Abstract
Objective:
To evaluate stages of chronic kidney disease (CKD) by apparent diffusion coefficient (ADC) obtained from diffusion weighted imaging (DWI) using a meta-analysis.
Methods:
Literature databases were searched from PubMed, Web of Science, Cochrane and Embase to identify relevant articles about DWI in CKD between 1999 and 2017. ADC values were extracted from the healthy group and CKD patients with different stages. Meta-analysis was conducted using STATA v. 12.0. A random-effects model was performed to acquire the effect estimate, which was expressed as a pooled weighted mean difference (WMD) with 95% confidence interval (CI). We performed comparisons of ADC values between the following groups: (1) the ADC values of the normal kidneys v s earlier Stage 1–2 of CKD; (2) Stage 3 v s the Stage 1–2 of CKD; (3) the Stage 4–5 v s the Stage 3.
Results:
Six studies were included in this meta-analysis. The CKD patients with earlier Stage 1–2 showed lower ADC values than the healthy subjects [WMD = −0.09, 95% CI(−0.12 to −0.06), p < 0.001]. However, no obvious difference in ADC values was found between the Stage 3 and Stage1–2 of CKD [WMD = −0.09, 95% CI (−0.18 to 0.01), p = 0.08]. The CKD Stage3 had higher ADC values than those of Stage4–5 [WMD = −0.21, 95% CI (−0.32 to −0.11), p = 0.01].
Conclusion:
DWI is an accurate and non-invasive imaging technique for early diagnosis and staging of CKD. Quantitative DWI may potentially play a role in making clinical decisions in the follow-up of CKD.
Advances in knowledge
DWI can be a valuable tool for staging of CKD.
Introduction
Chronic kidney disease (CKD) is a worldwide public health problem characterized by progressive decrease in kidney function.1 Early and accurate diagnosis of CKD patients is critical for early prediction of outcome and individualized therapies.2 CKD staging can benefit accurate diagnosis, therefore appropriate intervention can be adopted at early stage to delay the progression of CKD.3, 4
Serum markers such as creatinine and blood urea nitrogen levels and estimated glomerular rate (eGFR) are useful parameters for estimating renal function in clinical practice.5 However, these indicators only assess the global renal function and cannot reflect morphological changes of kidney. The routine radiological methods of detecting CKD, such as ultrasonography (US), CT and MRI, only provide anatomic images without functional information.6 With regard to contrast enhancement, contrast agents in CT and gadolinium-based MRI may cause nephrotoxicity and systemic nephrogenic fibrosis respectively, thereby limiting their use in CKD patients.7 Radioisotope scintigraphy is the only established imaging modality to assess renal function by measuring glomerular filtration rate (GFR), but it leads to radiation exposure and has low spatial resolution. So it is necessary to find non-invasive imaging method to quantitatively evaluate renal function of CKD patients.
Diffusion-weighted imaging (DWI) shows the Brownian motion of water molecules in biological tissue, which is usually quantified by the apparent diffusion coefficient (ADC) and provide information on diffusion and perfusion. DWI has been used as a promising modality to assess renal function.8–13 Some studies have indicated the relationship between ADC values and different stages of CKD, but the efficacy of ADC values to identify different stages of CKD remains unclear, with inconsistent results presented by different researchers. Yalcin-Safak et al13 and Xu Y et al14 reported no difference in ADC values between the healthy group and the early renal function impairment group. However, Goyal et al15 reported significant difference of ADC values at different stages of CKD.
The existing findings about the relationship between ADC values and CKD stage are controversial in the previous studies, and in order to address this issue, a meta-analysis was conducted based on high quality published studies to determine the potential value of DWI imaging in the staging of CKD.
methods and Materials
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)statement (Supplementary Material 1). This meta-analysis did not involve identifiable patient information, so no particular ethical consideration was required.
Literature search
Databases were searched from PubMed, Web of science, Cochrane and Embase to identify all the relevant articles characterizing the relationship between ADC and the staging of CKD between 1999 and 2017. The following search terms were used “DWI”, “diffusion weighted imaging”, “ADC” “apparent diffusion coefficient”, “CKD”, “chronic renal disease”, “chronic kidney disease”, “magnetic resonance imaging” and “MRI”.
Eligibility criteria for study selection
Two investigators screened the titles and abstracts from the databases.
The following inclusion criteria for studies were applied:
The population consisted of healthy subjects and CKD patients on native kidneys which based on the K/DOQI (kidney disease outcomes quality initiative) classification.16 ADC values based on DWI was assessed.
Diagnostic criteria for different stages of CKD were as follows: the normal kidney and Stage 1 of CKD (eGFR ≥ 90 ml/min/1.73 m2); Stage 2 of CKD (60 ml/min/1.73 m2 ≤ eGFR <90 ml/min/1.73 m2); Stage 3 of CKD (30 ml/min/1.73 m2 ≤ eGFR <60 ml/min/1.73 m2); Stage 4 of CKD (15 ml/min/1.73 m2 ≤ eGFR <30 ml/min/1.73 m2); Stage 5 of CKD (eGFR <15 ml/min/1.73 m2).
The search was limited to those printed in English.
The exclusion criteria were as follows:
Review articles, letters, and researches on animal models, comments and case reports.
Duplicate or irrelevant publications.
Studies without sufficient data.
Data extraction and assessment of quality
Two authors extracted data independently, and disagreements between them were solved by discussion and consultation with a third author. For accuracy analyses, data were extracted from included studies: such as authors, year of publication, baseline information about the patients, sample size, MR scanner, the equation of eGFR, and the ROI (region of interest) disposition.
We used the standard quality assessment of diagnostic studies (QUADAS-2) tool to assess the quality of included studies, which were classified as low risk of bias, unclear risk of bias or high risk of bias.17
Statistical analysis
To compare ADC values between different stages of CKD in different studies, the pooled mean and standard deviation (SD) of ADC were calculated by the following equations:18
M and SD are the pooled mean and standard deviation of Group 1 and Group 2 (grouped by stage of eGFR). N1, M1, and SD1 are the size, mean, and standard deviation of Group 1, respectively; N2, M2, and SD2 are the size, mean, and standard deviation of Group 2, respectively.
The ADC value was estimated by the weighted mean difference (WMD) with 95% confidence intervals (CI) by STATA 12.0 (USA). We evaluated the heterogeneity of the individual studies through Cochrane’s Q test and calculating the inconsistency index (I-squared, I2) statistics. If p < 0.1, it is considered significant heterogeneity between the statistics.19 It is assigned adjectives of low, moderate, and high to I2 values of 25%, 50%, and 75%. If I2 <25%, the fixed effect model was applied for meta-analysis; If 25% < I 2 < 50%, random effect model was applied; If I2 >50%, the heterogeneity was analyzed first, and the random effect model were used under the circumstances that the source of heterogeneity cannot be found. Egger’s test was performed to assess publication bias, and with existence of an inverted symmetrical funnel plot with p > 0.05 was considered evidence of insignificant publication bias.
Results
Study selection
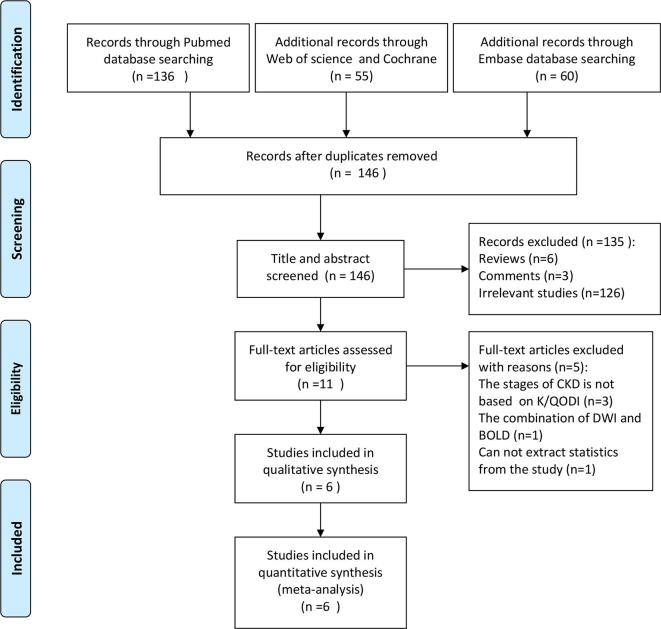
After reviewing the titles and abstracts of all searched articles, 146 articles were excluded. There were 11 full-text articles were assessed for eligibility, and 5 articles were excluded for the following reasons: the stages of CKD were not based on the eGFR at the basis of K/QODI; DWI was combined with other MRI techniques; data could not be extracted from the studies. Finally, 6 eligible studies were included in this meta-analysis10, 12,13,15,20,21 (Figure 1). The basic characteristics of included studies are shown in Table 1.
Figure 1.
Process of study selection.
Table 1.
Baseline characteristics of included studies
| Study | Year | design | Sample size | Age | Male/female | MR scanner | Equation of eGFR | b values (s mm−2) | ROI disposition (0 n ADC map) |
| Healthy | Healthy | Healthy | |||||||
| CKD | CKD | CKD | |||||||
| Yalcin-safak et al13 | 2016 | Retrospective | 15 | NA | NA | 1.5T Siemens | Japanese eGFR equation | 0.400 | ROIs were placed on renal parenchyma on both kidneys |
| 110 | 61.5 (19–85) | 45/65 | |||||||
| Feng et al12 | 2015 | Retrospective | 30 | NA | NA | 1.5T Siemens | MDRD | 0.600 | The parenchyma and three ROIs from the upper, middle, and lower pole |
| 75 | NA | 39/36 | |||||||
| Wang et al10 | 2014 | Retrospective | 20 | 31 | 10/10 | 3T Siemens | MDRD | 0.400 | ROIs were drawn on the upper, middle, and lower portions of bilateral cortex and medulla |
| 29 | 36 | 14/15 | |||||||
| Goyal et al15 | 2012 | Retrospective | 66 | 45.1 (18–85) | 55/33 | 1.5T Siemens | Cockcroft-Gault’s equation | 0.500 | ROI of size 1 cm2 were placed on the normal renal parenchyma |
| 22 | |||||||||
| Toya et al21 | 2010 | Retrospective | NA | NA | NA | 1.5TSiemens | Japanese eGFR equation | 50,1000 | ROIs were placed on the corticomedullary junction |
| 180 | 61.06 (20–89) | 113/67 | |||||||
| Carbone et al20 | 2007 | Prospective | NA | NA | NA | 1.5T Philips | Cockcroft-Gault’s equation | 0.600 | ROIs were placed on the parenchyma of each kidney |
| 14 | 49 (22–66) | 9/5 |
CKD, chronic kidney disease; GFR, glomerular filtration rate; MDRD, modification of diet in renal disease; NA, not available.
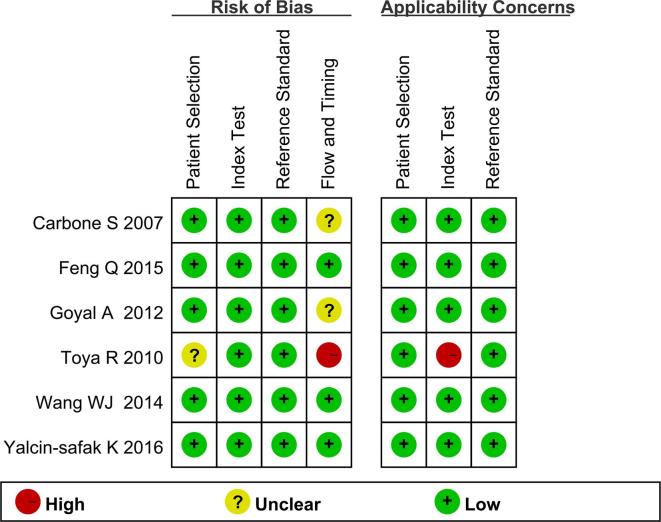
Assessment of study quality
The quality of included studies was assessed according to the QUADAS-2 items.17 The results of quality assessment are presented in Figure 2. The risk of all articles was low because the index test and the reference tests were mutually independent. Some unclear risks from several studies were caused by the different reference standards for CKD diagnosis, including the pathology, the clinical and laboratorial factors, and different equations of eGFR.
Figure 2.
Quality assessment of include studies.
Apparent diffusion coefficient distinguished stage 1–2 of CKD from the normal kidneys
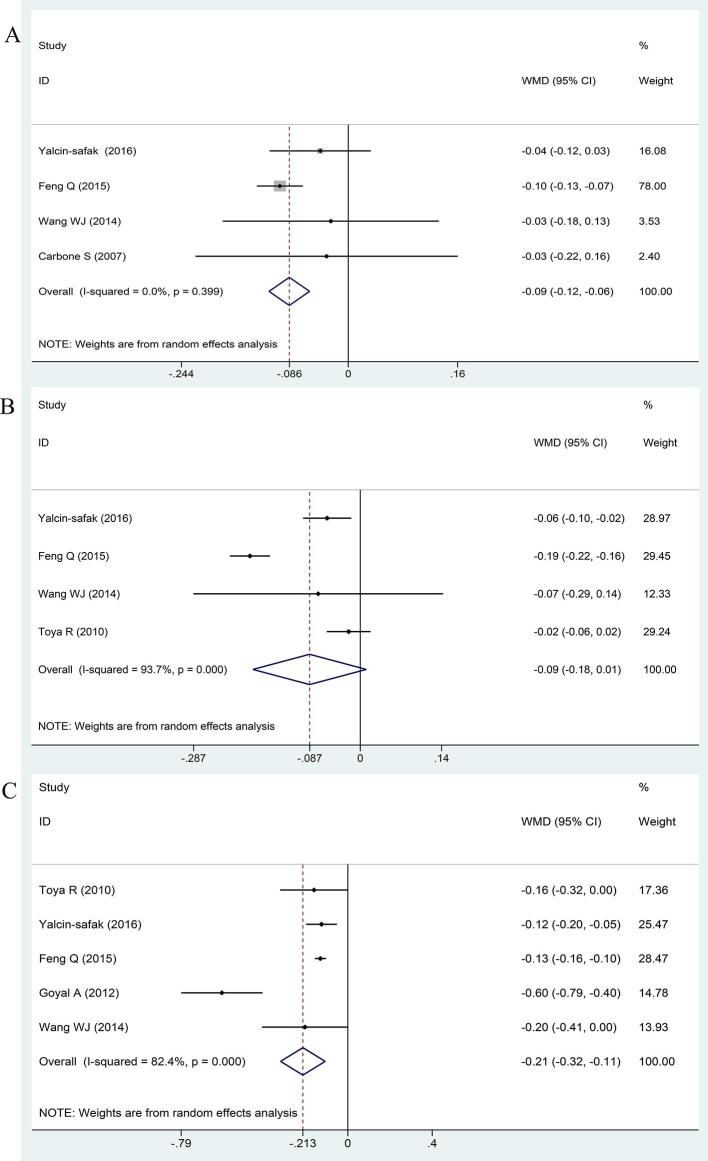
Detailed data of six studies for pooled weighted mean difference model were shown in Table 2. There were 561 subjects: 131 healthy volunteers and 430 CKD patients. The ADC values were compared between the healthy subjects and Stage 1–2 CKD patients, and the normal kidneys showed significantly higher ADC values than those of CKD Stage 1–2 [WMD:−0.086, 95% CI (−0.116 to −0.057), p < 0.001; I2 = 0.0%, p = 0.399] (Figure 3a).
Table 2.
Detailed data of included studies
| Groups | Author | Sample size | ROIs disposition | ADC values (mm s– 2) | ADC values (mm s–2) |
| Stage 1–2 vs Normal | Yalcin-safak et al13 | 38 vs 15 | Parenchyma | 1.1962 ± 0.0932 | 1.23713 ± 0.13415 |
| Feng et al12 | 30 vs 20 | Parenchyma | 2.23 ± 0.0865 | 2.33 ± 0.03 | |
| Carbone et al20 | 6 vs 5 | Parenchyma | 2.4083 ± 0.19343 | 2.44 ± 0.1294 | |
| Wang et al10 | 11 vs 20 | Parenchyma | 2.175 ± 0.2151 | 2.2005 ± 0.21478 | |
| Stage 3 vs Stage 1–2 | Yalcin-safak et al13 | 43 vs 38 | Parenchyma | 1.13916 ± 0.09761 | 1.1962 ± 0.0932 |
| Feng et al12 | 15 vs 30 | Parenchyma | 2.04 ± 0.03 | 2.23 ± 0.0865 | |
| Wang et al10 | 7 vs 11 | Parenchyma | 2.1025 ± 0.23399 | 2.175 ± 0.2151 | |
| Toya R21 | 47 vs 128 | Parenchyma | 1.87 ± 0.11 | 1.89 ± 0.12 | |
| Stage 4–5 vs Stage 3 | Yalcin-safak et al13 | 14 vs 43 | Parenchyma | 1.01436 ± 0.12794 | 1.13916 ± 0.09761 |
| Feng et al12 | 30 vs 15 | Parenchyma | 1.91 ± 0.0615 | 2.04 ± 0.03 | |
| Goyal et al15 | 9 vs 6 | Parenchyma | 1.6993 ± 0.2522 | 2.2964 ± 0.1248 | |
| Wang et al10 | 11 vs 7 | Parenchyma | 1.8997 ± 0.1829 | 2.1025 ± 0.23399 | |
| Toya et al21 | 5 vs 47 | Parenchyma | 1.71 ± 0.18 | 1.87 ± 0.11 |
ADC, apparent diffusion coefficient.
The value of apparent diffusion coefficient in CKD Stage 3 and Stage 1–2.
Figure 3.
Forest plots for the ADC values in different stages of CKD. (A) Stage 1–2 vs the healthy group; (B) Stage 3 vs Stage 1–2; (C) Stage 4–5 vs Stage 3. ADC, apparent diffusion coefficient; CKD, chronic kidney disease.
Four studies compared the ADC values of renal parenchyma between the CKD Stage 3 and the Stage 1–2 (Figure 3b). However, our study shows no obvious difference in ADC between CKD Stage 3 and Stage 1-2 [WMD:−0.087, 95% CI(−0.185 to 0.010),p = 0.080; I2 = 93.7%, p < 0.001].
Apparent diffusion coefficient distinguished Stage 4–5 of the CKD from Stage 3
Next, we explored whether ADC of CKD Stage 4–5 differed from Stage 3. The ADC values in CKD Stage 4–5 were significantly lower than those of Stage 3 [WMD:−0.213, 95% CI(−0.319 to −0.107), p = 0.01; I2 = 82.4%, p = 0.000] (Figure 3c).
Heterogeneity and risk of bias
There was no obvious heterogeneity in the ADC values when distinguishing CKD Stage 1–2 from normal kidneys. However, obvious heterogeneity was observed in the ADC values when distinguishing CKD Stage 3 from Stage 1–2, and distinguishing CKD Stage 4–5 from Stage 3.
The results of the Egger’s test showed no evidence of publication bias in the ADC values of CKD Stage 1–2 vs normal kidney (p = 0.147>0.05), CKD Stage 3 vs Stage 1–2 (p = 0.851>0.05), or CKD Stage 4–5 vs Stage 3 (p = 0.257 > 0.05).
Discussion
This meta-analysis showed that DWI is a useful imaging method to evaluate renal function. Furthermore, by comparing ADC values between normal kidneys and different stages of CKD, DWI can distinguish early stages of CKD from normal kidneys and staging CKD.
We observed significant heterogeneity in Stage 3 vs Stage 1–2 and Stage 4–5 vs Stage 3, and these heterogeneity may caused by basic information of included studies, the different b values, scanning parameters, and the method for defining ROIs. However, due to the limited sample size of included studies, meta-regression is not suitable to evaluate the factors which are associated with heterogeneity. The results of ADC calculation may be affected by b values.22 There is no standard DWI scanning method at present. Low b value (less than 200 s mm– 2) significantly affected signals by perfusion effects, leading to inaccurate reflection of water diffusion motion,23 while high b values carry the risk of distortion and susceptibility artifacts. Variability in b values makes it difficult to interpret its range, however, all meta-analyzed studies had high b values of at least 400 s mm– 2 at 1.5T. Therefore, heterogeneity in performance were unlikely resulted from perfusion effects, which were seen mostly at lower b values of 250 s mm–2 or less.24 Included studies in our meta-analysis were of high quality and showed no publication bias.
Our results showed that ADC values were different in CKD Stage 1–2 vs normal kidneys and in Stage 4–5 vs Stage 3. The kidneys of CKD patients were characterized by reduced blood flow, loss of the nephron, interstitial fibrosis, tubular atrophy, and scarring of glomeruli. These pathological changes always cause a decline of perfusion, as well as diffusion restriction due to fibrosis.25 Moreover, the reduced ADC values of early Stage 1–2 in CKD compared with the healthy group suggests that ADC value might reflect the changes of kidney function and further could serve as a non-invasive and effective index to guide therapy and monitor CKD patients in follow-up. Additionally, the pooled data did not suggest significant difference between Stage 3 and Stage 1–2, which is consistent with previous studies by Carbone et al20 These results might be attributed to the fact that the pathological changes of CKD Stage 1–2 are similar to those of Stage 3.12 Besides, our meta-analysis shows a significant decrease of ADC values along with the increase of CKD stages, which is consistent with the previous studies.12, 14 Hence, DWI might be a promising non-invasive technique to monitor the changes of renal function.
Causes of CKD include diabetes, high blood pressure, glomerulonephritis and various chronic renal inflammation. These lead to different renal pathology which is important in guiding therapy. Qing Li et al26 analyzed the renal ADC values of differentiate CKD pathology types, including IgA nephropathy, focal segmental proliferative glomerulonephritis, and membranous nephropathy. They reported no significant differences in ADC values among the various pathology types. Different pathology types of CKD might share similar pathogenic features, such as hypoxia, chronic renal inflammation and renal fibrosis at last. So the changes of ADC values reflect severity of renal pathology and have clinical potential for assessing the renal function. Meanwhile, DWI is regarded as a functional MRI technique and could obtain the single and spatial information of kidney function, especially in CKD patients with renal tumors who need nephro-sparing surgery. DWI technique not only could evaluate kidney function, but also could provide clinical benefit to choice of treatment strategy for CKD patients. However, various renal pathologies with acute renal infection and inflammatory nephritis could also cause the decrease of ADC values. Hueper et al27 researched the acute kidney injury in mice by T2 relaxation and ADC values. They reported that ADC values decreased significantly at the beginning (day 0–day 1) and then presented with an increasing trend (day 1–day 28). ADC values decreased with the severity of renal fibrosis 4 weeks after acute kidney injury. Measurement of the dynamic change in ADC values may provide more information to differentiate acute kidney injury from CKD. Further researches are needed to identify the variation of ADC values in human with acute kidney injury.
This meta-analysis has several limitations: First, the number of included literatures is limited and sample size is small, so it is not possible to calculate ROC curves and reliable threshold values. Second, considerable heterogeneity was identified among the included studies and the different measurement methods. Difference in MRI scanner vendors, magnetic field intensity and choice of b values may contribute to study heterogeneity, which should be clarified in further studies. Third, the eGFR is recognized as clinical indicator of renal function and used for CKD staging. The eGFR was calculated from several different equations in lots of studies, which may cause the heterogeneity.
In conclusion, this meta-analysis provides evidence for DWI as a non-invasive method in the assessment of renal function in CKD. DWI can differentiate early stage of CKD from normal kidney and determine stages of CKD quantitatively using ADC values.
Contributor Information
Haitian Liu, Email: 297944082@qq.com.
Zhangjian Zhou, Email: 1002558077@qq.com.
Xiang Li, Email: 752991600@qq.com.
Chenxia Li, Email: saphirli@sina.com.
Rong Wang, Email: 834806594@qq.com.
Yuelang Zhang, Email: 18991232590@163.com.
Gang Niu, Email: niugang369@126.com.
REFERENCES
- 1. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: third national health and nutrition examination survey. Am J Kidney Dis 2003; 41: 1–12. doi: 10.1053/ajkd.2003.50007 [DOI] [PubMed] [Google Scholar]
- 2. Kiberd BA, Clase CM. Cumulative risk for developing end-stage renal disease in the US population. J Am Soc Nephrol 2002; 13: 1635–44. doi: 10.1097/01.ASN.0000014251.87778.01 [DOI] [PubMed] [Google Scholar]
- 3. Nangaku M. Hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. Nephron Exp Nephrol 2004; 98: e8–e12. doi: 10.1159/000079927 [DOI] [PubMed] [Google Scholar]
- 4. Palm F, Nordquist L. Renal tubulointerstitial hypoxia: cause and consequence of kidney dysfunction. Clin Exp Pharmacol Physiol 2011; 38: 474–80. doi: 10.1111/j.1440-1681.2011.05532.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prigent A. Monitoring renal function and limitations of renal function tests. Semin Nucl Med 2008; 38: 32–46. doi: 10.1053/j.semnuclmed.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 6. Braley-Berthoumieux E, Gamé X, Marque P, de Boissezon X, Rischmann P, Castel-Lacanal E. Study of the sensitivity of renal ultrasonography as an indirect means of assessing renal function in patients with neurogenic bladder, from a cohort of 103 patients. Prog Urol 2014; 24: 1114–9. doi: 10.1016/j.purol.2014.09.044 [DOI] [PubMed] [Google Scholar]
- 7. Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol 2007; 2: 264–7. doi: 10.2215/CJN.03921106 [DOI] [PubMed] [Google Scholar]
- 8. Lu L, Sedor JR, Gulani V, Schelling JR, O'Brien A, Flask CA, et al. Use of diffusion tensor MRI to identify early changes in diabetic nephropathy. Am J Nephrol 2011; 34: 476–82. doi: 10.1159/000333044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaudiano C, Clementi V, Busato F, Corcioni B, Orrei MG, Ferramosca E, et al. Diffusion tensor imaging and tractography of the kidneys: assessment of chronic parenchymal diseases. Eur Radiol 2013; 23: 1678–85. doi: 10.1007/s00330-012-2749-y [DOI] [PubMed] [Google Scholar]
- 10. Wang WJ, Pui MH, Guo Y, Wang LQ, Wang HJ, Liu M. 3T magnetic resonance diffusion tensor imaging in chronic kidney disease. Abdom Imaging 2014; 39: 770–5. doi: 10.1007/s00261-014-0116-y [DOI] [PubMed] [Google Scholar]
- 11. Zhao J, Wang ZJ, Liu M, Zhu J, Zhang X, Zhang T, et al. Assessment of renal fibrosis in chronic kidney disease using diffusion-weighted MRI. Clin Radiol 2014; 69: 1117–22. doi: 10.1016/j.crad.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 12. Feng Q, Ma Z, Wu J, Fang W. DTI for the assessment of disease stage in patients with glomerulonephritis--correlation with renal histology. Eur Radiol 2015; 25: 92–8. doi: 10.1007/s00330-014-3336-1 [DOI] [PubMed] [Google Scholar]
- 13. Yalçin-Şafak K, Ayyildiz M, Ünel SY, Umarusman-Tanju N, Akça A, Baysal T. The relationship of ADC values of renal parenchyma with CKD stage and serum creatinine levels. Eur J Radiol Open 2016; 3: 8–11. doi: 10.1016/j.ejro.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu Y, Wang X, Jiang X. Relationship between the renal apparent diffusion coefficient and glomerular filtration rate: preliminary experience. J Magn Reson Imaging 2007; 26: 678–81. doi: 10.1002/jmri.20979 [DOI] [PubMed] [Google Scholar]
- 15. Goyal A, Sharma R, Bhalla AS, Gamanagatti S, Seth A. Diffusion-weighted MRI in assessment of renal dysfunction. Indian J Radiol Imaging 2012; 22: 155–9. doi: 10.4103/0971-3026.107169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(2 Suppl 1): S1–266. [PubMed] [Google Scholar]
- 17. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–36. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 18. Zhang B, Liang L, Dong Y, Lian Z, Chen W, Liang C, et al. Intravoxel incoherent motion MR imaging for staging of hepatic fibrosis. PLoS One 2016; 11: e0147789. doi: 10.1371/journal.pone.0147789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carbone SF, Gaggioli E, Ricci V, Mazzei F, Mazzei MA, Volterrani L. Diffusion-weighted magnetic resonance imaging in the evaluation of renal function: a preliminary study. Radiol Med 2007; 112: 1201–10. doi: 10.1007/s11547-007-0217-6 [DOI] [PubMed] [Google Scholar]
- 21. Toya R, Naganawa S, Kawai H, Ikeda M. Correlation between estimated glomerular filtration rate (eGFR) and apparent diffusion coefficient (ADC) values of the kidneys. Magn Reson Med Sci 2010; 9: 59–64. doi: 10.2463/mrms.9.59 [DOI] [PubMed] [Google Scholar]
- 22. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 2007; 188: 1622–35. doi: 10.2214/AJR.06.1403 [DOI] [PubMed] [Google Scholar]
- 23. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988; 168: 497–505. doi: 10.1148/radiology.168.2.3393671 [DOI] [PubMed] [Google Scholar]
- 24. LeBihan D. IVIM method measures diffusion and perfusion. Diagn Imaging 1990; 12: 6. [PubMed] [Google Scholar]
- 25. Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Tang Y, Takahashi M. Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging 1999; 9: 832–7. doi: [DOI] [PubMed] [Google Scholar]
- 26. Li Q, Li J, Zhang L, Chen Y, Zhang M, Yan F. Diffusion-weighted imaging in assessing renal pathology of chronic kidney disease: A preliminary clinical study. Eur J Radiol 2014; 83: 756–62. doi: 10.1016/j.ejrad.2014.01.024 [DOI] [PubMed] [Google Scholar]
- 27. Hueper K, Rong S, Gutberlet M, Hartung D, Mengel M, Lu X, et al. T2 relaxation time and apparent diffusion coefficient for noninvasive assessment of renal pathology after acute kidney injury in mice: comparison with histopathology. Invest Radiol 2013; 48: 834–42. doi: 10.1097/RLI.0b013e31829d0414 [DOI] [PubMed] [Google Scholar]