Abstract
We describe the use of Cytosorb™, a synthetic extracorporeal haemoperfusion adsorption column, in the management of two patients with drug induced cholestasis and a third with alcoholic hepatitis and subsequent acute on chronic liver failure. Cytosorb was used in these patients to remove bilirubin and bile acids by supporting impaired excretory hepatic function, alleviating symptoms with the intention of serving as a bridge to endogenous recovery. The first two cases demonstrate favourable biochemical and symptomatic responses; the third case demonstrated a good biochemical response but subsequently died from the complications of multiple organ failure. These cases suggest Cytosorb™ be evaluated as an adjunct to support liver excretory functions in other arenas, such as acute liver failure or overdose. It remains unclear whether extracorporeal therapies removing liver toxins allow faster or more complete spontaneous recovery of endogenous function.
Keywords: Cholestasis, liver failure, Cytosorb, haemoperfusion, haemabsorption, pruritis, extra-corporeal
Introduction
Cytosorb™ (Cytosorbent, Monmouth Junction, USA) is a synthetic adsorption column composed of highly porous biocompatible polymer beads that are able to capture and absorb molecules smaller than 55 kDa.1
Cytosorb has been primarily studied and marketed for cytokine adsorption in the treatment of sepsis,2,3 for which there is a UK National Institute of Clinical Excellence (NICE) Medtech Innovation Briefing,4 and other inflammatory syndromes such as pancreatitis and burns. Ongoing research is defining it’s place in manipulating inflammation in a variety of settings, much of which has been reported through registry data.5 Observations during Cytosorb use noted significant reductions in plasma bilirubin, a therapeutic approach our group have explored previously using a complex high-flux haemofiltration system.6 Our experience with Cytosorb in managing sepsis along with early published reports of similar effects and the desire for a simpler system to clear the toxins of liver failure7,8 led us to evaluate the device to support liver failure secondary to drug-induced cholestasis and acute alcoholic hepatitis. These were felt appropriate disease models as spontaneous liver recovery was expected over a period of months but these patients experienced very severe symptoms (e.g. pruritis, malaise, anorexia) or complications (e.g. severe jaundice, shock and encephalopathy), which could not be controlled by alternative means.
The Cytosorb column can be used by incorporation into an extra-corporeal circuit, which in intensive care unit (ICU) would most frequently be a conventional continuous veno-venous haemodiafilter as would be used for renal replacement therapy, but could equally be extra-corporeal membrane oxygenation or cardiopulmonary bypass. Among the range of ‘liver toxins’ which accumulate in liver failure (including bilirubin, bile acids, mercaptans, phenols and nonapeptides), conventional renal replacement therapy offers almost universally poor clearance, principally as they are largely protein bound and/ or lipid soluble.
Case 1
A 51-year-old man was referred to the Royal Derby Hospital (RDH) Hepatology service with a two-week history of increasing jaundice, dark urine, grey stools, pruritis, anorexia, nausea and weight loss. He admitted to recent use of anabolic steroids for 10 weeks at his local gymnasium for the purpose of body building. On presentation, bilirubin was 55 µmol/L, alkaline phosphatase (ALP) 272 U/L and alanine transaminase (ALT) 142 U/L.
Over the course of one month, his symptoms progressively worsened and he was admitted with severe drug-induced cholestasis with confusion, grade 2–3 hepatic encephalopathy and hepatic flap. Bilirubin had risen to 254 µmol/L. A liver biopsy supported the diagnosis of drug-induced liver injury and cholestatic hepatitis. Over the following month, the bilirubin continued to rise to 538 µmol/L. At this point, he had lost over 30 kg in weight and the severity of pruritis had led to skin scarring, insomnia and depression to the point of suicidal ideation.
A range of symptomatic treatments had been tried including cetirizne, cyclizine, mometasone cream, chlorphenaramine, ondansetron, zopicolne, temazepam, loratadine, colestyramine, rifampicin, naltrexone and naloxone infusion without success in alleviating his distress.
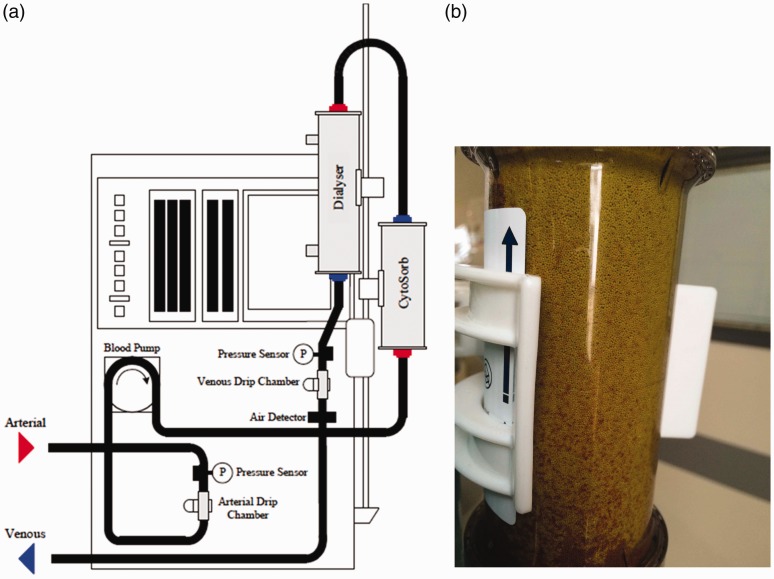
He was admitted to the ICU for trial of Cytosorb therapy as a bridge to recovery of endogenous liver function. The Intensive Care National Audit and Research Centre (ICNARC) score was 15 and estimated mortality by ICNARCH2015 was 12.4%. The patient gave informed consent and via a right internal jugular co-axial veno-venous haemodialysis line the extracorporeal circuit was established to a Prismaflex™ machine (Baxter, Deerfield, USA) delivering continuous veno-venous haemodiafiltration (CVVHDF). After ensuring satisfactory flow, a Cytosorb column was incorporated into this circuit, immediately proximal to the air trap as illustrated in Figure 1(a). Figure 1(b) shows the yellow discolouration of the Cytosorb unit at the end of therapy after adsorption of bilirubin and bile acids. The prescribed flow filtration parameters are outlined in Table 1; as the aim was not to support renal function but to use the extracorporeal set the diafiltration rates are low.
Figure 1.
(a) Schematic diagram of the Cytosorb unit integrated into Prismaflex extra-corporeal circuit. Diagram courtesy of Cytosorb. (b) A Cytosorb unit at the end of therapy after adsorption of bilirubin and bile acids.
Table 1.
Prismaflex extracorporeal circuit parameters for cases 1 and 2.
| Parameter | Setting |
|---|---|
| Therapy mode | CVVHDF |
| Access | 200–300 mL/min |
| Replacement | 500 mL/h |
| PBP | 500 mL/h |
| Dialysis | 100 mL/h |
| Removal | Nil |
| Anticoagulation | Unfractionated heparin 700 iu/h |
CVVHDF: continuous veno-venous haemodiafiltration; PBP: pre blood pump.
The patient received 41 h of Cytosorb therapy in total over two sessions (4 h on, 4 h off, 37 h on) during a total period of 52 h in ICU with a 4-h disruption due to vascular access kinking and circuit clotting. Table 2 shows the change in blood parameters during therapy. The lowest levels of bilirubin (164 µmol/L) and bile acids (16 µmol/L) were on day 2 of therapy on the day of ICU discharge. Figure 2 shows the change in bilirubin and bile acid blood concentrations during the period of therapy and Figure 3 shows the change in protein levels during this period.
Table 2.
Case 1 – Blood results during therapy.
| Time post therapy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | −6 h | 0 | 4 h | 7 h | 16 h | 22 h | 1 d 5 h | 1 d 16 h | 2 d 2 h | 2 d 23 h |
| Bilirubin | 480 | 451 | 365 | 363 | 258 | 240 | 177 | 164 | 166 | 206 |
| Bile acids | 161 | 77 | 107 | 37 | 32 | 22 | 16 | 20 | 48 | |
| Ammonia | 11 | 17 | <8 | |||||||
| ALP | 446 | 413 | 452 | 439 | 413 | 400 | 297 | 287 | 286 | 299 |
| ALT | 58 | 55 | 51 | 51 | 45 | 46 | 31 | 34 | 39 | 53 |
| GGT | 48 | 41 | 54 | 53 | 48 | 47 | 46 | 25 | 25 | 33 |
| Total protein | 58 | 54 | 54 | 53 | 48 | 47 | 46 | 42 | 47 | 48 |
| Albumin | 18 | 17 | 16 | 16 | 13 | 13 | 19 | 16 | 16 | 17 |
| Globulins | 40 | 37 | 38 | 37 | 35 | 34 | 27 | 26 | 31 | 31 |
| PT | 13.0 | 15.0 | ||||||||
| INR | 1.2 | 1.4 | ||||||||
| APTT | 29 | 31 | ||||||||
| APTT ratio | 1 | 1 | ||||||||
ALP: alkaline phosphatase; ALT: alanine aminotransferase; APTT: activated partial thromboplastin time; GGT gamma glutamyl transferase; INR: international normalised ratio; PT: prothrombin time.
Figure 2.
Case 1 – Change in concentrations of bilirubin and bile acids during therapy.
Figure 3.
Case 1 – Change in concentrations of total protein, albumin and globulins during therapy.
After 24 h of commencing therapy, the patient reported improvement in symptoms of pruritis, nausea and insomnia. After stopping therapy, these symptoms did return, albeit to a lesser extent than pre-therapy. Over the next seven days, bilirubin increased to 228 µmol/L and bile acids to 87 µmol/L. However, when seen as an outpatient five weeks later, his markers of cholestasis had improved, his appetite improved and he had gained over 6 kg. Bile acids had plateaued and he was started of hydroxyzine which improved pruritis, which was much milder than at presentation. Blood parameters over a period of five months of follow-up are shown in Table 3 and Figure 4 shows the change in bilirubin and bile acids during this period.
Table 3.
Case 1 – Blood results during five month follow-up after therapy.
| Number of days after therapy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | 1 | 2 | 3 | 5 | 7 | 9 | 14 | 20 | 34 | 135 |
| Bilirubin | 232 | 241 | 225 | 228 | 228 | 225 | 188 | 136 | 52 | 9 |
| Bile acids | 48 | 63 | 87 | 86 | 153 | 159 | 36 | 9 | ||
| ALP | 327 | 306 | 321 | 314 | 283 | 278 | 269 | 266 | 267 | 74 |
| ALT | 82 | 67 | 62 | 55 | 53 | 52 | 56 | 59 | 160 | 21 |
| GGT | 50 | 57 | 69 | 79 | 81 | 69 | 67 | 55 | 125 | 20 |
| Total protein | 52 | 61 | 60 | 64 | 68 | 63 | 67 | 68 | 75 | 80 |
| Albumin | 18 | 26 | 25 | 28 | 33 | 29 | 29 | 28 | 32 | 44 |
| Globulins | 34 | 35 | 35 | 36 | 35 | 34 | 38 | 40 | 43 | 36 |
| PT | 10 | 11 | 11 | |||||||
| INR | 0.9 | 1.2 | 1.0 | |||||||
| APTT | 26 | |||||||||
| APTT ratio | 1.0 | |||||||||
| Urea | 4.4 | 4.2 | 4.4 | 5.2 | 5.0 | 4.3 | 6.3 | |||
| Creatinine | 120 | 108 | 121 | 111 | 100 | 104 | 85 | |||
| Haemoglobin | 88 | 89 | 90 | 94 | 101 | 99 | 109 | |||
Figure 4.
Case 1 – Change in concentrations of bilirubin and bile acids during five-month follow-up.
At five months, the patient reported feeling well, had regained his appetite and had resumed weight training without the ‘assistance’ of anabolic steroids.
Case 2
A 71-year-old man was referred to the Hepatology Department RDH with a history of jaundice, dark urine, pruritus, lethargy and decreased appetite. He had recently completed a five-day course of intravenous ceftriaxone for an infected hand injury. On initial presentation bilirubin was 275 µmol/L, ALP 339 U/L, ALT 263 U/L and international normalised ratio (INR) 1.1. Imaging of his liver showed no focal lesion or biliary duct dilatation. An initial diagnosis of ceftriaxone induced biliary cholestasis was made and a transjugular liver biopsy showed features of cholestasis with non-specific mild portal inflammation and the most likely aetiological factor was a reaction to medication.
Over the course of next month, the bilirubin increased to 608 µmol/L, the patient lost weight and pruritis worsened so he was admitted to ICU for trial of Cytosorb therapy, having provided informed consent. The ICNARC score was 14 and risk of death 10.8%. Through a right femoral co-axial haemodialysis line a continuous haemodiafiltration extra-corporeal circuit was used via the Prismaflex system with a Cytosorb column attached in the extracorporeal circuit as described Figure 1 and therapy as detailed in Table 1.
The patient received a total of 49 h of therapy over two sessions (23 h on, 4 h off, 26 h on). In addition to routine bloods, bile acids and interleukin (IL)-6 were measured during therapy and these results are shown in Table 4. Two hours following removal of the femoral catheter, the patient experienced localised bleeding while straining on the lavatory. This was treated with external compression and fluid resuscitation. The following day, on the day of discharge from ICU, the haemoglobin had dropped from 115 to 82 g/L and there had been a rise in creatinine from 127 to 157 µmol/L. The patient was discharged from hospital the following day.
Table 4.
Case 2 – Blood results during therapy.
| Time post therapy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | −1 d | 1 h | 3 h | 9 h | 14 h | 20 h | 1 d 7 h | 1 d 17 h | 1 d 23 h | 2 d 19 h |
| Bilirubin | 608 | 528 | 417 | 326 | 322 | 378 | 226 | 215 | 236 | 237 |
| Bile acids | 202 | 88 | 58 | 39 | 63 | 23 | 17 | 21 | 33 | |
| Ammonia | 11 | 17 | <8 | |||||||
| ALP | 456 | 404 | 391 | 365 | 399 | 470 | 377 | 387 | 397 | 355 |
| ALT | 92 | 84 | 76 | 69 | 73 | 93 | 68 | 76 | 90 | 96 |
| GGT | 27 | 22 | 21 | 21 | 18 | 24 | 18 | 16 | 17 | 19 |
| Total protein | 53 | 49 | 46 | 41 | 45 | 55 | 41 | 43 | 47 | 44 |
| Albumin | 22 | 21 | 18 | 17 | 17 | 21 | 14 | 16 | 17 | 16 |
| Globulins | 31 | 28 | 28 | 24 | 28 | 34 | 27 | 27 | 30 | 28 |
| PT | 11.0 | 11.0 | 13.0 | 12.0 | 12.0 | 13.0 | 12.0 | 12.0 | 11.0 | |
| INR | 1.1 | 1.1 | 1.2 | 1.1 | 1.2 | 1.2 | 1.1 | 1.1 | 1.0 | |
| APTT | 22 | 57 | 71 | 164 | 56 | 65 | 61 | 25 | ||
| APTT ratio | 0.8 | 2.2 | 2.7 | 6.2 | 2.1 | 2.5 | 2.3 | 1.0 | ||
| IL-6 | 7.7 | 10.8 | 10.3 | 8.5 | 9.7 | 15.1 | ||||
IL: interleukin.
Table 5 shows the changes to blood parameters over a three-month period of follow-up and Figure 5 shows the changes in bilirubin and bile acids over this period. Despite a transient increase in bilirubin and bile acids post therapy, they never reached the pre-therapy levels and when reviewed on 10 weeks after discharge the liver function had returned to normal levels and patient had made a full recovery.
Table 5.
Case 2 – Blood results during three month follow-up after therapy.
| Number of days after therapy |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 4 | 7 | 14 | 19 | 25 | 33 | 40 | 54 | 78 |
| Bilirubin | 340 | 314 | 260 | 224 | 185 | 95 | 64 | 34 | 17 |
| Bile acids | 87 | 112 | 150 | 135 | 53 | 15 | |||
| ALP | 295 | 314 | 325 | 338 | 381 | 273 | 182 | 122 | 101 |
| ALT | 116 | 97 | 77 | 69 | 72 | 57 | 48 | 34 | 22 |
| GGT | 33 | 32 | 28 | 30 | 34 | 29 | 34 | 35 | 22 |
| Total protein | 46 | 50 | 53 | 53 | 60 | 57 | 58 | 64 | 73 |
| Albumin | 17 | 19 | 18 | 20 | 23 | 24 | 27 | 34 | 43 |
| Globulins | 29 | 31 | 35 | 33 | 37 | 33 | 31 | 30 | 30 |
| PT | 10 | ||||||||
| INR | 1.0 | ||||||||
| APTT | 21 | ||||||||
| APTT ratio | 0.8 | ||||||||
| Urea | 9.5 | 7.9 | 7.3 | 6.6 | 6.7 | ||||
| Creatinine | 150 | 146 | 116 | 103 | 109 | ||||
Figure 5.
Case 2 – Change in concentration of bilirubin over time following therapy.
Case 3
A 54-year-old patient was admitted to the ICU with first presentation of rapidly progressing acute alcoholic hepatitis and acute on chronic liver failure and secondary multiple organ failure (encephalopathic, hypotensive and oliguric). The patient required tracheal intubation and mechanical ventilation, vasoactive support and was treated with systemic corticosteroids while receiving broad spectrum antimicrobial therapy, in the hope that recovery of endogenous liver function might occur. The admission Modelling in End Stage Liver Disease (MELD-UNOS) score suggested 83% mortality at three months. The patient’s renal function deteriorated on the basis of hepatorenal syndrome and so continuous renal replacement therapy (CRRT) was commenced using our unit standard prescription of 1800 mL h−1 paired haemodialysis and filtration.
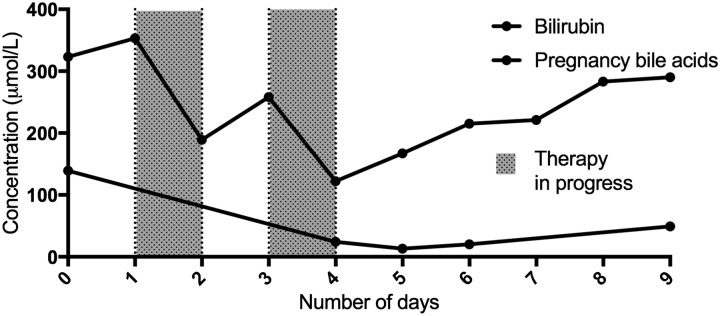
Considering established liver failure with severe jaundice and encephalopathy, and that the patient was already on an extracorporeal CRRT circuit, we included the Cytosorb column into the CRRT circuit for tow therapies, each therapy session lasting approximately 24 h, using methodology described above. The main biochemical changes are detailed below in Table 6 and shown in Figure 6. Unfortunately, despite optimal supportive care, the patient never recovered renal function when observed off CRRT and remained profoundly hypotensive despite antimicrobial cover, fluid therapy and vasoactives. Active therapy was discontinued at day 15 of intensive care admission.
Table 6.
Case 3 – Biochemical changes over the course of Cytosorb therapy.
| Timeline in ICU admission | Bilirubin (µmol/L) | Bile acids (µmol/L) |
|---|---|---|
| Baseline | 353 | 139 |
| Post Cytosorb therapy 1 (24 h) | 189 | No sample |
| Pre Cytosorb therapy 2 (72 h) | 258 | 24 |
| Post Cytosorb therapy 2 (96 h) | 122 | 12 |
| Four days post Cytosorb therapy | 290 | 49 |
ICU: intensive care unit.
Figure 6.
Case 3 – Change in concentrations of bilirubin and bile acids with therapy.
Discussion
We present the use of the Cytosorb haemoperfusion column in the setting of clearing liver toxins during optimal supportive care while awaiting recovery of endogenous liver function. Our first two patients with cholestasis were treated for general malaise, anorexia and severe pruritus with no known pre-existing liver disease and our third patient was critically ill with acute alcoholic hepatitis and acute on chronic liver failure. In all three cases, meaningful reductions in bilirubin (typically 50% or so with 24 h Cytosorb therapy) and even more impressive reductions in bile acids were observed and in conscious patients were associated with improvement of symptoms.
At present, such support should only be seen as a supportive intervention allowing a level of biochemical control and it would be premature to suggest Cytosorb may allow recovery of endogenous liver function or as a bridge to transplant; this would require further evaluation in appropriate prospective, randomised trials. However, clearing liver toxins is likely to be directly and indirectly beneficial to the failing liver e.g. by reducing cerebral oedema. This construct is consistent with animal models suggesting an improved internal milieu and reduced ‘liver toxins’ may be associated with more rapid recovery of liver function and this hypothesis retains widespread support amongst clinicians.9 Acute liver failure exemplifies a complex interaction of dysregulated inflammation, vasoparesis and bacterial translocation compounding ‘simple’ accumulation of liver toxins. Consequently, improved outcomes in acute liver failure will reflect timely decisions around transplant in conjunction with optimal multiple organ supportive care and effective therapeutic interventions, rather than a single ‘magic bullet’ such as extracorporeal support.10–12
However, the ability to support the excretory functions of the failing liver would represent intervention, which has previously not been widely available on ICU. Much ICU supportive care supports excretory functions e.g. mechanical ventilation for respiratory failure carbon dioxide and renal replacement therapy in renal failure. It is perhaps not intuitive the respective organ systems require dedicated excretory support (e.g. mechanical ventilation cannot treat renal failure acidosis and vice versa) and currently in the setting of liver failure there is no readily available therapy which can support excretory function. As discussed the large range of ‘liver toxins’ which accumulate in liver failure are very poorly cleared by renal replacement therapy, principally as they are largely protein bound and/ or lipid soluble. The one directly injurious compound of note which is well cleared by CRRT is ammonia. Consequently, the ability to convert an extracorporeal circuit from renal replacement to ‘hepatorenal replacement’ is conceptually attractive and we would say has been hitherto unavailable to most clinicians. Such a circuit cannot support liver synthetic function; it simply replaces a proportion of excretory capacity. However, just as extracorporeal carbon dioxide removal, if demonstrated to be effective and beneficial,13 would be a significant shift in ICU philosophy then so it is tempting to foresee the combined delivery of extracorporeal respiratory, renal and liver excretory support with relatively minor modifications to each individual modality. There is currently only experimental potential for replacing liver synthetic function and cultured hepatocyte technology systems are undergoing trial evaluation and likely to be prohibitively expensive (typically £80,000 per therapy) and require dedicated perfusionist support.14
Case three describes the application of Cytosorb in the setting of alcoholic hepatitis with acute kidney injury (AKI). This patient was already being managed with an extracorporeal CRRT circuit when Cytosorb was considered. Cytosorb therapy was offered as full care was already in place, including corticosteroid therapy, and spontaneous recovery of endogenous liver function was the primary therapeutic target. Having commenced CRRT, we felt it inconsistent to observe accumulating liver toxins when a relatively simple addition to the existing CRRT circuit offers useful clearance. Similarly, while the prognosis for case three was poor the backdrop to treating liver failure has undergone significant changes in recent years. The Stopah trial demonstrated the 28-day mortality in the control group of alcoholic hepatitis was 17%, although this exceeded 50% death or transplantation at one year.15 The use of corticosteroids in alcoholic hepatitis may reduce early mortality, and it is this therapy which the Cytosorb was intended to support and buy time, particularly as this patient was deemed unsuitable for liver transplantation. Currently, there are limited therapies which clinicians may pursue while supportive care is offered (potentially including extracorporeal liver support using Cytosorb) including corticosteroids,15 multiple organ and vasoactive support (e.g. terlipressin and albumin)16 or rifaximin for encephalopathy.17 In practice, many of these therapies are combined in the setting of liver failure, as multiple organ failure often complicates liver failure.
A comprehensive review of extracorporeal therapies is beyond the scope of this report and has been undertaken elsewhere.18 Commercially available extracorporeal liver support is restricted to a few complex and expensive systems. The Molecular Adsorption Recirculation System (MARS Gambro™) is the best evaluated and most familiar but is a highly specialised therapy delivering combined renal and liver support, costing typically £2000 day−1. While this therapy is believed to be largely safe, it’s use is subject to UK NICE guidance around cost and uncertain efficacy and benefit.19 Alternative proprietary systems e.g. Prometheus (Fresenius) and non-proprietary alternatives e.g. plasma exchange or single pass albumin dialysis (SPAD) have undergone even less complete evaluation. Our group in Derby has previously investigated high flux albumin filtration, and the clearance rates of bilirubin appear broadly comparable to Cytosorb and MARS.20 The Cytosorb column was originally marketed for sepsis management and we have described our early experiences in this setting.2 However, the polymer bead technology shares similarities with the anion exchange column component of the MARS circuit and the Cytosorbent team suspected liver toxin clearance would be occur in clinical use (personal communication, Christian Steiner, Cytosorbents). The current costs of an extracorporeal CRRT circuit are approximately £70 and the Cytosorb column pricing is approximately £1250, but will be influenced by volume of useage (personal communication Stephen Bailey, Linc Medical UK).
The Cytosorb will require longer evaluation for safety. Cytosorb therapy requires an extracorporeal circuit and the inherent risks of this should be carefully considered. Our conscious patients were explicitly consented; where an extracorporeal circuit (e.g. AKI and CRRT) is already employed this may be less hazardous. Case 2 experienced significant bleeding from the femoral catheter site, rather than a complication of Cytosorb therapy per se, a complication of intravenous access and severe haemorrhoids and straining. Both cases 1 and 2 had transient rises in creatinine, but these resolved spontaneously in both cases within two months. The mechanism behind this is speculative and the impact of enhanced muscle catabolism with enforced bed rest cannot be discounted. However, it is entirely conceivable that renal injury occurred e.g. due to reduced renal blood flow or altered tubuloglomerular feedback, and future Cytosorb evaluations would need to be vigilant for renal consequences.
In summary for patients with liver impairment where recovery might be expected or where transplantation is not clinically appropriate, and especially where an existing CRRT extracorporeal circuit is in use perhaps for AKI, clinicians may wish to consider the Cytosorb as a convenient and effective means of reducing jaundice and clearing bile acids. The ultimate impact on recovery liver function is speculative and requires evaluation in larger studies. The safety profile of the Cytosorb in an existing extracorporeal circuit appears good but also requires more complete evaluation.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Cytosorb units were purchased for use. Clinicians were supported in the clinical use of the Cytocorb column with technical advice but Cytosorbents played no part in dictating patient therapy or the writing of this manuscript. No authors have received financial support or otherwise from Cytosobents.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Patient Consent
Published with written consent from cases 1 and 2, and provided by next of kin for case 3.
References
- 1.http://cytosorb-therapy.com/ (accessed 3 October 2017).
- 2.Morris C, Gray L, Giovannelli M. Early report: the use of Cytosorb™ haemabsorption column as an adjunct in managing severe sepsis: initial experiences, review and recommendations. J Intensive Care Soc 2015; 16: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kogelmann K, Jarczak D, Scheller M, et al. Hemoadsorption by CytoSorb in septic patients: a case series. Crit Care 2017; 21: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.https://www.nice.org.uk/advice/mib87/ (2016, accessed 30 September 2017).
- 5.http://www.cytosorb-registry.org/publikationen-2/?lang=en (accessed 3 October 2017).
- 6.http://vitaltherapies.com/elad/technology/ (accessed 03 October 2017).
- 7.Tomescu DR, Olimpia Dima S, Ungureanu D, et al. First report of cytokine removal using CytoSorb® in severe noninfectious inflammatory syndrome after liver transplantation. Int J Artif Organs 2016; 39: 136–140. [DOI] [PubMed] [Google Scholar]
- 8.Faltlhauser A, Kullmann F. Use of hemoadsorption in a case of severe hepatic failure and hyperbilirubinemia. Blood Purif 2017; 44: 98–99. [DOI] [PubMed] [Google Scholar]
- 9.Zieve L, Shekleton M, Lyftogt C, et al. Ammonia, octanoate and a mercaptan depress regeneration of normal rat liver after partial hepatectomy. Hepatology 1985; 5: 28–31. [DOI] [PubMed] [Google Scholar]
- 10.Kramer L, Kodras K. Detoxification as a treatment goal in hepatic failure. Liver Int 2011; 31: S1–S4. [DOI] [PubMed] [Google Scholar]
- 11.Hernaez R, Sola E, Moreau R. Acute on chronic liver failure: an update. Gut 2017; 66: 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbeke L, Nevens F, Laleman W. Bench to bedside review: acute on chronic liver failure. Linking the gut, liver and systemic circulation. Crit Care 2011; 15: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.https://clinicaltrials.gov/ct2/show/NCT02654327 (accessed 3 October 2017).
- 14.https://www.organ-assist.nl/products/liver-assist (accessed 3 October 2017).
- 15.Thrursz MR, Richardson P, Allison M, et al. for the Stopah Trial. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015; 372: 1619–1628. [DOI] [PubMed] [Google Scholar]
- 16.Gludd LL, Christensen K, Christensen E, et al. Terlipressin for hepatorenal syndrome. Cochrane Database Syst Rev 2012; 9: CD005162. [DOI] [PubMed] [Google Scholar]
- 17.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010; 362: 1071–1081. [DOI] [PubMed] [Google Scholar]
- 18.Stange J. Extracorporeal liver support. Organogenesis 2011; 7: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Extracorporeal albumin dialysis for acute liver failure. Interventional procedures guidance [IPG316], https://www.nice.org.uk/guidance/ipg316/chapter/1-Guidance (2009, accessed 3 October 2017).
- 20.Morris C, Rogerson D. The use of high-flux albumin haemofiltration (HFAF) with Evaclio EC-2C™ in the management of liver failure as a bridge to transplantation. J Intensive Care Soc 2011; 12: 228–233. [Google Scholar]