Abstract
Although use of the term “theranostic” is relatively recent, the concept goes back to the earliest days of nuclear medicine, with the use of radioiodine for diagnosis and therapy of benign and malignant thyroid disease being arguably the most successful molecular radiotherapy in history. A diagnostic scan with 123I-, 124I-, or a low activity of 131I-iodide is followed by therapy with high activity 131I-iodide. Similarly, adrenergic tumours such as phaeochromocytoma and neuroblastoma can be imaged with 123I-metaiodobenzylguanidine and treated with 131I-metaiodobenzylguanidine. Bone scintigraphy can be used to select patients with painful bone metastases from prostate cancer who may benefit from treatment with beta- or alpha-particle emitting bone seeking agents, the most recent and successful of which is 223Ra radium chloride. Anti-CD20 monoclonal antibodies can be used to image and treat non-Hodgkins lymphoma, though this has not been as commercially successful as initially predicted. More recently established theranostics include somatostatin receptor targeting peptides for diagnosis and treatment of neuroendocrine tumours with agents such as 68Ga-DOTATATE and 177Lu-DOTATATE, respectively. Finally, agents which target prostate-specific membrane antigen are becoming increasingly widely available, despite the current lack of a commercial product. With the recent licensing of the somatostatin peptides and the rapid adoption of 68Ga- and 177Lu-labelled prostate-specific membrane antigen targeting agents, we have built upon the experience of radioiodine and are already seeing a great expansion in the availability of widely accepted theranostic radiopharmaceuticals.
Introduction
Although use of the term “theranostic” is relatively recent, and also applies to therapies which do not involve radionuclides, the concept goes back to the earliest days of nuclear medicine, with the use of radioiodine for diagnosis and therapy of benign and malignant thyroid disease being arguably the most successful molecular radiotherapy in history. A definition of theranostic involves the administration of a diagnostic agent:
to determine localisation in the site or disease state under study as a surrogate for a potential therapeutic agent with similar chemical properties;
to examine its biodistribution as predictive of off-target (adverse) effects of the potential therapeutic agent;
as an aid in determining the optimal therapeutic dosage or activity to be administered, based on the anticipated tumoricidal doses measured in the tumour site (i.e. dosimetry); and/or
to monitor the response to this treatment.1
Molecular radiotherapy or radionuclide therapy can be highly effective in treatment of tumours, but it is not without its dangers, making it important to select patients who are most likely to benefit from the administration of the therapeutic agent. Indeed, it can be argued that some chemotherapeutic and radiotherapeutic agents have failed in clinical trials due to inadequate patient selection; e.g. the patient does not express the target of the drug and/or the systemically administered drug does not reach the target in sufficient quantities.
Although common sense (and regulations in some countries) suggests that the quantity of a radiotherapeutic agent to be administered should be optimised for an individual patient, in practice this rarely happens.2, 3 However, in many cases it is possible to do this with a theranostic agent. Just as chemotherapeutic agents are administered to the maximum tolerated dose in terms of normal tissue toxicity, molecular radiotherapy is often administered in an activity chosen to result in the highest acceptable whole body dose or critical tissue radiation dose.
Theranostics is an active area of radiopharmaceutical research, as evidenced by other papers in this issue of the journal. However, we will review some long established examples of the theranostic concept which provide the underpinning for current research. Before discussing specific agents we must first address several general issues.
Challenges in theranostics
Chemical or biological equivalence?
How similar must the diagnostic and therapeutic agents be? In the ideal form, theranostics would involve chemically and therefore, biologically identical compounds. Radioiodine and metaiodobenzylguanidine (MIBG) meet this ideal (see below) but few others do. Although the diagnostic radiometals, 111In and 68Ga, and their therapeutic counterparts, 90Y and 177Lu, are all trivalent metals which can be attached to a targeting moiety with the same chelator, such as DOTA, there are subtle differences in their chemical structures which can affect their biological properties.4, 5 To this end, 86Y has been used for diagnostic PET imaging prior to therapy with 90Y labelled compounds, but there is very limited availability of 86Y making its use not very widely applicable.6–8
H-J Wester has used the expression “twins in spirit” for a pair of theranostic molecules which are not chemically or biologically identical but which are similar enough in biodistribution that the diagnostic moiety adequately predicts the biodistribution of the therapeutic analogue.9 The reasons for using these twins are mainly practical, as discussed below. The patent status of a molecule may limit its availability for clinical use. The labelling conditions may differ between, e.g. 68Ga and 177Lu; labelling with 68Ga is best performed with a chelator which labels rapidly at room temperature, such as NOTA or tris (hydropyridinone) ligands,10–12 whereas 177Lu labelling requires a more stable chelator such as DOTA.
Use of licensed products
The licensing/registration status of the diagnostic or therapeutic agent may limit its use, though the importance of this varies between countries. For example, in the international Phase III clinical trial of 177Lu-DOTATATE (Lutathera®) in neuroendocrine tumours, it could be argued that PET imaging with 68Ga-DOTATATE should have been used for patient selection and monitoring of response.13 However, the much inferior agent 111In-pentetreotide (Octreoscan®) was used with lower-resolution SPECT imaging because it was a commercially available licensed agent.14
Cost of licensing a theranostic pair
Bringing a new drug to market is an expensive proposition.15 A theranostic pair can be twice as costly as a single agent because each may require a separate licensing application. In the above example of neuroendocrine tumours, this has happened, with separate licensing applications for 68Ga-DOTATATE (NETSPOT®) and 177Lu-DOTATATE (Lutathera) being submitted by the same company in the USA. However, for the European market a different agent, 68Ga-DOTATOC (SomaKit-TOC®), was licensed for the diagnostic portion of the procedure prior to therapy with 177Lu-DOTATATE.
Established clinical use
Radioiodine in thyroid disease
The use of radioiodine in benign and malignant thyroid disease has long been the standard of practice around the world.16 In benign thyroid disease, for many years a low activity of 131I-iodide was administered for a diagnostic scan or determination of percentage uptake using a collimated probe. Because of the poor dosimetry and imaging characteristics of 131I, 123I-iodide is being used increasingly in its place, though often a 99mTc-pertechnetate scan will suffice. The therapeutic activity of 131I-iodide to be administered is then individualised based on the size of the patient’s gland and the percentage uptake of iodide in order to deliver a radiation dose of 100–150 Gy to the thyroid.17
Differentiated thyroid carcinoma is initially treated surgically, followed by a diagnostic scan using a low activity of 131I-iodide or, increasingly, 123I-iodide or 124I-iodide.18 The purpose of the diagnostic scan is to identify residual functioning tissue in the thyroid bed and to document the extent of metastases and their avidity for iodide. However, despite 70 years’ experience with radioiodine therapy, there is no generally accepted dosage regimen for individually optimised therapy.19, 20 Although activities of 1–5 GBq are recommended, most commonly 3.7 or 5.55 GBq are administered. Indeed, the value of 3.7 GBq (100 mCi) was originally proposed for 130I-iodide and translated to 131I-iodide on an empirical basis.16 A few years ago, two large studies showed equivalent results with 1.1 GBq compared to the standard 3.7 GBq in low risk thyroid carcinoma.21, 22 Thus, a therapy which has been in use for 70 years is still being optimised.
Figure 1 shows an example of serial treatment of a patient with differentiated thyroid carcinoma.
Figure 1.
Serial whole body anterior planar gamma camera images in a 64-year-old male with differentiated thyroid carcinoma who was initially treated surgically. (a) Imaging following first ablation dose of 5940 MBq 131I-iodide show multifocal tracer uptake in the neck, particularly on the left side confirmed by SPECT/CT (not shown), suggestive of residual functioning thyroid tissue. (b) Imaging following second dose of 6785 MBq 131I-iodide 6 months later shows no evidence of iodine-avid disease.
Metaiodobenzylguanidine (MIBG) in adrenergic tumours
131I-MIBG(iobenguane) was developed by the Ann Arbor group for imaging adrenergic nerve terminals in the adrenal medulla.23 Its chemical structure is an amalgam of the neuron blocking drugs bretylium and guanethidine. It was quickly shown to be useful in detection of phaeochromocytoma24 and soon thereafter, its first therapeutic use was reported.25 Since then, it has had a chequered history because of limited availability due to lack of a commercial sponsor and a dearth of randomised controlled trials.26–28
Nonetheless, 131I-MIBG is an effective agent and a true theranostic in that the diagnostic and therapeutic products are chemically identical.29 A diagnostic scan is performed using 131I- or 123I-MIBG to demonstrate “adequate uptake” in the lesion(s), though there is little agreement on a definition of this criterion. In general, for therapy with 131I-MIBG fixed activities between 3.7 and 11.1 GBq are administered rather than using a dosimetric approach, though the latter has been attempted.30 Patient preparation includes withdrawal of a long list of potentially interfering medications and provision of adequate blockade of the thyroid gland.29 In recent years, a high specific activity preparation of 131I-MIBG (Azedra®) has been investigated which may allow administration of higher activities with fewer side effects attributable to the cold drug;31 however, this is not yet commercially available.
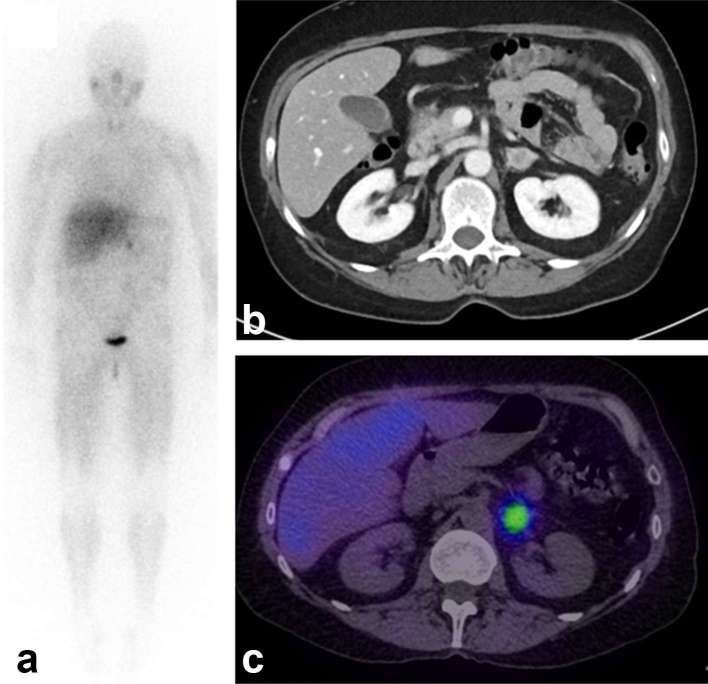
Figure 2 shows an example of a patient with phaeochromocytoma undergoing a diagnostic scan with 123I-MIBG prior to therapy with 131I-MIBG.
Figure 2.
Images following administration of 400 MBq 123I-MIBG in a 62-year-old female with suspected phaeochromocytoma. (a) Anterior whole body planar gamma camera image shows an intense focus of activity in the abdomen. (b) Contrast-enhanced CT scan shows a nodule in the left adrenal gland. (c) Fused SPECT/CT image shows colocalisation of activity to the nodule in keeping with phaeochromocytoma. MIBG, metaiodobenzylguanidine.
Bisphosphonate bone scintigraphy prior to palliation of painful bone metastases
Palliative treatment of painful bone metastases from prostate and breast cancer became more widely available in the 1990s with the commercial introduction of 89Sr chloride (Metastron®), 153Sm-lexidronam (EDTMP; Quadramet®), and 186Re-etidronate (HEDP).32–34 For all of these agents, bone scintigraphy with 99mTc-bisphosphonates (medronate, oxidronate) is used in patient selection by documenting sites of increased osteoblastic activity. For 153Sm-lexidronam and 186Re-etidronate, the targetting mechanism is the same as 99mTc-bisphosphonates, i.e. binding to hydroxyapatite. In contrast, 89Sr chloride localises by a different mechanism as an analogue of calcium, though the bone scan still provides an adequate prediction of localisation. However, the diagnostic scan is not used to tailor an individual patient’s dose and the standard activities administered are 150 MBq 89Sr chloride, 37 MBq kg –1 153Sm-lexidronam, and 1295 MBq 186Re-etidronate.34 A recent study has highlighted the potential role of patient specific dosimetry in 186Re-editronate therapy.35
In 2013, 223Ra chloride (Alpharadin; Xofigo®) became available for treatment of patients with castration resistant metastatic prostate cancer; indeed, the Phase III clinical trial was terminated early due to the survival advantage of the patients who received the active drug, the first bone seeking radionuclide to produce such an effect.36 Bone scintigraphy is used to select patients who may benefit from 223Ra chloride; however, the activity administered is calculated based on the patient’s weight, not degree of accumulation of activity on the diagnostic scan.37 Recent work has shown the feasibility of imaging based dosimetry with 223Ra chloride.38
Targeting the CD20 antigen in non-Hodgkins lymphoma
The first commercially available radioimmunotherapeutic agents were 90Y-ibritumomab tiuxetan (Zevalin®, USA and Europe) and 131I-tositumomab (Bexxar®, USA, now withdrawn).39, 40 Both targetted the CD20 antigen in non-Hodgkins lymphoma and a pre-therapy diagnostic scan was required in the USA. For ibritumomab, 185 MBq of the 111In analogue was used with imaging with 2–3 scans over 3–5 days, though this was only to confirm normal biodistribution and was not used in dose adjustment. Indeed, the standard activity of 15 MBq kg–1 90Y-ibritumomab is only reduced in patients with platelet counts between 100,000 and 150,000 mm– 3 who receive 11 MBq kg–1. The maximum activity to be administered is 1200 MBq. The requirement for the 111In scan has been removed because of its cost, inconvenience and rare utility; it was never required in Europe.41
For tositumomab, a low activity (185 MBq) of 131I-tositumomab is administered for the diagnostic scan, with three sets of images acquired over 7 days.42 These images are used to confirm normal distribution and to estimate the activity required to deliver a total body dose of 0.75, or 0.65 Gy in patients with platelet counts between 100,000 and 150,000 mm– 3.43, 44 The therapeutic activity is typically 2000–6000 MBq.
In the early days, it was posited that the difference in beta particle energy, and hence path length in tissue, might determine the choice of agent; i.e. 90Y-ibritumomab with its higher energy and path length being suited to larger tumours and the lower energy 131I-tositumomab for smaller ones.42 The two agents might even be used sequentially, switching from ibritumomab to tositumomab as tumours shrank. However, in the end, it was convenience and commercial issues which resulted in the continuing availability of ibritumomab, albeit with modest usage, while tositumomab has been withdrawn from the market.45
With the expiry of the patent on ibritumomab, there is the potential for generic biosimilars to become available, though current interest centres on agents such as rituximab labelled with 131I, 177Lu, or even the alpha emitter 227Th.46–49
Recently established clinical use
Somatostatin peptide therapy
Probably, the greatest recent success story in theranostics is the development of radiopeptides which bind to the somatostatin receptor for diagnosis and treatment of patients with neuroendocrine tumours, culminating with the international licensing of a radiotherapeutic agent in 2017. The story started with the investigation of 123I-Tyr-octreotide for proof of principle,50 followed by the commercial introduction of 111In-pentetreotide (Octreoscan).51 Because of the limitations of planar and SPECT imaging, analogues labelled with the positron emitter 68Ga were developed for PET imaging (DOTATOC and DOTATATE), which in turn were the impetus for the expanded availability of 68Ge/68Ga generators.52, 53 Despite the recent licensing of kits for use with 68Ga, numerous technical, regulatory, and financial challenges limit the utilisation of these agents. In parallel with this, therapy was evaluated, first with high activities of auger electron emitting 111In-pentetreotide, then the beta particle emitters 90Y-DOTATOC and 177Lu-DOTATATE.54–57 The path of these agents to market has been a circuitous one, with various false starts due to commercial issues. Though both 90Y and 177Lu agents have been studied extensively, 177Lu is preferred because of a more favourable side effect profile, in particular, less renal toxicity.57 Eventually, a company used the Rotterdam data to initiate an international Phase III clinical trial of 177Lu-DOTATATE (Lutathera) leading to its licensing in the USA and Europe.14 However, nothing remains the same for very long, as now there is evidence that alpha particle emitters such as 213Bi- and 225Ac-DOTATATE may be even more effective than 177Lu.58, 59
Figure 3 shows an example of response in a patient with a carcinoid tumour.
Figure 3.
Serial PET images (maximum intensity projections) following administration of ~150 MBq 68Ga-HA-DOTATATE in a 66-year-old male with an atypical pulmonary carcinoid with liver and bone metastases. (a) Baseline anterior projection shows multiple hepatic and bony metastases. (b) Follow-up imaging 1 year later after four cycles of 7400 MBq 177Lu-DOTATATE shows reduction in size and avidity of the lesions in the liver, with some being no longer visible, and only low grade bone uptake.
Prostate specific membrane antigen
A somewhat more direct story is the development of agents to image and target the prostate-specific membrane antigen (PSMA) receptor which is overexpressed in prostate cancer,60 lthough this too has faced commercial challenges. Indeed, the initial forays by Molecular Insight Pharmaceuticals were sidetracked by bankruptcy.
More than 20 years ago, a monoclonal antibody which binds to PSMA was developed, 111In capromab pendetide (ProstaScint®), but it struggled commercially due to poor image quality.61 Utility was somewhat enhanced by hybrid imaging with SPECT/CT.62 An important problem was that capromab binds to an internal epitope of PSMA and membrane permeability is insufficient to allow adequate quantities of the tracer to accumulate. More recently, alternative antibodies such as J591 which bind to an external domain of PSMA have been studied with somewhat greater success.63, 64 However, the size of an antibody results in prolonged distribution and clearance kinetics, limiting the choice of radiolabel.65, 66
Molecular Insight Pharmaceuticals chose to target PSMA expression using peptide–urea small molecule inhibitors of the enzyme N-acetylaspartylglutamate peptidase, a structural and functional homologue of PSMA. These small molecules should have a more favourable pharmacokinetic profile than an intact antibody or its fragments. The purest theranostic pair would use 123I (SPECT) or 124I (PET) for diagnosis followed by 131I for therapy, though more convenient 99mTc-labelled diagnostic agents were also developed.67–69 In parallel with this, a 68Ga-labelled analogue for PET imaging was developed and popularised by the Heidelberg group and has quickly been adopted around the world. In addition to the initial compound, DKFZ-PSMA-11, a variety of analogues are under investigation,70–73 and a clinical procedure guideline has been published.74
The remarkable degree of targeting and high tissue contrast made this an ideal candidate for therapy and a variety of 177Lu labelled analogues have been developed.72, 75 Although no commercial product is available yet, investigator sponsored studies are ongoing and compassionate usage is already widespread, although the UK is lagging behind other countries.76, 77 Promising results have also been reported with the alpha-emitter 225Ac-PSMA-617.78
Future prospects
The theranostic concept goes back to the earliest days of nuclear medicine. Indeed, with a looser definition of theranostic, many nuclear medicine procedures are used to select the optimal treatment for individual patients. We have recently seen the first licensing of a companion diagnostic, 99mTc-etarfolatide (EC20, Folcepri®), for selection of patients for treatment of lung and ovarian cancers; both agents target the folate receptor and the therapeutic counterpart (vintafolide, EC145) is coupled to a vinca alkyloid.79 With the licensing in 2017 of 68Ga-DOTATOC, 68Ga-DOTATATE, and 177Lu-DOTATATE and with the rapid adoption of 68Ga- and 177Lu-labelled PSMA targeting agents, we have built upon the experience of radioiodine and are already seeing a great expansion in the availability of widely accepted theranostic radiopharmaceuticals (Table 1).
Table 1.
Established theranostic agents in current use in the clinic
| Clinical indication | Diagnostic agent | Therapeutic agent |
| Hyperthyroidism or thyroid cancer | 123I-iodide | 131I-iodide |
| Adrenergic tumours | 123I-iobenguane | 131I-iobenguane |
| Bone metastases from prostate cancer | 99mTc-MDP | 223Ra chloride |
| Non-Hodgkins lymphoma | 111In-ibritumomab | 90Y-ibritumomab |
| Neuroendocrine tumours | 68Ga-DOTATATE | 177Lu-DOTATATE |
| Prostate cancer | 68Ga-DKFZ-PSMA-11 | 177Lu-DKFZ-PSMA-617 |
ACKNOWLEDGMENTS
The author would like to thank Dr Fahim Hassan, Consultant in Nuclear Medicine at Guy’s and St Thomas’ Hospital, London, UK for providing the clinical images and Dr Greg Mullen for useful discussion.
REFERENCES
- 1. Del Vecchio S, Zannetti A, Fonti R, Pace L, Salvatore M. Nuclear imaging in cancer theranostics. Q J Nucl Med Mol Imaging 2007; 51: 152–63. [PubMed] [Google Scholar]
- 2. Strigari L, Konijnenberg M, Chiesa C, Bardies M, Du Y, Gleisner KS, et al. The evidence base for the use of internal dosimetry in the clinical practice of molecular radiotherapy. Eur J Nucl Med Mol Imaging 2014; 41: 1976–88. doi: 10.1007/s00259-014-2824-5 [DOI] [PubMed] [Google Scholar]
- 3. Chiesa C, Sjogreen Gleisner K, Flux G, Gear J, Walrand S, Bacher K, et al. The conflict between treatment optimization and registration of radiopharmaceuticals with fixed activity posology in oncological nuclear medicine therapy. Eur J Nucl Med Mol Imaging 2017; 44: 1783–6. doi: 10.1007/s00259-017-3707-3 [DOI] [PubMed] [Google Scholar]
- 4. Deshmukh MV, Voll G, Kühlewein A, Mäcke H, Schmitt J, Kessler H, et al. NMR studies reveal structural differences between the gallium and yttrium complexes of DOTA-D-Phe1-Tyr3-octreotide. J Med Chem 2005; 48: 1506–14. doi: 10.1021/jm0496335 [DOI] [PubMed] [Google Scholar]
- 5. Maurin M, Garnuszek P, Baran P, Pawlak D, Mikołajczak R. The radiometal makes a difference. Synthesis and preliminary characterisation of DOTA-minigastrin analogue complexes with Ga, Lu and Y. Nucl Med Rev Cent East Eur 2015; 18: 51–5. doi: 10.5603/NMR.2015.0014 [DOI] [PubMed] [Google Scholar]
- 6. Förster GJ, Engelbach MJ, Brockmann JJ, Reber HJ, Buchholz HG, Mäcke HR, et al. Preliminary data on biodistribution and dosimetry for therapy planning of somatostatin receptor positive tumours: comparison of 86Y-DOTATOC and 111In-DTPA-octreotide. Eur J Nucl Med 2001; 28: 1743–50. doi: 10.1007/s002590100628 [DOI] [PubMed] [Google Scholar]
- 7. Lopci E, Chiti A, Castellani MR, Pepe G, Antunovic L, Fanti S, et al. Matched pairs dosimetry: 124I/131I metaiodobenzylguanidine and 124I/131I and 86Y/90Y antibodies. Eur J Nucl Med Mol Imaging 2011; 38(suppl 1): S28–40. doi: 10.1007/s00259-011-1772-6 [DOI] [PubMed] [Google Scholar]
- 8. Rösch F, Herzog H, Qaim S. The beginning and development of the theranostic approach in nuclear medicine, as exemplified by the radionuclide pair 86Y and 90Y. Pharmaceuticals 2017; 10: 56. doi: 10.3390/ph10020056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schottelius M, Šimeček J, Hoffmann F, Willibald M, Schwaiger M, Wester HJ. Twins in spirit - episode I: comparative preclinical evaluation of [68Ga]DOTATATE and [68Ga]HA-DOTATATE. EJNMMI Res 2015; 5: 22. doi: 10.1186/s13550-015-0099-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Velikyan I, Maecke H, Langstrom B. Convenient preparation of 68Ga-based PET-radiopharmaceuticals at room temperature. Bioconjug Chem 2008; 19: 569–73. doi: 10.1021/bc700341x [DOI] [PubMed] [Google Scholar]
- 11. Berry DJ, Ma Y, Ballinger JR, Tavaré R, Koers A, Sunassee K, et al. Efficient bifunctional gallium-68 chelators for positron emission tomography: tris(hydroxypyridinone) ligands. Chem Commun 2011; 47: 7068–70. doi: 10.1039/c1cc12123e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Young JD, Abbate V, Imberti C, Meszaros LK, Ma MT, Terry SYA, et al. 68Ga-THP-PSMA: a PET imaging agent for prostate cancer offering rapid, room-temperature, 1-step kit-based radiolabeling. J Nucl Med 2017; 58: 1270–7. doi: 10.2967/jnumed.117.191882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deppen SA, Blume J, Bobbey AJ, Shah C, Graham MM, Lee P, et al. 68Ga-DOTATATE compared with 111In-DTPA-octreotide and conventional imaging for pulmonary and gastroenteropancreatic neuroendocrine tumors: a systematic review and meta-analysis. J Nucl Med 2016; 57: 872–8. doi: 10.2967/jnumed.115.165803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med 2017; 376: 125–35. doi: 10.1056/NEJMoa1607427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nunn AD. The cost of bringing a radiopharmaceutical to the patient's bedside. J Nucl Med 2007; 48: 169. [PubMed] [Google Scholar]
- 16. Seidlin SM, Marinelli LD, Oshry E. Radioactive iodine therapy; effect on functioning metastases of adenocarcinoma of the thyroid. J Am Med Assoc 1946; 132: 838–47. [DOI] [PubMed] [Google Scholar]
- 17. Stokkel MP, Handkiewicz Junak D, Lassmann M, Dietlein M, Luster M. EANM procedure guidelines for therapy of benign thyroid disease. Eur J Nucl Med Mol Imaging 2010; 37: 2218–28. doi: 10.1007/s00259-010-1536-8 [DOI] [PubMed] [Google Scholar]
- 18. Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJ, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 2008; 35: 1941–59. doi: 10.1007/s00259-008-0883-1 [DOI] [PubMed] [Google Scholar]
- 19. Reiners C, Hänscheid H, Luster M, Lassmann M, Verburg FA. Radioiodine for remnant ablation and therapy of metastatic disease. Nat Rev Endocrinol 2011; 7: 589–95. doi: 10.1038/nrendo.2011.134 [DOI] [PubMed] [Google Scholar]
- 20. Sun F, Gerrard GE, Roberts JK, Telford T, Namini S, Waller M, et al. Ten year experience of radioiodine dosimetry: is it useful in the management of metastatic differentiated thyroid cancer? Clin Oncol 2017; 29: 310–5. doi: 10.1016/j.clon.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 21. Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med 2012; 366: 1674–85. doi: 10.1056/NEJMoa1109589 [DOI] [PubMed] [Google Scholar]
- 22. Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med 2012; 366: 1663–73. doi: 10.1056/NEJMoa1108586 [DOI] [PubMed] [Google Scholar]
- 23. Wieland DM, Wu J, Brown LE, Mangner TJ, Swanson DP, Beierwaltes WH. Radiolabeled adrenergi neuron-blocking agents: adrenomedullary imaging with [131I]iodobenzylguanidine. J Nucl Med 1980; 21: 349–53. [PubMed] [Google Scholar]
- 24. Sisson JC, Frager MS, Valk TW, Gross MD, Swanson DP, Wieland DM, et al. Scintigraphic localization of pheochromocytoma. N Engl J Med 1981; 305: 12–17. doi: 10.1056/NEJM198107023050103 [DOI] [PubMed] [Google Scholar]
- 25. Sisson J, Shapiro B, Beierwaltes WH, Nakajo M, Glowniak J, Mangner T, et al. Treatment of malignant pheochromocytoma with a new radiopharmaceutical. Trans Assoc Am Physicians 1983; 96: 209–17. [PubMed] [Google Scholar]
- 26. Postema EJ, McEwan AJ. Radioiodinated metaiodobenzylguanidine treatment of neuroendocrine tumors in adults. Cancer Biother Radiopharm 2009; 24: 519–25. doi: 10.1089/cbr.2009.0672 [DOI] [PubMed] [Google Scholar]
- 27. Wilson JS, Gains JE, Moroz V, Wheatley K, Gaze MN. A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer 2014; 50: 801–15. doi: 10.1016/j.ejca.2013.11.016 [DOI] [PubMed] [Google Scholar]
- 28. Parisi MT, Eslamy H, Park JR, Shulkin BL, Yanik GA. 133I-metaiodobenzylguanidine theranostics in neuroblastoma: historical perspectives; practical applications. Semin Nucl Med 2016; 46: 184–202. doi: 10.1053/j.semnuclmed.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 29. Giammarile F, Chiti A, Lassmann M, Brans B, Flux G, EANM. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur J Nucl Med Mol Imaging 2008; 35: 1039–47. doi: 10.1007/s00259-008-0715-3 [DOI] [PubMed] [Google Scholar]
- 30. George SL, Falzone N, Chittenden S, Kirk SJ, Lancaster D, Vaidya SJ, et al. Individualized 131I-mIBG therapy in the management of refractory and relapsed neuroblastoma. Nucl Med Commun 2016; 37: 466–72. doi: 10.1097/MNM.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coleman RE, Stubbs JB, Barrett JA, de la Guardia M, Lafrance N, Babich JW. Radiation dosimetry, pharmacokinetics, and safety of ultratrace Iobenguane I-131 in patients with malignant pheochromocytoma/paraganglioma or metastatic carcinoid. Cancer Biother Radiopharm 2009; 24: 469–75. doi: 10.1089/cbr.2008.0584 [DOI] [PubMed] [Google Scholar]
- 32. Lewington VJ. Bone-seeking radiopharmaceuticals. J Nucl Med 2005; 46(Suppl 1): 38S–47. [PubMed] [Google Scholar]
- 33. Finlay IG, Mason MD, Shelley M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review. Lancet Oncol 2005; 6: 392–400. doi: 10.1016/S1470-2045(05)70206-0 [DOI] [PubMed] [Google Scholar]
- 34. Bodei L, Lam M, Chiesa C, Flux G, Brans B, Chiti A, et al . EANM procedure guideline for treatment of refractory metastatic bone pain. Eur J Nucl Med Mol Imaging 2008; 35: 1934–40. doi: 10.1007/s00259-008-0841-y [DOI] [PubMed] [Google Scholar]
- 35. Denis-Bacelar AM, Chittenden SJ, Dearnaley DP, Divoli A, O’Sullivan JM, McCready VR, et al. Phase I/II trials of186Re-HEDP in metastatic castration-resistant prostate cancer: post-hoc analysis of the impact of administered activity and dosimetry on survival. Eur J Nucl Med Mol Imaging 2017; 44: 620–9. doi: 10.1007/s00259-016-3543-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–23. doi: 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 37. Du Y, Carrio I, De Vincentis G, Fanti S, Ilhan H, Mommsen C, et al. Practical recommendations for radium-223 treatment of metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2017; 44: 1671–8. doi: 10.1007/s00259-017-3756-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Flux GD. Imaging and dosimetry for radium-223: the potential for personalized treatment. Br J Radiol 2017; 90: 20160748. doi: 10.1259/bjr.20160748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wagner HN, Wiseman GA, Marcus CS, Nabi HA, Nagle CE, Fink-Bennett DM, et al. Administration guidelines for radioimmunotherapy of non-Hodgkin's lymphoma with 90Y-labeled anti-CD20 monoclonal antibody. J Nucl Med 2002; 43: 267–72. [PubMed] [Google Scholar]
- 40. Koral KF, Dewaraja Y, Li J, Lin Q, Regan DD, Zasadny KR, et al. Update on hybrid conjugate-view SPECT tumor dosimetry and response in 131I-tositumomab therapy of previously untreated lymphoma patients. J Nucl Med 2003; 44: 457–64. [PubMed] [Google Scholar]
- 41. Hagenbeek A, Lewington V. Report of a European consensus workshop to develop recommendations for the optimal use of 90Y-ibritumomab tiuxetan (Zevalin) in lymphoma. Ann Oncol 2005; 16: 786–92. doi: 10.1093/annonc/mdi148 [DOI] [PubMed] [Google Scholar]
- 42. Goldsmith SJ. Radioimmunotherapy of lymphoma: Bexxar and Zevalin. Semin Nucl Med 2010; 40: 122–35. doi: 10.1053/j.semnuclmed.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 43. Wahl RL. The clinical importance of dosimetry in radioimmunotherapy with tositumomab and iodine I 131 tositumomab. Semin Oncol 2003; 30(suppl 4): 31–8. doi: 10.1053/sonc.2003.23799 [DOI] [PubMed] [Google Scholar]
- 44. Dewaraja YK, Schipper MJ, Roberson PL, Wilderman SJ, Amro H, Regan DD, et al. 131I-tositumomab radioimmunotherapy: initial tumor dose-response results using 3-dimensional dosimetry including radiobiologic modeling. J Nucl Med 2010; 51: 1155–62. doi: 10.2967/jnumed.110.075176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schaefer NG, Huang P, Buchanan JW, Wahl RL. Radioimmunotherapy in non-hodgkin lymphoma: opinions of nuclear medicine physicians and radiation oncologists. J Nucl Med 2011; 52: 830–8. doi: 10.2967/jnumed.110.085589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Calais PJ, Turner JH. Standard operating procedure for prospective individualised dosimetry for [131]I-rituximab radioimmunotherapy of non-hodgkin’s lymphoma. World J Nucl Med 2012; 11: 110–6. doi: 10.4103/1450-1147.103409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kameswaran M, Pandey U, Dhakan C, Pathak K, Gota V, Vimalnath KV, et al. Synthesis and preclinical evaluation of 177Lu-CHX-A”-DTPA-rituximab as a radioimmunotherapeutic agent for non-hodgkin’s lymphoma. Cancer Biother Radiopharm 2015; 30: 240–6. doi: 10.1089/cbr.2015.1836 [DOI] [PubMed] [Google Scholar]
- 48. Repetto-Llamazares AH, Larsen RH, Giusti AM, Riccardi E, Bruland ØS, Selbo PK, et al. 177Lu-DOTA-HH1, a novel anti-CD37 radio-immunoconjugate: a study of toxicity in nude mice. PLoS One 2014; 9: e103070. doi: 10.1371/journal.pone.0103070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Melhus KB, Larsen RH, Stokke T, Kaalhus O, Selbo PK, Dahle J. Evaluation of the binding of radiolabeled rituximab to CD20-positive lymphoma cells: an in vitro feasibility study concerning low-dose-rate radioimmunotherapy with the alpha-emitter 227Th. Cancer Biother Radiopharm 2007; 22: 469–79. doi: 10.1089/cbr.2007.371 [DOI] [PubMed] [Google Scholar]
- 50. Becker W, Marienhagen J, Scheubel R, Saptogino A, Bakker WH, Breeman WA, et al. Octreotide scintigraphy localizes somatostatin receptor-positive islet cell carcinomas. Eur J Nucl Med 1991; 18: 924–7. doi: 10.1007/BF02258458 [DOI] [PubMed] [Google Scholar]
- 51. Krenning EP, Bakker WH, Kooij PP, Breeman WA, Oei HY, de Jong M, et al. Somatostatin receptor scintigraphy with indium-111-DTPA-D-Phe-1-octreotide in man: metabolism, dosimetry and comparison with iodine-123-Tyr-3-octreotide. J Nucl Med 1992; 33: 652–8. [PubMed] [Google Scholar]
- 52. Hofmann M, Maecke H, Börner R, Weckesser E, Schöffski P, Oei L, et al. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: preliminary data. Eur J Nucl Med 2001; 28: 1751–7. doi: 10.1007/s002590100639 [DOI] [PubMed] [Google Scholar]
- 53. Mojtahedi A, Thamake S, Tworowska I, Ranganathan D, Delpassand ES. The value of 68Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imaging 2014; 4: 426–34. [PMC free article] [PubMed] [Google Scholar]
- 54. Caplin ME, Mielcarek W, Buscombe JR, Jones AL, Croasdale PL, Cooper MS, et al. Toxicity of high-activity 111In-Octreotide therapy in patients with disseminated neuroendocrine tumours. Nucl Med Commun 2000; 21: 97–102. doi: 10.1097/00006231-200001000-00016 [DOI] [PubMed] [Google Scholar]
- 55. Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, et al. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med 1999; 26: 1439–47. doi: 10.1007/s002590050476 [DOI] [PubMed] [Google Scholar]
- 56. Kwekkeboom DJ, Bakker WH, Kooij PP, Konijnenberg MW, Srinivasan A, Erion JL, et al. [177Lu-DOTA0Tyr3]octreotate: comparison with [111In-DTPAo]octreotide in patients. Eur J Nucl Med 2001; 28: 1319–25. doi: 10.1007/s002590100574 [DOI] [PubMed] [Google Scholar]
- 57. Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O'Dorisio MS, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2013; 40: 800–16. doi: 10.1007/s00259-012-2330-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chan HS, de Blois E, Morgenstern A, Bruchertseifer F, de Jong M, Breeman W, et al. In vitro comparison of 213Bi- and 177Lu-radiation for peptide receptor radionuclide therapy. PLoS One 2017; 12: e0181473. doi: 10.1371/journal.pone.0181473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Graf F, Fahrer J, Maus S, Morgenstern A, Bruchertseifer F, Venkatachalam S, et al. DNA double strand breaks as predictor of efficacy of the alpha-particle emitter Ac-225 and the electron emitter Lu-177 for somatostatin receptor targeted radiotherapy. PLoS One 2014; 9: e88239. doi: 10.1371/journal.pone.0088239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yao D, Trabulsi EJ, Kostakoglu L, Vallabhajosula S, Joyce MA, Nanus DM, et al. The utility of monoclonal antibodies in the imaging of prostate cancer. Semin Urol Oncol 2002; 20: 211–8. [DOI] [PubMed] [Google Scholar]
- 61. Sodee DB, Nelson AD, Faulhaber PF, Maclennan GT, Resnick MI, Bakale G. Update on fused capromab pendetide imaging of prostate cancer. Clin Prostate Cancer 2005; 3: 230–8. [DOI] [PubMed] [Google Scholar]
- 62. Manyak MJ. Indium-111 capromab pendetide in the management of recurrent prostate cancer. Expert Rev Anticancer Ther 2008; 8: 175–81. doi: 10.1586/14737140.8.2.175 [DOI] [PubMed] [Google Scholar]
- 63. Pandit-Taskar N, O'Donoghue JA, Durack JC, Lyashchenko SK, Cheal SM, Beylergil V, et al. A Phase I/II study for analytic validation of 89Zr-J591 immunoPET as a molecular imaging agent for metastatic prostate cancer. Clin Cancer Res 2015; 21: 5277–85. doi: 10.1158/1078-0432.CCR-15-0552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vallabhajosula S, Nikolopoulou A, Jhanwar YS, Kaur G, Tagawa ST, Nanus DM, et al. Radioimmunotherapy of metastatic prostate cancer with ¹⁷⁷Lu-DOTAhuJ591 anti prostate specific membrane antigen specific monoclonal antibody. Curr Radiopharm 2016; 9: 44–53. [DOI] [PubMed] [Google Scholar]
- 65. Nawaz S, Mullen GED, Blower PJ, Ballinger JR. A 99mTc-labelled scFv antibody fragment that binds to prostate-specific membrane antigen. Nucl Med Commun 2017; 38: 666–71. doi: 10.1097/MNM.0000000000000698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nawaz S, Mullen GED, Sunassee K, Bordoloi J, Blower PJ, Ballinger JR. Simple, mild, one-step labelling of proteins with gallium-68 using a tris(hydroxypyridinone) bifunctional chelator: a68Ga-THP-scFv targeting the prostate-specific membrane antigen. EJNMMI Res 2017; 7: 86. doi: 10.1186/s13550-017-0336-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barrett JA, Coleman RE, Goldsmith SJ, Vallabhajosula S, Petry NA, Cho S, et al. First-in-man evaluation of 2 high-affinity PSMA-avid small molecules for imaging prostate cancer. J Nucl Med 2013; 54: 380–7. doi: 10.2967/jnumed.112.111203 [DOI] [PubMed] [Google Scholar]
- 68. Vallabhajosula S, Nikolopoulou A, Babich JW, Osborne JR, Tagawa ST, Lipai I, et al. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen: pharmacokinetics and biodistribution studies in healthy subjects and patients with metastatic prostate cancer. J Nucl Med 2014; 55: 1791–8. doi: 10.2967/jnumed.114.140426 [DOI] [PubMed] [Google Scholar]
- 69. Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, et al. Radiation dosimetry and first therapy results with a 124I/ 131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging 2014; 41: 1280–92. doi: 10.1007/s00259-014-2713-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging 2013; 40: 486–95. doi: 10.1007/s00259-012-2298-2 [DOI] [PubMed] [Google Scholar]
- 71. Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015; 42: 197–209. doi: 10.1007/s00259-014-2949-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med 2015; 56: 1169–76. doi: 10.2967/jnumed.115.158550 [DOI] [PubMed] [Google Scholar]
- 73. Hofman MS, Eu P, Jackson P, Hong E, Binns D, Iravani A, et al. Cold Kit PSMA PET Imaging: Phase I study of68Ga-THP-PSMA PET/CT in patients with prostate cancer. J Nucl Med 2017: jnumed.117.199554: jnumed.117.199554. doi: 10.2967/jnumed.117.199554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging 2017; 44: 1014–24. doi: 10.1007/s00259-017-3670-z [DOI] [PubMed] [Google Scholar]
- 75. Delker A, Fendler WP, Kratochwil C, Brunegraf A, Gosewisch A, Gildehaus FJ, et al. Dosimetry for 177Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging 2016; 43: 42–51. doi: 10.1007/s00259-015-3174-7 [DOI] [PubMed] [Google Scholar]
- 76. Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the bad berka experience since 2013. J Nucl Med 2016; 57(suppl 3): 97S–104. doi: 10.2967/jnumed.115.170167 [DOI] [PubMed] [Google Scholar]
- 77. Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med 2017; 58: 85–90. doi: 10.2967/jnumed.116.183194 [DOI] [PubMed] [Google Scholar]
- 78. Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, et al. Targeted alpha therapy of mCRPC with225actinium-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor-control. J Nucl Med 2018. [Epub ahead of print]. doi: 10.2967/jnumed.117.203539 [DOI] [PubMed] [Google Scholar]
- 79. Ledermann JA, Canevari S, Thigpen T. Targeting the folate receptor: diagnostic and therapeutic approaches to personalize cancer treatments. Ann Oncol 2015; 26: 2034–43. doi: 10.1093/annonc/mdv250 [DOI] [PubMed] [Google Scholar]