quantity of hepatic steatosis directly. In fact, the use of MR mDIXON-Quant sequence for the accurate quantification of hepatic steatosis has been described in the literature.18, 19 This method yields high diagnostic and fat-grading accuracy, without the need for any invasive procedure or radiation exposure. Furthermore, the MR mDIXON-Quant method employs a 3D-FFE sequence with multiple acquired echoes, thus generating water and fat images, as well as in-phase and opposed-phase images that are synthesized from the water-fat images. The sensitivity and specificity of mDIXON-Quant MR imaging in detecting histologic steatosis were found to be 95.0 and 100%, respectively.18
In clinical practice, CT is reasonably accurate in the detection of moderate-to-severe hepatic steatosis;15 however, no cutoff value for the quantification of mild hepatic steatosis has been established, and the use of standard criteria is not appropriate for the accurate quantification of mild hepatic steatosis. Furthermore, only a few validation studies have compared the quantification of hepatic steatosis between CT and mDIXON-Quant.
In the present study, we aimed to determine the diagnostic performance of unenhanced CT in the quantitative assessment of mild hepatic steatosis, while using mDIXON-Quant MR as the reference standard. In addition, we sought to explore the clinical applications of CT and MR mDIXON-Quant sequence in the quantification of mild hepatic steatosis.
Methods and materials
Study population and clinical data
This was a prospective study, a total of 169 individuals living in Beijing (90 males and 79 females; age range, 21–52 years) who underwent unenhanced CT and mDIXON-Quant MR examinations were examined; these participants were recruited via advertisement for an ongoing study started in June 2014 on spine and knee degeneration (Figure 1). The study protocol was approved by the ethics committee of our hospital (Beijing Jishuitan Hospital), China. Informed consent was obtained from all the subjects prior to the examinations. None of these patients had a history of alcoholism or signs of other liver diseases. Furthermore, any hypolipidemic drugs or drugs that could cause steatosis were not being taken by the patients.
Figure 1.
Flow diagram of participants.
CT examinations
The volunteers were scheduled to have a liver unenhanced CT scans in our department. A 80-row multislice spiral CT scanner (TOSHIBA Inc., Aquilion PRIME ESX-302A, Japan) was used with the following parameters: tube voltage, 120 kV; tube current, 250 mA; slice thickness and interval, 5 mm; and field of view (FOV), 40 cm. For estimating the hepatic attenuation values, a total of 6 regions of interest (ROIs) were drawn in transverse sections through the right hepatic portal vein and below the second hepatic portal vein. All the ROIs were distributed in the hepatic parenchyma, and any biliary, vascular, and extrahepatic structures were excluded. The area of each ROI was 3 cm2. In each section, one ROI was located in the left liver lobe, another ROI was located in the middle of the right liver lobe, and the third ROI was located in the posterior portion of the right liver lobe. At the same time, to estimate the splenic attenuation values, 2 ROIs were drawn in transverse sections through the right hepatic portal vein. We used a RadiAnt DICOM Viewer software (Medixant, Poznan, Poland) to measure the hepatic and splenic attenuation values. We calculated the average values for hepatic attenuation from the 6 ROIs and for splenic attenuation from the 2 ROIs. CT L was defined as the hepatic attenuation value expressed in Hounsfield units, CT L/S was defined as the hepatic-to-splenic attenuation ratio, and CT L-S was defined as the difference between hepatic and splenic attenuation. Hepatic steatosis was diagnosed based on the following standard criteria: CT L ≤ 48 HU; CT L/S ≤ 1.0 HU; CT L-S ≤ 5 HU.20–22
Measurement of hepatic fat content using MR mDIXON-Quant sequence imaging
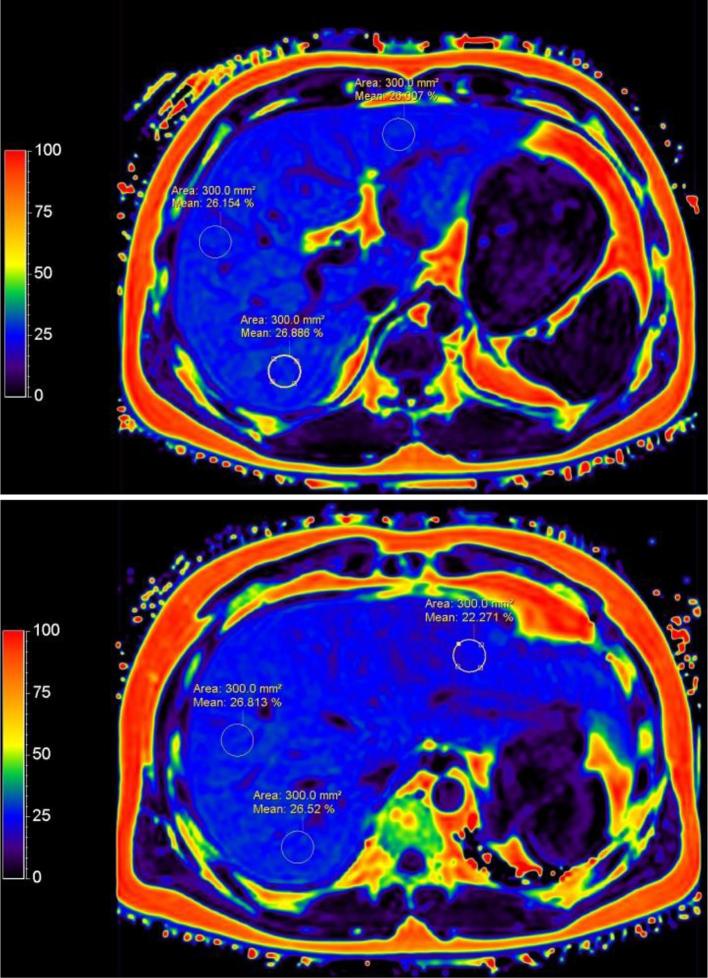
On the same day with QCT examination, liver mDIXON-Quant MR imaging was performed by an experienced radiologist using a 3.0 T MR scanner in all subjects (Ingenia, Philips, Healthcare, Best, Netherlands) in our department. In mDIXON-Quant, 3D-FFE with multiple acquired echoes was used to generate water, fat, T 2*, R2* images, along with in-phase and opposed-phase images that were synthesized from the water-fat images. The scan parameters were as follows: TR, 6.2 ms; TE1, 0.95 ms; 6 echoes with delta echo time (TE) 0.8 ms; FOV, 360 × 330 × 120 mm; FA, 3°; resolution, 2.5 × 2.5 × 3.0; SENSE, 2; NSA, 2; and scan time, 12.5 ms. After MRI acquisition, we measured the hepatic fat fraction and R2* value from the 6 ROIs in the transverse sections through the right hepatic portal vein and below the second portal vein from fat and R2* images, corresponding to the CT images (Figure 2). The area of each ROI was 3 cm2, and the average fat content and R2* value from 6 ROIs was recorded. The radiologist analyzing the CT scans and the radiologist analyzing the MRI scans were blinded to each other findings.
Figure 2.
Sample ROIs used for calculating the fat fraction of the liver in transverse sections through the right hepatic portal vein (A) and below the second hepatic portal vein (B). All the ROIs are distributed in the hepatic parenchyma, and any biliary, vascular, and extrahepatic structures are excluded. The area of each ROI is 3 cm2. In each section, one ROI is located in the left liver lobe, another ROI is located in the middle of the right liver lobe, and a third ROI is located in the posterior portion of the right liver lobe. ROI, region of interest.
According to the literature, a liver fat content of 50 mg/g (5% by wet weight) is diagnostic as hepatic steatosis. To determine the diagnostic performance of unenhanced CT in the quantitative assessment of mild hepatic steatosis, we also set 10 and 15% as reference standards.
Statistical analysis
Continuous data are expressed as mean ± standard deviation. McNemar’s test and linear correlation analysis were used, when appropriate. Correlative analysis were used between CT and mDIXON-quant MR imaging in the measurement of hepatic steatosis and R2*. The diagnostic accuracy of CT L, CT L/S and CT L-S was estimated by using standard criteria for diagnosing hepatic steatosis ≥5%. A receiver operating characteristic (ROC) curve was established to determine the best cut-off values for the detection of steatosis via CT L, CT L/S and CT L-S. The optimal threshold value was used as the cut-off point to determine the sensitivity and specificity of CT-L, CT-L/S, and CT-L-S for detecting the presence of liver fat content in reference to mDIXON-Quant MR imaging in cases with at least 5%, 10%, and 15% liver fat. Statistical analysis was performed using Statistical Package for Social Sciences v. 23.0 software (SPSS, Chicago, IL) and MedCalc 9.3 (MedCalc Software, Mariakerke, Belgium). A p value of < 0.05 was considered statistically significant.
Results
Clinical and radiologic imaging characteristics of the study population
The clinical and radiologic imaging characteristics of the study population are summed up in Table 1. The study included 169 subjects [90 (53.3%) males and 79 (46.7%) females], with a mean age of 34.27 years (range, 21–52 years) and body mass index of 24.51 kg m− 2 (range, 16.14–36.73 kg m− 2). The extent of hepatic steatosis determined via mDIXON-Quant MR imaging ranged from 1.13 to 27.42% (mean value, 6.15%). The extent of R2* ranged from 29.47 to 103.22 Hz (mean value, 49.41 Hz).
Table 1.
Clinical and radiologic imaging characteristics of the study population
| Characteristics |
Study population
(n = 169) |
| Sex (male: female) | 90:79 |
| Age (years) | 34.12 ± 6.97 (21–52) |
| Height (cm) | 166.99 ± 7.82 (147–187) |
| Weight (Kg) | 69.51 ± 13.49 (45-120) |
| Body mass index (BMI, kg m− 2) | 24.80 ± 3.60 (16.14–38.31) |
| CT L (Hu) | 60.03 ± 7.93 (30.22–71.22) |
| CT S (Hu) | 53.44 ± 4.26 (33.38–61.01) |
| CT L/S | 1.13 ± 0.16 (0.56–1.51) |
| CT L-S (Hu) | 6.59 ± 8.26 (-25.03–21.74) |
| R2* (HZ) | 49.41 ± 13.44 (29.47–103.22) |
| Fat content measured by MR (%) | 6.15 ± 5.10 (1.13–27.42) |
CT L, hepatic attenuation; CT S, splenic attenuation; CT L/S, the ratio of hepatic attenuation to splenic attenuation; CT L-S, hepatic-splenic attenuation difference.
Continuous data are expressed as means ± SD.
Correlation of hepatic steatosis measurement between CT and MR imaging
Linear correlation analyses indicated a strong correlation between the results of the 2 imaging modalities, even after adjusted by R2*, and there is a positive correlation between R2* and iron deposition (Table 2 and Figure 3)
Table 2.
Correlation coefficients between CT and mDIXON-quant MR imaging in the measurement of hepatic steatosis and R2*
| CT L | CT L/S | CT L-S | R2 a | |
| Fat content measured by MR | −0.862 a | −0.825 a | −0.867 a | 0.556 a |
| Fat content measured by MR (Adjusted by R2 a ) | −0.854 a | −0.780 a | −0.830 a | |
| R2 a | −0.360 a | −0.434 a | −0.453 a | |
| R2 a (Adjusted by fat content) | 0.283 a | 0.054b | 0.071b |
p< 0.001.
p > 0.05.
Figure 3.
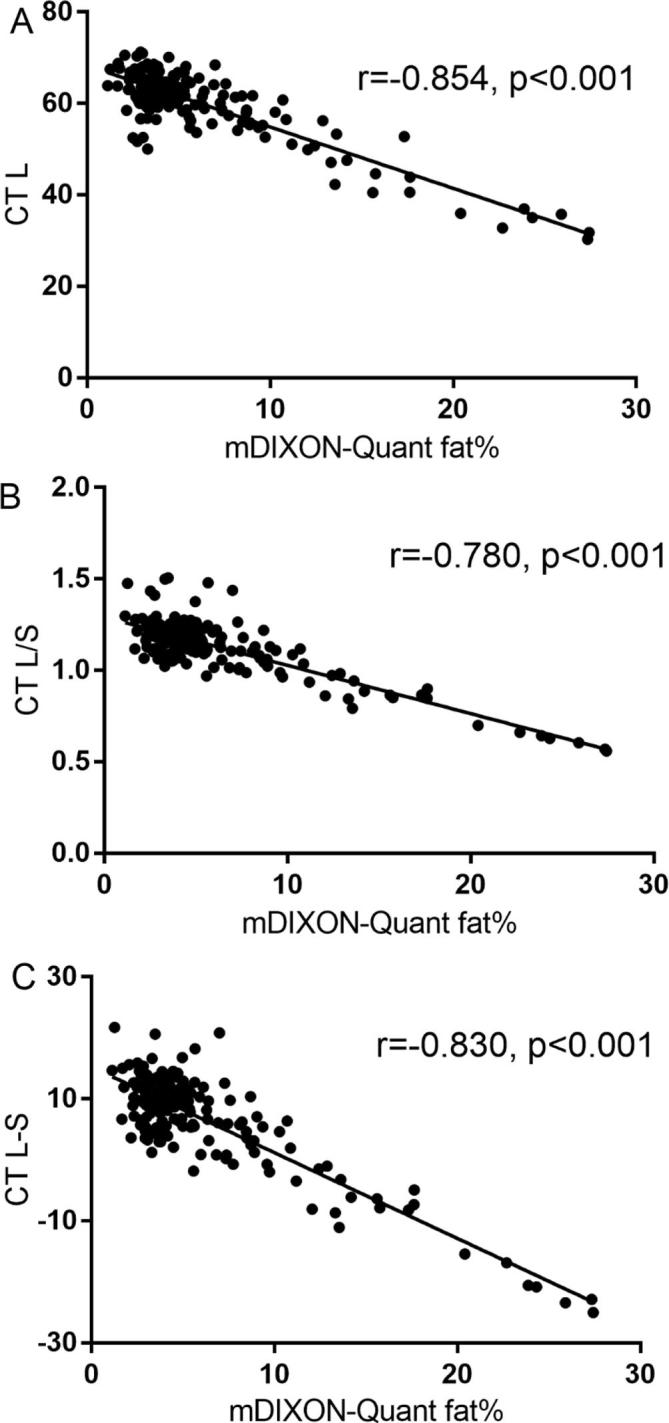
Correlation coefficients between CT and mDIXON-quant MR imaging in the measurement of hepatic steatosis (adjusted by R2*). (A) Hepatic attenuation (CT L); (B) the ratio of hepatic attenuation to splenic attenuation (CT L/S); (C) hepatic-splenic attenuation difference (CT L-S).
Diagnosis of hepatic steatosis using standard criteria
Based on the standard criteria, the detection rate of hepatic steatosis was found to be 8.3% (14/169), 14.2% (24/169), and 28.4% (48/169) for CT L, CT L/S, and CT L-S, respectively. Moreover, the detection rate of hepatic steatosis with mDIXON-Quant MR imaging was 38.4% (65/169), which was higher than that of the 3 CT methods. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the diagnosis of hepatic steatosis ≥5% with CT L, CT L/S, and CT L-S using the standard criteria are summarized in Table 3. The kappa value for was 0.253–0.461 for the 3 CT methods, in comparison with mDIXON-Quant MR imaging.
Table 3.
Diagnostic performance of CT using standard criteria for the diagnosis of hepatic steatosis ≥5%, in comparison with that of mDIXON-Quant MRI of hepatic fat content
| CT L | CT L/S | CT L-S | |
| Standard criterion | ≤48.0 | ≤1.0 | ≤5.0 |
| Sensitivity (%) | 21.5 (14/65) | 36.9 (24/65) | 55.4 (36/65) |
| Specificity (%) | 100.0 (104/104) | 100.0 (104/104) | 88.5 (92/104) |
| PPV (%) | 100.0 (14/14) | 100.0 (24/24) | 75.0 (36/48) |
| NPV (%) | 67.1 (104/155) | 71.7 (104/145) | 86.0 (92/121) |
| McNemar test (P value) | 0.000 | 0.000 | 0.012 |
| Kappa | 0.253 | 0.419 | 0.461 |
NPV, negative predictive value; PPV, positive predictive value.
Diagnosis of hepatic steatosis using the cut-off values
The diagnostic performance was calculated for the different CT methods in the cases with hepatic steatosis ≥5, ≥10, and ≥15%, and is summarized in Table 4. We performed ROC curve fitting for CT L, CT L/S, and CT L-S. We observed that the area under the ROC curve (AUC) was 0.82, 0.97, and 0.99 for CT L; 0.79, 0.98, and 0.99 for CT L/S; and 0.80, 0.98, and 0.99 for CT L-S in the diagnosis of hepatic steatosis ≥5, ≥10, and ≥15%, respectively (Figure 4). Furthermore, the Youden index was found to be 0.55, 0.81, and 0.93 for CT L; 0.47, 0.86, and 0.97 for CT L/S; and 0.49, 0.86, and 0.97 for CT L-S in the diagnosis of hepatic steatosis ≥5, ≥10, and ≥15%, respectively.
Table 4.
Diagnostic performance of CT using the cut-off values for diagnosing hepatic steatosis ≥5, ≥10, and ≥15%, in comparison with that of mDIXON-Quant MRI of hepatic fat content
| CT L | CT L/S | CT L-S | |
| Hepatic steatosis (≥5%, and <10%) | |||
| Cutoff Value | ≤58.9 | ≤1.06 | ≤6.21 |
| Sensitivity (%) | 64.6 (51.8, 76.1) | 50.8 (38.1, 63.4) | 67.7 (54.9, 78.8) |
| Specificity (%) | 90.4 (83.0,95.3) | 96.2 (90.4, 98.9) | 81.7 (72.9, 88.6) |
| McNemar test (p value) | 0.035 | 0.000 | 0.875 |
| Kappa | 0.571 | 0.495 | 0.497 |
| Youden index | 0.55 | 0.47 | 0.49 |
| AUC | 0.82 (0.75, 0.87) | 0.79 (072, 0.85) | 0.80 (0.72, 0.86) |
| Hepatic steatosis (≥10%, and <15%) | |||
| Cutoff value | ≤56.5 | ≤0.98 | ≤−1.04 |
| Sensitivity (%) | 91.3 (72.0, 98.9) | 87.0 (66.4, 97.2) | 87.0 (66.4, 97.2) |
| Specificity (%) | 89.7 (83.6, 94.1) | 98.6 (95.1, 99.8) | 98.6 (95.1, 99.8) |
| McNemar test (p value) | 0.001 | 1.000 | 1.000 |
| Kappa | 0.639 | 0.872 | 0.872 |
| Youden index | 0.81 | 0.86 | 0.86 |
| AUC | 0.97 (0.93, 0.99) | 0.98 (0.94, 0.99) | 0.98 (0.94, 0.99) |
| Hepatic steatosis (≥15%, and <30%) | |||
| Cutoff Value | ≤52.8 | ≤0.90 | ≤−4.93 |
| Sensitivity (%) | 100.0 (73.5, 100.0) | 100.0 (73.5, 100.0) | 100.0 (73.5, 100.0) |
| Specificity (%) | 93.0 (87.8, 96.5) | 97.5 (93.6, 99.3) | 97.5 (93.6, 99.3) |
| McNemar test (p value) | 0.001 | 0.125 | 0.125 |
| Kappa | 0.653 | 0.845 | 0.845 |
| Youden index | 0.93 | 0.97 | 0.97 |
| AUC | 0.99 (0.97, 1.00) | 0.99 (0.97, 1.00) | 0.99 (0.96, 1.00) |
AUC, area under the ROC curve. 95% CI in the brackets.
Figure 4.
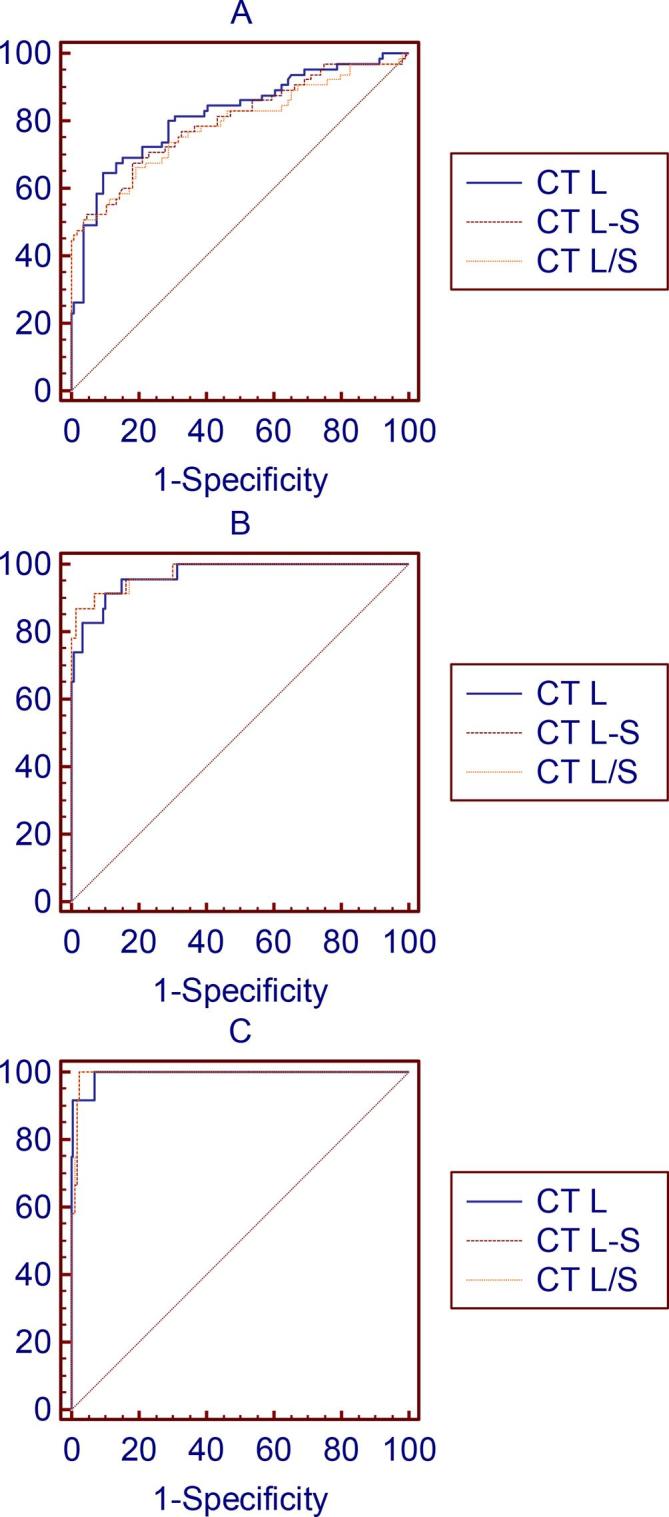
Receiver operating characteristic curve of hepatic attenuation (CT L), the ratio of hepatic attenuation to splenic attenuation (CT L/S), and hepatic-splenic attenuation difference (CT L-S), established using the cut-off values for the diagnosis of steatosis (hepatic fat content of 5–10% (A), 10–15% (B), and 15–30% (C) on mDIXON-Quant MRI).
Discussion
NAFLD is one of the most frequent kinds of chronic liver disease in Western countries and China, and affects more than one-third of the patients with chronic liver disease. The working hypothesis of the pathogenesis of non-alcoholic steatohepatitis indicates that a first hit results in the development of steatosis.23 Thus, hepatic steatosis is actually a key element of steatohepatitis. Although the quantification and assessment of steatosis does not provide a total indication of the severity of NAFLD, these factors could play an important role in the screening and surveillance of NAFLD, monitoring of steatosis following treatment, and preoperative evaluation of hepatic surgery and transplantation. Considering the high prevalence of NAFLD as well as its hepatic and extrahepatic consequences, the evaluation of hepatic steatosis has become an extremely important topic. Hence, there is a crucial necessity to found accurate, effective, and noninvasive methods for diagnosing hepatic steatosis.
In the present study, we assessed the effectiveness of the 3 different CT approaches (liver attenuation only, liver/spleen attenuation ratio, liver minus spleen attenuation) for the quantification of hepatic fat content, measured using mDIXON-Quant MR imaging. Our results indicate that there is a good association in hepatic steatosis measurement between mDIXON-Quant and CT L, CT L/S, and CT L-S. When standard criteria were used to diagnose hepatic steatosis >5%, we found that the sensitivity varied from 21.5 to 55.4% and the specificity ranged from 88.5 to 100%. Thus, the sensitivity of those criteria is low, consistent with the findings of previous studies.24–26 Moreover, the application of the standard criteria with the 3 different CT methods showed low agreement, which means that it would underestimate mild hepatic steatosis. In addition, the standard criteria were not very suitable for the quantitative evaluation of mild hepatic steatosis. Nevertheless, the use of a cut-off value for the diagnosis of hepatic steatosis >5% yielded a better diagnostic performance; in that case, the sensitivity ranged from 50.8 to 67.7% and the specificity ranged from 81.7 to 96.2%.
Our results showed that, with the mDIXON-Quant method, the use of cut-off values for the diagnosis of hepatic steatosis >10% and>15% was relatively more appropriate than the use of cut-off values for the diagnosis of hepatic steatosis >5%. In cases with hepatic steatosis >10%, the sensitivity of CT-L, CT-L/S, and CT-L-S was 97.3, 87.0, and 87.0%, respectively, whereas in cases with hepatic steatosis >15%, the sensitivity of CT L, CT L/S, and CT L-S was 93.0, 97.5, and 97.5%, respectively. The AUC was 0.82, 0.97, and 0.99 for CT L; 0.79, 0.98, and 0.99 for CT L/S; and 0.80, 0.98, and 0.99 for CT L-S in the diagnosis of hepatic steatosis >5, >10, and >15%, respectively. Based on the ROC curves, we found that the cut-off values for diagnosing hepatic steatosis >5, >10, and >15% were 58.9, 56.5, and 52.8 HU for CT L; 1.06, 0.98, and 0.90 HU for CT L/S; and 6.21,–1.04, and −4.93 HU for CT L-S, respectively. Thus, the close agreement between the 3 different CT methods (with our cut-off values) and the mDIXON-Quant method indicated that CT could be suitable for diagnosing hepatic steatosis >10%.
Patients with NAFLD frequently exhibit a mild grade of steatosis, there are some studies support it.27, 28 In the study of Kühn JP, 2561 white participants (1336 females) were prospectively recruited to the Study of Health in Pomerania (SHIP), liver steatosis was classified as mild (fat content 5–14%) in 27.2% (696 participants).27 The frequency of the mild grade is the largest. And of the 2287 members of the cohort in Szczepaniak LS’s study, The frequency of fat content 5–10% is the largest for hepatic triglyceride content exceeding 5%.28 Moreover, the split of the data into >5, >10, >15% thresholds helps to quantitative estimation of hepatic fat content in potential living liver donors29 and diagnosis metabolic syndrome.30 In a study of Ducluzeau PH, hepatic fat fraction increased gradually with the number of MetS components. Among patients with fewer than two MetS criteria, hepatic fat fraction showed a median of 4.4% and a mean of 6.7%, whereas the medians with two, three and four criteria were5.12%, 7.7 and 17.9%, respectively.30
Although liver biopsy remains to be the gold standard for the quantification of hepatic steatosis, it does not appear to be suitable for screening and repeated monitoring of NAFLD, as it is associated with certain complications, occasionally requires hospitalization, and results in significant bleeding.30
CT has proven to be a useful method for noninvasively diagnosing the presence and quantifying the severity of liver fat. Steatosis leads to the reduced attenuation of the liver and manifests as hypodense liver parenchyma, which can be expressed as HU. Liver attenuation alone upon unenhanced CT, without having comparison with splenic attenuation, is an useful forecaster of hepatic fat content and is also highly specific for the diagnosis of moderate/severe hepatic steatosis.31 The hepatic attenuation index, liver to spleen attenuation ratio, and difference between liver and spleen attenuation can be used for the evaluation of steatosis. Since the spleen is devoid of fat, it can be used as an internal control for the degree of penetrance of the scan and for image quality.32
Furthermore, mDIXON-Quant MR imaging could enable more accurate and efficient measurements of tissue fat content in a single sequence. Previous studies have compared the accuracy of this MR technique with that of histologic grading.33, 34 A previous study showed that the histological fat quantification was excellently correlated between six-echo mDIXON-Quant and MRS (R = 0.984, 0.967, respectively).33 mDIXON-Quant is a faster imaging method, with a higher resolution, as compared to other MR methods. Depending on the original approach applying addition and periodical MR signal cancelation throughout spin precession in lipids as well as water, the modified Dixon sequences (mDIXON-Quant) enable flexible TE, without any specific restriction to exact in-phase/opposed-phase values. In addition to the inclusion of a multipeak spectral model with a number of lipid components, mDIXON-Quant could be expanded to six-echo mDIXON-Quant (6E-mDixon-Quant) to enable corrections or estimations of T 2* decay in the liver.35 Unlike CT and US, which assess hepatic steatosis by means of proxy parameters, mDIXON-Quant can directly gauge the quantity of hepatic fat. These findings suggest that mDIXON-Quant is more effective than ultrasound and CT in the detection of separate disease grades, particularly for mild disease (<30% steatosis). Compared to the mDIXON-Quant method, the threshold values of the CT indices for the evaluation of hepatic steatosis varied, depending on the methods and population used.36 In addition, CT has limitations in patients with hepatic iron overload, acute hepatitis, acute toxic hepatic injury, or cirrhosis.37 Furthermore, the potential hazard involving ionizing radiation tends to make CT inappropriate for use in children or for the longitudinal monitoring of patients with NAFLD.15 In MR images, R2* value is well known to have a linear relationship to hepatic iron concentration and R2* value is preferred because it directly correlate with iron content.27, 38 The larger the R2 or R2* value, the higher the iron content. MR mDIOXN-Quant imaging method also demonstrated excellent promise for quantifying liver iron content through estimation of R2*.27, 38 In our study, after adjusted by R2*, linear correlation analyses also indicated a strong correlation of the results of liver fat content between the 2 imaging modalities. In addition, changes in the CT values in the spleen due to disease would affect the results of CT-L/S and CT-L- S.38
A homogenous distribution of the hepatic fat infiltration may cause sampling error during liver biopsy,38 and the utilizing of multiple ROIs in both the left and right liver lobes may help decrease CT number variability because of the heterogeneity. Therefore, in the present study, the mean values from the 6 ROIs in the left and right lobes of the liver would yield a more representative quantification.
The present study has certain limitations. First, liver biopsy was not applied as the standard in the present study; instead, mDIXON-Quant MR imaging was used as the reference standard. Second, the number of subjects with hepatic steatosis >5%, as measured by the mDIXON-Quant method, was not large (n = 65). Third, our study primarily included subjects who were young, healthy, and relatively lean.
In conclusion, when standard l criteria published in the literature were used, we found that the 3 CT methods exhibited moderate agreements with mDIXON-Quant in the diagnosis of hepatic steatosis >5%. However, when the cut-off values from the present study were used, these 3 methods exhibited better agreement with mDIXON-Quant in the diagnosis of hepatic steatosis >10%. Thus, CT and the MR mDIXON-Quant sequence could be suitable for the accurate quantification of mild hepatic steatosis in clinical practice.
Footnotes
Funding: This work was supported by Beijing Municipal Bureau of Health of 215 program [grant number 2009-02-03]; and Capital Health Research and Development of Special [grant number 2014-2-1122]. The funding agencies had no role in the study design, the collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication.
Disclosure: The results and conclusions of this study are those of the authors.
Contributor Information
Yong Zhang, Email: jinxiabilan@163.com.
Chao Wang, Email: chaowangjst@163.com.
Yangyang Duanmu, Email: 569456884@qq.com.
Chenxin Zhang, Email: 359136653@qq.com.
Wei Zhao, Email: 670030762@qq.com.
Ling Wang, Email: 1988yisheng@163.com.
Xiaoguang Cheng, Email: xiao65@263.net.
Nicola Veronese, Email: ilmannato@gmail.com.
Giuseppe Guglielmi, Email: giuseppe.guglielmi@unifg.it.
REFERENCES
- 1. Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women's heart and health study and meta-analysis. Diabetes Care 2009; 32: 741–50. doi: 10.2337/dc08-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zelber-Sagi S, Webb M, Assy N, Blendis L, Yeshua H, Leshno M, et al. Comparison of fatty liver index with noninvasive methods for steatosis detection and quantification. World J Gastroenterol 2013; 19: 57–64. doi: 10.3748/wjg.v19.i1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol 2013; 28(Suppl 1): 11–17. doi: 10.1111/jgh.12036 [DOI] [PubMed] [Google Scholar]
- 4. Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int 2006; 26: 856–63. doi: 10.1111/j.1478-3231.2006.01311.x [DOI] [PubMed] [Google Scholar]
- 5. Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, et al. Prevalence of fatty liver in Japanese children and relationship to obesity. Dig Dis Sci 1995; 40: 2002–9. doi: 10.1007/BF02208670 [DOI] [PubMed] [Google Scholar]
- 6. Omagari K, Kadokawa Y, Masuda J, Egawa I, Sawa T, Hazama H, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol 2002; 17: 1098–105. doi: 10.1046/j.1440-1746.2002.02846.x [DOI] [PubMed] [Google Scholar]
- 7. Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut 2009; 58: 1538–44. doi: 10.1136/gut.2008.171280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 2010; 59: 969–74. doi: 10.1136/gut.2009.205088 [DOI] [PubMed] [Google Scholar]
- 9. Selzner M, Clavien P-A. Fatty liver in liver transplantation and surgery. Paper presented at: Seminars in liver disease 2000. [DOI] [PubMed] [Google Scholar]
- 10. Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005; 42: 132–8. doi: 10.1016/j.jhep.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 11. Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol 2007; 102: 399–408. doi: 10.1111/j.1572-0241.2006.01041.x [DOI] [PubMed] [Google Scholar]
- 12. Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, et al. Risk factors for primary dysfunction after liver transplantation-a multivariate analysis. Transplantation 1993; 55: 807–13. doi: 10.1097/00007890-199304000-00024 [DOI] [PubMed] [Google Scholar]
- 13. Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ 2005; 172: 899–905. doi: 10.1503/cmaj.045232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fitzpatrick E, Dhawan A. Noninvasive biomarkers in non-alcoholic fatty liver disease: current status and a glimpse of the future. World J Gastroenterol 2014; 20: 10851–63. doi: 10.3748/wjg.v20.i31.10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SS, Park SH. Radiologic evaluation of nonalcoholic fatty liver disease. World J Gastroenterol 2014; 20: 7392–402. doi: 10.3748/wjg.v20.i23.7392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 2009; 51: 1061–7. doi: 10.1016/j.jhep.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karcaaltincaba M, Akhan O. Imaging of hepatic steatosis and fatty sparing. Eur J Radiol 2007; 61: 33–43. doi: 10.1016/j.ejrad.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 18. Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology 2009; 251: 67–76. doi: 10.1148/radiol.2511080666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reeder SB, Sirlin CB. Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am 2010; 18: 337–57. doi: 10.1016/j.mric.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yajima Y, NARUI T, ISHII M, ABE R, OHTSUKI M, GOTO Y, et al. Computed tomography in the diagnosis of fatty liver: total lipid content and computed tomography number. Tohoku J Exp Med 1982; 136: 337–42. doi: 10.1620/tjem.136.337 [DOI] [PubMed] [Google Scholar]
- 21. Association ALDSGoCLD Fatty Liver and Alcoholic Liver Disease Study Group of Chinese Liver Disease Association, ALDSGoCLD A. Diagnostic criteria of nonalcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi 2003; 11: 71. [PubMed] [Google Scholar]
- 22. Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology 2004; 230: 276–80. doi: 10.1148/radiol.2301021176 [DOI] [PubMed] [Google Scholar]
- 23. McCullough AJ. Update on nonalcoholic fatty liver disease. J Clin Gastroenterol 2002; 34 255–62. doi: 10.1097/00004836-200203000-00013 [DOI] [PubMed] [Google Scholar]
- 24. Lee SS, Park SH, Kim HJ, Kim SY, Kim MY, Kim DY, et al. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol 2010; 52: 579–85. doi: 10.1016/j.jhep.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 25. Lee JY, Kim KM, Lee SG, Yu E, Lim YS, Lee HC, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol 2007; 47: 239–44. doi: 10.1016/j.jhep.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 26. Palmentieri B, de Sio I, La Mura V, Masarone M, Vecchione R, Bruno S, Sio D I, Mura L V, et al. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis 2006; 38: 485–9. doi: 10.1016/j.dld.2006.03.021 [DOI] [PubMed] [Google Scholar]
- 27. Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005; 288: E462–E468. doi: 10.1152/ajpendo.00064.2004 [DOI] [PubMed] [Google Scholar]
- 28. Maruzzelli L, Parr AJ, Miraglia R, Tuzzolino F, Luca A. Quantification of hepatic steatosis: a comparison of computed tomography and magnetic resonance indices in candidates for living liver donation. Acad Radiol 2014; 21: 507–13. doi: 10.1016/j.acra.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 29. Ducluzeau PH, Boursier J, Bertrais S, Dubois S, Gauthier A, Rohmer V, et al. MRI measurement of liver fat content predicts the metabolic syndrome. Diabetes Metab 2013; 39: 314–21. doi: 10.1016/j.diabet.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 30. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001; 344: 495–500. doi: 10.1056/NEJM200102153440706 [DOI] [PubMed] [Google Scholar]
- 31. Pickhardt PJ, Park SH, Hahn L, Lee SG, Bae KT, Yu ES, . Specificity of unenhanced CT for non-invasive diagnosis of hepatic steatosis: implications for the investigation of the natural history of incidental steatosis. Eur Radiol 2012; 22: 1075–82. doi: 10.1007/s00330-011-2349-2 [DOI] [PubMed] [Google Scholar]
- 32. Zeb I, Li D, Nasir K, Katz R, Larijani VN, Budoff MJ. Computed tomography scans in the evaluation of fatty liver disease in a population based study: the multi-ethnic study of atherosclerosis. Acad Radiol 2012; 19: 811–8. doi: 10.1016/j.acra.2012.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013; 58: 1930–40. doi: 10.1002/hep.26455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 2008; 60: 1122–34. doi: 10.1002/mrm.21737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ma X, Holalkere NS, Kambadakone R A, Mino-Kenudson M, Hahn PF, Sahani DV. Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics 2009; 29: 1253–77. doi: 10.1148/rg.295085186 [DOI] [PubMed] [Google Scholar]
- 36. Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 2009; 51: 433–45. doi: 10.1016/j.jhep.2009.05.023 [DOI] [PubMed] [Google Scholar]
- 37. Rabushka LS, Kawashima A, Fishman EK. Imaging of the spleen: CT with supplemental MR examination. Radiographics 1994; 14: 307–32. doi: 10.1148/radiographics.14.2.8190956 [DOI] [PubMed] [Google Scholar]
- 38. Tchelepi H, Ralls PW, Radin R, Grant E. Sonography of diffuse liver disease. J Ultrasound Med 2002; 21: 1023–32. doi: 10.7863/jum.2002.21.9.1023 [DOI] [PubMed] [Google Scholar]