Abstract
The global incidence of invasive meningococcal disease due to serogroup W (MenW) has risen over the last decade. The following case emphasises the atypical features of MenW meningococcaemia, which included myocarditis, a rare but important complication. It also highlights the potential novel role that cardiac magnetic resonance imaging can provide in the diagnosis of MenW myocarditis. Complications of these infections can be avoided with early recognition and susceptibility testing to prevent the use of inappropriate antibiotics and treatment failure.
Keywords: Myocarditis, Neisseria meningitidis, serogroup W, bacteraemia, magnetic resonance imaging
Introduction
The global incidence of invasive meningococcal disease (IMD) due to serogroup W (MenW) has risen over the last decade.1 MenW disease is a subtype of meningococcal disease associated with high mortality and may present with atypical symptoms not characteristic of other meningococcal disease.2 Importantly, its atypical presentation can lead to delays in time critical interventions. We report a case of MenW myocarditis that presented to our intensive care unit (ICU).
Case report
A 56-year-old woman presented to the Emergency Department by ambulance, with a 5-h history of progressively worsening pleuritic chest pain and dyspnoea. In the prior four days, she had non-specific viral symptoms including sore throat, myalgias, rigors, fatigue, diarrhoea and fevers of 39℃. She had no medical history. Initial observations included a blood pressure of 70/50 mmHg, heart rate 95 beats/min, normal respiratory rate and oxygen saturation and temperature 36.5℃. Examination demonstrated marked pallor, dry mucous membranes and cool peripheries. Her conscious state was normal with no meningismus or sensorimotor impairment. Initial laboratory investigations revealed a high C-reactive protein (369 mg/L), leukopenia (3.5 × 109/L), thrombocytopenia (64 × 109/L) and raised troponin T (72 ng/mL). Other routine biochemical tests were otherwise unremarkable. Electrocardiography demonstrated saddle-sloped sequence-type (ST)-elevation anterolaterally with a raised J point. Bed-side transthoracic echocardiogram (TTE) showed reduced biventricular contractile function and a well-filled inferior vena cava. No cerebral spinal fluid was collected, given the absence of meningeal signs.
Her systemic hypotension was initially treated with intravenous (IV) crystalloid but only improved once an adrenaline infusion was commenced. Piperacillin/tazobactam (4.5 g, IV, four times daily) was administered 5 h after initial presentation to emergency department for empiric treatment of septic cardiomyopathy. Formal TTE on ICU admission demonstrated an improvement in ventricular function following the commencement of adrenaline. No other abnormalities were detected. The infusion was weaned within 48 h as haemodynamics stabilised with troponin T peaking at 30,000 ng/mL.
On day 2, Neisseria meningitidis was isolated from an aerobic blood culture bottle taken at admission (BD BACTEC FX blood culture system (BD Diagnostics, Sparks, MD), identification by VITEK MS MALDI-TOF MS; bioMerieux, Marcy L’Etoile, France). The isolate was sent to the reference laboratory for serogrouping and was identified as serogroup W. Variable region typing (finetyping) for PorA and FetA genes and multilocus sequence typing determined the final identification of the isolate as N. meningitidis W:P1.5,2:F1-1:ST-11(cc11). The isolate was sensitive to ceftriaxone, ciprofloxacin and rifampicin using the agar dilution method of the National Neisseria Network of Australia3 but less susceptible to penicillin with a minimum inhibitory concentration (MIC) of 0.5 mg/L. Piperacillin/tazobactam was ceased, and the patient was commenced on high-dose ceftriaxone (2 g/twice a day). A repeat TTE post-cessation of adrenaline showed normal left ventricle (LV) size and global systolic function but reduced global longitudinal strain (previously normal) with a small circumferential pericardial effusion.
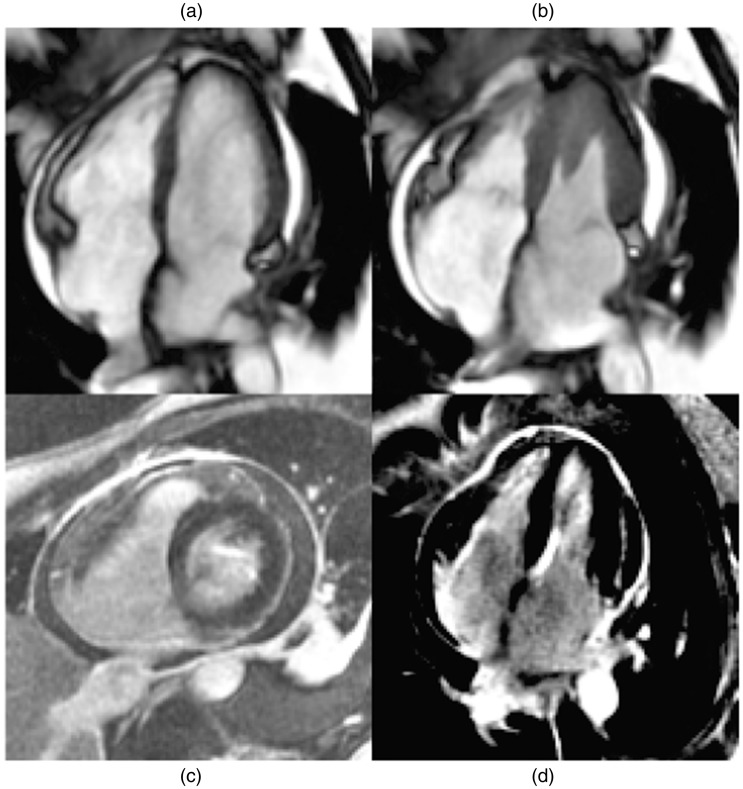
She continued to convalesce with gradual resolution of electrocardiogram changes and troponin. Urine and faecal culture, nasopharyngeal swab for respiratory viral polymerase chain reaction and serological results for atypical organisms (human immunodeficiency virus, influenza viruses, cytomegalovirus and hepatitis B and C) were negative. Auto-immune screening tests were also negative. Cardiovascular magnetic resonance imaging (MRI) was performed day 9 of her admission and demonstrated normal LV morphology and function, normal valvular function, a small pericardial effusion and post-contrast imaging consistent with acute myopericarditis (Figure 1).
Figure 1.
Cardiovascular magnetic resonance imaging performed day 9 of admission post-diagnosis of meningococcaemia (a) and (b) showing 4Ch view of normal left ventricular mass, size and systolic function. Normal global short-tau inversion recovery ratio suggests absence of myocardial oedema. (c) SAX view (T1-weighted image) late phase post-gadolinium showing a circumferential pericardial effusion. (d) 4Ch view with delayed gadolinium enhancement of visceral and parietal pericardium with probable subpericardial involvement consistent with myocyte injury and scaring caused by myocardial inflammation. 4Ch: four chamber; SAX: short axis.
Patient was discharged home 10 days after her presentation where she received two weeks in total of parental ceftriaxone (dose adjusted to 2 g/daily when susceptibility results were available, MIC < 0.008). Outpatient TTE at three months post-admission demonstrated mildly impaired biventricular function concurring with mildly reduced exercise capacity.
Discussion
This case highlights the following:
Patients with MenW infection may present to clinicians in atypical ways.
Myopericarditis can, rarely, be a dominant feature of MenW sepsis.
Cardiac MRI may provide important diagnostic information in cases of MenW infection.
The early recognition and susceptibility testing of MenW infections are essential to avoid inappropriate antibiotic use and treatment failure.
The epidemiology of meningococcal infections is changing as a result of changes to the vaccine composition and availability.
Neisseria meningitidis is a gram-negative diplococcus that has been identified with 13 serogroups based on unique capsular polysaccharides; 6 of the serogroups cause virtually all human disease (A, B, C, W, X, Y).4 Serogroups causing IMD vary geographically; in Europe, the Americas and Australia serogroups B, C and Y account for majority of the cases, while serogroup A predominately occurs in Africa.5 The introduction of meningococcal C or quadrivalent meningococcal ACWY conjugate vaccines have led to control of serogroup C disease in countries with high rates of immunisation. In Australia, this has led to an 82% decline in IMD over a 10-year period, from 3.5 per 100,000 in 2002 to 0.6 per 100,000 in 2013.2,5 However, there has also been a concurrent rise in the incidence of MenW strain infections over the last 15 years, which has now become a major cause of meningococcal disease.1
The MenW was thought to have little epidemic potential until the first international outbreak occurred at the Hajj in the year 2000. There have now been epidemics described in South Africa, Saudi Arabia, South America and the UK.1,6 Historically, compared to other strains, MenW incidence has been low of 1%–2%, but from 2009 onwards, England and Wales have seen a year-on-year increase in MenW accounting for 15% of all IMD cases in 2014.7 In Australia, IMD caused by MenW increased from 4% of all cases in 2013 to the predominant meningococcal serogroup in Australia with a total of 109 cases reported to the National Notifiable Diseases Surveillance System in 2016, overtaking serogroup B as the commonest cause of meningococcal disease.2 The rising incidence of MenW infections is important, as this serogroup has been associated with atypical presentations and higher mortality.7 The MenW ST 11 clonal complex (cc11) appears to be more virulent and is associated with higher case fatality rates in some studies8,9 and higher frequency of ICU admission.6
It is important for clinicians to recognise the unique characteristics of MenW infections that differentiate this capsular group from the meningococcaemia and acute meningitis presentations typically associated with other serogroups. The common presentation of a prodromal non-specific vial like upper respiratory infection followed by classical symptoms of non-blanching rash, neck stiffness, high fever and altered conscious state are predominantly due to serogroups B and C. A primary pneumonia is most frequently associated with the rarer serogroup Y as its clinical presentation.10 In contrast, MenW patients are more likely to develop non-meningeal manifestations such as arthritis, pericarditis and pneumonia.11 An analysis of all cases of meningococcal disease from 1999 to 2002 in France showed MenW was significantly more likely to be associated with meningococcal arthritis and meningococcal pneumonia.12 Furthermore, unlike in other serogroups which tend to present in younger patients, the age distribution of MenW is more evenly distributed with the highest prevalence aged 65 years and over.13 Very rarely, as demonstrated in the above case, patients can present with primary meningococcal myopericarditis. Such atypical features can lead to delays in potentially time critical interventions, such as the administration of antibiotics and definitive cardiac imaging, which have been associated with significant harm to the patient.14
MenW-associated primary meningococcal myopericarditis is an extremely rare manifestation of meningococcal disease.15,16 Furthermore, the frequency of myocardial involvement in IMD is likely underestimated. In an autopsy series of 31 children with fatal meningococcal disease, 41% were found to have evidence of myocarditis.17
We believe this is the first report to show that MenW myopericarditis can be confirmed using cardiac MRI. Post-contrast cardiac MRI confirmed early myocardial enhancement consistent with inflammation and hyperaemia as well as delayed enhancement of visceral and parietal pericardium suggestive of subpericardial involvement, findings that are consistent with myopericarditis. The normal global short-tau inversion recovery ratio establishes that no myocardial oedema was present. We did not confirm the diagnosis with endomyocardial biopsy or pericardial aspirate due to a risk:benefit ratio assessment in the setting of this patient having a positive microbiological diagnosis and clinical improvement with appropriate antimicrobials. In previous case reports of MenW myopericarditis, myocardial inflammation was confirmed with non-specific investigations such as cardiac biomarkers, electrocardiography and echocardiography. These features are only supportive of the diagnosis and do not definitively rule out septic cardiomyopathy as an alternative diagnosis. The speculated pathogenesis of myocarditis is either related to early bacteraemic seeding of the pericardium13 or a delayed immunological process as previous studies have discovered high levels of cytokines in the pericardial fluid.18
IMD is a medical emergency, and early antibiotic treatment should be the primary goal as effective antibiotics immediately stops the proliferation of N. meningitidis. In Australia, patients suspected of bacterial meningitis are empirically commenced on ceftriaxone or cefotaxime, as per national guidelines, and often combined with vancomycin until causative agent is found. When N. meningitdis is isolated, antibiotic treatment is continued with benzylpenicillin alone or a third-generation cephalosporin.19 Penicillin resistance in N. meningitidis is mediated by two known mechanisms: plasmid-mediated ß-lactamase production (MIC > 2 mg/L), which is extremely rare, and altered forms of penicillin-binding proteins (PBPs) that impair peptidoglycan biosynthesis and confer intermediate resistance to penicillin (MIC 0.12–0.25 mg/L). Although penicillin treatment is still effective against penicillin-intermediate strains, low-dose treatment regimens may fail for cases involving penicillin-resistant isolates (MIC ≥ 0.5 mg/L).20 Isolates conferring intermediate resistance to penicillin are uncommon, but the frequency of these isolates varies geographically and incidence has been rising in Australia, with rates of 78%–88% between 2010 and 2015.13 Recent data from 19 MenW:cc11 strains isolated in Western Australia between 2013 and 2016 demonstrated that 9/19 (47.4%) isolates were penicillin resistant (MIC ≥ 0.5 mg/L) and 2/19 (10.5%) were less susceptible to penicillin (MIC 0.12–0.25 mg/L) according to Clinical Laboratory Standard Institute (http://clsi.org) breakpoints.20 Core-genome analysis of these MenW:cc11 strains in Western Australia identified the emergence of a phylogenetically related penicillin-resistant cluster which contained an identical allele (penA_253) within the penA gene that encodes PBP2. This allele was found to play a major role in penicillin resistance, conferring a four-fold increase in MIC when transformed into penicillin-sensitive isolates.
The outcomes following MenW induced myopericarditis are variable. Early mortality rate remains high at approximately 25%.21 However, the longer term prognosis of survivors was also found to be excellent: 9/12 survivors had normal ejection fractions at one year. Other cases have been reported the reversal of rapidly progressive cardiac failure when correct antimicrobial therapy was promptly implemented.22 In our patient, there was rapid early improvement, although her TTE at three months demonstrated some ongoing mild reduction in biventricular function which suggest some ongoing myocardial impairment.
The case has highlighted the changing IMD epidemiology. The incidence of MenW infections is rising, and this is affecting all age groups. MenW infections may behave differently to other serogroups, including atypical clinical presentations and emerging reports of increased penicillin resistance. The atypical clinical presentation of IMD can lead to delays in the diagnosis until it has been isolated from a sterile site culture, usually 24–72 h after admission.11 Due to its low incidence and requirement of invasive investigations for tissue diagnosis, there is an underappreciation of meningococcal myopericarditis. Cardiac MRI may have a role in the diagnosis of such cases. This delayed diagnosis can have significant ramifications on the outcomes of patients with primary meningococcal myopericarditis due to the adverse impacts on myocardium. Timely treatment of primary meningococcal myopericarditis, as demonstrated in our case, may result in rapid resolution and possible avoidance of long-term morbidity or mortality.
Consent
This report is published with the written consent of the patient.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Mustapha MM, Marsh JW, Harrison LH. Global epidemiology of capsular group W meningococcal disease (1970-2015): multifocal emergence and persistence of hypervirulent sequence type (ST)-11 clonal complex. Vaccine 2016; 34: 1515–1523. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health. Invasive meningococcal disease national surveillance report with a focus on MenW, www.health.gov.au/internet/main/publishing.nsf/Content/5FEABC4B495BDEC1CA25807D001327FA/$File/w14-Aug-2017-IMD-Surveillance-report.docx (2017, accessed December 2017).
- 3.Tapsall J. Members of the National Neisseria Network of Australia. Antimicrobial susceptibility testing methods and practice with an Australian perspective. In: Merlino J. (eds). Antimicrobial testing and applications in the pathogenic Neisseria, Sydney: Australian Society for Microbiology, Antimicrobial Special Interest Group, 2004, pp. 175–188. [Google Scholar]
- 4.Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal disease. N Engl J Med 2001; 344: 1378–1388. [DOI] [PubMed] [Google Scholar]
- 5.Halperin SA, Bettinger JA, Greenwood B, et al. The changing and dynamic epidemiology of meningococcal disease. Vaccine 2012; 30: B26–B36. [DOI] [PubMed] [Google Scholar]
- 6.Carville KS, Stevens K, Sohail A, et al. Increase in meningococcal serogroup W disease, Victoria, Australia, 2013-2015. Emerg Infect Dis 2016; 22: 1785–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell H, Parikh SR, Borrow R, et al. Presentation with gastrointestinal symptoms and high case fatality associated with group W meningococcal disease (MenW) in teenagers, England, July 2015 to January 2016. Euro Surveill 2016; 21: 11–14. [DOI] [PubMed] [Google Scholar]
- 8.Ladhani SN, Beebeejaun K, Lucidarme J, et al. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis 2015; 60: 578–585. [DOI] [PubMed] [Google Scholar]
- 9.Wilder-Smith A, Goh KT, Barkham T, et al. Hajj-associated outbreak strain of Neisseria meningitidis serogroup W135: estimates of the attack rate in a defined population and the risk of invasive disease developing in carriers. Clin Infect Dis 2003; 36: 679–683. [DOI] [PubMed] [Google Scholar]
- 10.Batista RS, Gomes AP, Dutra Gazineo JL, et al. Meningococcal disease, a clinical and epidemiological review. Asian Pac J Trop Med 2017; 10: 1019–1029. [DOI] [PubMed] [Google Scholar]
- 11.Bethea J, Makki S, Gray S, et al. Clinical characteristics and public health management of invasive meningococcal group W disease in the East Midlands region of England, United Kingdom, 2011 to 2013. Euro Surveill 2016; 21: 12–16. [DOI] [PubMed] [Google Scholar]
- 12.Vienne P, Ducos-Galand M, Guiyoule A, et al. The role of particular strains of Neisseria meningitidis in meningococcal arthritis, pericarditis, and pneumonia. Clin Infect Dis 2003; 37: 1639–1642. [DOI] [PubMed] [Google Scholar]
- 13.Lahra MM, Enriquez RP. Australian Meningococcal Surveillance Programme annual report, 2015. Commun Dis Intell Q Rep 2016; 40: E503–E511. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 15.Brasier AR, Macklis JD, Vaughan D, et al. Myopericarditis as an initial presentation of meningococcemia. Unusual manifestation of infection with serotype W135. Am J Med 1987; 82: 641–644. [DOI] [PubMed] [Google Scholar]
- 16.Ejlertsen T, Vesterlund T, Schmidt EB. Myopericarditis with cardiac tamponade caused by Neisseria meningitidis serogroup W135. Eur J Clin Microbiol Infect Dis 1988; 7: 403–404. [DOI] [PubMed] [Google Scholar]
- 17.Garcia NS, Castelo JS, Ramos V, et al. Frequency of myocarditis in cases of fatal meningococcal infection in children: observations on 31 cases studied at autopsy. Rev Soc Bras Med Trop 1999; 32: 517–522. [DOI] [PubMed] [Google Scholar]
- 18.Taldir G, Parize P, Arvis P, et al. Acute right-sided heart failure caused by Neisseria meningitidis. J Clin Microbiol 2013; 51: 363–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephens DS, Apicella MA. Neisseria meningitidis . In: Bennett J, Dolin R, Blaser M. (eds). Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, New York: Elselvier, 2015, pp. 2425–2445. [Google Scholar]
- 20.Mowlaboccus S, Jolley KA, Bray JE, et al. Clonal expansion of new penicillin-resistant clade of Neisseria meningitidis serogroup W clonal complex 11, Australia. Emerg Infect Dis 2017; 23: 1364–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madelaine T, Cour M, Bohe J, et al. Invasive meningococcal disease-induced myocarditis in critically ill adult patients: initial presentation and long-term outcome. Intensive Care Med 2016; 43: 279–281. [DOI] [PubMed]
- 22.Razminia M, Salem Y, Elbzour M, et al. Importance of early diagnosis and therapy of acute meningococcal myocarditis: a case report with review of literature. Am J Ther 2005; 12: 269–271. [PubMed] [Google Scholar]