Abstract
Background
Elevated levels of cardiac troponin T are associated with poor outcome in critically ill patients and have been proposed as a prognostic marker in major trauma. This study investigated the relationship between cardiac troponin T levels on admission to intensive care unit (ICU) and all-cause mortality in major trauma patients.
Methods
A retrospective database analysis of cardiac troponin T levels on admission to the ICU in major trauma patients between 1 August 2015 and 31 December 2016 at a UK Major Trauma Centre was performed.
Results
Of the 243 patients, 69 (28.4%) died. Cardiac troponin T levels were significantly higher in patients who died compared to survivors: 42 vs. 13 ng/L, respectively (p < 0.0001); the odds of all-cause mortality increased significantly as troponin increased, independent of age or Acute Physiology and Chronic Health Evaluation score.
Discussion
This confirms cardiac troponin T at ICU admission as a marker of mortality in major trauma. Elevated cardiac troponin T may be seen in patients without evidence of direct cardiac trauma.
Keywords: Troponin T, multiple trauma, mortality
Introduction
Major trauma accounts for over 5 m deaths each year worldwide1 and remains the leading cause of death in patients under the age of 46 years.2 The use of biomarkers as a tool in outcome prediction for these patients is a relatively novel concept for which there is an expanding evidence base. Cardiac smooth muscle troponin (cTn) is well established as a sensitive and early indicator of cardiac injury3 but further to this cardiac role, studies in critically ill patients have demonstrated an association between elevated cTn levels and increased morbidity, mortality and length of stay in both unselected medical and non-cardiac surgical patients.4–7 It remains unclear whether the different cTn subunits, troponin I (cTnI), troponin T (cTnT) and C (cTnC), are of equivalent diagnostic and prognostic significance in the non-acute coronary syndrome setting. Studies have suggested that cTnI performs better at predicting cardiovascular mortality and cTnT may be better at predicting all-cause mortality.8 The majority of studies in major trauma report the use of the TnI as the measured subunit and focus on elevation of this biomarker in chest trauma or traumatic brain injury.9–13 There are little data evaluating the role of cTnT as a prognostic indicator for all-cause mortality in major trauma and no published data on cTnT in trauma from the UK, where injury patterns and trauma care systems differ from other countries. This retrospective study investigated the relationship between cTnT levels on admission to intensive care unit (ICU) and all-cause mortality in major trauma patients presenting to a UK Major Trauma Centre. Subgroup analysis was performed to identify any correlation between elevated troponin and traumatic chest or brain injury. The identification of early prognostic markers in major trauma may improve outcomes in this global patient group by guiding treatment decisions and risk stratification for ongoing care.14
Methods
The Trauma Audit and Research Network (TARN) database for Southmead MTC, Bristol was used retrospectively to identify major trauma patients admitted to the ICU between 1 August 2015 and 31 December 2016. The Intensive Care database (Wardwatcher, Critical Care Audit Ltd) was reviewed to determine the cTnT level taken on admission to the ICU. Other data identified for each patient included patient demographics, injury severity score (ISS), a description of the injuries sustained, Acute Physiology and Chronic Health Evaluation (APACHE) II score and patient outcome.
Blood samples were taken from each patient on first admission to the ICU. cTnT levels were analysed in an on-site laboratory using a third-generation immunoassay (Elecsys, Roche Diagnostics, Rotkreuz, Switzerland; 99th percentile upper reference limit 14 ng/L, 10% coefficient of variation precision 13 ng/L).
The primary outcome measure was all-cause hospital mortality with cTnT level as the main explanatory variable. Secondary analysis assessed cTnT levels in different injury patterns to determine if any effect was due to primary cardiac injury or was a secondary phenomenon. Three sub-groups were pre-defined: neurological injury (brain and spinal cord), chest injury and any ‘other’ injury without evidence of chest or neurological trauma.
Statistical analysis was conducted to determine any associations between initial cTnT levels and patient outcome. Median values with an interquartile range were used to report continuous data. Patients were categorised into those who survived and those who died; chi-square was used to compare frequencies between the two groups. The Mann-Whitney U test was used to compare distributions for continuous data where appropriate. In addition, multivariable logistic regression was used with troponin grouped into quartiles and variables with a p value < 0.05 were considered statistically significant. Receiver operating characteristic (ROC) curves were included to assess the ability of cTnT to discriminate between survivors and non-survivors. Risk stratification was assessed using the continuous net reclassification index (NRI) to assess the net proportion of patients correctly assigned a higher probability if they died and correctly assigned a lower probability if they survived when cTnT is added in above APACHE II. An interaction term was fitted to test for differences in the effect for those with different injury types. Data analysis was performed using Stata Version 14 (StataCorp, Texas).
Results
During the study period, there were 333 major trauma patients admitted to the ICU. Of these, 243 (73.0%) patients had a complete dataset and were included in the final data analysis. The median age of the patients was 51.7 years (16.4, 90.5 years); 170 patients were male and 73 patients were female. Patient demographics are reported in Table 1. The median ISS for the patient group was 25 (range 1.75); 170 (70.0%) patients had an ISS > 15.
Table 1.
Patient demographics.
| Survived | Died | p value | |
|---|---|---|---|
| Number of patients | 174 | 69 | |
| Age | 50.25 (16.4, 90.4) | 63.3 (17.8, 90.5) | 0.002 |
| Gender | 125:49 | 45:24 | 0.31 |
| ISS | 25 (1.66) | 25 (1.75) | 0.06 |
Of the 243 patients, 174 patients survived to hospital discharge and 69 patients died. The cTnT level was higher in those who died compared to survivors (p < 0.0001). The median cTnT level for survivors was 13 ng/L (5 and 1259 ng/L) and for non-survivors was 42 ng/L (2 and 948 ng/L), p < 0.0001. The area under the ROC curve (95% CI) for troponin was 0.73 (0.65, 0.80) (Figure 1). The odds of dying were significantly increased as cTnT increased, with 53.3% dying if the cTnT level was in the highest quartile (>55 ng/L) compared to 11.5% in the lowest quartile (≤7 ng/L). The effect is independent of age or APACHE II score (Table 2). If a cTnT > 55 ng/L is taken as a positive test to predict hospital mortality the sensitivity (95% CI) is 46% (34.2, 58.8), specificity 83.9% (77.6, 89.0), positive predictive value (95% CI) 53% (40.0, 63.3) and negative predictive value (95% CI) 80% (73.2, 85.3). The continuous net reclassification index = 0.487 (0.215–0.759), p = 0.0005; 35.6% of survivors and 13% of those who died were reassigned to a more accurate probability of death by adding in cTnT to the APACHE II score giving an overall NRI value of 48.7%.
Figure 1.
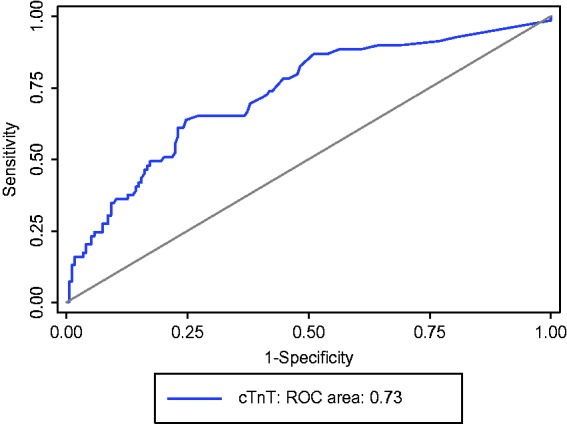
ROC curve for troponin analysis.
Table 2.
Mortality by quartile of troponin distribution.
| Quartile of troponin | Percentage dying | Unadjusted odds ratio | Age-adjusted odds ratio | OR adjusted for APACHE score |
|---|---|---|---|---|
| 1 (≤7) | 11.5% (7/61) | 1.00 | 1.00 | 1.00 |
| 2 (8–20) | 20.3% (13/64) | 1.97 (0.73–5.32) | 1.51 (0.54–4.18) | 2.11 (0.70–6.36) |
| 3 (21–55) | 29.3% (17/58) | 3.20 (1.21–8.43) | 2.20 (0.80–6.09) | 1.91 (0.65–5.60) |
| 4 (>55) | 53.3% (32/60) | 8.82 (3.46–22.49) | 7.68 (2.97–19.86) | 5.79 (2.03–16.55) |
| p value | <0.0001 | 0.002 | 0.004 |
APACHE: Acute Physiology and Chronic Health Evaluation; OR: odds ratio.
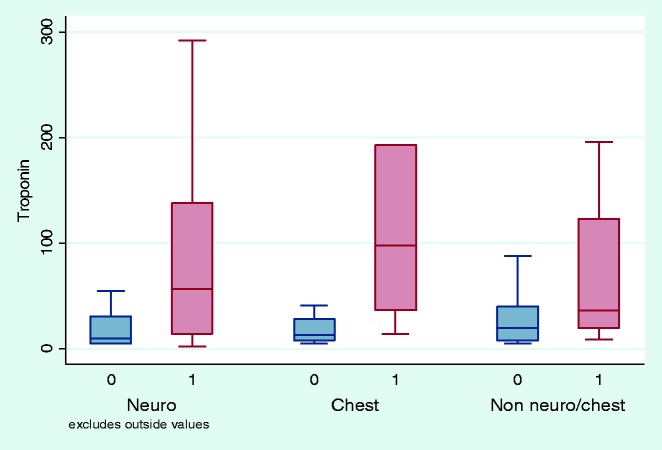
When classified by the three groups of types of injuries sustained, the non-survivors had elevated cTnT levels compared with survivors (Figure 2). Elevated cTnT levels were significantly associated with death in neurological, chest and other injury groups (Table 3). The adjusted odds ratio for mortality was significant for patients with traumatic brain injury but not for those with chest trauma or for other injuries sustained where the power was lower. The interaction p value is non-significant and therefore the association of cTnT with death does not differ significantly between the three groups (Table 4). The ROC areas did not differ significantly by injury type (p = 0.45); however, discrimination was excellent for chest trauma (ROC area (95% CI) = 0.83 (0.67, 0.99)) compared to fair for neurological trauma and other injuries (0.73 (0.63, 0.83) and 0.71 (0.59, 0.83), respectively) (Figure 3).
Figure 2.
Box and whisker plot showing median troponin values and interquartile range for survivors and non-survivors classified by type of injuries sustained, 0 = survivors, 1 = non-survivors.
Table 3.
Patient outcome and troponin levels (median (IQR)) for subgroups of neurological trauma (brain or spinal cord trauma), chest trauma and any other injuries.
| Survivors | Non-survivors | p value | |
|---|---|---|---|
| Neurological trauma (brain or spinal cord) (N = 120) | 10 (5–31) N = 78 | 56.5 (14–138) N = 42 | 0.00003 |
| Chest trauma (N = 35) | 13 (8–28) N = 29 | 98 (37–193) N = 6 | 0.01 |
| Any other injuries (N = 88) | 20 (8–40) N = 67 | 36 (20–123) N = 21 | 0.004 |
IQR: interquartile range.
Table 4.
Adjusted odds ratio for mortality for the type of injuries sustained.
| Adjusted for age |
Adjusted for APACHE |
|||
|---|---|---|---|---|
| ORa (95% CI) | p value | ORa (95% CI) | p value | |
| Neurological trauma (brain or spinal cord) (n = 120) | 2.16 (1.49–3.13) | 0.00005 | 1.90 (1.25–2.88) | 0.003 |
| Chest trauma (n = 35) | 3.44 (0.95–12.49) | 0.060 | 3.11 (0.84–11.50) | 0.10 |
| Any other injuries (n = 88) | 1.76 (0.99–3.11) | 0.053 | 1.20 (0.59–2.41) | 0.62 |
| p value interaction | 0.56 | 0.43 | ||
APACHE: Acute Physiology and Chronic Health Evaluation; CI: confidence interval; OR: odds ratio.
Per quartile increase in troponin level.
Figure 3.
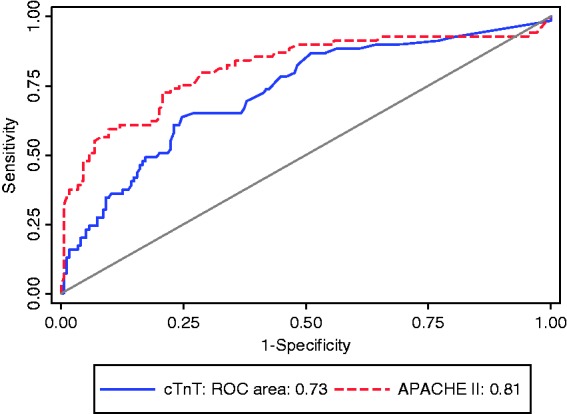
ROC curves for cTnT by injury group.
Discussion
This study showed a significant association between elevated cTnT levels in major trauma patients on admission to a UK major trauma centre critical care unit and all-cause hospital mortality. This association remained following adjustment for age, APACHE score and ISS. When the data were further analysed by the type of injury sustained, there was a statistically significant association between cTnT level and mortality for patients with traumatic brain injury and those who sustained any other types of injuries, in addition to those patients with chest trauma. Similar associations of cTnI elevation have also been reported in traumatic brain injury patients10,15,16 with correlation demonstrated between the level of troponin and the severity of head injury.10 The results of this study suggest that cTnT may assist in the prognostication of major trauma patients at an early stage following hospital admission and is unlikely to be exclusively due to direct myocardial trauma.
Whilst this study supports an association between cTn elevation and all-cause mortality in major trauma patients, it differs from previous studies of this patient population by analysing the cTnT rather than the cTnI subunit as predominantly utilised in other studies.9,11,12 Previous studies utilising cTnT assays in non-UK trauma cohorts have subdivided patients by TnT positivity rather than value (Mahmood: 2016) or as a predictor of direct cardiac injury.13 Despite similarities in the association between the troponin subunits and mortality, it cannot be assumed that cTnT and cTnI are equivalent. The availability of a single cTnT assay means that results may be directly comparable across sites, whereas this is not the case for cTnI. In addition, differences observed between the prognostic discrimination of cTnT and cTnI in the non-major trauma critical care population have led to suggestions that cTnT may be better at predicting all-cause mortality8 and that discordant results may be due to differing cellular distribution of the cTn subunits or post-translational cTn modification.17 These factors may suggest that cTnT may represent a more universally applicable prognostic marker in major trauma than the more studied cTnI.
The underlying mechanism of cTn elevation in non-cardiac major trauma or critically ill patients without evidence of acute coronary syndrome remains unclear. The association observed for direct chest injury in this and other studies9,11,12,18 may be partially accounted for by direct myocardial injury but this is unlikely to be the case for all mechanisms of injury, particularly given the strong association with mortality in the isolated traumatic brain injury cohort. Identification of increased troponin levels in trauma patients without significant chest trauma supports the theory that the secondary cardiac injury is related more to the degree of physiological stress sustained by the patient, than the initial traumatic insult itself.12 Heart–brain interactions are well-reported in other forms of non-traumatic neurological injury including subarachnoid haemorrhage and stroke.19,20 Secondary cardiac injury may result from an increase in myocardial oxygen demand as a consequence of the underlying disease process.21 Further insult may occur if myocardial oxygen supply is reduced in hypovolaemia, anaemia, tachycardia, hypoxaemia and impaired tissue perfusion.22 Exposure to catecholamines, either endogenous or exogenous may precipitate membrane leak and microscopic circulatory thrombosis of the myocardium.21 In other settings, for example subarachnoid haemorrhage, it is accepted that a catecholamine surge results in myocardial dysfunction and cTn release: otherwise known as stress cardiomyopathy.23
Inflammatory changes and oxidative stress may also contribute to the cTn rise. Tumour necrosis factor can depress myocardial function and induce cardiomyocyte apoptosis, causing reduced flow in the coronary arteries and decreased ejection fraction with subsequent cardiomyocyte necrosis.22 Reperfusion injury, free radical production and adrenergic stress may also play a role in secondary cardiac injury following trauma24 with mitochondrial failure increasingly recognised as the hallmark of organ failure in critical illness.25 Increased serum levels of TNF α and interleukin (IL)-6 have been demonstrated to correlate with elevated cTn levels leading to speculation that these, and other, cytokines may increase the permeability of the cardiomyocyte membrane permitting release of cardiac enzymes.7 These and other inflammatory mediators including IL-4, IL-8 and cytokines have all been implicated in the underlying pathophysiology of cardiomyocyte necrosis associated with traumatic injury.24,26 Elevated levels of IL-4 have been demonstrated in one study to be a significant predictor of death and are associated the highest relative risk of dying in trauma patients.26 Finally, although cTn release is generally assumed to represent cardiac myocyte cell death, it may occur secondary to ischaemia without cell death. Up to 8% of cardiac myocyte, cTnT is cytosolic, rather than bound to myofibrils, and may be released as a result of increased membrane permeability or stretch without irreversible protein damage.
There is ongoing debate regarding the utility of cTn as a predictive tool in critical illness. This study demonstrated that using the cTn and APACHE II score – both easily obtained values, over one-third of survivors and 13% of those who died were reassigned to a more accurate probability of death. Some argue that the lack of additional discriminatory value when troponin is added to established population risk prediction scores (e.g. APACHE II) mean it should not be used in this fashion.27 Others think differently22 and there is a case to be made for this simple and widely available test that does not require a detailed patient history, subjective assessment, calculation according to complex algorithm before a result is available, to assist in decision-making soon after hospital admission.
Of relevance to all biomarker association studies, and in particular those with retrospective methodology, there is potential for bias from multiple sources.28 In this case, 90/333 patients did not have cTnT measured on admission to the ICU, introducing the potential for significant selection bias. In those major trauma patients who did have cTn levels measured, there is potential for false positive results due to assay cross-reactivity with the high levels of skeletal muscle troponin T due to extensive tissue injury.29 Despite these important limitations, there are reasons to believe that the association described exists in the population studied. Adapting the Hill criteria,30 there is consistency, strength, a biological gradient, analogy and plausibility. First, the finding is consistent with other studies in both trauma and critical care patients in diverse settings. Second, the unadjusted odds ratio for death in the fourth quartile is high at 8. Third, there is a gradient of effect, with the odds ratio for death increasing quartile by quartile. Fourth, there is analogy with other biomarkers of severity of illness such as lactate. Finally, there is a potential mechanism, or mechanisms, to explain troponin elevation after trauma that plausibly reflects the severity of the injury sustained.
Limitations
The study was a retrospective review of data collected in a single centre receiving major trauma patients. Missing data in this study is likely to be related to the introduction and upgrading of computer-based data-recording systems. The study focussed on critical care patients and therefore data from patients who were not admitted to the ICU were not included which may skew the results. The study was designed using a pragmatic approach to provide critical care clinicians with more information on the outcome of major trauma patients using readily available and relatively inexpensive tests. Another flaw is that the study is unable to rule out an overall effect of increasing age on troponin elevation and mortality but the increased odds of dying if troponin was elevated into the highest quartile were found to be independent of age.
Summary
The study demonstrates a significant correlation between admission TnT levels in mortality for major trauma patients admitted to a critical care unit. The majority of studies to date have focussed on TnI levels and specific patterns of traumatic injury. In contrast, this study presents data for all injury patterns in major trauma, mirroring the findings in non-traumatic critical illness. The results obtained strongly support the need for prospective verification that may suggest the incorporation of cTnT into widely used major trauma risk prediction models.
Authors’ contributions
KC contributed to initial data analysis and co-authored the first and subsequent drafts. MT conceived the study, collected the data and co-authored the first and subsequent drafts. JT contributed to data analysis and co-authored the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship and/or publication of this article.
References
- 1.Haagsma JA, Graetz N, Bolliger I, et al. The global burden of injury: incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj Prev 2016; 22: 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhee P, Joseph B, Pandit V, et al. Increasing trauma deaths in the United States. Ann Surg 2014; 260: 13–21. [DOI] [PubMed] [Google Scholar]
- 3.Bass A, Patterson JH, Adams KF. Perspective on the clinical application of troponin in heart failure and states of cardiac injury. Heart Fail Rev 2010; 15: 305–317. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton MA, Toner A, Cecconi M. Troponin in critically ill patients. Minerva Anestesiol 2012; 78: 1039–1045. [PubMed] [Google Scholar]
- 5.Lim W, Holinski P, Devereaux PJ, et al. Detecting myocardial infarction in critical illness using screening troponin measurements and ECG recordings. Crit Care 2008; 12: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bessière F, Khenifer S, Dubourg J, et al. Prognostic value of troponins in sepsis: a meta-analysis. Intensive Care Med 2013; 39: 1181–1189. [DOI] [PubMed] [Google Scholar]
- 7.Ammann P, Maggiorini M, Bertel O, et al. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J Am Coll Cardiol 2003; 41: 2004–2009. [DOI] [PubMed] [Google Scholar]
- 8.Árnadóttir Á, Falk KC, Iversen K. Head-to-head comparison of cardiac troponin T and troponin I in patients without acute coronary syndrome: a systematic review. Biomarkers 2017; 22: 701–708. [DOI] [PubMed] [Google Scholar]
- 9.Edouard AR, Felten ML, Hebert JL, et al. Incidence and significance of cardiac troponin I release in severe trauma patients. Anesthesiology 2004; 101: 1262–1268. [DOI] [PubMed] [Google Scholar]
- 10.Salim A, Hadjizacharia P, Brown C, et al. Significance of Troponin elevation after severe traumatic brain injury. J Trauma 2008; 64: 46–52. [DOI] [PubMed] [Google Scholar]
- 11.Lippi G, Buonocore R, Mitaritonno M, et al. Cardiac troponin I is increased in patients with polytrauma and chest or head trauma. Results of a retrospective case-control study. J Med Biochem 2016; 35: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin M, Mullenix P, Rhee P, et al. Troponin increases in the critically injured patient: mechanical trauma or physiologic stress? J Trauma 2005; 59: 1086–1091. [DOI] [PubMed] [Google Scholar]
- 13.Kalbitz M, Pressmar J, Stecher J, et al. The role of troponin in blunt cardiac injury after multiple trauma in humans. World J Surg 2017; 41: 162–169. [DOI] [PubMed] [Google Scholar]
- 14.Brockamp T, Maegele M, Gaarder C, et al. Comparison of the predictive performance of the BIG, TRISS, and PS09 score in an adult trauma population derived from multiple international trauma registries. Crit Care 2013; 17: R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai SS, Bonds BW, Hu PF, et al. The role of cardiac troponin I in prognostication of patients with isolated severe traumatic brain injury. J Trauma 2016; 80: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasanin A, Kamal A, Amin S, et al. Incidence and outcome of cardiac injury in patients with severe head trauma. Scand J Trauma Resusc Emerg Med 2016; 24: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaze DC, Collinson PO. Multiple molecular forms of circulating cardiac troponin: analytical and clinical significance. Ann Clin Biochem 2008; 45: 349–355. [DOI] [PubMed] [Google Scholar]
- 18.Mahmood I, El-Menyar A, Dabdoob W, et al. Troponin T in patients with traumatic chest injuries with and without cardiac involvement: insights from an observational study. North Am J Med Sci 2016; 8: 17–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James P, Ellis CJ, Whitlock RM, et al. Relation between troponin T concentration and mortality in patients presenting with an acute stroke: observational study. BMJ 2000; 320: 1502–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Temes RE, Tessitore E, Schmidt JM, et al. Left ventricular dysfunction and cerebral infarction from vasospasm after subarachnoid hemorrhage. Neurocrit Care 2010; 13: 359–365. [DOI] [PubMed] [Google Scholar]
- 21.Ostermann M, Lo J, Toolan M, et al. A prospective study of the impact of serial troponin measurements on the diagnosis of myocardial infarction and hospital and six-month mortality in patients admitted to ICU with non-cardiac diagnoses. Crit Care 2014; 18: R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu TT, Yuan A, Chen CY, et al. Cardiac troponin I levels are a risk factor for mortality and multiple organ failure in noncardiac critically ill patients and have an additive effect to the APACHE II score in outcome prediction. Shock 2004; 22: 95–101. [DOI] [PubMed] [Google Scholar]
- 23.Richard C. Stress-related cardiomyopathies. Ann Intensive Care 2011; 1: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De'Ath HD, Rourke C, Davenport R, et al. Clinical and biomarker profile of trauma-induced secondary cardiac injury. Br J Surg 2012; 99: 789–797. [DOI] [PubMed] [Google Scholar]
- 25.Brown DA, Perry JB, Allen ME, et al. Expert consensus document: mitochondrial function as a therapeutic target in heart failure. Nature Rev Cardiol 2016; 14: 238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hranjec T, Swenson BR, Dossett LA, et al. Diagnosis-dependent relationships between cytokine levels and survival in patients admitted for surgical critical care. J Am Coll Surg 2010; 210: 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Docherty AB, Sim M, Oliveira J, et al. Early troponin I in critical illness and its association with hospital mortality: a cohort study. Crit Care 2017; 21: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet 2002; 359: 248–252. [DOI] [PubMed] [Google Scholar]
- 29.Apple FS, Collinson PO. for the IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012; 58: 54–61. [DOI] [PubMed] [Google Scholar]
- 30.Hill AB. The environment and disease: association or causation? J R Soc Med 2015; 108: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]