Abstract
Objective:
We want to explore the safety and technical feasibility of MRI-guided stereotactic radiotherapy for locally advanced pancreatic cancer.
Methods:
A custom-made abdominal corset was manufactured to reduce breathing induced tumour motion. Delineation of the tumour and organs at risk (OARs) was performed on CT and multiparametric MRI. Tumour motion was quantified with cine MRI. After treatment planning, the static dose distribution was convolved with the cine MRI-based motion trajectory to simulate the delivered dose to the tumour and OARs. Stereotactic body radiation therapy (SBRT) was carried out up to a dose of 24 G in three fractions in 1 week.
Results:
From July 2013 to January 2016, 20 patients were included. Tumours and OARs were clearly visible with contrast-enhanced CT and MRI. After simulation of the delivered dose taking the motion into account, an adequate target coverage was achieved with acceptable dose in the OARs. No Grade3 or higher treatment related toxicity was observed.
Conclusion:
MRI-guided SBRT for pancreatic cancer is technical feasible and safe, with no treatment related grade ≥3 toxicity. New strategies are applied, including an individual corset to reduce breathing motion, MRI-based delineation and simulation of motion-integrated dose distributions.
Advances in knowledge:
This article is the first to describe an MRI-integrated workflow in SBRT for locally advanced pancreatic cancer. In addition, it demonstrated that SBRT with an abdominal corset to reduce tumour motion is feasible and safe.
Trial registration:
This trial was registered at www.clinicaltrials.gov (NCT01898741) on July 9, 2013.
Introduction
40 to 50% of pancreatic cancer patients present with locally advanced disease.1, 2 Patients with locally advanced pancreatic cancer (LAPC), in the absence of distant metastases have a median survival of 8–14 months.2 Unfortunately, effective local treatment options are lacking.
Radiotherapy may delay the development of metastasis and physical discomfort and it may lead to better palliation and possibly increase survival. Up to now, there is no evidence for a survival benefit of radiotherapy for LAPC.3 However, mostly three-dimensional (3D) conformal radiotherapy techniques have been used with a total dose of 50.4 Gy.4, 5 There is a rationale for higher dose levels, as this potentially leads to higher local control and higher survival rates.6 Modern randomized controlled trials investigating chemoradiation for LAPC indicate good tolerance of the combined modality regimens.4, 7
In addition to standard chemoradiation, stereotactic body radiation therapy (SBRT) has also been explored for LAPC.8–10 SBRT offers the advantage of shorter overall treatment times for this frail patient group with a poor prognosis than a more protracted regimen. Several non-randomized studies delivering a dose of 24–36 Gy in three fractions showed good survival rates of 10.6–20 months in LAPC.9–14 In addition, high local control rates between 82 and 91.7% were reported.9, 12,13 However, substantial severe (grade ≥3) toxicity was seen, ranging from 6 to 22%. The toxicity reported was often due to duodenal toxicity in the initial studies treated with cyberknife where fiducials were used as surrogate for tumour position with tracking. Target definition has been CT-based in these SBRT series. Our aim was to integrate MRI in the workflow of pancreas SBRT under free breathing conditions, with an abdominal cast to reduce breathing induced motion. We used the superior soft tissue contrast of MRI for optimal target and organ at risk (OAR) definition.15–17 This could lead to smaller treated volumes, and subsequently a decreased toxicity. A custom abdominal corset was used to decrease the breathing induced pancreatic motion. Residual motion was quantified using MRI and patient-specific motion was prospectively integrated into the treatment planning.
Here, we report the safety and technical feasibility of this novel strategy of MRI-guided stereotactic radiotherapy for inoperable pancreatic cancer patients.
MEthods and Materials
Patients
All patients with LAPC as defined by the Dutch Pancreatic Cancer Group (2012) or medically inoperable resectable pancreatic cancer patients were eligible for this trial. Patients with distant metastases, Eastern Cooperative Oncology Group performance score ≥3, life expectancy of <3 months, age <18 years, previous chemotherapy or pancreatic surgery, or contra indications for contrast enhanced (CE) CT or MRI were excluded. Patients received a proton pump inhibitor from the day before treatment up to 6 months after treatment. This trial was approved by our institutional review board and registered at www.clinicaltrials.gov (NCT01898741). All patients provided written informed consent.
Preparatory work
Fiducial markers (0.4 × 5 mm gold fiducial marker, QLRAD inc, Miami, FL or 0.35 × 10 mm Visicoil, IBA Dosimetry, Schwarzenbruck, Germany) were placed during an endoscopic ultrasonography procedure inside the tumour. In addition, pathology was obtained during this endoscopic ultrasonography. A custom abdominal corset was manufactured (Neofrakt®, Spronken Orthopedie NV, Genk, Belgium). The corset was pulled tight in such a way that the abdominal breathing was restricted, but still with reasonable comfort.
Simulation
CT scanning
1 week after fiducial marker placement, patients underwent CT scanning with the custom-made abdominal corset in place. No restrictions in dietary intake prior to scanning or irradiation were placed. The CT protocol consisted of a four-dimensional (4D) CT and an intravenous CECT with an arterial and a portal venous phase with a slice thickness of 3 mm.
Following CT simulation, a simulation took place in the treatment room to evaluate marker visibility and cone beam CT (CBCT) quality. During simulation, a 4D CBCT was executed. When the fiducial marker peak-to-peak amplitude was less than 5 mm in all directions, 3D CBCTs were standard during treatment. If the amplitude was more than 5 mm, 4D CBCTs were performed during treatment.
MRI scanning
MRI scanning was performed on the same day as CT scanning, on a 1.5 T MR scanner (Achieva, Philips Healthcare, Best, Netherlands) using a 16-channel phased array torso coil. Patients were positioned with their arms down at their sides, on a diagnostic table top with the corset in place. No alignment with the CT simulation position was performed. Immediately before scanning, patients drank 300 ml of tap water to increase the contrast between the pancreas and duodenum and stomach. Scanning included T 1 weighted (T 1W) imaging, T 2 weighed (T 2W) imaging, diffusion-weighted imaging (DWI), multiphase CE MRI, and a cine MRI (Table 1).
Table 1.
MRI protocol
| T1W | T2W | CE-T1 | DWI | 2D cine-MRI | |
| Motion management | Breath-hold at end expiration | Inspiratory triggered, 400 ms delay |
Breath-hold at end expiration | Free breathing | Free breathing |
| Scan mode | 2D | Multi slice | 3D | Multislice | 2D |
| Sequence type | Spoiled gradient echo | Spin echo | Spoiled gradient echo | Spin echo | Steady state free precession |
| TE (ms) | 4.2 | 80 | 2.1 | 46 | 1.44 |
| TR (ms) | 8.5 | 588 | 4.5 | 3897 | 2.9 |
| T 1 prepulse delay (ms) | 735 | n/a | n/a | n/a | n/a |
| FOV (mm3) | 350 × 350×150 | 400 × 299 × 262 | 395 × 294 × 100 | 350 × 350 × 109 | 320 × 301 |
| Acquired voxel size (mm3) | 2.0 × 2.8 × 5.0 | 1.0 × 1.3 × 3.5 | 1.8 × 1.8 × 4.0 | 2.5 × 2.7 × 5.0 | 7.0 × 1.5 × 1.5 |
| Reconstructed voxel size (mm3) | 1.6 × 1.6 × 5.0 | 0.8 × 0.8 × 3.5 | 1.5 × 1.5 × 2.0 | 1.4 × 1.4 × 5.0 | 7.0 × 1.4 × 1.4 |
| Orientation | Transverse | Transverse | Transverse | Transverse | Sagittal |
| Flip angle (°) | 10 | 90 | 10 | 90 | 50 |
| TSE | n/a | 74 | n/a | n/a | n/a |
| R factor (SENSE) | None | 2 | 2 | 2 | None |
| Half scan factor | None | 0.635 | 0.625 | None | None |
| B-values (s mm− 2) | n/a | n/a | n/a | 10, 200, 600, 800 | n/a |
2D, two-dimensional; 3D, three-dimensional; CE-T1, contrast enhanced T 1 weighted imaging; DWI, diffusion weighted imaging; FOV, field of view; n/a, not; applicable; T 1W, T 1 weighted imaging; T 2W, T 2 weighted imaging; TE, echo time; TR, repetition time; TSE, turbo spin echo.
Motion characterization
Cine MRI scanning was performed with the corset in place in the sagittal direction with the scan plane positioned through the center of the tumour. The two-dimensional cine MRI was collected over the course of 1 min, at a rate of 2 Hz. Cine MRI-based tumour tracking was performed with a Minimum Output Sum of Squared Error adaptive correlation filter, as described previously.18 Peak-to-peak motion in craniocaudal and anteroposterior direction was calculated.
Treatment planning
Delineation
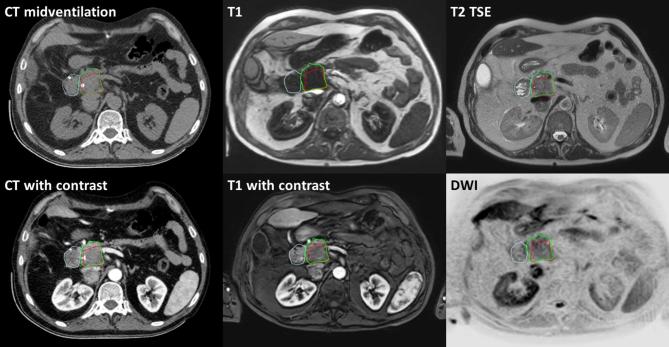
Registration of the CT and MRI was based on anatomy as the fiducial markers were not visible on MRI. Delineation of the gross tumour volume (GTV) and the OARs was performed at the 20% phase of the 4DCT with the aid of the rigidly co-registered CECT, DWI, T 1W MRI, T 2W and CE T 1W MRI (Figure 1). This 20% phase was empirically proven to be the best representative of the midventilation phase of the 4DCT, but as an online correction protocol was applied during treatment delivery, (small) deviations with respect to the real midventation phase were not critical here.
Figure 1.
Delineation with the aid of MRI and CT. Red: GTV. Green, pancreatic head. Blue, duodenum. GTV, gross tumour volume.
Dose prescription
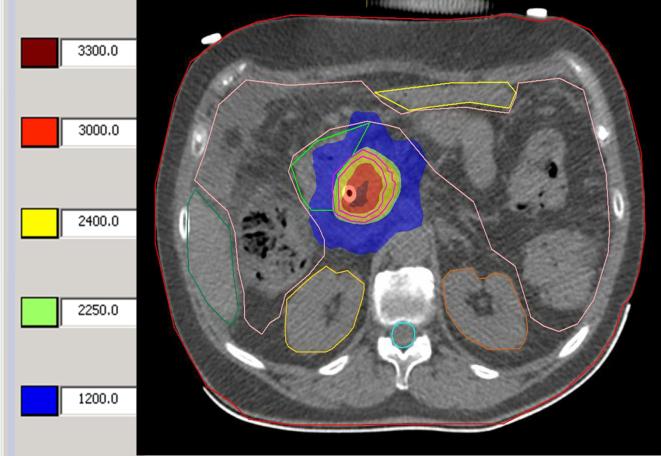
The planning target volume (PTV) was defined as a 3 mm margin around the GTV. A total dose of 24 Gy in three fractions was prescribed to the PTV. Preferably, at least 95% of the PTV received 24 Gy. Heterogeneity within the tumour was desired and the maximum dose (Dmax) was allowed to go up to 150% of the prescribed dose (Figure 2). The following dose constraints were used:12, 19,20
Figure 2.
Example treatment planning. Different dose levels are shown in centi gray at the left. Inner circle line: GTV. Outer circle line: PTV. In addition, the duodenum, kidneys, liver, spinal cord and bowel are contoured.
liver ≥700 ml less than 15 Gy;
spinal cord: maximum point dose ≤22.5 Gy;
small bowel, large bowel, stomach, and duodenum: maximum point dose <30 Gy and D5cc < 22.5 Gy;
Both kidneys mean dose <11.1 Gy
Planning organ at risk volumes (PRVs) were created around the small and large bowel, stomach, and duodenum with a 2 mm margin. Dose constraints were applied to the PRV for these organs. A dual arc VMAT plan was created using Monaco versions 3.2 and 5.1 (Elekta Corporation, Atlanta, GA).
Dosimetric assessment of respiratory motion patterns
After treatment planning, the planned dose distribution was convolved with the 3D motion trajectory around the midventilation position. The respiratory induced motion was measured by cine MRI during 1 min at a rate of 2 Hz. This resulted in 120 tumour and OAR positions. The static dose distribution was shifted for each of the 120 positions. This leads to an accumulated dose distribution in which the effect of the motion on the dose distribution was simulated for the GTV, PTV, and OARs before start of treatment. Before the start of the study, we performed a simulation experiment in which the craniocaudal breathing amplitude was increased, up to 3–10 times the original tumour motion. In this way, we evaluated the effect of unexpected larger tumour motions on the dose distribution.
Treatment delivery
Patients were treated with a linear accelerator (Elekta Synergy, Stockholm, Sweden). In case of a 4D CBCT, each of the individual frames was automatically registered to the midventilation phase of the planning CT based on the fiducial markers. In case of a 3D CBCT, the CBCT was automatically registered to the midventilation phase of the planning CT. Set-up corrections were carried out accordingly. A 3D CBCT scan was carried out after the translations to verify the setup correction. The third CBCT was performed after dose delivery to monitor the intrafraction motion.
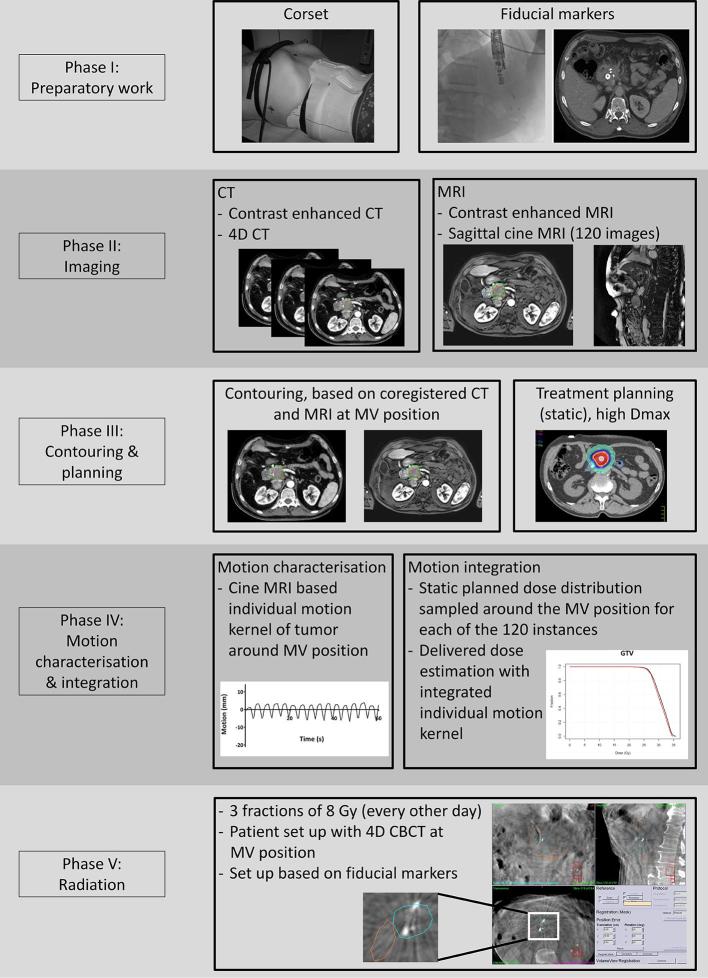
The whole workflow of the MRI-guided SBRT treatment is summarized in Figure 3.
Figure 3.
Workflow of MRI guided SBRT treatment. 4D, four-dimensional; CBCT, cone-beam CT; Dmax, maximum dose; MV, midventilation; SBRT, stereotactic body radiation therapy.
Follow up
Follow-up was scheduled at 1, 3, 6, and 12 months after SBRT. Tumour response was assessed by Response Evaluation Criteria In Solid Tumours v. 1.1 at 3 months after SBRT by CT and MRI scan.21
Quality-of-life
Quality-of-life (QOL) questionnaires were completed before treatment and at 1, 3, 6, and 12 months after SBRT. The questionnaires consisted of the general health-related RAND-36, the cancer-specific EORTC QLQ-C30, and pancreatic cancer specific EORTC QLQ PAN26.22–24 Items range from 0 to 100 points. A high score on the RAND-36 items, functional items and general QOL on the EORTC questionnaires indicate a good QOL. A high score on the symptom items on the EORTC questionnaires indicate a high degree of complaints, and thus, a poor QOL. A clinically relevant difference was defined as a 10% change on the item compared to baseline.25 As statistical analysis in this small proportion of patients is futile, QOL was described in a descriptive way.
Statistics
The primary outcome of this study was safety, expressed in study related toxicity grade ≥3 according to the Common Terminology Criteria of Adverse Events v. 4.0 within 90 days of radiotherapy. Fiducial marker placement, radiotherapy and CT and MRI scanning were defined as study procedures. To determine whether an event was study-related, an independent expert panel was generated, consisting of a radiation oncologist, gastroenterologist, and medical oncologist. All events grade ≥3 were evaluated by the expert panel. An event was considered study related when two out of three experts determined this event to be (very) likely study related.
This study was continuously monitored, i.e. an analysis was performed after every treatment-related grade 3 or higher toxicity, by using an established, sequential testing safety model.26, 27 This model was constructed at an expected toxicity rate of 10% and an unacceptable toxicity rate of 20%, according to previous SBRT studies.8–12,28 The p-value was set at 0.05 one-sided for the safety monitoring.
Results
Patient and treatment characteristics
From July 2013 until January 2016, 24 pancreatic cancer patients were enrolled in this prospective Phase II trial (Table 2 for patient characteristics). Four of these patients were not irradiated after signing informed consent, due to rapid disease progression.
Table 2.
Patient characteristics
| N (%) | |
| Age (year, range) | 70.3 (50–85) |
| Male/female | 14/6 (70/30%) |
| Location | |
| Head | 17 (85%) |
| Body | 2 (10%) |
| Body/tail | 1 (5%) |
| Performance score | |
| 0 | 7 (35%) |
| 1 | 9 (45%) |
| 2 | 3 (15%) |
| Missing | 1 (5%) |
| Classification | |
| Locally advanced | 18 (90%) |
| Medically inoperable/ refused surgery | 2 (10%) |
| Chemotherapy (after SBRT) | |
| FOLFIRINOX | 2 (10%) |
| Gemcitabine/nab-paclitaxel | 2 (10%) |
| None | 16 (80%) |
SBRT, stereotactic body radiation therapy.
Technical feasibility
Fiducial markers
QLRAD markers were placed in 15 patients. In the first five patients, a combination of two Visicoils and two QLRAD markers were placed. Placement of fiducial markers was uncomplicated in all but one patient (grade 2 post-puncture pancreatitis, requiring administration of analgesics and intravenously administered fluids). In two patients, three markers were placed, for all other patients four markers were placed.
Corset and tumour motion
All patients tolerated the abdominal corset well during MRI scanning and treatment. With the application of the corset, the average 100% craniocaudal tumour motion as calculated from the sagittal cine MRI was 8.2 mm (range 2.7–23.8 mm). In anteroposterior direction, the average 100% motion was 3.8 mm (range 0.8–12.6 mm).
Treatment planning
Contouring was based on the midventilation scan of the 4DCT. We found the multiparametric MRI helpful for contouring. The DWI and contrast-enhanced T 1W imaging were used for GTV determination. Diffusion restriction in DWI is very sensitive for tumour detection, although it is not specific to tumour in pancreatic cancer. Therefore, tumour delineation was based on DWI in combination with arterial T 1W imaging. Predominantly in the arterial phase, the GTV appears as a hypointense area. T 2W imaging was used for discrimination of tumour and OARs as stomach and duodenum. In case of geometric discrepancies, the midventilation CT scan was leading in contouring, as this was the imaging that was also used for treatment.
All patients received 24 Gy in three fractions every other day. Mean dose to the GTV was on average 29.8 Gy (range 24.7–32.3 Gy) with a maximum dose of on average 34.5 Gy (range 30.4–36.2 Gy) (Table 3). After blurring the dose with the individual respiratory motion kernels obtained from the cine MRI scans, the mean GTV dose only slightly reduced to an average dose of 29.6 Gy (range 24.6–31.8 Gy). The same pattern was observed for the PTV dose: here, the D99 of the PTV was on average 22.3 Gy (range 20.4–24.6 Gy) and after motion simulation it was 22.0 Gy (range 20.3–24.1 Gy). Even in the patient with the largest tumour motion of 23.8 mm, the mean dose to the GTV decreased with only 0.8 Gy. These findings support that the planned stereotactic dose distributions were very robust against motion oscillations around the midventilation position.
Table 3.
Treatment characteristics
| Mean | Range | |
| GTV | ||
| Volume (cm3) | 52.30 | 6.92–134.4 |
| Min dose (Gy) | 22.58 | 18.86–26.51 |
| Mean dose (Gy) | 29.55 | 25.07–32.41 |
| Max dose (Gy) | 34.70 | 32.57–36.16 |
| PTV | ||
| Volume (cm3) | 81.98 | 14.92–197.59 |
| Min dose (Gy) | 19.98 | 15.16–22.78 |
| Mean dose (Gy) | 28.05 | 23.83–30.40 |
| Duodenum | ||
| Max point dose (Gy) | 25.85 | 23.82–28.39 |
| D5cc (Gy) | 19.91 | 13.27–21.89 |
| Stomach | ||
| Max point dose (Gy) | 16.21 | 0.15–29.58 |
| D5cc (Gy) | 9.54 | 0.10–19.59 |
| Bowel | ||
| Max point dose (Gy) | 23.79 | 15.09–30.75 |
| D5cc (Gy) | 17.21 | 10.85–20.99 |
GTV, gross tumour volume; PTV, planning target volume.
The simulation experiment in which the tumour motion was increased by 3–10 times the original tumour motion demonstrated that adequate tumour coverage was still reached while meeting the OAR constraints at larger tumour motions (Supplementary Figure 1).
Treatment delivery
After the radiation treatment, a third CBCT was performed to determine the intrafraction motion. 19 patients were available for analysis. The intrafraction motion was modest, with a mean vector length over all patients of 1.7 mm (standard deviation 1.0 mm, range 0.4–4.0 mm). In one patient, the PTV margin was increased to 10 mm only for the last fraction, as there was an extreme rotation of 7° seen at the second fraction.
Clinical outcomes
The median overall survival of irradiated patients was 8.5 months (range 3.7–19 months), calculated from the first fraction. 1 May 2016, five patients are still alive at 3, 3, 4, 5, and 19 months from the first SBRT fraction. At 3 months, radiological evaluation of the treatment response took place in 18 patients. Three patients did not undergo follow-up scanning per protocol, due to a poor performance status. However, one of them had an abdominal ultrasound which demonstrated liver metastases at three months. According to Response Evaluation Criteria In Solid Tumours, no patients had a complete or partial response, 7 showed stable disease and 11 demonstrated disease progression at three months. Progression was local alone in one patient, whereas distant metastases without local progression developed in six patients. In three patients, there was both local and distant progression. Three patients demonstrated progressive disease at 3 months and had a good performance score. Therefore, these patients received palliative chemotherapy. One patient demonstrated progression at 6 months and had a good performance score; this patient received chemotherapy 6 months after SBRT. The other patients had no signs of disease progression, were in a poor physical condition or refused chemotherapy.
Safety
No acute or late treatment related grade 3 or higher toxicity was seen in this trial. A one-sided Pearson-Klopper analysis revealed a toxicity rate of 0% (95% confidence interval 0–14%). There were several non-study related grade 3 or higher toxicities, as was expected in this fragile patient category. Acute grade ≥3 toxicities were: pneumonia, asymptomatic pulmonary embolism, infected ascites, bile duct stenosis, morphine associated constipation (all grade 3). Late toxicities were: grade 3 bleeding duodenal varices due to portal hypertension at 6 months, grade 3 liver abscess at 4 months, grade 3 gastroparesis at 5 months, perforated cholecystitis causing abdominal sepsis grade 4 at 9 months, grade 5 bleeding of the SMA at 5 months due to tumour progression. For an overview of all toxicities, see Supplementary Table 1.
Quality-of-life
Pre-SBRT, all 20 irradiated patients completed the QOL questionnaires. At time point 1, 3, 6, and 12 months after SBRT, 18, 15, 8 and 3 patients completed the questionnaires. See Table 4 for the results. Overall QOL after SBRT was equal or improved compared to baseline in 69, 60, 43, and 33 percent of patients at 1, 3, 6, and 12 months, respectively. After 1, 3, 6, and 12 months, patients rated their general health improved or equal to baseline in 88, 67, 38 and 33 percent, respectively.
Table 4.
Quality of life before and after SBRT
| Pre-SBRT | 1 month | 3 months | 6 months | 12 months | |
| Number of returned questionnaires | 20 | 18 | 15 | 8 | 3 |
| Percentage of expected completiona | 100 | 90 | 88 | 80 | 100 |
| RAND-36 | |||||
| Physical functioning | 63.3 (27.5) | 60.0 (34.7) | 70.3 (28.1) | 71.9 (22.0) | 50.0 (42.7) |
| Social functioning | 51.9 (27.3) | 61.8 (30.5) | 70.0 (18.8)b | 67.2 (27.5) | 41.7 (26.0) |
| Physical role restriction | 30.0 (40.2) | 38.9 (42.2) | 45.0 (38.0) | 31.3 (32.0) | 8.3 (14.4) |
| Emotional role restriction | 42.6 (45.5) | 51.9 (43.1) | 47.6 (40.7) | 45.8 (43.4) | 33.3 (57.7) |
| Mental health | 64.6 (17.7) | 69.3 (22.2) | 72.3 (16.9) | 68.0 (17.1) | 68.0 (14.4) |
| Vitality | 53.3 (19.6) | 51.4 (22.1) | 57.3 (20.4) | 60.6 (24.7) | 26.7 (12.6) |
| Pain | 55.6 (25.1) | 74.8 (20.1)b | 72.9 (23.0) | 67.3 (17.9) | 51.7 (23.2) |
| General health | 45.1 (15.4) | 45.3 (19.9) | 44.7 (13.2) | 42.9 (17.8) | 26.7 (14.4) |
| Change in health | 20.0 (13.1) | 33.3 (29.7) | 35.0 (32.5) | 34.4 (39.9) | 16.7 (14.4) |
| EORTC QLQ-C30 | |||||
| Physical functioning | 71.4 (22.3) | 70.4 (27.8) | 76.9 (23.5) | 79.2 (16.9) | 53.3 (37.1) |
| Role functioning | 55.0 (23.6) | 63.9 (33.5) | 65.6 (20.4) | 56.3 (30.8) | 38.9 (34.7) |
| Emotional functioning | 67.1 (17.8) | 71.3 (26.1) | 77.2 (18.2) | 61.5 (24.4) | 61.1 (12.7) |
| Cognitive functioning | 81.7 (21.6) | 75.9 (30.9) | 81.1 (22.6) | 85.4 (18.8) | 77.8 (25.5) |
| Social functioning | 68.3 (24.1) | 69.6 (34.0) | 81.1 (20.8)b | 72.9 (26.6) | 55.6 (25.5) |
| Global health/QOL | 58.5 (16.2) | 62.3 (26.0) | 65.6 (18.3) | 57.3 (25.4) | 44.4 (12.7) |
| Fatigue | 40.0 (22.6) | 37.7 (24.1) | 37.0 (25.1) | 47.2 (21.2) | 74.1 (25.7) |
| Nausea and vomiting | 11.7 (16.3) | 14.8 (27.9) | 8.9 (13.9) | 22.9 (33.3) | 22.2 (19.2) |
| Pain | 35.0 (26.4) | 23.1 (23.0) | 26.7 (24.2) | 33.3 (21.8) | 50.0 (16.7) |
| Dyspnea | 15.8 (25.1) | 11.1 (22.9) | 15.6 (30.5) | 20.8 (24.8) | 22.2 (19.2) |
| Insomnia | 25.0 (35.7) | 22.2 (22.9) | 20.0 (27.6) | 37.5 (27.8) | 66.7 (33.3) |
| Appetite loss | 41.7 (34.0) | 33.3 (37.9) | 28.9 (35.3) | 33.3 (35.6) | 33.3 (33.3) |
| Constipation | 23.3 (30.8) | 20.4 (34.6) | 15.6 (21.3) | 8.3 (23.6) | 0.0 (0.0) |
| Diarrhea | 21.7 (29.2) | 27.8 (36.6) | 28.9 (33.0) | 57.1 (37.1)b | 55.6 (50.9) |
| Financial difficulties | 6.7 (17.4) | 7.8 (14.6) | 8.9 (15.3) | 12.5 (24.8) | 0.0 (0.0) |
| EORTC QLQ-PAN26 | |||||
| Pain | 32.4 (24.9) | 23.5 (17.5) | 29.4 (21.6) | 35.4 (18.2) | 22.2 (9.6) |
| Eating related items | 31.7 (25.3) | 33.3 (34.4) | 24.4 (26.6) | 35.4 (27.4) | 27.8 (19.2) |
| Cachexia | 36.7 (22.7) | 35.3 (23.5) | 32.2 (23.1) | 56.3 (33.3) | 27.8 (25.5) |
| Hepatic | 18.3 (21.6) | 7.8 (14.6) | 3.3 (9.3)b | 14.6 (22.6) | 11.1 (9.6) |
| Body image | 20.8 (22.2) | 26.0 (26.5) | 21.1 (24.0) | 43.8 (28.1) | 27.8 (25.5) |
| Side effects | 22.8 (20.5) | 28.8 (28.3) | 23.0 (25.0) | 30.6 (25.7) | 25.9 (23.1) |
| Health-care satisfaction | 74.2 (21.9) | 69.8 (24.5) | 80.0 (20.1) | 76.2 (23.3) | 75.0 (35.4) |
| Altered bowel habit | 35.8 (26.6) | 35.3 (17.6) | 44.4 (24.1) | 47.9 (18.8) | 55.6 (9.6) |
| Sexuality | 41.7 (39.7) | 57.8 (36.9) | 47.6 (45.2) | 52.8 (40.0) | 38.9 (41.9) |
| Ascites | 35.0 (29.6) | 31.4 (22.0) | 37.8 (27.8) | 37.5 (33.0) | 44.4 (19.2) |
| Indigestion | 26.7 (31.7) | 29.2 (34.2) | 31.1 (34.4) | 28.6 (40.5) | 33.3 (33.3) |
| Flatulence | 48.3 (31.5) | 43.1 (30.7) | 42.2 (34.4 | 38.1 (23.0) | 44.4 (50.9) |
| Fear of future health | 61.7 (27.1) | 51.0 (29.1) | 51.1 (27.8) | 62.5 (21.4) | 55.6 (19.2) |
| Ability to plan future | 45.0 (27.1) | 39.2 (33.8) | 37.8 (33.0) | 41.7 (29.5) | 44.4 (50.9) |
EORTC, European Organization for Research and Treatment of Cancer; QOL, quality of life; SBRT, stereotactic body radiation therapy.
Values are mean (SD) unless indicated otherwise.
Values are percentage of expected replies after exclusion of censored patients (those who had died or within 1 month of dying) or not yet reached time point due to short follow- up.
p < 0.050 and clinically relevant (more than 10-point change in score) difference between baseline and postoperative time point (paired t test).
Table 4 Quality of life before and after SBRT (see end of manuscript).
Discussion
This study shows that MRI-guided stereotactic delivery of at least 24 Gy was safe and feasible, with no treatment related grade 3 or higher acute and late toxicity. A high dose delivered with high precision in free breathing conditions and good target coverage was achieved while sparing OAR. A dose of 24 Gy to the PTV was prescribed, however, a higher dose in the tumour was feasible with an average Dmean of 29.8 Gy and Dmax of 34.5 Gy to the GTV.
MRI was integrated in the treatment planning. MRI was used for delineation and motion simulation. As MRI is capable of demonstrating functional information in addition to anatomical information, we found the contribution of multiparametric MRI in addition to CT in delineating pancreatic tumours useful. Up to now, no evidence about the best way to delineate a pancreatic tumour exists. In a previous radiology vs pathology study, MRI underestimated the tumour diameter by 4 mm.29 However, in contrast to our study, only a single imaging sequence was used. Moreover, accurate pathology orientation related to the MRI measurements was lacking. Therefore, these results have to be approached with caution. With CT, discrepancies are also seen between the largest diameter on pathology and on CT. In one study, an underestimation of 7 mm was observed, and another study demonstrated an underestimation by 8 mm of tumours larger than 3 cm and an overestimation of 4 mm when tumours were smaller than 3 cm.30, 31 Hall describes an interobserver study that compared MRI to CT in contouring pancreatic tumours.32 This study demonstrated that volumes contoured on MRI are smaller compared to CT contoured volumes.
Besides its role in tumour delineation, motion data derived from MRI were incorporated into treatment planning. Cine MRI is able to visualize tumour motion, instead of intratumoural fiducials and, similarly, OAR motion can be characterized. 4DCT averages the motion amplitude over multiple breathing cycles by retrospectively binning into different phases. Therefore, the fourth dimension reflects rather “phase” than “time”. Therefore, organ and tumour motion might be underestimated with 4DCT in comparison to motion observed with cine MRI. Another advantage of cine MRI over 4DCT is that it is possible to explore multiple respiration cycles over a long time span, or over different days to quantify day-to-day variation without being exposed to ionizing radiation. In this study, patients were imaged for 1 min with 2 images per second, covering on average 12–16 breathing cycles. After integration of the motion trajectory into the treatment planning the GTV-to-PTV margin of 3 mm was considered sufficient for dose coverage in all patients. Patients were treated on a modern linear accelerator with a VMAT technique in free breathing conditions. Although it was previously demonstrated that the breathing amplitude on a planning 4DCT is not always representative of the amplitude during the course of treatment,33 dose distributions were robust against changes in the breathing amplitude as patients were positioned at the midventilation position. Furthermore, the advantage of a stereotactic dose distribution is that when the tumour is mobile, the maximum dose is blurred over a larger area. This results in a compensation of under- and overdosage areas. This beneficial strategy results in feasibility of smaller margins compared to application of an internal target volume. In addition, the use of the custom made corset decreased the breathing amplitude of the tumour and the surrounding tissues, as previously described.34 Overall in this study, an average craniocaudal tumour motion of 8 mm was observed, while in other studies larger tumour motions were seen, i.e. 15 mm, 20 mm, and 24 mm.20, 35,36 Moreover, we performed a simulation experiment in which the craniocaudal breathing amplitude was increased, up to 3 and 10 times the original tumour motion, demonstrating adequate tumour coverage with this increased tumour motion.
The toxicity profile of our stereotactic delivery of 24 Gy was less when compared to other studies. This might be partly a result of the lack of concurrent chemotherapy. In addition, this fractionation schedule has a lower biologically effective dose (BED) than the 25 Gy in one fraction prescription of other groups: 43.2 vs 87.5 Gy, respectively, when calculated with an α/β of 10.8, 30,37 However, as the average mean dose in the GTV was 29.8 Gy, the average BED was 59.4 Gy in this study. Previous literature has demonstrated a survival benefit for patients treated with a BED >70 Gy compared to patients treated with a BED <70 Gy.38 Our study has a low local control rate and low overall survival. This might be due to the relatively low BED, the older patient group and the higher percentage of distant metastases documented at 3 months. Only four patients received systemic therapy with disease progression 3 or 6 months after SBRT, as other patients were too frail, refused chemotherapy or had no progressive disease. This was mainly due to the frail and elderly patient population. However, QOL was good in this study, with an overall QOL equal to or improved compared to baseline in 69, 60, 43, and 33 percent of patients at 1, 3, 6, and 12 months, respectively.
A drawback of this study is the high percentage of early distant metastases. This might be a result of the presence of occult metastases at the initiation of treatment and this could bias our results. In a future study, patient selection might be beneficial. Biomarkers such as DPC4 could identify patients that are prone to distant metastases or local destructive disease only.39 In addition, induction chemotherapy could select patients that do not metastasize early as 30–35% of patients scheduled for local treatment develop distant metastases after neoadjuvant systemic therapy for localized disease.40, 41 Another downside is the difference in scanning position between CT and MRI and the difference in fasting protocol between imaging and treatment. However, as registration of both images was on the local area (i.e. tumour and duodenal area), discrepancies in GTV and closeby OAR between CT and MRI were minor. In addition, a PRV margin was applied to increase safety.
A future perspective includes real time MRI during treatment. This could increase accuracy as registration can be performed on both tumour and the organs at risk. This holds premise for adaptive radiotherapy and dose escalation without adding toxicity.42, 43
Conclusion
MRI guided SBRT for pancreatic cancer is technical feasible and safe, with no treatment related grade ≥3 toxicity. New strategies are applied, including an individual corset to reduce breathing induced motion, MRI based delineation, and evaluation of motion-integrated dose distributions.
Footnotes
Ethics approval: This trial was approved bythe institutional review board of the University Medical Center Utrecht (12-628) andregistered at www.clinicaltrials.gov (NCT01898741). All patients providedwritten informed consent.
Availability of data and material: The dataset/information supporting the conclusions of this article is included within the article.
Availability of data and material: The dataset/information supporting the conclusions of this article is included within the article.
Contributor Information
Hanne D Heerkens, Email: h.heerkens@umcutrecht.nl.
Marco van Vulpen, Email: m.van.vulpen@hollandptc.nl.
Beth Erickson, Email: berickson@mcw.edu.
Onne Reerink, Email: o.reerink@isala.nl.
Martijn PW Intven, Email: m.intven@umcutrecht.nl.
Cornelis AT van den Berg, Email: c.a.t.vandenberg@umcutrecht.nl.
I Quintus Molenaar, Email: i.q.molenaar@umcutrecht.nl.
Frank P Vleggaar, Email: f.vleggaar@umcutrecht.nl.
Gert J Meijer, Email: g.j.meijer@umcutrecht.nl.
REFERENCES
- 1. Willett CG, Czito BG, Bendell JC, Ryan DP. Locally advanced pancreatic cancer. J Clin Oncol 2005; 23: 4538–44. doi: 10.1200/JCO.2005.23.911 [DOI] [PubMed] [Google Scholar]
- 2. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011; 378: 607–20. doi: 10.1016/S0140-6736(10)62307-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huguet F, Girard N, Guerche CS, Hennequin C, Mornex F, Azria D. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol 2009; 27: 2269–77. doi: 10.1200/JCO.2008.19.7921 [DOI] [PubMed] [Google Scholar]
- 4. Mukherjee S, Hurt CN, Bridgewater J, Falk S, Cummins S, Wasan H, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol 2013; 14: 317–26. doi: 10.1016/S1470-2045(13)70021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loehrer PJ, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 2011; 29: 4105–12. doi: 10.1200/JCO.2011.34.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moraru IC, Tai A, Erickson B, Li XA. Radiation dose responses for chemoradiation therapy of pancreatic cancer: an analysis of compiled clinical data using biophysical models. Pract Radiat Oncol 2014; 4: 13–19. doi: 10.1016/j.prro.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 7. Huguet F, Hammel P, Vernerey D, Goldstein D, Van Laethem J, Glimelius B. Impact of chemoradiation on local control and time without treatment in patients with locally advanced pancreatic cancer included in the international phase III LAP 07 study. J Clin Oncol 2014; 32: Abstract 4001. [Google Scholar]
- 8. Chang DT, Schellenberg D, Shen J, Kim J, Goodman KA, Fisher GA, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009; 115: 665–72. doi: 10.1002/cncr.24059 [DOI] [PubMed] [Google Scholar]
- 9. Didolkar MS, Coleman CW, Brenner MJ, Chu KU, Olexa N, Stanwyck E, et al. Image-guided stereotactic radiosurgery for locally advanced pancreatic adenocarcinoma results of first 85 patients. J Gastrointest Surg 2010; 14: 1547–59. doi: 10.1007/s11605-010-1323-7 [DOI] [PubMed] [Google Scholar]
- 10. Goyal K, Einstein D, Ibarra RA, Yao M, Kunos C, Ellis R, et al. Stereotactic body radiation therapy for nonresectable tumors of the pancreas. J Surg Res 2012; 174: 319–25. doi: 10.1016/j.jss.2011.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahadevan A, Jain S, Goldstein M, Miksad R, Pleskow D, Sawhney M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2010; 78: 735–42. doi: 10.1016/j.ijrobp.2009.08.046 [DOI] [PubMed] [Google Scholar]
- 12. Mahadevan A, Miksad R, Goldstein M, Sullivan R, Bullock A, Buchbinder E, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys 2011; 81: e615–e622. doi: 10.1016/j.ijrobp.2011.04.045 [DOI] [PubMed] [Google Scholar]
- 13. Polistina F, Costantin G, Casamassima F, Francescon P, Guglielmi R, Panizzoni G, et al. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol 2010; 17: 2092–101. doi: 10.1245/s10434-010-1019-y [DOI] [PubMed] [Google Scholar]
- 14. Su TS, Liang P, Lu HZ, Liang JN, Liu JM, Zhou Y, et al. Stereotactic body radiotherapy using CyberKnife for locally advanced unresectable and metastatic pancreatic cancer. World J Gastroenterol 2015; 21: 8156–62. doi: 10.3748/wjg.v21.i26.8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saisho H, Yamaguchi T. Diagnostic imaging for pancreatic cancer: computed tomography, magnetic resonance imaging, and positron emission tomography. Pancreas 2004; 28: 273–8. [DOI] [PubMed] [Google Scholar]
- 16. Vellet AD, Romano W, Bach DB, Passi RB, Taves DH, Munk PL. Adenocarcinoma of the pancreatic ducts: comparative evaluation with CT and MR imaging at 1.5 T. Radiology 1992; 183: 87–95. doi: 10.1148/radiology.183.1.1312736 [DOI] [PubMed] [Google Scholar]
- 17. Heerkens HD, Hall WA, Li XA, Knechtges P, Dalah E, Paulson ES, et al. Recommendations for MRI-based contouring of gross tumor volume and organs at risk for radiation therapy of pancreatic cancer. Pract Radiat Oncol 2017; 7: 126–36. doi: 10.1016/j.prro.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 18. Heerkens HD, van Vulpen M, van den Berg CA, Tijssen RH, Crijns SP, Molenaar IQ, et al. MRI-based tumor motion characterization and gating schemes for radiation therapy of pancreatic cancer. Radiother Oncol 2014; 111: 252–7. doi: 10.1016/j.radonc.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 19. Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 2010; 76: S94–S100. doi: 10.1016/j.ijrobp.2009.06.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kavanagh BD, Pan CC, Dawson LA, Das SK, Li XA, Ten Haken RK, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys 2010; 76(3 Suppl): S101–S107. doi: 10.1016/j.ijrobp.2009.05.071 [DOI] [PubMed] [Google Scholar]
- 21. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 22. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–83. [PubMed] [Google Scholar]
- 23. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–76. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 24. Fitzsimmons D, Johnson CD, George S, Payne S, Sandberg AA, Bassi C, et al. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Cancer 1999; 35: 939–41. doi: 10.1016/S0959-8049(99)00047-7 [DOI] [PubMed] [Google Scholar]
- 25. Osoba D, Bezjak A, Brundage M, Zee B, Tu D, Pater J, et al. Analysis and interpretation of health-related quality-of-life data from clinical trials: basic approach of the National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer 2005; 41: 280–7. doi: 10.1016/j.ejca.2004.10.017 [DOI] [PubMed] [Google Scholar]
- 26. Hirdes MM, van Hooft JE, Wijrdeman HK, Hulshof MC, Fockens P, Reerink O, et al. Combination of biodegradable stent placement and single-dose brachytherapy is associated with an unacceptably high complication rate in the treatment of dysphagia from esophageal cancer. Gastrointest Endosc 2012; 76: 267–74. doi: 10.1016/j.gie.2012.04.442 [DOI] [PubMed] [Google Scholar]
- 27. Whitehead J. The design and analysis of sequential clinical trials, rev. 2nd ed Chichester: The British Institute of Radiology.; 1997. [Google Scholar]
- 28. Rwigema JC, Parikh SD, Heron DE, Howell M, Zeh H, Moser AJ, et al. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol 2011; 34: 63–9. doi: 10.1097/COC.0b013e3181d270b4 [DOI] [PubMed] [Google Scholar]
- 29. Hall WA, Mikell JL, Mittal P, Colbert L, Prabhu RS, Kooby DA, et al. Tumor size on abdominal MRI versus pathologic specimen in resected pancreatic adenocarcinoma: implications for radiation treatment planning. Int J Radiat Oncol Biol Phys 2013; 86: 102–7. doi: 10.1016/j.ijrobp.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 30. Arvold ND, Niemierko A, Mamon HJ, Fernandez-del Castillo C, Hong TS. Pancreatic cancer tumor size on CT scan versus pathologic specimen: implications for radiation treatment planning. Int J Radiat Oncol Biol Phys 2011; 80: 1383–90. doi: 10.1016/j.ijrobp.2010.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qiu H, Wild AT, Wang H, Fishman EK, Hruban RH, Laheru DA, et al. Comparison of conventional and 3-dimensional computed tomography against histopathologic examination in determining pancreatic adenocarcinoma tumor size: implications for radiation therapy planning. Radiother Oncol 2012; 104: 167–72. doi: 10.1016/j.radonc.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hall WA, Heerkens HD, Paulson ES, Meijer GJ, Kotte AN, Knechtges P, et al. Pancreatic gross tumor volume contouring on computed tomography (CT) compared with magnetic resonance imaging (MRI): Results of an international contouring conference. Pract Radiat Oncol 2018; 8: 107–15. doi: 10.1016/j.prro.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 33. Lens E, van der Horst A, Kroon PS, van Hooft JE, Dávila Fajardo R, Fockens P, et al. Differences in respiratory-induced pancreatic tumor motion between 4D treatment planning CT and daily cone beam CT, measured using intratumoral fiducials. Acta Oncol 2014; 53: 1257–64. doi: 10.3109/0284186X.2014.905699 [DOI] [PubMed] [Google Scholar]
- 34. Heerkens HD, Reerink O, Intven MPW, Hiensch RR, van den Berg CAT, Crijns SPM, et al. Pancreatic tumor motion reduction by use of a custom abdominal corset. Phys Imaging Radiat Oncol 2017; 2: 7–10. doi: 10.1016/j.phro.2017.02.003 [DOI] [Google Scholar]
- 35. Feng M, Balter JM, Normolle D, Adusumilli S, Cao Y, Chenevert TL, et al. Characterization of pancreatic tumor motion using cine MRI: surrogates for tumor position should be used with caution. Int J Radiat Oncol Biol Phys 2009; 74: 884–91. doi: 10.1016/j.ijrobp.2009.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bussels B, Goethals L, Feron M, Bielen D, Dymarkowski S, Suetens P, et al. Respiration-induced movement of the upper abdominal organs: a pitfall for the three-dimensional conformal radiation treatment of pancreatic cancer. Radiother Oncol 2003; 68: 69–74. doi: 10.1016/S0167-8140(03)00133-6 [DOI] [PubMed] [Google Scholar]
- 37. Schellenberg D, Goodman KA, Lee F, Chang S, Kuo T, Ford JM, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2008; 72: 678–86. doi: 10.1016/j.ijrobp.2008.01.051 [DOI] [PubMed] [Google Scholar]
- 38. Krishnan S, Chadha AS, Suh Y, Chen HC, Rao A, Das P, et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol Biol Phys 2016; 94: 755–65. doi: 10.1016/j.ijrobp.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009; 27: 1806–13. doi: 10.1200/JCO.2008.17.7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huguet F, André T, Hammel P, Artru P, Balosso J, Selle F, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007; 25: 326–31. doi: 10.1200/JCO.2006.07.5663 [DOI] [PubMed] [Google Scholar]
- 41. Krishnan S, Rana V, Janjan NA, Varadhachary GR, Abbruzzese JL, Das P, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer 2007; 110: 47–55. doi: 10.1002/cncr.22735 [DOI] [PubMed] [Google Scholar]
- 42. Lagendijk JJ, Raaymakers BW, Raaijmakers AJ, Overweg J, Brown KJ, Kerkhof EM, et al. MRI/linac integration. Radiother Oncol 2008; 86: 25–9. doi: 10.1016/j.radonc.2007.10.034 [DOI] [PubMed] [Google Scholar]
- 43. Fallone BG, Murray B, Rathee S, Stanescu T, Steciw S, Vidakovic S, et al. First MR images obtained during megavoltage photon irradiation from a prototype integrated linac-MR system. Med Phys 2009; 36: 2084–8. doi: 10.1118/1.3125662 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of data and material: The dataset/information supporting the conclusions of this article is included within the article.