Abstract
Theranostics and its principles: pre-treatment selection of patients who are most likely to benefit from treatment by the use of a related, specific diagnostic test are integral to the treatment of patients with neuroendocrine tumours (NETs). This is due to NETs' important, but variable, somatostatin receptor (SSTR) expression, their heterogeneity and variation in site of primary and rate of progression. Only patients whose tumours have sufficient expression of SSTRs will benefit from SSTR-based radionuclide therapy and demonstrating this expression prior to therapy is essential. This article provides a relevant overview of NETs and the multiple facets of SSTR based theranostics, including imaging and therapy radionuclides; clinical efficacy and toxicity; patient selection and treatment and finally emerging radiopharmaceuticals and newer clinical applications.
Introduction
Neuroendocrine tumours (NETs) are a heterogeneous group of tumours in which the cells display neuroendocrine characteristics. NETs can originate from pancreatic islet cells (pNETs), gastroenteric tissue [together: gastroenteropancreatic (GEP)] and respiratory epithelium.1 Two of the strongest prognostic markers are tumour differentiation and proliferation. NETs can be well- or poorly-differentiated (depending on how closely the tumour resembles neuroendocrine cells). Tumour grade refers to the proliferative activity of the tumour, measured by the Ki-67 proliferative index and/or the mitotic rate and is divided into Grade 1 (G1): Ki-67 ≤2%, Grade 2 (G2): Ki-67 3–20% and Grade 3 (G3): Ki-67 > 20%2 with G1 being the least proliferative and G3 the most proliferative2–4
Another characteristic of NETs is their ability to secrete a large number of bioactive substances, e.g. chromogranin A (CgA), serotonin, gastrin and insulin.1 The classic carcinoid syndrome results predominantly in patients with liver metastases that produce serotonin which, when not broken down by the liver, produce symptoms including diarrhoea, wheezing and flushing which can have a significant negative impact on a patient’s quality of life (QoL).5
When NET is suspected investigations include biochemical testing (e.g. for serum CgA and urinary 5-hydroxyindoleacetic acid, the breakdown product of serotonin) and imaging such as ultrasound, CT and MRI. In addition to conventional radiology, functional/molecular or nuclear imaging is of particular importance in the diagnosis, staging and treatment decisions of well differentiated NETs. A large percentage of NETs tend to retain the properties of neuroendocrine cells and express somatostatin receptors (SSTRs) on their surface. The well differentiated tumours typically express SSTRs, of which there are five different subtypes, in particular SSTR subtype 2 (SSTR2) is (over)expressed by NETs.6 The over expression of SSTRs is exploited by using radiolabelled somatostatin (SST) peptide analogues resulting in a highly sensitive and specific imaging modality.
There are several licenced and unlicensed therapeutic options for progressive metastatic NETs where no surgical cure is possible. Some of the more commonly employed treatments include long-acting somatostatin analogues (SSAs), local liver therapies, chemotherapy, molecular targeted treatments (everolimus and sunitinib), interferon and peptide receptor radionuclide therapy (PRRT). Of these therapies, PRRT appears to provide the greatest benefit in terms of progression-free survival (PFS) with the recent NETTER 1 trial reporting a PFS of approximately 40 months in patients with progressive midgut NETs.7 In keeping with the principles of theranostics both imaging and therapeutic radionuclides can be bound to the SST analogue via the same mechanism allowing targeted treatment of the previously imaged disease. The diagnostic and therapeutic agents form a theranostic pair.
Molecular imaging of NETs
Somatostatin receptor Scintigraphy
There are a number of different SST peptide analogues which can be used to bind to SSTRs and image NETs. One of the first and most widely used radiopharmaceuticals was 111-Indium-DTPA-octreotide. Octreotide is a synthetic peptide that binds to SSTRs and 111-Indium (111In) is a gamma emitter. 111In has a half-life of 67h and delayed imaging (24 or 48 h) is usually required to ensure reduction in background activity caused by clearance via the renal and hepatobiliary system. SST analogues have also been radiolabelled with 99m-Technetium (99mTc), which has imaging and logistic advantages (can be performed on a single day) over 111In. These include 99mTc-EDDA/HYNIC-octreotate,8 or 99mTc-EDDA/HYNIC-octreotide.9 However, these tracers are currently less widely used than 111In-octreotide.
Newer DOTA-coupled peptides which are bound to the positron emitter 68-Gallium (68Ga) have been developed which have advantages over octreotide. DOTATOC (DOTA-Tyr3-octreotide) is lipophilic, with better affinity for SSTR2 and higher uptake in SSTR2 positive tumours than octreotide.10 Another analogue is DOTATATE (DOTA-D-Phe1-Tyr3-octreotide) and newer still is DOTANOC (DOTA, 1-Nal3-octreotide) which binds avidly to SSTR2 and also has higher affinity for SSTR3 and SSTR5 than DOTATATE.11 There are also pansomatostatin analogues which target multiple SSTR subtypes e.g. DOTA0-lanreotide.12
In addition to higher receptor affinity, there are other pharmacological advantages of the DOTA peptides over octreotide. These include maximum tumour activity occurring about 70 mins after injection and excretion almost entirely through the kidneys.13 This is in contrast to 111In-pentetreotide where 2% is excreted through the hepatobiliary system and then via the bowel and 1% of injected activity remains in the blood pool 20 h after injection.14 These differences confer advantages both to scanning protocols and image interpretation. The chelating of the DOTA peptides to the positron emitter 68Ga results in superior sensitivity and resolution of these images compared to images acquired with 111In-octreotide. The characteristics of the commonly used 68Ga labelled peptides for imaging NETs are displayed in Table 1.13,15–20
Table 1.
Common 68Ga-labelled peptides for imaging NETs
| 68Ga-DOTATOC | 68Ga-DOTATATE | 68Ga-DOTANOC | |
| Half life | 68 min | 68 min | 68 min |
| Production | Generator | Generator | Generator |
| Injected activity | Ranges from 100 to 150 MBq | Ranges from 100 to 150 MBq | Ranges from 100 to 150 MBq |
| Route of synthesis | Chelation of generator-produced 6 8Ga chloride by DOTATOC at elevated temperature. May be preceded or followed by purification step. | Chelation of generator-produced 68Ga chloride by DOTATATE at elevated temperature. May be preceded or followed by purification step. | Chelation of generator-produced 68Ga chloride by DOTANOC at elevated temperature. May be preceded or followed by purification step. |
| Radiation dosimetry | Effective dose equivalent (mSv/MBq): 0.021 (2 mSv/100 MBq) Organ doses (mGy/MBq): urinary bladder wall, 0.119; spleen, 0.108; kidney, 0.082; adrenal gland, 0.077 |
Effective dose equivalent (mSv/MBq): 0.021 (2 mSv/100 MBq) Organ doses (mGy/MBq): spleen, 0.109; urinary bladder wall, 0.098; kidney, 0.093; adrenal gland, 0.086 |
Effective dose equivalent (mSv/MBq): 0.025 (2.5 mSv/100 MBq) Organ doses (mGy/MBq): kidney, 0.090; urinary bladder wall, 0.084; spleen, 0.073; liver, 0.034 |
| Normal biodistribution | Activity initially high in liver, spleen and kidneys. | Activity initially high in liver, spleen and kidneys. | Activity initially high in liver, spleen and kidneys. |
| Excretion | Gradual clearance with 16% urinary excretion over 4 h. | Gradual clearance with 12% urinary excretion over 4 h. | Gradual clearance with 25% urinary excretion over 4 h. |
| Affinity profile | 68Ga-DOTA-TOC binds to SSTR2 also binds to SSTR5 | 68Ga-DOTA-TATE has a predominant affinity for SSTR2 | 68Ga-DOTA-NOC binds to SSTR2 also shows a good affinity for SSTR3 and 5 |
| Clinical application | Assessment of SSTR status of NETs and selection of patients for PRRT | Assessment of SSTR status of NETs and selection of patients for PRRT | Assessment of SSTR status of NETs and selection of patients for PRRT |
NETs, neuroendocrine tumours; PRRT, peptidereceptor radionuclide therapy;
Systematic reviews and meta-analyses have demonstrated SSTR PET-CT’s high accuracy, high rates of change in patient management and its superiority to 111In- octreotide. For example a meta-analysis of the use of 68Ga-DOTATOC found it had a sensitivity of 92% and specificity of 82%. It demonstrated an impact on subsequent NET patient management in 51%. The sensitivity of DOTATOC on a per-lesion basis was 100%, and for 111In-octreotide it was 78%.21 A systematic review and meta-analysis of 68Ga-DOTATATE compared with 111In-DTPA-octreotide found there were three studies which compared the two radiopharmaceuticals in the same patient, finding 68Ga-DOTATATE had an estimated sensitivity of 90.9% and specificity of 90.6% and was more sensitive than octreotide.22
Another advantage of the PET tracers is that quantification of tumour uptake of tracer can be performed which is not possible with SPECT.23 A close correlation has been found between maximum standardised uptake value (SUVmax) and quantitative assessment of the density of subtypes of SSTR using immunohistochemical scores.24 Also, SUVmax of the tumour of uptake of 68Ga-DOTATOC has been found to correlate with response to PRRT.25
More recently. SSTR antagonists are starting to enter clinical use, these are discussed below.
FDG
18-F-fludeoxyglucose (FDG) PET-CT does not have a therapeutic counterpart, however its role in imaging is still relevant when discussing SSTR theranostics. Typically, as Ki-67 and grade of tumour increases, the intensity/extent of FDG uptake in the tumour increases, although low grade and well differentiated NETs can still be FDG avid.26
FDG positivity is a very strong negative prognostic factor. A prospective study of 38 patients found that patients with a negative FDG PET-CT had overall survival (OS) of 119.5 vs 15 months for those with positive FDG PET. Even in patients with a low grade GEPNET and a positive SSTR scintigraphy, PFS and OS were significantly lower for patients with a positive FDG PET.27 A different study found that FDG positivity conferred a hazard ratio of 10.3 exceeding the prognostic value of Ki-67, CgA and liver metastases.28
In terms of the current use of FDG PET-CT in NET: it is usually the preferred tracer for G3 NETs, as well as for some high-grade G2 tumours. Its use in G1 and low-grade G2 tumours is not yet fully defined, a Ki-67 >10% is often considered as a cut-off.29 FDG-PET-CT should also be considered if lesions are identified on anatomical imaging (CT or MRI), which are not SSTR tracer avid or if they are progressing fast as these could represent de-differentiated disease.30
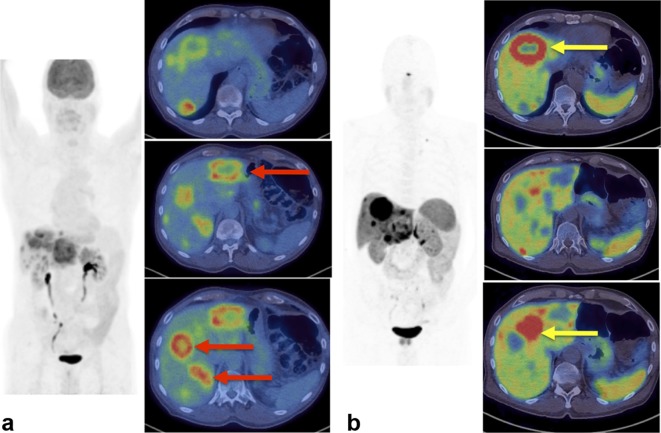
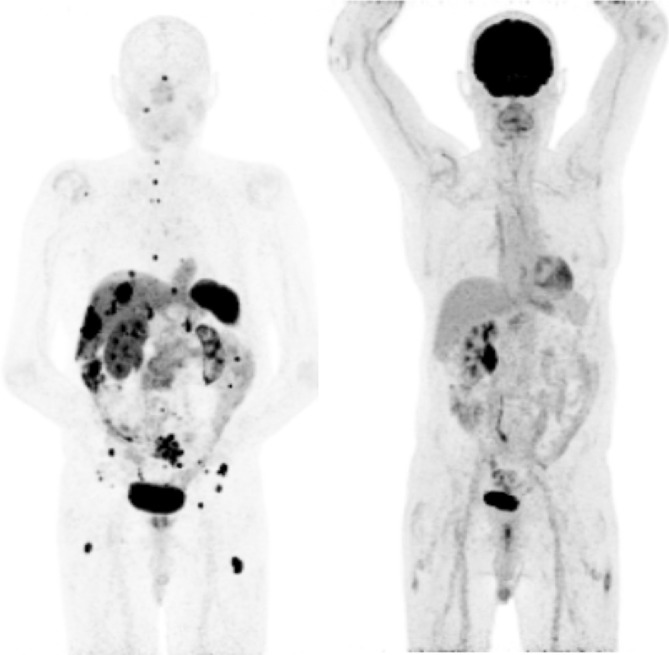
Currently, the combination of FDG and 68Ga somatostatin receptor scintigraphy (SRS) is often performed together in patients with G2/G3 tumours or if there has been relatively quick progression of disease to determine if there is discordant disease, i.e. FDG positive, SRS negative disease. The combination of these two scans helps plan treatment appropriately: if patients have lesions that are FDG-positive, SRS-negative, these patients should not be treated with PRRT as the first option, but treatment of the FDG-positive lesions should be prioritised (chemotherapy/local treatments). Figures 1 and 2 show two patients, one of which is suitable for PRRT (68Ga DOTATATE+, FDG–), the second of which is not suitable for PRRT (68Ga+; FDG+ but with discordant lesions). Figure 3 shows that tracer distribution on pre-therapy SRS (68Ga-DOTATATE) and post-therapy imaging (177Lu-DOTATATE) is the same.
Figure 1. .
A 64-year-old patient with G2 metastatic midgut NET who underwent staging with 18F-FDG and 68Ga-DOTATATE. The 68Ga-DOTATATE demonstrated multiple sites of avid disease within a mesenteric mass, abdominal nodes, liver and skeletal deposits. All of these lesions were not avid on 18F-FDG. 18-fludeoxyglucose; NETs, neuro endocrine tumours.
Figure 2. .
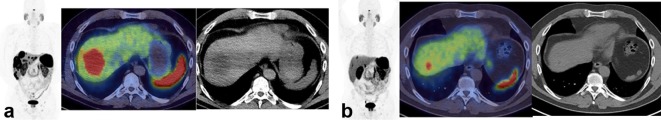
A 72-year-old gentleman with G2 pancreatic NET who had PD on CT, underwent staging with 18F-FDG (a) and 68Ga-DOTATATE (b), consideration for PRRT. There are several tracer avid liver metastases. The yellow arrows demonstrate two lesions that are DOTATATE avid and to a lesser degree FDG avid. The three red arrows demonstrate lesions that are FDG avid but 68Ga negative. As this patient has discordant disease that is progressive, he is not suitable for PRRT. 18-fludeoxyglucose; NETs, neuroendocrine tumours; PRRT, peptide receptor radionuclide therapy.
Figure 3. .
A 71-year-old male with midgut NET, small bowel resection, had pre-therapy 68Ga-DOTATATE (a) and post-therapy 177Lu-DOTATATE (b) imaging, demonstrating similar tracer distribution with avid liver and mesenteric node. NETs, neuro endocrine tumours.
There has been recent interest in the more systematic use of FDG PET-CT in patients with NET, this is due to the variable outcome of patients within the same category of NET (e.g. grade and primary) and the recognition of the strong negative association between FDG avidity and prognosis described above. A “NETPET” score has been developed which combines the uptake on both SSTR and FDG PET-CT into a single reportable value which has prognostic significance.31 In future, more routine use of dual tracer imaging might be used to decide on when to use a watch and wait strategy vs SSTR analogues vs PRRT vs chemotherapy in addition to guiding sites for biopsy.26
Selection of patients for PRRT based on molecular imaging
In line with the theranostic principle, compounds which bind to SSTRs can also be labelled with therapeutic radionuclides, allowing treatment of the previously imaged disease. This is often referred to as PRRT. The key factor in assessing suitability of a patient for PRRT is SRS. Sufficient uptake within tumours on the SSTR scan provides evidence that the tumours are likely to concentrate radioactivity in sufficient quantities to achieve tumour damage (the theranostic principle, illustrated in Figure 3). Based on SSTR imaging, the liver is commonly used as the reference point (the liver having tracer uptake related to hepatic peptide metabolism). Uptake greater than that seen in the background liver in the majority of disease (i.e. >90%) is generally considered sufficient to proceed with PRRT, this criterion is used by many centres32,33 and was used in a recently published randomised controlled trial (RCT) on the use of PRRT, the NETTER-1 trial.7 Partial volume effects and the resolution of the imaging system (e.g. single photon versus PET) should be taken into account when assessing small lesions as it can result in an underestimate of the true tracer uptake of the tumour.
As imaging with 68Ga- DOTA-peptides is more sensitive than 111In-octreotide, patients who do not show sufficient uptake within their tumour on 111In-octreotide should ideally be re-imaged with 68Ga-DOTA peptides. This is because there can still be sufficient uptake (≥liver) of the 68Ga-DOTA peptides to make these patients eligible for PRRT.34 Patients who still demonstrate only low-grade uptake on 68Ga-DOTA peptides (<liver) in their tumour sites are not suitable for PRRT as they are unlikely to concentrate sufficient radiation in their tumours. It has been shown that reduced tumoral uptake on SRS correlates with reduced response rates.29
Additionally, SSTR expression within tumours and metastases can decline as the disease progresses and so, it is necessary to ensure there is up-to-date SRS if there has been a long time interval between the initial SRS and the decision to treat with PRRT or if there has been a marked change in tumour behaviour.
Treatment of NETs
PRRT is a treatment option for patients with metastatic, inoperable, well-differentiated NETs that show good uptake of the radiolabelled peptide on diagnostic imaging.35,36 For patients with localised disease or with resectable hepatic metastases, potentially curative surgical resection is offered when possible.1,3,36–38 There are other systemic treatments for metastatic or locally advanced disease which depend on factors including site of primary, grade and differentiation. These include SSAs, cytotoxic chemotherapy, molecular targeted therapies including sunitinib or everolimus (particularly for pNET), interferon and liver targeted treatments (embolization, radioembolisation, radiofrequency ablation). Regarding chemotherapy, response rates are highly variable depending on site and grade of tumour. Poorly-differentiated G3 tumours are usually the most chemosensitive and well-differentiated midgut NETs the most chemoresistant.
Characteristics of the therapeutic radionuclides used in PRRT
The two most frequently used therapeutic radionuclides are 90-Yttrium (90Y) and 177-Lutetium (177Lu). Both are βbeta particle emitters with path lengths well-suited for killing tumour cells with restricted damage to neighbouring normal tissue. The half-lives are appropriate for molecular radiotherapy agents, allowing particle emission during the cell cycle of malignant cells and (hopefully) ensuring cell death. The other emissions can be used to image the distribution of the radionuclide. Since 177Lu also emits gamma rays, these can be used for dosimetry and monitoring of tumour response. Table 2 below, describes the physical characteristics of 90Y and 177Lu.
Table 2.
The physical characteristics of 90Y and 177Lu
| Radionuclide | Half life (days) | β-particle path length (mean, max) mm | Other emissions |
| 177Lu | 6.75 | 0.23, 1.7 | 113keV and 208 keV gamma rays |
| 90Y | 2.67 | 3.9, 11 | Bremsstrahlung and positrons |
Indications and patient selection for PRRT
The current timing of PRRT in the treatment of patients with incurable, well differentiated NET is not well defined and the European Neuroendocrine Tumour Society (ENETS) guidelines are not proscriptive on its use. In patients with positive SRS, SSAs should be considered as first-line treatment.35,39 In patients with midgut NET who require systemic therapy having progressed through SSAs, PRRT is usually the preferred next treatment option.35 It is also considered in patients with NETs of other primary sites, particularly when there is extensive disease, extrahepatic disease and good uptake on functional imaging.35 In pNET, there are more therapeutic options including cytotoxic chemotherapy, everolimus or sunitinib and PRRT. The treatment options for NETs, including suitability for PRRT, should be discussed in a multidisciplinary team meeting. Inclusion and exclusion criteria, below, are adapted from recent ENETs guidelines on PRRT.30
Inclusion criteria
Inoperable/metastatic well-differentiated (G1/2) NET
Well-differentiated G3 NET can be considered if it shows sufficient uptake on SSTR imaging
Sufficient tumour uptake on the diagnostic SRS (defined as tumour uptake >liver) ± FDG PET/CT demonstrating no discordant sites of uptake.
Sufficient bone marrow reserves (Grades 1–2 haematological toxicity usually accepted)
Creatinine clearance >50 mL/min
Karnofsky Performance Status > 50 or ECOG 1 or 2
Expected survival >3 months
Following MDT discussion
Exclusion criteria
Significant sites of active disease identified as contrast-enhancing lesions on CT or MRI that lack SSTR expression (i.e. negative SRS).
Moderate to severe renal impairment (i.e. creatinine clearance <50 ml min)1
Impaired haematological function2
Severe hepatic impairment3
Severe cardiac impairment4
Moderate to severe right heart valvular disease (valve replacement is encouraged prior to PRRT)
Pregnancy or ongoing lactation
Significant sites of active disease identified as contrast-enhancing lesions on CT or MRI that lack SSTR expression (i.e. negative SRS).
Moderate to severe renal impairment (i.e. creatinine clearance <50 ml min) 1
1 (patients on dialysis can be treated with a significantly reduced administered activity to account for lack of urinary excretion with dialysis delayed for 24 h after treatment, following liaison with the renal team)
2, i.e. Hb <5 mmol l−1 (8 g dl−1); platelets < 75 × 10 9/l; white blood cell count <2×10 9/l
3, i.e. total bilirubin >3. upper limit of normal or both an albumin <25 g l−1 and prothrombin time increased >1.5. upper limit of normal, indicating biosynthetic liver failure *
4 NYHA Grade 3 or 4
Clinical efficacy
90Y-DOTATOC was first used in 1996.36 Since then, multiple papers reporting on the outcomes of patients with incurable NET-treated with PRRT have been published, although the inclusion and disease response criteria are quite variable limiting direct comparison between studies. A recent meta-analysis of the use of 177Lu-DOTATATE demonstrated a pooled effect disease objective response (OR) rate (which they defined as complete response (CR) and partial response (PR)) of 29% and an average disease control rate (OR and stable disease (SD)) of 81%.40 Median PFS with PRRT has been reported as 33 months,33 26 months41 and 36 months.42
Unfortunately, in the above studies, there has been no true control population, limiting assessment of the significance of these results. This changed with the publication of the NETTER-1 trial, an RCT of 177Lu-DOTATATE vs high-dose SSAs7 in patients with incurable G1/2 midgut NET. It found that median PFS for the 177Lu-DOTATATE group was not yet reached (estimated at 40 months) and was 8.4 months for the high-dose SSA group, giving a statistically significant hazard ratio of 0.21 and a 79% reduction in the risk of disease progression/death. Figure 4 demonstrates an example of an excellent response to 177Lu-DOTATATE.
Figure 4.
A 67-year-old gentleman who had four cycles of 177Lu-DOTATATE imaging. Pre-therapy 68Ga-DOTATATE (a) imaging demonstrates multiple sites of tracer avid disease in the liver. Post-therapy 68Ga-DOTATATE imaging (b) shows an excellent response to treatment with significant reduction in size and avidity in multiple lesions (including a target lesion shown).
PRRT has also been shown to improve QoL which is an important measure given the severe symptoms which secretion of the bioactive substances can cause. A study of 265 patients43 found QoL, insomnia, appetite loss, and diarrhoea improved significantly in patients with inoperable or metastatic GEP or bronchial NET treated with 177Lu-DOTATATE.
Toxicity
The critical organs are the bone marrow and kidneys.44 The kidneys are usually the dose limiting organ, the radiopeptide is renally excreted and also reabsorbed in the proximal tubules45 and therefore retained within renal parenchyma. Bone marrow is irradiated from circulating radiopeptide and irradiation from absorbed radiopeptide into bone metastases. The most common subacute side-effect of PRRT, occurring within 4–6 weeks after therapy, is haematologic toxicity.44
Renal protection can be provided by administering intravenous lysine and arginine prior to PRRT which reduces reabsorption of the radiopeptide (competitive inhibition) but also causes nausea and, occasionally, vomiting. With adequate renal amino acid protection, Grade 3–4 renal toxicity occurs in <3% of patients.44 There are currently no forms of myeloprotection available and myelodysplastic syndromes and leukaemia have been reported.44 Table 3 (adapted from recent ENETS guidelines)30 lists the acute and subacute side effects from PRRT.
Table 3.
Side effects from PRRT
| Nausea and vomiting Abdominal pain (after 10% of administrations) Temporary mild hair loss in 60% of patients after 177Lu-DOTATATE Grade 3/4 haematological toxicity (<15% of patients) Hormonal crises due to release of bioactive substances (<1% of patients) |
PRRT, peptidereceptor radionuclide therapy;
177Lu, Lutetium-177.
There are no RCTs comparing optimal administered activity per treatment cycle, optimal cycle interval, or optimal cumulative administered activity for either 90Y- or 177Lu-labelled SST analogues. The NETTER-1 trial used up to four cycles of a fixed administered activity of 7.4 GBq per cycle, administering each cycle every 8 weeks. Renoprotection is provided with infused solutions containing lysine and arginine and intravenous fluids together with anti emetics. Blood counts, renal and liver function are checked before and between cycles. Post-treatment imaging of Bremsstrahlung for 90Y or gamma rays for 177Lu is often performed to confirm distribution of disease and uptake of PRRT and, when required, for dosimetry. Treatment is usually stopped after the pre-determined number of cycles (usually four) or if the patient progresses during treatment, identified either by imaging or symptomatic deterioration. The recent ENETs guidelines30 provide further, detailed guidance on PRRT administration requirements.
Comparison with other treatments
Table 4, below, shows that the reported PFS is better with PRRT than other treatments. Radiological response with PRRT is similar to chemotherapy, but much better than that seen in molecular targeted treatments. A study comparing 90Y and 177Lu-DOTA-peptides53 showed them to be broadly similar in terms of overall efficacy although 177Lu produces less nephrotoxicity and severe haematological toxicity than 90Y.53,54
Table 4.
Comparison of the efficacy of different treatment options for metastatic NET
| Study | Treatment | Patient group | Outcome |
| Strosberg et al7 (NETTER-1 trial) n = 229 | 177Lu-DOTATATE versus SSAs | Advanced progressive midgut NET | PFS after 20 months: 65.2 in the 177Lu-Dotatate group and 10.8 in the SSA group. OR 18% in the 177Lu-Dotatate group versus 3% in the SSA group |
| Kwekkeboom et al33(n = 310 2008 |
177Lu-DOTA-peptides | Patients with metastatic GEP NET (not necessarily progressive) | Median PFS 33 months. Radiological response: 46% OR, 35% SD. |
| Imhof et al46
n = 1109 2011 |
90Y-DOTA-peptides | Progressive metastatic NET of any primary | Median survival 45 months in those who responded, 18 months in those who progressed. Radiological response: 34% OR, 5% SD. Biochemical response 15.5%. |
| Kong et al47
n = 68 2014 |
177Lu-DOTA-peptides with 5-FU | Patients with progressive or uncontrolled symptomatic, metastatic NET of any primary site | OS 72 and 52% at 2 and 5 years. PFS not reported. Radiological response: 68% OR. Biochemical response 56% |
| Ezziddin et al48
n = 68 2014 |
177Lu-DOTATATE | Metastatic inoperable Grade 1/2 pNET | Median PFS 34 months, OS 53 months. Radiological response 72% OR, 13% SD |
| Strosberg et al49
n = 30 2011 |
Capecitabine with temozolomide | Metastatic pNET | Median PFS 18 months. Radiological response 70% OR |
| Sun et al50
n = 249 2005 |
RCT of fluorouracil with either streptozocin or doxorubicin | Metastatic carcinoid tumours | PFS 4.5–5.3 months. Radiological response 16% |
| Yao et al51
n = 410 2011 |
RCT of Everolimus v. placebo | Metastatic radiologically progressive pNET | PFS 11 months. Radiological response: 5% OR 73% SD |
| Raymond et al52 n = 171 2011 | RCT sunitinib v placebo | Metastatic progressive pNET | PFS 11.4 months. Radiological response: 8% OR 54% SD |
177Lu, Lutetium-177; 5-FU, 5 fluorouracil; GEP, gastro entero pancreatic; NET, neuroendocrine tumour; OR, objective response; PFS, progression- free survival; pNET, pancreatic neuro endocrine tumour; RCT, randomised controlled trial; SD, stable disease; SSAs, somato statin analogues.
In addition to patient selection, another important aspect of the theranostic approach is individualised patient dosimetry using pre- or post-therapy imaging. The goal of pre-therapeutic dosimetry is to maximise radiation dose to the tumour whilst ensuring the dose to the kidneys and bone marrow is kept below a toxic threshold.
For 177Lu compounds, the emitted γ-rays allow individual imaging and dosimetry of the same compound using the first cycle of post-therapy images. Due to 90Y’s emission of Bremsstrahlung rather than gamma rays, post-therapy dosimetry for 90Y is more challenging but can be performed, either with the use of complex corrections or simulations using 111In-DOTATOC, the positron emitter 86Y36 or the (low rates of) positron emission from 90Y.55
Planar scans, SPECT imaging, blood and urine collections can all be used for dosimetry calculations and the dosimetric methods/calculations are described in multiple publications including those by Bodei et al36 and Bison et al.56 The calculation of internal dosimetry is complex for a number of reasons. These include that tumour cells are irradiated not for seconds or minutes, but continuously, over a long period of time with permanently changing dose rate. Additionally it can be difficult, or impossible, to always define relevant, accurate regions of interest, e.g. around bone marrow or around multiple different tumour sites with variable uptake
In practice, very few centres routinely follow dosimetry-guided administration but use a fixed activity scheme instead. This is at least, in part, because it is labour intensive and also because of the relatively low toxicity of PRRT. In clinical practice, dosimetry is usually performed in those with pre-existing renal impairment to help predict and limit renal toxicity, where the dosimetric results from the first cycle of treatment can help determine how many cycles can be safely administrated.
In the future, particularly if the development of new software allows more efficient dosimetry calculations, it may be possible to deliver even more personalised therapy by routinely performing dosimetry thus ensuring that the radiation dose delivered to the tumour can be safely maximised for every patient.
PRRT variants
As described above, PRRT is typically used for patients with incurable NET, however, PRRT has been used in other circumstances, although generally in much smaller patient numbers and with less published evidence on its efficacy. These variants are outlined below.
Combination PRRT
In theory, 90Y has advantages for larger tumours due to its longer β-particle path length whereas 177Lu would be better suited to smaller lesions and there is some evidence combined PRRT may be more effective than either agent alone.57 An analysis58 of data from three previously published studies46,53,59 also found that combined therapy with 90Y and 177Lu PRRT may be associated with prolonged survival than treatment with either radiopeptide alone. More recent data using 90Y-DOTA-octreotate followed by 177Lu-DOTA-octreotate for patients with bulky disease demonstrated relatively high response rates and survival.60 However, prospective, randomized controlled studies are needed to ultimately prove that PFS is better when using a combination of radionuclides.
Chemoradionuclide therapy
Chemotherapeutic agents particularly those with radiosensitising effects such as 5-fluorouracil (5-FU) and its prodrug capecitabine and have been combined with PRRT, sometimes referred to as peptide receptor chemoradionuclide therapy (PRCRT). A study of combined 5-FU infusion with 177Lu-DOTATATE reported similar response and toxicity rates to PRRT alone.47 A Phase 2 study evaluating capecitabine with Lu-177-octreotate in 33 patients demonstrated radiological regression or stabilisation in 94% with no significant increase in toxicity, survival at 1 and 2 years was 91 and 88% respectively.61 The same authors reported on the results of a Phase I-II study of 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade NETs. This showed median PFS was 31 months, and median OS has not been reached with 90% surviving at 24 months, OR (regression or stabilisation) occurred in 94% of patients.62
While apparently safe and efficacious, there is currently no data confirming whether PRCRT is superior to PRRT. Therefore, the use of PRCRT rather than PRRT alone could be more appropriate for those with higher grade NETs, the shorter survival associated with these tumours means the potential benefits of chemotherapy are more likely to outweigh the risks. As described above, a group of patients with known poorer prognoses are those with FDG avidity, therefore, this has been used as a criterion for selecting patients for PRCRT. This could be considered a different form of theranostic. A study of 52 patients with FDG avid NETs treated with 5-FU and 177Lu DOTATATE reported radiological response rates of OR in 30%, SD in 68% and PD in only 2%, PFS was 48 months.63
In vivo and in vitro studies have also been performed combining PRRT with a number of other chemotherapeutic agents including gemcitabine, camptothecin, mitomycin C, cisplatin, doxorubicin and everolimus.56
Salvage
Repeat or “salvage” treatment in patients who have already received cycles of PRRT and later progress is also administered. A retrospective analysis of 33 patients who received salvage treatment reported radiological regression or stabilisation of disease in 77%. Median PFS from the start of salvage therapy was 13 months and patients with a history of a durable PFS after initial PRRT tended to have long-lasting PFS after salvage treatment (p = 0.04). There was no severe nephrotoxicity but reversible severe haematotoxicity was seen in 21% of patients.64
Future directions
The scope for PRRT is growing, through the development of new SSTR agents and the introduction of new radionuclides. Additionally, the clinical indications for PRRT could expand and, as the results of randomised trials are published, better evidence regarding its current role in the treatment of NETs and its potential for combination with other therapies will emerge. These areas are outlined below.
SSTR antagonists
More recently SSTR antagonists have been developed and are entering clinical use. They have the advantages of higher tumour uptake and better tumour to background ratio than the agonists.65 An antagonist named JR11 ((Cpa-c[D-Cys-Aph(Hor)-D-Aph(Cbm)-Lys-Thr-Cys]-D-Tyr-NH2) has been developed as a PET tracer labelled with 68Ga using the chelator NODAGA (68Ga-OPS202)65 and as a therapeutic agent labelled with 177Lutetium using the chelator DOTA (177Lu OPS201).
A Phase1/2 trial comparing 68Ga-OPS202 with 68Ga-DOTATOC PET-CT found that the antagonist showed lower uptake than 68Ga-DOTATOC in the normal liver, the pancreas and the gastrointestinal tract but similar uptake in malignant lesions, this significantly improved lesion contrast. On a lesion-basis, the diagnostic accuracy of 68Ga-DOTATOC vs 68Ga-OPS202 was 42 and 92% respectively.66
Pilot studies have shown 1.7–10.6 times higher tumour dose with Lu-177-DOTA-JR11 when compared to Lu-177-DOTATATE.67 Additionally, renal retention of SSTR antagonists is lower, resulting in a 5.2 times higher tumour-to-kidney ratio in favour of the receptor antagonist.56 These characteristics mean that PRRT with SSTR antagonists could have significant advantages over the agonists. There is currently a clinical Phase I/II study underway to investigate the safety and tolerability, biodistribution, dosimetry and preliminary efficacy of 177Lu-OPS201.68
α emitters
Some radionuclides decay to release α particles (comprised of two protons and two neutrons). These have high energy, e.g. 8.32 MeV for Bismuth-213 (213Bi) and a small particle range of 50–80 μm. This gives them a higher relative biological effectiveness and potential to induce cell death. When alpha emitters are stably complexed to their SST analogue peptide and receptor density in normal tissue is low, radiotoxicity in non-targeted normal tissues should be minimal, based on the short path length of the emitted particle.56
A pre-clinical study showed that, at the same absorbed dose, 213Bi-DOTATOC is therapeutically more effective in decreasing survival of human pancreatic adenocarcinoma cells than is 177Lu-DOTATOC.69 A study in patients refractory to treatment with 90Y/177Lu-DOTATOC of intra arterial (n = 7) and systemic administration (n = 1) of 213Bi-DOTATOC found that the biodistribution of 213Bi-DOTATOC was evaluable with 440 keV gamma emission scans, and demonstrated specific tumour binding. Prolonged responses were observed in all treated patients, there was moderate chronic kidney toxicity but haematotoxicity was less severe than with the preceding βbeta therapies.70
Scandium
Scandium-44 (44Sc) is a cyclotron produced positron emitting radionuclide with a half life of 3.97 h (vs 68 min for 68Ga). It can therefore be more easily transported over long distances compared to 68Ga. A report on 44Sc-DOTATOC in four patients found that five additional metastases were detected in one patient, and one new metastasis was revealed in another patient, compared to the previous 68Ga-DOTATOC PET/CT study. No adverse effects were observed in any of the patients. This tracer could be an excellent theranostic agent as pre-therapeutic imaging and dosimetry by 44Sc-DOTATOC may be followed by radiopeptide therapy using the beta-emitting Scandium-47.71
Adjuvant and neo-adjuvant use
PRRT could, in future, be used as an adjuvant treatment after surgery, either to kill micrometastases already present or to prevent tumour development after spread during manipulation of the tumour during surgery. This potential use stems from an animal model where therapy with 177Lu-octreotate prevented or significantly reduced the growth of tumour deposits in the liver after injection of tumour cells via the portal vein, mimicking preoperative tumour spill.72 Neo-adjuvant use is also a possibility as there have been a few case reports of the neo-adjuvant use of PRRT to render initially inoperable pancreatic and duodenal NETs73,74 and hepatic metastases75 resectable.
Intra-arterial
Direct administration of radiolabelled DOTA peptides into hepatic arteries supplying liver metastases has been investigated. A study comparing standardised uptake values after intra-arterial administration of 68Ga-DOTATOC vs intravenous administration in 15 NET patients found that SUVs were 3.75 times higher after intra-arterial administration.76 The same authors performed a small study in which 90Y- or 177Lu-DOTATOC was infused via the hepatic artery in 15 patients with liver metastases arising from GEP-NETs. This resulted in a higher rate of objective radiological responses than typically reported for intravenous treatment (60 vs 30 %, respectively).77 However long-term responses and toxicity are not available yet. There is an early Phase 1 study currently underway where some study participants will receive a single dose of 90Y-DOTATOC via the hepatic artery and other participants will receive 68Ga-DOTATOC together with the 90Y-DOTATOC dose and then have additional imaging and assessment.78
Combined PRRT and other agents
The combination of PRRT with other agents, apart from just chemotherapy, is a potential area for development for example a Phase 1 study demonstrated that 177Lu-octreotate could be combined with everolimus.79
There has been also been recent interest in the use of immunotherapy for NET. The results of pembrolizumab in patients with NET has been reported80 and there is a clinical trial underway of nivolumab and ipilimumab81 in patients with NET. Given the emerging interest, efficacy and possible synergy of combining radiotherapy and immunotherapy82,83 combined PRRT and immunotherapy may be an additional future treatment option.
PRRT and RCTs
In the past, there has been criticism of PRRT that it was used in the absence of good quality evidence of its effectiveness, most data coming from observational or cohort studies. This is changing, both with the publication of the preliminary results of the NETTER-1 trial7 and because other randomised trials are underway. These include:
A randomized Phase II, parallel group study/trial of patients with GEP NET that is SRS and FDG positive. Patients will be randomly assigned to two different arms: 177Lu PRRT either with or without capecitabine.84
A Phase II randomised trial comparing PRCRT with CAPTEM (capecitabine plus temozolomide) with either CAPTEM for patients with pNET or PRRT for patients with midgut NET85
A prospective randomised controlled open Phase 3 study of 177Lu-Edotreotide (DOTATOC) compared to everolimus in patients with or GEPNET85
The final outcomes of the NETTER-1 trial
In line with this move towards more trial based research of PRRT, the recent ENETs guidelines include a section on “recommendations to improve comparability of trials of PRRT”.30 However, until the results of these trials, and potentially others which may still be necessary, are known there is inadequate evidence to clearly define where in the treatment pathway PRRT should lie. Current guidelines place it as an option after other treatments have failed, however, in the future PRRT may be earlier in the treatment pathway and, in the further future still, may be able to cure patients who are currently deemed incurable.
Conclusion
NETs are a heterogeneous collection of tumours and the ability to apply the theranostic principle of appropriately identifying patients who are most likely to benefit from the therapeutic arm of SSTR-based therapy, by using the diagnostic arm of SSTR scintigraphy, is a key treatment strategy for patients with NETs. There are multiple different imaging and therapeutic radionuclides available with newer generation ones entering clinical use or in development. Improving evidence in the form of clinical trials is consolidating PRRT’s current place in the treatment of incurable NETs and it is likely this role will expand over time, either by PRRT moving earlier in the treatment algorithm or by combining it with other therapies.
Footnotes
Acknowledgements: Evidence search: Radio-labelled somatostatin peptide agonists and antagonists in the imaging and treatment of patients with neuroendocrine tumours. Tom Roper. (16 October, 2017). BRIGHTON, UK: Brighton and Sussex Library and Knowledge Service.
Contributor Information
Deborah Pencharz, Email: deborah.pencharz@bsuh.nhs.uk.
Gopinath Gnanasegaran, Email: g.gnanasegaran@nhs.net.
Shaunak Navalkissoor, Email: s.navalkissoor@nhs.net.
REFERENCES
- 1. Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut 2012; 61: 6–32. doi: 10.1136/gutjnl-2011-300831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, et al. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2007; 451: 757–62. doi: 10.1007/s00428-007-0452-1 [DOI] [PubMed] [Google Scholar]
- 3. Pape UF, Perren A, Niederle B, Gross D, Gress T, Costa F, et al. ENETS Consensus Guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology 2012; 95: 135–56. doi: 10.1159/000335629 [DOI] [PubMed] [Google Scholar]
- 4. Cives M, Strosberg J. An update on gastroenteropancreatic neuroendocrine tumors. Oncology 2014; 28: 749–56. [PubMed] [Google Scholar]
- 5. Bushnell DL, O'Dorisio TM, O'Dorisio MS, Menda Y, Hicks RJ, Van Cutsem E, et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol 2010; 28: 1652–9. doi: 10.1200/JCO.2009.22.8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 1999; 20: 157–98. doi: 10.1006/frne.1999.0183 [DOI] [PubMed] [Google Scholar]
- 7. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med 2017; 376: 125–35. doi: 10.1056/NEJMoa1607427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hubalewska-Dydejczyk A, Fröss-Baron K, Mikołajczak R, Maecke HR, Huszno B, Pach D, et al. 99mTc-EDDA/HYNIC-octreotate scintigraphy, an efficient method for the detection and staging of carcinoid tumours: results of 3 years' experience. Eur J Nucl Med Mol Imaging 2006; 33: 1123–33. doi: 10.1007/s00259-006-0113-7 [DOI] [PubMed] [Google Scholar]
- 9. Gabriel M, Muehllechner P, Decristoforo C, von Guggenberg E, Kendler D, Prommegger R, et al. 99mTc-EDDA/HYNIC-Tyr(3)-octreotide for staging and follow-up of patients with neuroendocrine gastro-entero-pancreatic tumors. Q J Nucl Med Mol Imaging 2005; 49: 237–44. [PubMed] [Google Scholar]
- 10. Al-Nahhas A, Win Z, Szyszko T, Singh A, Nanni C, Fanti S, et al. Gallium-68 PET: a new frontier in receptor cancer imaging. Anticancer Res 2007; 27: 4087–94. [PubMed] [Google Scholar]
- 11. Baum RP, Kulkarni HR, Carreras C. Peptides and receptors in image-guided therapy: theranostics for neuroendocrine neoplasms. Semin Nucl Med 2012; 42: 190–207. doi: 10.1053/j.semnuclmed.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 12. Putzer D, Kroiss A, Waitz D, Gabriel M, Traub-Weidinger T, Uprimny C, et al. Somatostatin receptor PET in neuroendocrine tumours: 68Ga-DOTA0,Tyr3-octreotide versus 68Ga-DOTA0-lanreotide. Eur J Nucl Med Mol Imaging 2013; 40: 364–72. doi: 10.1007/s00259-012-2286-6 [DOI] [PubMed] [Google Scholar]
- 13. Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging 2010; 37: 2004–10. doi: 10.1007/s00259-010-1512-3 [DOI] [PubMed] [Google Scholar]
- 14. Bombardieri E, Ambrosini V, Aktolun C, Baum RP, Bishof-Delaloye A, Del Vecchio S, et al. 111In-pentetreotide scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging 2010; 37: 1441–8. doi: 10.1007/s00259-010-1473-6 [DOI] [PubMed] [Google Scholar]
- 15. Kagna O, Pirmisashvili N, Tshori S, Freedman N, Israel O, Krausz Y. Neuroendocrine tumor imaging with 68Ga-DOTA-NOC: physiologic and benign variants. AJR Am J Roentgenol 2014; 203: 1317–23. doi: 10.2214/AJR.14.12588 [DOI] [PubMed] [Google Scholar]
- 16. Sandström M, Velikyan I, Garske-Román U, Sörensen J, Eriksson B, Granberg D, et al. Comparative biodistribution and radiation dosimetry of 68Ga-DOTATOC and 68Ga-DOTATATE in patients with neuroendocrine tumors. J Nucl Med 2013; 54: 1755–9. doi: 10.2967/jnumed.113.120600 [DOI] [PubMed] [Google Scholar]
- 17. Pettinato C, Sarnelli A, Di Donna M, Civollani S, Nanni C, Montini G, et al. 68Ga-DOTANOC: biodistribution and dosimetry in patients affected by neuroendocrine tumors. Eur J Nucl Med Mol Imaging 2008; 35: 72–9. doi: 10.1007/s00259-007-0587-y [DOI] [PubMed] [Google Scholar]
- 18. Brogsitter C, Zöphel K, Hartmann H, Schottelius M, Wester HJ, Kotzerke J. Twins in spirit part II: DOTATATE and high-affinity DOTATATE--the clinical experience. Eur J Nucl Med Mol Imaging 2014; 41: 1158–65. doi: 10.1007/s00259-014-2690-1 [DOI] [PubMed] [Google Scholar]
- 19. Hartmann H, Freudenberg R, Oehme L, Zöphel K, Schottelius M, Wester HJ, et al. Dosimetric measurements of (68)Ga-high affinity DOTATATE: twins in spirit - part III. Nuklearmedizin 2014; 53: 211–6. doi: 10.3413/Nukmed-0667-14-05 [DOI] [PubMed] [Google Scholar]
- 20. Bodei L, Kidd M, Prasad V, Baum RP, Drozdov I, Modlin IM. The future of nuclear medicine imaging of neuroendocrine tumors: on a clear day one might see forever.. Eur J Nucl Med Mol Imaging 2014; 41: 2189–93. doi: 10.1007/s00259-014-2836-1 [DOI] [PubMed] [Google Scholar]
- 21. Graham MM, Gu X, Ginader T, Breheny P, Sunderland JJ. 68Ga-DOTATOC imaging of neuroendocrine tumors: a systematic review and metaanalysis. J Nucl Med 2017; 58: 1452–8. doi: 10.2967/jnumed.117.191197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deppen SA, Blume J, Bobbey AJ, Shah C, Graham MM, Lee P, et al. 68Ga-DOTATATE compared with 111In-DTPA-octreotide and conventional imaging for pulmonary and gastroenteropancreatic neuroendocrine tumors: a systematic review and meta-analysis. J Nucl Med 2016; 57: 872–8. doi: 10.2967/jnumed.115.165803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taïeb D, Garrigue P, Bardiès M, Abdullah AE, Pacak K. Application and dosimetric requirements for gallium-68-labeled somatostatin analogues in targeted radionuclide therapy for gastroenteropancreatic neuroendocrine tumors. PET Clin 2015; 10: 477–86. doi: 10.1016/j.cpet.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaemmerer D, Peter L, Lupp A, Schulz S, Sänger J, Prasad V, et al. Molecular imaging with ⁶⁸Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2011; 38: 1659–68. doi: 10.1007/s00259-011-1846-5 [DOI] [PubMed] [Google Scholar]
- 25. Kratochwil C, Stefanova M, Mavriopoulou E, Holland-Letz T, Dimitrakopoulou-Strauss A, Afshar-Oromieh A, et al. SUV of [68Ga]DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol Imaging Biol 2015; 17: 313–8. doi: 10.1007/s11307-014-0795-3 [DOI] [PubMed] [Google Scholar]
- 26. Hindié E. The NETPET score: combining FDG and somatostatin receptor imaging for optimal management of patients with metastatic well-differentiated neuroendocrine tumors. Theranostics 2017; 7: 1159–63. doi: 10.7150/thno.19588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bahri H, Laurence L, Edeline J, Leghzali H, Devillers A, Raoul JL, et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long-term evaluation. J Nucl Med 2014; 55: 1786–90. doi: 10.2967/jnumed.114.144386 [DOI] [PubMed] [Google Scholar]
- 28. Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res 2010; 16: 978–85. doi: 10.1158/1078-0432.CCR-09-1759 [DOI] [PubMed] [Google Scholar]
- 29. Deroose CM, Hindié E, Kebebew E, Goichot B, Pacak K, Taïeb D, et al. Molecular imaging of gastroenteropancreatic neuroendocrine tumors: current status and future directions. J Nucl Med 2016; 57: 1949–56. doi: 10.2967/jnumed.116.179234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hicks RJ, Kwekkeboom DJ, Krenning E, Bodei L, Grozinsky-Glasberg S, Arnold R, et al. ENETS consensus guidelines for the standards of care in neuroendocrine neoplasia: peptide receptor radionuclide therapy with radiolabeled somatostatin analogues. Neuroendocrinology 2017; 105: 295–309. doi: 10.1159/000475526 [DOI] [PubMed] [Google Scholar]
- 31. Chan DL, Pavlakis N, Schembri GP, Bernard EJ, Hsiao E, Hayes A, et al. Dual somatostatin receptor/FDG PET/CT imaging in metastatic neuroendocrine tumours: proposal for a novel grading scheme with prognostic significance. Theranostics 2017; 7: 1149–58. doi: 10.7150/thno.18068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hicks RJ. Use of molecular targeted agents for the diagnosis, staging and therapy of neuroendocrine malignancy. Cancer Imaging 2010; 10: 83–91. doi: 10.1102/1470-7330.2010.9007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008; 26: 2124–30. doi: 10.1200/JCO.2007.15.2553 [DOI] [PubMed] [Google Scholar]
- 34. Srirajaskanthan R, Kayani I, Quigley AM, Soh J, Caplin ME, Bomanji J. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J Nucl Med 2010; 51: 875–82. doi: 10.2967/jnumed.109.066134 [DOI] [PubMed] [Google Scholar]
- 35. Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012; 95: 157–76. doi: 10.1159/000335597 [DOI] [PubMed] [Google Scholar]
- 36. Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O'Dorisio MS, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2013; 40: 800–16. doi: 10.1007/s00259-012-2330-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caplin M, Sundin A, Nillson O, Baum RP, Klose KJ, Kelestimur F, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology 2012; 95: 88–97. doi: 10.1159/000335594 [DOI] [PubMed] [Google Scholar]
- 38. Falconi M, Bartsch DK, Eriksson B, Klöppel G, Lopes JM, O'Connor JM, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology 2012; 95: 120–34. doi: 10.1159/000335587 [DOI] [PubMed] [Google Scholar]
- 39. Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014; 371: 224–33. doi: 10.1056/NEJMoa1316158 [DOI] [PubMed] [Google Scholar]
- 40. Kim SJ, Pak K, Koo PJ, Kwak JJ, Chang S. The efficacy of (177)Lu-labelled peptide receptor radionuclide therapy in patients with neuroendocrine tumours: a meta-analysis. Eur J Nucl Med Mol Imaging 2015; 42: 1964–70. doi: 10.1007/s00259-015-3155-x [DOI] [PubMed] [Google Scholar]
- 41. Ezziddin S, Attassi M, Yong-Hing CJ, Ahmadzadehfar H, Willinek W, Grünwald F, et al. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med 2014; 55: 183–90. doi: 10.2967/jnumed.113.125336 [DOI] [PubMed] [Google Scholar]
- 42. Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with ¹⁷⁷Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging 2011; 38: 2125–35. doi: 10.1007/s00259-011-1902-1 [DOI] [PubMed] [Google Scholar]
- 43. Khan S, Krenning EP, van Essen M, Kam BL, Teunissen JJ, Kwekkeboom DJ. Quality of life in 265 patients with gastroenteropancreatic or bronchial neuroendocrine tumors treated with [177Lu-DOTA0,Tyr3]octreotate. J Nucl Med 2011; 52: 1361–8. doi: 10.2967/jnumed.111.087932 [DOI] [PubMed] [Google Scholar]
- 44. van der Zwan WA, Bodei L, Mueller-Brand J, de Herder WW, Kvols LK, Kwekkeboom DJ. GEPNETs update: Radionuclide therapy in neuroendocrine tumors. Eur J Endocrinol 2015; 172: R1–R8. doi: 10.1530/EJE-14-0488 [DOI] [PubMed] [Google Scholar]
- 45. Melis M, Krenning EP, Bernard BF, Barone R, Visser TJ, de Jong M. Localisation and mechanism of renal retention of radiolabelled somatostatin analogues. Eur J Nucl Med Mol Imaging 2005; 32: 1136–43. doi: 10.1007/s00259-005-1793-0 [DOI] [PubMed] [Google Scholar]
- 46. Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011; 29: 2416–23. doi: 10.1200/JCO.2010.33.7873 [DOI] [PubMed] [Google Scholar]
- 47. Kong G, Thompson M, Collins M, Herschtal A, Hofman MS, Johnston V, et al. Assessment of predictors of response and long-term survival of patients with neuroendocrine tumour treated with peptide receptor chemoradionuclide therapy (PRCRT). Eur J Nucl Med Mol Imaging 2014; 41: 1831–44. doi: 10.1007/s00259-014-2788-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ezziddin S, Khalaf F, Vanezi M, Haslerud T, Mayer K, Al Zreiqat A, et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2014; 41: 925–33. doi: 10.1007/s00259-013-2677-3 [DOI] [PubMed] [Google Scholar]
- 49. Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen DT, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011; 117: 268–75. doi: 10.1002/cncr.25425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG, Eastern Cooperative Oncology Group. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol 2005; 23: 4897–904. doi: 10.1200/JCO.2005.03.616 [DOI] [PubMed] [Google Scholar]
- 51. Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011; 364: 514–23. doi: 10.1056/NEJMoa1009290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011; 364: 501–13. doi: 10.1056/NEJMoa1003825 [DOI] [PubMed] [Google Scholar]
- 53. Romer A, Seiler D, Marincek N, Brunner P, Koller MT, Ng QK, et al. Somatostatin-based radiopeptide therapy with [177Lu-DOTA]-TOC versus [90Y-DOTA]-TOC in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2014; 41: 214–22. doi: 10.1007/s00259-013-2559-8 [DOI] [PubMed] [Google Scholar]
- 54. Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging 2015; 42: 5–19. doi: 10.1007/s00259-014-2893-5 [DOI] [PubMed] [Google Scholar]
- 55. Walrand S, Flux GD, Konijnenberg MW, Valkema R, Krenning EP, Lhommel R, et al. Dosimetry of yttrium-labelled radiopharmaceuticals for internal therapy: 86Y or 90Y imaging? Eur J Nucl Med Mol Imaging 2011; 38(Suppl 1): 57–68. doi: 10.1007/s00259-011-1771-7 [DOI] [PubMed] [Google Scholar]
- 56. Bison SM, Konijnenberg MW, Melis M, Pool SE, Bernsen MR, Teunissen JJ, et al. Peptide receptor radionuclide therapy using radiolabeled somatostatin analogs: focus on future developments. Clin Transl Imaging 2014; 2: 55–66. doi: 10.1007/s40336-014-0054-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de Jong M, Breeman WA, Valkema R, Bernard BF, Krenning EP. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J Nucl Med 2005; 46(Suppl 1): 13s–17. [PubMed] [Google Scholar]
- 58. Radojewski P, Dumont R, Marincek N, Brunner P, Mäcke HR, Müller-Brand J, et al. Towards tailored radiopeptide therapy. Eur J Nucl Med Mol Imaging 2015; 42: 1231–7. doi: 10.1007/s00259-015-3030-9 [DOI] [PubMed] [Google Scholar]
- 59. Villard L, Romer A, Marincek N, Brunner P, Koller MT, Schindler C, et al. Cohort study of somatostatin-based radiopeptide therapy with [(90)Y-DOTA]-TOC versus [(90)Y-DOTA]-TOC plus [(177)Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol 2012; 30: 1100–6. doi: 10.1200/JCO.2011.37.2151 [DOI] [PubMed] [Google Scholar]
- 60. Kong G, Callahan J, Hofman MS, Pattison DA, Akhurst T, Michael M, et al. High clinical and morphologic response using 90Y-DOTA-octreotate sequenced with 177Lu-DOTA-octreotate induction peptide receptor chemoradionuclide therapy (PRCRT) for bulky neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2017; 44: 476–89. doi: 10.1007/s00259-016-3527-x [DOI] [PubMed] [Google Scholar]
- 61. Claringbold PG, Brayshaw PA, Price RA, Turner JH. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2011; 38: 302–11. doi: 10.1007/s00259-010-1631-x [DOI] [PubMed] [Google Scholar]
- 62. Claringbold PG, Price RA, Turner JH. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother Radiopharm 2012; 27: 561–9. doi: 10.1089/cbr.2012.1276 [DOI] [PubMed] [Google Scholar]
- 63. Kashyap R, Hofman MS, Michael M, Kong G, Akhurst T, Eu P, et al. Favourable outcomes of (177)Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2015; 42: 176–85. doi: 10.1007/s00259-014-2906-4 [DOI] [PubMed] [Google Scholar]
- 64. Sabet A, Haslerud T, Pape UF, Sabet A, Ahmadzadehfar H, Grünwald F, et al. Outcome and toxicity of salvage therapy with 177Lu-octreotate in patients with metastatic gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2014; 41: 205–10. doi: 10.1007/s00259-013-2547-z [DOI] [PubMed] [Google Scholar]
- 65. Fani M, Nicolas GP, Wild D. Somatostatin receptor antagonists for imaging and therapy. J Nucl Med 2017; 58(Supplement 2): 61S–6. doi: 10.2967/jnumed.116.186783 [DOI] [PubMed] [Google Scholar]
- 66. Nicolas G, Schreiter N, Kaul F, Uiters J, Mena R, Bouterfa H, et al. PET/CT with the somatostatin receptor antagonist 68Ga-OPS202 is twice as accurate as with the agonist 68Ga-DOTATOC for detecting liver metastases: results of a phase 1/2 study in gastroenteropancreatic NET patients. J Nucl Med 2016; 57(supplement 2): 154. [Google Scholar]
- 67. Wild D, Fani M, Fischer R, Del Pozzo L, Kaul F, Krebs S, et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J Nucl Med 2014; 55: 1248–52. doi: 10.2967/jnumed.114.138834 [DOI] [PubMed] [Google Scholar]
- 68. Study to Evaluate the Safety and Preliminary Efficacy of 177Lu-OPSC001 in NETs. 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT02592707 [3rd September. doi: 10.2214/ajr.181.3.1810679 [DOI]
- 69. Nayak TK, Norenberg JP, Anderson TL, Prossnitz ER, Stabin MG, Atcher RW. Somatostatin-receptor-targeted alpha-emitting 213Bi is therapeutically more effective than beta(-)-emitting 177Lu in human pancreatic adenocarcinoma cells. Nucl Med Biol 2007; 34: 185–93. doi: 10.1016/j.nucmedbio.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 70. Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R, et al. ²¹³Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging 2014; 41: 2106–19. doi: 10.1007/s00259-014-2857-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Singh A, Baum R, Klette I, van der Meulen N, Mueller C, Tuerler A. Scandium-44 DOTATOC PET/CT: First in-human molecular imaging of neuroendocrine tumors and possible perspectives for Theranostics. J Nucl Med 2015; 56(supplement 3): 267. [Google Scholar]
- 72. Breeman WA, Mearadji A, Capello A, Bernard BF, van Eijck CH, Krenning EP, et al. Anti-tumor effect and increased survival after treatment with [177Lu-DOTA0,Tyr3]octreotate in a rat liver micrometastases model. Int J Cancer 2003; 104: 376–9. doi: 10.1002/ijc.10952 [DOI] [PubMed] [Google Scholar]
- 73. Barber TW, Hofman MS, Thomson BN, Hicks RJ. The potential for induction peptide receptor chemoradionuclide therapy to render inoperable pancreatic and duodenal neuroendocrine tumours resectable. Eur J Surg Oncol 2012; 38: 64–71. doi: 10.1016/j.ejso.2011.08.129 [DOI] [PubMed] [Google Scholar]
- 74. Kaemmerer D, Prasad V, Daffner W, Hörsch D, Klöppel G, Hommann M, et al. Neoadjuvant peptide receptor radionuclide therapy for an inoperable neuroendocrine pancreatic tumor. World J Gastroenterol 2009; 15: 5867–70. doi: 10.3748/wjg.15.5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stoeltzing O, Loss M, Huber E, Gross V, Eilles C, Mueller-Brand J, et al. Staged surgery with neoadjuvant 90Y-DOTATOC therapy for down-sizing synchronous bilobular hepatic metastases from a neuroendocrine pancreatic tumor. Langenbecks Arch Surg 2010; 395: 185–92. doi: 10.1007/s00423-009-0520-x [DOI] [PubMed] [Google Scholar]
- 76. Kratochwil C, Giesel FL, López-Benítez R, Schimpfky N, Kunze K, Eisenhut M, et al. Intraindividual comparison of selective arterial versus venous 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors. Clin Cancer Res 2010; 16: 2899–905. doi: 10.1158/1078-0432.CCR-10-0004 [DOI] [PubMed] [Google Scholar]
- 77. Kratochwil C, López-Benítez R, Mier W, Haufe S, Isermann B, Kauczor HU, et al. Hepatic arterial infusion enhances DOTATOC radiopeptide therapy in patients with neuroendocrine liver metastases. Endocr Relat Cancer 2011; 18: 595–602. doi: 10.1530/ERC-11-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Clinical Trials.gov. 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03197012?term=neuroendocrine+tumors&type=Intr&draw=4&rank=28 [cited 2017 24th November].
- 79. Claringbold PG, Turner JH. NeuroEndocrine Tumor Therapy with Lutetium-177-octreotate and Everolimus (NETTLE): A Phase I Study. Cancer Biother Radiopharm 2015; 30: 261–9. doi: 10.1089/cbr.2015.1876 [DOI] [PubMed] [Google Scholar]
- 80. ESMO. First KEYNOTE-028 report of pembrolizumab activity in patients with PD-L1-positive pNETs and carcinoid tumours. 2017. Available from: https://www.esmo.org/Conferences/Past-Conferences/ESMO-2017-Congress/News-Articles/First-KEYNOTE-028-Report-of-Pembrolizumab-Activity-in-Patients-with-PD-L1-positive-pNETs-and-Carcinoid-Tumours [cited 2018 31st July].
- 81. An Open-Label. Single arm phase II study of nivolumab in combination with ipilimumab in subjects with advanced neuroendocrine tumors. 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03420521 [cited 2018 31st July].
- 82. Salama AK, Postow MA, Salama JK. Irradiation and immunotherapy: From concept to the clinic. Cancer 2016; 122: 1659–71. doi: 10.1002/cncr.29889 [DOI] [PubMed] [Google Scholar]
- 83. Pilones KA, Vanpouille-Box C, Demaria S. Combination of radiotherapy and immune checkpoint inhibitors. Semin Radiat Oncol 2015; 25: 28–33. doi: 10.1016/j.semradonc.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 84. 177Lutetium. Peptide receptor radionuclide therapy (Lu-PRRT) plus capecitabine versus Lu-PRRT in FDG positive, gastro-entero-pancreatic neuroendocrine tumors (Lu-Ca-S). 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT02736448 [cited 2017 6th November].
- 85. Clinical trials. 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03049189 [cited 2017 30th November].