Abstract
According to embodied simulation theories, others' emotions are recognized by the unconscious mimicking of observed facial expressions, which requires the implicit activation of the motor programs that produce a specific expression. Motor responses performed during the expression of a given emotion are hypothesized to be directly linked to autonomic responses associated with that emotional behavior. We tested this hypothesis in 9 children (Mage = 5.66) affected by Moebius syndrome (MBS) and 15 control children (Mage = 6.6). MBS is a neurological congenital disorder characterized by underdevelopment of the VI and VII cranial nerves, which results in paralysis of the face. Moebius patients' inability to produce facial expressions impairs their capacity to communicate emotions through the face. We therefore assessed Moebius children's autonomic response to emotional stimuli (video cartoons) by means of functional infrared thermal (fIRT) imaging. Patients showed weaker temperature changes compared to controls, suggesting impaired autonomic activity. They also showed difficulties in recognizing facial emotions from static illustrations. These findings reveal that the impairment of facial movement attenuates the intensity of emotional experience, probably through the diminished activation of autonomic responses associated with emotional stimuli. The current study is the first to investigate emotional responses in MBS children, providing important insights into the role of facial expressions in emotional processing during early development.
1. Introduction
Moebius syndrome (MBS) is a rare congenital syndrome affecting approximately 1 in 50000 to 1 in 500000 live births [1], with no gender predominance [2]. The disorder presents with varying phenotypes and severity and is characterized by unilateral or bilateral facial paralysis, as well as impaired bilateral movement of the eyes. This is due to maldevelopment of the VI and VII cranial nerve nuclei early in prenatal life [3–7]. The VI and VII cranial nerves control, respectively, the abduction of the eyes and the muscles used to generate facial expressions, lip speech, and eye closure. V, IX, X, and XII cranial nerves can also be affected [8–10]. Other congenital abnormalities are sometimes associated with the syndrome, including sensorineural hearing loss, craniofacial malformations, limb anomalies, Poland syndrome (underdevelopment of the pectoralis muscle and hand malformation), hypoglossia, and poor coordination [10, 11]. Most patients are of normal intelligence, while approximately 9-15% present mild mental retardation, and another 0-5% are diagnosed with autistic-like behaviors [12–14].
One of the most prominent features of MBS patients is their inability to smile or produce any facial movement, which limits their capacity to communicate emotions through the face [15–19].
Evidence has shown that the motor component of emotional facial expressions is associated with an involuntary autonomic nervous system (ANS) response [20]. It has been proposed that the coding of emotional stimuli in macaque monkeys is mediated by the activity of brain networks including both cortical motor and specific limbic regions [21]. Human neuroimaging studies have demonstrated that, in addition to motor regions, the observation and direct experience of an emotion activate specific brain areas (i.e., the anterior insula, the anterior cingulate cortex (ACC), and the amygdala) [22], which are important not only in the control of the motor components of emotions but also in orchestrating the complex visceromotor responses associated with an emotional state (increase/decrease in heart rate (HR), changes in blood pressure, pupil dilation, piloerection, metabolic changes, etc.) [21, 23–25]. Emotional processing therefore relies on a complex network of brain regions in which some structures, such as the insular cortex, the amygdala, and the ACC, could coordinate the autonomic responses typical of the limbic system with the motor modifications associated with the expression of an emotion [26, 27]. This tight connection between motor and autonomic responses is therefore of utmost importance when investigating disturbances involving the motor commands controlling emotional expressions.
Several studies posit that the same motor regions involved in the generation of a particular facial expression of emotion are also implicated in recognizing that emotion in others [28–30]. The neuronal basis of this process is underpinned by a mirror mechanism, implemented by a parietal-premotor cortical network known as the “mirror neuron system” (MNS) [31, 32]. The MNS in humans has been proposed to support not only the understanding of others' action intentions [33–35] but also the recognition of others' emotions through activation in the observer of a neural motor representation similar to that expressed by the observed individual [25–27, 34–40]. Emotion recognition therefore occurs via unconscious mimicking of the observed expression, which requires the implicit activation of those motor programs responsible for the production of a particular facial expression and associated physiological responses (also named reverse simulation model) [41–44]. According to embodied simulation theories [41, 45–49], the perception of an emotional facial expression is accompanied by the simulation of that specific emotional state in the motor, somatosensory, affective, and reward systems of the perceiver [44, 50, 51].
In light of these premises, facial motor impairment in MBS patients could impact several processes related to emotions. A few studies have shown that adult Moebius patients can recognize others' emotions to some degree, but the results are mixed. This is likely due to certain methodological limitations including patient sample size, lack of clinical evaluation, nonobjective assessment (i.e., self-evaluation), and variations in the measures and tasks used [52–55]. In addition, previous studies have centered on adults, who may have developed alternative strategies throughout their lifespan in order to cognitively recognize facial expressions. These supportive strategies, whereby specific facial cues of emotion expression (e.g., the mouth corners turned up or down) are extracted [56, 57], could have positively affected their ability to discern different emotions later in life. Finally, the above-mentioned studies focused on Moebius patients' emotion recognition abilities without investigating the autonomic component of emotional processing.
Bearing in mind that the motor and autonomic components associated with an emotional expression interact with each other, one could say that the congenital absence of facial muscle activity and relative proprioceptive feedback could result in a dysfunctional autonomic response to emotional stimuli and difficulties in recognizing others' emotions [52, 55]. MBS patients therefore represent an interesting population to investigate this.
The measurement of the autonomic component of emotional processing during childhood would enable the constraints linked to cognitive processing of emotional information to be bypassed. In this sense, the lack of facial expressivity in Moebius children makes them an ideal subgroup to study emotional processing during the early phases of development, when complex cognitive strategies have yet to emerge.
We hypothesized that the lack of facial motor activity in MBS children during the decoding of emotions could induce an altered autonomic response while watching emotional videos, as well as difficulties in deciphering emotional facial stimuli. To this end, we monitored participants' autonomic response during observation of emotional stimuli using functional infrared thermal (fIRT) imaging, a dynamic and noninvasive method of measuring skin temperature distribution [58]. Facial skin thermal patterns depend on subcutaneous vessels transporting blood heat. These vessels regulate blood flow via local vascular resistance (vasodilation and vasoconstriction) and arterial pressure [59]. Therefore, by recording the dynamics of facial cutaneous temperature, it is possible to assess ANS activity and infer the subject's emotional state [60–64].
fIRT has been shown to be effective in detecting several affective states, including extreme stress [63], startle [65], fear [66], arousal [67], and happiness [68]. For example, fear experienced during a threatening and distressing situation [62, 69–71], as well as the experience of stress [71, 72] or guilt [61], is related to a decrease in nasal tip temperature due to subcutaneous adrenergic vasoconstriction [73]. On the contrary, social interaction [62, 74] and sexual arousal [75] produce an increase in nasal tip temperature, caused by the vasodilation effect of the parasympathetic nervous system on the autonomic state of the individual. Crucially, due to its low invasiveness and versatility, fIRT results are particularly suitable for use with younger individuals, as well as clinical populations [61, 62, 76, 77].
In the present study, we expected to observe a weaker thermal modulation in MBS participants compared to control subjects, and we hypothesized that the motor impairment of MBS patients would result in an impaired autonomic response during emotion observation.
2. Materials and Methods
2.1. Participants
We recruited 9 children (5 males) with MBS aged 4 to 8 years (mean age 5.66, SD = 1.78). Moebius participants exhibited unilateral or bilateral facial paralysis, as well as related neurological symptoms (see Table 1); all were referred to as cognitively able in the study, and all were attending mainstream schools at a level appropriate to their age. Moebius children were recruited through the clinical center at the University of Parma, which specializes in the diagnosis of MBS and therapeutic intervention. Only patients without cognitive disability or diagnosis of autism were included in the experiments. We also recruited 15 healthy children (control group) (9 males) in the same age range (mean age 6.6, SD = 1.79). All participants were informed that they would be videotaped by means of a thermal camera and a webcam. All parents gave their informed written consent after full explanation of the procedure, which is in accordance with the 1964 Declaration of Helsinki. The study was approved by the Ethics Committee of the University of Parma.
Table 1.
Moebius subjects' medical cases. The term “laterality” refers to the kind of facial paralysis that can be unilateral or bilateral; the sixth and seventh cranial nerves are usually involved, but other nerves may also be affected. “Associated pathologies” linked to Moebius syndrome can involve possible hand and foot anomalies, muscle hypotonia, hypoacusis, swallowing and speech problems, and Poland syndrome.
| ID no. | Sex | Laterality | Cranial nerves involved | Additional functional deficits and associated pathologies |
|---|---|---|---|---|
| 1 | M | Unilateral left | VI, VII | — |
| 2 | M | Bilateral | VI, VII, III, IV | Strabismus, hypotonia, hypoacusia of the right ear, speech deficit (articulation-phonetic disorders), right plagiocephaly, psychomotor delay, epileptic seizures, cardiac crisis |
| 3 | F | Bilateral | VI, VII, XII | Foot malformations |
| 4 | F | Unilateral left | VI, VII, XII | Speech deficit, club feet |
| 5 | M | Bilateral | VI, VII, XII | Club foot, brain stem atrophy with enlargement of the fourth ventricle, hand deformities |
| 6 | F | Unilateral right | VI, VII, XII right | Micrognathia, tongue hypoplasia |
| 7 | F | Bilateral | VI, VII | Bilateral mixed hypoacusia, hypotonia, delayed growth, laryngomalacia, palatal schisis, coloboma of the right optic nerve |
| 8 | M | Bilateral | VI, VII, XII left | Respiratory difficulties, micrognathia, hypotonia, psychomotor delay, club foot |
| 9 | M | Bilateral | VII | No ocular deficits, speech delay |
2.2. Materials
Thermal IR imaging was performed by means of a digital thermal camera FLIR T450sc (IR resolution: 320 × 240 pixels; spectral range: 7.5–13.0 μm; thermal sensitivity/NETD: <30 mK at 30°C). The frame rate was set to 5 Hz (5 frames/sec). A remote-controlled webcam (Logitech webcam C170) was used to film the participants' behavior, so as to record their level of attention while watching video stimuli.
2.3. Procedure and Stimuli
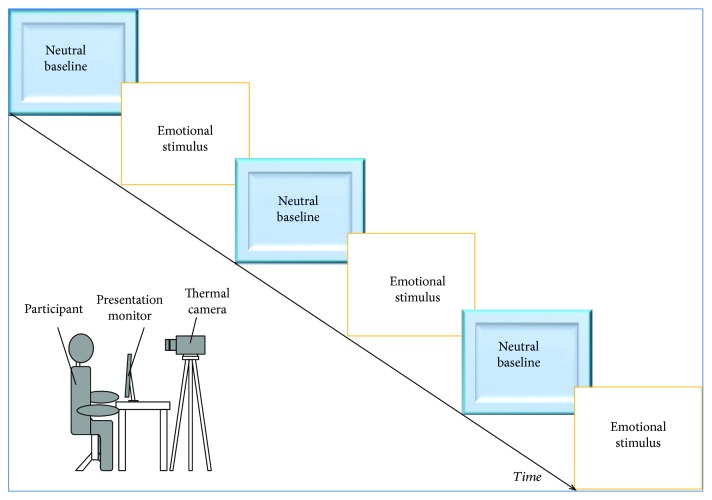
Prior to testing, each participant was left to acclimatize for 10 minutes to the experimental room and to allow their skin temperature to stabilize. The recording room was set at a standardized temperature (23°C) and humidity (50–60%) and was not subject to direct sunlight, ventilation, or airflow. During an initial neutral interaction, the experimenter asked the child to answer questions related to personal data (e.g., name and age). The child was then invited to watch a series of video stimuli displayed on a computer monitor (32.5 × 22.7 cm) placed 60 cm far from the chair where the child was sitting. According to other thermal imaging studies using video stimuli [60, 78, 79], our sequences included 6 different video clips (neutral baseline-happiness-neutral baseline-sadness-neutral baseline-fear), with each emotional video preceded by a neutral video. Stimuli were comprised of short clips taken from the Internet in which the main character of the scene was in a happy, sad, or scary situation. The emotional video clips varied in their duration (mean = 81.38 sec; SD = 43.49), while neutral video clips (ones with no emotional content) lasted about 30 sec (mean = 28.83 sec; SD = 3.69) (Figure 1). Chosen stimuli represented the kind of videos that children of this age are familiar with.
Figure 1.
Experimental paradigm. Schematic overview of the experimental paradigm.
Video clips were validated before the experiment in order to ensure that they were easily comprehensible and represented the specific emotion deemed appropriate for the age range of interest here. To do this, we presented neutral (baseline), happy, sad, and scary videos to a separate group of 16 children (8 males) with a mean age of 7.5 years; participants were asked to categorize the video clip as evoking feelings of “happiness,” “sadness,” “fear,” or “neutral baseline.” The average percentage of correct recognition was 95.83%. Based on our validation study, we randomly presented two video sequences from a list of six. The choice to present two sequences only was based on expected fatigue, habituation, and difficulty in sustaining children's attention for long periods of time.
During the experimental session, thermal and video cameras were placed above the monitor, one meter away from the participant. Cameras were automatically calibrated and manually fixed to capture a frontal view of the child's face. Facial thermal images and videotapes were recorded during each video presentation.
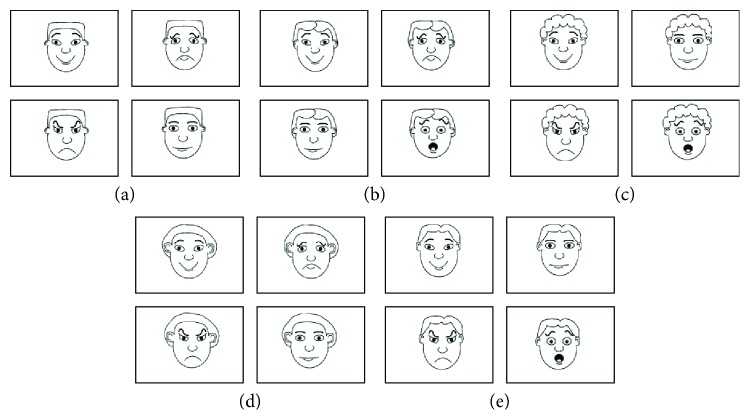
At the end of each short video clip, participants were asked a series of questions concerning (1) the emotional state of the main characters depicted in the video cartoon and (2) the child's own emotional involvement while watching each video clip. Unfortunately, in most cases, the children did not reply to the questions and therefore it was not possible to apply statistical analyses. To overcome the difficulty of this assessment, we also administered the Italian standardized version (see [80]) of the Test of Emotion Comprehension (TEC-1) [81], so as to obtain an index of the individual's capacity to discriminate different emotions. MBS children were administrated component I of the TEC-1, which assesses emotion recognition by means of facial expression discrimination (Figure 2). Four simple drawings were presented on an A4 sheet of paper, which included four out of five possible emotions depicted by cartoon facial expressions. The children were asked to indicate which of the facial expressions was happy, sad, angry, scared, or “just alright” (i.e., neutral component). Figure 2 illustrates the items used to assess children's emotion recognition. Five successive items were used to test children's recognition of emotions. Depending on the participant's own gender, a corresponding version of the drawings (i.e., female or male) was presented. Component I of the TEC-1 was also used to test the emotion recognition ability in 15 healthy subjects in the same age range as Moebius participants. The full experimental session, including both emotional sequences and TEC-1, lasted a maximum of 45 minutes.
Figure 2.
Example of cartoon pictures presented during TEC-1 (component I, emotion recognition).
2.4. Data Analysis
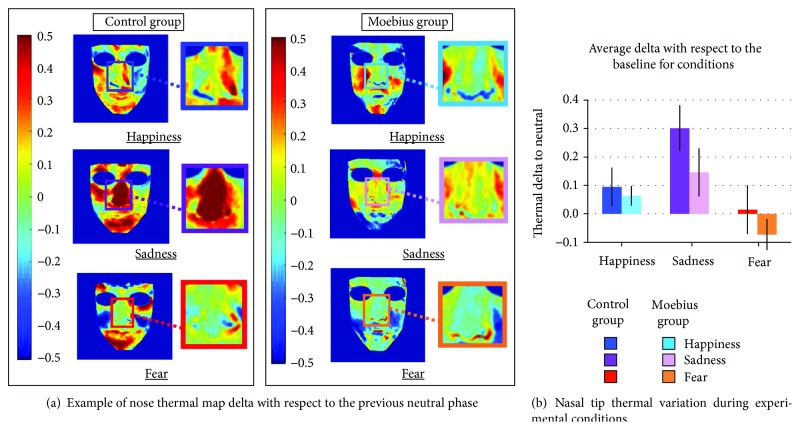
A quantitative analysis was carried out to measure temperature variations of participants' nasal tips. Elliptic regions of interest (ROIs) with identical shape and dimensions (A = 297 pixels; MajorAxisLength = 20.35 pixels; MinorAxisLength = 18.64 pixels) were utilized. We focused on this ROI for two main reasons. First, given the relatively low incidence of MBS, the specific age sample of interest, and the pioneering nature of the current study, we decided to include patients with unilateral or bilateral facial paralysis. The nasal tip is a nonlateralized ROI, so its temperature should not be modulated by the lateralization of nerve impairment. Second, the nasal tip has been shown to be particularly sensitive to emotional state transitions [62, 65, 70, 82]. This area of the face is indeed highly innervated by adrenergic fibers, resulting in a privileged window on a participant's autonomic state. More specifically, sympathetic nervous responses to emotional and distressing stimulation produce a decrease in nasal tip temperature whereas parasympathetic responses result in a temperature increase in this ROI [62, 65, 68, 71, 77, 82, 83]. Thermal signals were extracted through the use of the software Morphing GUI, developed with customized MATLAB algorithms (The Mathworks Inc., Natick, MA). This analysis procedure is more extensively described in [84]. Due to the high computational load associated with the morphing procedure, we decided to downsample the collected dataset. Given the slow nature of thermal responses, such a processing choice did not affect the precision of temperature change detection [62, 84]. For each video stimulus presented to the child, three thermal images were extracted (one frame at the beginning, one in the middle, and one at the end of each video) and morphed. These particular frames were selected in order to minimize the effect of the respiratory cycle on the thermal imprinting of the subject [71]. The three frames selected within each condition (emotional or neutral) were averaged. In order to interpret any affective response, the selection of an appropriate baseline represents the starting point for defining the directionality of the physiological change during emotional arousal [76]. For this reason, we selected video clips with no emotional content (see Procedure and Stimuli) to eliminate the interindividual variability in the subjects' temperature and to minimize the effect of participants' circadian variations on our data. We followed a typical procedure for thermal data analysis [62]: subtraction of the mean thermal value of each neutral condition from the mean thermal value of its following experimental condition (happiness, sadness, and fear). In this way, we obtained a dataset of thermal variation for each emotional condition relative to the neutral condition. The thermal variations for the two trials belonging to the same condition were then averaged to obtain a mean value for each emotion (happiness, sadness, and fear) (see Figure 3(a)). This was used as the variable of interest in our statistical analyses, including the comparison of MBS participants and the control group.
Figure 3.
(a) Thermal modulation in an example control participant and Moebius patient during the “happiness,” “sadness,” and “fear” conditions. In the figure, the inlays present the entire nasal area, but elliptic nasal tip ROIs were used for analyses (A = 297 pixels; MajorAxisLength = 20.35 pixels; MinorAxisLength = 18.64 pixels) [62, 65]. The control participant shows stronger thermal variation during the sadness condition than the Moebius patient. (b) Mean temperature values during each of the experimental conditions, baseline-corrected with respect to the neutral condition. Both control and Moebius participants show a significant nasal tip temperature increase during the “sadness” condition (∗p ≤ 0.001). Means and standard errors (SE) are reported for each condition in both control and Moebius groups.
During TEC-1 administration, participants' answers were noted on the answer sheet by the experimenter and subsequently coded (1 point for each correct answer and 0 for each wrong answer).
2.5. Statistical Data Analysis
A repeated measures ANOVA (6 × 2) was performed on the neutral baseline temperature values in order to confirm that baseline temperature did not significantly differ between the Moebius and control groups (p > 0.05). A repeated measures ANOVA (3 × 2) was performed on the mean nasal tip values (emotion compared to neutral baseline) for all participants [76]. The emotion condition (happiness, sadness, and fear) was set as a within-subjects factor, while the group (Moebius and controls) was set as a between-subjects factor [85, 86]. Bonferroni post hoc tests (Bonferroni corrected) followed the two-way ANOVA. Assumptions of residual normality and homogeneity of variance were investigated using Shapiro-Wilk and Levene's tests, respectively. Normality and equal variance were confirmed. If data violated the sphericity assumption, Greenhouse-Geisser (ε < 0.75) or Huynh-Feldt (ε > 0.75) corrected values were reported.
A nonparametric Mann-Whitney U test for independent samples was used to compare Moebius and control group answers from the TEC-1. One Moebius subject did not complete the TEC-1 and was excluded from the analysis. Finally, we correlated the thermal values for each emotion condition with the TEC-1 scores. Data were analyzed by means of Statistica 8.0 (StatSoft, Tulsa, OK, USA).
3. Results
3.1. Thermal Data: Group Temperature Variations in relation to Conditions
A repeated measures ANOVA (3 × 2) was performed on the resampled variations of mean nasal tip temperatures. We did not find any differences between the two groups (p = 0.432). The results highlighted a significant effect of emotion condition (F(1.53, 33.71) = 10.99; p ≤ 0.001∗; η2 = 0.325); post hoc tests showed that nasal tip temperature during the sadness condition significantly increased compared with that during the happiness (p = 0.013) and fear (p ≤ 0.001∗) conditions (for descriptive statistics, see Table 2). No significant difference was observed between the fear and happiness conditions (p = 0.133) (Figure 3(b)). The group × emotion condition interaction was not statistically significant (p = 0.447).
Table 2.
Descriptive statistics for each group and condition.
| Group | Happiness | Sadness | Fear | |
|---|---|---|---|---|
| Mean | Control | 0.199 | 0.364 | 0.216 |
| Moebius | 0.144 | 0.212 | 0.118 | |
| Std. error mean | Control | 0.033 | 0.075 | 0.042 |
| Moebius | 0.030 | 0.057 | 0.023 | |
| Standard deviation | Control | 0.128 | 0.290 | 0.163 |
| Moebius | 0.090 | 0.171 | 0.069 | |
| Variance | Control | 0.016 | 0.084 | 0.026 |
| Moebius | 0.008 | 0.029 | 0.005 |
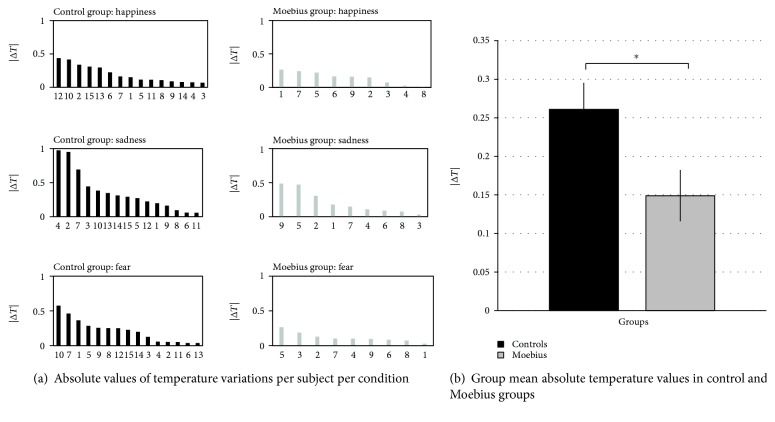
As shown in Figure 3(a), during all of the experimental conditions, Moebius participants exhibited a less appreciable thermal modulation compared to control participants while watching emotional stimuli. To measure any possible differences in the intensity of thermal modulation between the two groups, we considered the absolute value of change in temperature from baseline. As previously suggested, control participants exhibited a larger thermal response than Moebius participants during each experimental phase (Figure 4(a)).
Figure 4.
(a) Absolute value of the change in temperature from baseline per participant during each of the experimental conditions. Moebius participants exhibit a lower thermal modulation compared with control participants. (b) Group mean absolute temperature values in control and Moebius participants. Control participants have significantly more intense thermal modulation compared with Moebius participants. Means and standard errors (SE) are reported for both control and Moebius groups.
A one-way ANOVA performed on the absolute value of the change in temperature from baseline revealed a significant effect of the group (F(1, 22) = 4.732; p = 0.041; η2 = 0.177), with control participants having higher absolute changes in temperature (0.261 ΔT) than Moebius participants (0.149 ΔT) (Figure 4(b)).
3.2. Test of Emotion Comprehension (TEC-1)
Mann-Whitney U tests were performed to assess if control and Moebius participants' scores significantly differed during the Test of Emotion Comprehension (TEC-1) administration. The results showed that the Moebius group had a lower level of facial emotion recognition (mean = 2.63; SD = 1.69) than the control group (mean = 4.80; SD = 0.41) (p = 0.002) (Figure 5). The autonomic responses and TEC-1 scores were not significantly correlated (p > 0.05).
Figure 5.
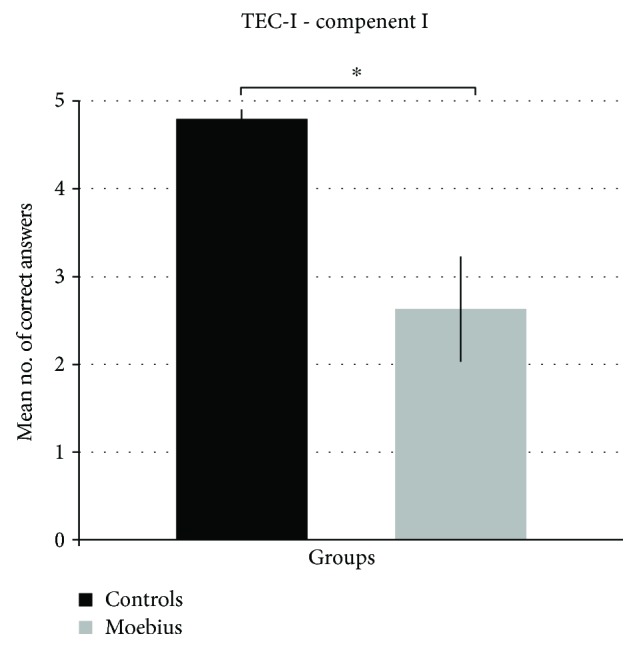
Mean number of correct answers from both control and Moebius participants. During the emotion recognition task (TEC-1), control participants performed better than Moebius participants. Means and standard errors (SE) are reported for both control and Moebius groups.
4. Discussion
The purpose of our study was to detect psychophysiological responses in children affected by MBS by means of a fIRT camera. Moebius and control participants were asked to observe two sequences of emotional cartoon video stimuli representing three main emotions: happiness, sadness, and fear. Changes in nasal tip temperature were measured during the observation of the stimuli, and the results showed a significant difference between emotional conditions. Both MBS and control participants showed an increase in nasal tip temperature during the “sadness” condition [73], but Moebius participants were characterized by a less pronounced change in nasal tip temperature across all three of the experimental conditions. Recent studies investigating the ANS response specificity in emotion found a dual sympathetic-parasympathetic coactivation in response to “sadness” [73]. Several studies using video clip stimuli to induce feelings of sadness have found that crying is associated with sympathetic activation, while parasympathetic activation is typical of sadness without crying. Specifically, an activating sadness response (crying) appears to be typified by increased cardiovascular sympathetic response and changed respiratory activity, while a deactivating sadness response (noncrying) is distinguished by a decrease in sympathetic activation. Furthermore, noncrying sadness is characterized by decreased HR associated with decreased electrodermal activity [73]. These results are in line with our thermal findings. Although nasal tip temperature increased in both groups during the sadness condition, Moebius patients exhibited a generally weaker thermal response. The reason why it was not possible to highlight a significant differential response to emotional conditions between MBS and control participants is probably due to the interindividual variability of participants' thermal response to each emotion. For this reason, we considered the absolute values of participants' thermal responses (independently of the direction of thermal variation with respect to a neutral baseline) in order to identify differences between groups. Our data revealed a weaker, nonspecific thermal response of Moebius children while watching emotional stimuli, with respect to control participants.
The diminished temperature changes observed in Moebius patients could be ascribed to a minor modulation of the autonomic system in response to emotional stimuli. This differential intensity of thermal change could be interpreted in terms of the tight link between an action-perception mechanism, which contributes to sensorimotor simulation and to the process of recognition of others' emotions, and coordinated changes in the autonomic system which control visceral responses associated with emotions [23, 31, 87].
Neuroimaging studies have shown that the observation and production of emotional facial expressions activate similar networks of brain areas [26, 38]. More specifically, in addition to the temporo-parietal-frontal areas, which are the core of the action-observation network, other regions such as the amygdala, the ACC, and the anterior insula show an overlapping activation during both imitation and observation of emotional facial expressions [26]. These regions are involved not only in processing the emotional content of a stimulus but also in coordinating the physiological responses associated with the emotion [21, 22, 25, 38]. Electrical stimulation of the anterior insula in the monkey has revealed that this region is composed of several sectors which generate different autonomic responses and facial motor patterns when stimulated [21]. This strengthens the proposal of a strict link between the production of emotional facial expressions and the physiological modifications associated with experience of them. Our results suggest that the autonomic response related to the observation of emotional stimuli is reduced in children with congenital facial palsy. Previous brain imaging studies have provided support for the crucial role of corticolimbic circuits in the regulation of emotions [88]; however, so far, none has investigated the effects of the lack of peripheral feedback on autonomic responses to emotional stimuli.
Although this was not the main purpose of our study, we also wanted to assess children's explicit comprehension of the emotions expressed by video cartoons. The difficulty in acquiring these behavioral measures (e.g., participants' identification of the emotion depicted by the characters of the videos and participants' feelings during the presentation of the cartoons) led us to administer a less complex task in order to assess children's ability to explicitly recognize basic emotions. We therefore examined the emotion recognition ability in Moebius children by means of a standardized test, TEC-1. Compared to control participants, Moebius participants showed impairments on the emotion recognition task, with lower scores than healthy children of comparable age. These findings suggest that the impairment of facial muscles involved in the emotional display could affect not only the autonomic response but also facial expression recognition [47, 89, 90]. These results, though preliminary, are also compatible with the reverse simulation model, which proposes that the preservation of cortical control of the facial muscles is necessary to fully comprehend the emotional state of the other [35].
A few reports have tested the capacity of Moebius patients to recognize emotions, and the results are inconsistent [51, 53–55]. Most of these studies tested a small group of adult patients, with significant interindividual variability. One study utilized a considerable number of adult patients [54], and the authors did not find any evidence of facial emotion recognition deficits. However, it must be noted that this study suffers from some critical methodological limitations, such as the indirect assessment of participants' performance and of their neurological deficits.
Our study is the first to use a relatively large sample of very young patients to investigate the effects of facial muscle paralysis on both autonomic responses and emotion recognition. The investigation of these issues early in development is critical for the detection of emotional processing mechanisms at a stage where more complex cognitive strategies might not yet compensate for their deficits. In this regard, a large amount of literature has focused on how and when children's decoding of facial emotions develops [91, 92]. In the early stages of postnatal development, infants discriminate between different facial expressions and respond appropriately to different emotions displayed by their caregiver [93]. Furthermore, even if the debate revolving around the existence, prevalence, and meaning of neonatal imitation is still underway (see [94, 95], but also see a reexamination of this study [96] by Meltzoff and colleagues, which led to opposite results), much of the literature suggests that newborns are capable of mimicking certain facial expressions, such as smiles, indicating an early capacity to match own and others' facial expressions [96–99].
Considering that MBS facial paralysis is present since birth, we can hypothesize that MBS patients will exhibit mild deficits in the development of a fully functional MNS during the early stages of life. According to a theoretical developmental account [100, 101], after birth, facial expression synchronization with caregivers is critical to creating a link between the “self” and the “other” and to ensuring the shaping of the mirror mechanism supporting social communicative functions. Indeed, neonates are able to engage in reciprocal and emotional face-to-face interactions with their mothers. These exchanges, including facial and vocal expressions and gestures, are present immediately after birth and in the first month of life [102] and can be important for the development and function of the MNS [97, 103–105]. Recent studies have shown that based on such mother-infant face-to-face exchanges, the capacity of neonates to develop social expressiveness is related to their ability to produce appropriate emotional facial expressions and is correlated with the mother's skill in mirroring or marking such expressions [102, 105]. We do not know how this type of early experience could impact brain and emotional development, and this requires further investigations related to brain activity in cortical motor regions during mother-infant interactions in early development. However, children with MBS, due to their inability to express emotions through the face, might experience reduced quality of social interactions. It has been suggested that Moebius children might receive diminished facial responses from other individuals who, not perceiving a clear facial response during interactions, are less encouraged to socially engage and interact with them facially [106]. These hypothetical reduced inputs from both caregivers and other children, especially during early developmental periods, could have occurred from birth through childhood, resulting in an overall lower exposure to facial stimuli and consequent biased responses compared to healthy control participants.
Despite our findings that Moebius children have some deficits in recognizing emotions, they are still capable of understanding the emotional content of complex stimuli. The ANS response results, showing a similar, though less intense, thermal response in Moebius children compared with control participants, suggest that several cognitive processes may be used by Moebius subjects in order to understand the emotional content of complex stimuli. It is possible that although subtle aspects of emotion recognition are impaired as a consequence of altered facial mimicry, brain plasticity during development and the exploitation of other cognitive strategies could be employed by Moebius patients to compensate for the early deficits.
At this point of the discussion, it should be mentioned that the role of the MNS in action understanding has been debated and discussions are still ongoing (see [107, 108], but also see [32, 109]). According to Hickok [110], action understanding and motor system function could be dissociated. In contrast to this view, a meta-analysis found impairments in recognizing actions associated with lesions in MNS regions [111]. These results are further supported by a study by Michael and colleagues [112] where participants received theta-burst stimulation to temporarily create a lesion on the premotor cortex, causing clear impairments in understanding actions performed by others. Our study does not allow us to support the hypothesis that facial mimicry is the only process involved in emotion understanding; in fact, other mechanisms could be exploited when automatic peripheral facial feedback is absent. However, a diminished autonomic response in Moebius patients makes us propose that, in line with embodied theories [48], facial mimicry could represent a key mechanism for emotional processing.
A few methodological limitations of our study should be mentioned. Cartoon stimuli differed in length because of our specific aim to present participants with an authentic content able to induce a particular emotion. Since emotional content is the actual variable expected to influence thermal values, the differential duration of the stimuli alone would not have affected the thermal results, given the slow dynamic of thermal response. This is further confirmed by our main result showing a difference between the experimental and control groups that was independent of stimuli duration.
Additionally, Moebius patients' impaired ocular abduction could be considered one limit of the current study; however, as discussed by Carta and colleagues [113], these patients compensate for their lack of lateral version with large movements of the head.
It has also to be pointed out that the extreme rarity of the syndrome, the limited age range taken into account, and the exclusion of patients with autism or mental retardation let us to include only a limited number of participants (9 participants), which did not permit further analysis. Despite the challenges involved in acquiring a sample large enough to study this syndrome, it would be worthwhile for future studies to explore the emotion recognition ability at different ages and/or gender differences.
Lastly, although fIRT is at the forefront of the techniques allowing ANS recording in a naturalistic setting, the thermal signal as a result of perspiration and muscle activity and the time course of metabolic responses are rather sluggish. Nevertheless, the reliability and feasibility of fIRT have been confirmed by several comparisons with other standard methods of ANS measurement such as electrocardiography (ECG) and skin conductance or galvanic skin response (GSR) [70]. As this technology is still in development, there is a need to determine if heat patterns indicate discrete emotions [114] or dimensional responses [115]. It would therefore be useful to integrate this method with other techniques to compare ANS measurements within the same experimental paradigm.
5. Conclusions
MBS patients' decreased capacity to activate a motor simulation process during the decoding of emotions could have led to a diminished thermal variation and ANS response during the observation of complex emotional stimuli. It is possible that patients' impairments in mimicking could have affected not only their cognitive emotion recognition processes but also the way in which they are related to ANS changes associated with emotions. If the absence or reduction of motor representations resulted in deficiencies in their early facial expression recognition mechanism (as a consequence of having limited control of facial muscles), MBS individuals might learn during development to cognitively deduce the emotional states of others by using a number of visual cues related to the face and the environmental context [116, 117]. By exploiting such cues, MBS patients can extract regularities and develop conceptual knowledge of an emotion [89]. Further studies are crucial in order to address the relationship between the level of emotion recognition deficits and the magnitude of the autonomic response, in order to better understand their possible causal relationship.
Acknowledgments
We are very grateful to all the children and their families for their patience and their incredible efforts to help our research through numerous visits and long trips from all over Italy to reach the lab. We would also like to thank the Associazione Italiana Sindrome di Moebius for their continuous work and support to our research. We are also grateful to Guido Dalla Rosa Prati for supporting our research and to Livia Garavelli for her contribution to our data collection. We also wish to thank Leonardo De Pascalis for the statistical consultation and to Holly Rayson and James Bonaiuto for their helpful comments and careful English proofreading on an earlier draft of this manuscript. Finally, special thanks are due to the specialist surgeon Dr. Andrea Ferri for his excellent expertise and cooperation. This research was supported by the Centro Diagnostico Europeo Dalla Rosa Prati (Parma).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Rasmussen L. K., Rian O., Korshoej A. R., Christensen S. Fatal complications during anaesthesia in Moebius syndrome: a case report and brief discussion of relevant precautions and preoperative assessments. International Journal of Anesthesiology & Research. 2015;3(6):116–118. doi: 10.19070/2332-2780-1500030. [DOI] [Google Scholar]
- 2.Lindsay R. W., Hadlock T. A., Cheney M. L. Upper lip elongation in Möbius syndrome. Otolaryngology-Head and Neck Surgery. 2010;142(2):286–287. doi: 10.1016/j.otohns.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 3.Abramson D. L., Cohen M. M., Jr, Mulliken J. B. Möbius syndrome: classification and grading system. Plastic and Reconstructive Surgery. 1998;102(4):961–967. doi: 10.1097/00006534-199809020-00004. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi B., Copelli C., Ferrari S., Ferri A., Sesenna E. Facial animation in children with Moebius and Moebius-like syndromes. Journal of Pediatric Surgery. 2009;44(11):2236–2242. doi: 10.1016/j.jpedsurg.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 5.Cattaneo L., Chierici E., Bianchi B., Sesenna E., Pavesi G. The localization of facial motor impairment in sporadic Möbius syndrome. Neurology. 2006;66(12):1907–1912. doi: 10.1212/01.wnl.0000219766.96499.6c. [DOI] [PubMed] [Google Scholar]
- 6.Terzis J. K., Noah E. M. Dynamic restoration in Möbius and Möbius-like patients. Plastic and Reconstructive Surgery. 2003;111(1):40–55. doi: 10.1097/00006534-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Terzis J. K., Anesti K. Developmental facial paralysis: a review. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2011;64(10):1318–1333. doi: 10.1016/j.bjps.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni A., Madhavi M. R., Nagasudha M., Bhavi S. A rare case of Moebius sequence. Indian Journal of Ophthalmology. 2012;60(6):558–560. doi: 10.4103/0301-4738.103798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shashikiran N. D., Subba Reddy V. V., Patil R. ‘Moebius syndrome’: a case report. Journal of the Indian Society of Pedodontics and Preventive Dentistry. 2004;22(3):96–99. [PubMed] [Google Scholar]
- 10.Terzis J. K., Noah E. M. Möbius and Möbius-like patients: etiology, diagnosis, and treatment options. Clinics in Plastic Surgery. 2002;29(4):497–514. doi: 10.1016/S0094-1298(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 11.Verzijl H. T. F. M., van der Zwaag B., Cruysberg J. R. M., Padberg G. W. Möbius syndrome redefined: a syndrome of rhombencephalic maldevelopment. Neurology. 2003;61(3):327–333. doi: 10.1212/01.WNL.0000076484.91275.CD. [DOI] [PubMed] [Google Scholar]
- 12.Bogart K. R. ‘People are all about appearances’: a focus group of teenagers with Moebius syndrome. Journal of Health Psychology. 2013;20(12):1579–1588. doi: 10.1177/1359105313517277. [DOI] [PubMed] [Google Scholar]
- 13.Briegel W., Schimek M., Kamp-Becker I., Hofmann C., Schwab K. O. Autism spectrum disorders in children and adolescents with Moebius sequence. European Child & Adolescent Psychiatry. 2009;18(8):515–519. doi: 10.1007/s00787-009-0003-1. [DOI] [PubMed] [Google Scholar]
- 14.Briegel W., Schimek M., Kamp-Becker I. Moebius sequence and autism spectrum disorders--less frequently associated than formerly thought. Research in Developmental Disabilities. 2010;31(6):1462–1466. doi: 10.1016/j.ridd.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Ekman P., Sorenson E. R., Friesen W. V. Pan-cultural elements in facial displays of emotion. Science. 1969;164(3875):86–88. doi: 10.1126/science.164.3875.86. [DOI] [PubMed] [Google Scholar]
- 16.Ekman P., Friesen W. V. Constants across cultures in the face and emotion. Journal of Personality and Social Psychology. 1971;17(2):124–129. doi: 10.1037/h0030377. [DOI] [PubMed] [Google Scholar]
- 17.Ekman P., Friesen W. V. A new pan-cultural facial expression of emotion. Motivation and Emotion. 1986;10(2):159–168. doi: 10.1007/BF00992253. [DOI] [Google Scholar]
- 18.Matsumoto D., Willingham B. Spontaneous facial expressions of emotion of congenitally and noncongenitally blind individuals. Journal of Personality and Social Psychology. 2009;96(1):1–10. doi: 10.1037/a0014037. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari P. F., Barbot A., Bianchi B., et al. A proposal for new neurorehabilitative intervention on Moebius Syndrome patients after ‘smile surgery’. Proof of concept based on mirror neuron system properties and hand-mouth synergistic activity. Neuroscience and Biobehavioral Reviews. 2017;76:111–122. doi: 10.1016/j.neubiorev.2017.01.050. [DOI] [PubMed] [Google Scholar]
- 20.Critchley H. D., Rotshtein P., Nagai Y., O'Doherty J., Mathias C. J., Dolan R. J. Activity in the human brain predicting differential heart rate responses to emotional facial expressions. NeuroImage. 2005;24(3):751–762. doi: 10.1016/j.neuroimage.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Caruana F., Jezzini A., Sbriscia-Fioretti B., Rizzolatti G., Gallese V. Emotional and social behaviors elicited by electrical stimulation of the insula in the macaque monkey. Current Biology. 2011;21(3):195–199. doi: 10.1016/j.cub.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 22.van der Gaag C., Minderaa R. B., Keysers C. Facial expressions: what the mirror neuron system can and cannot tell us. Social Neuroscience. 2007;2(3-4):179–222. doi: 10.1080/17470910701376878. [DOI] [PubMed] [Google Scholar]
- 23.Panksepp J. On the embodied neural nature of core emotional affects. Journal of Consciousness Studies. 2005;12(8–10):158–184. [Google Scholar]
- 24.Jezzini A., Caruana F., Stoianov I., Gallese V., Rizzolatti G. Functional organization of the insula and inner perisylvian regions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(25):10077–10082. doi: 10.1073/pnas.1200143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caruana F., Avanzini P., Gozzo F., Pelliccia V., Casaceli G., Rizzolatti G. A mirror mechanism for smiling in the anterior cingulate cortex. Emotion. 2017;17(2):187–190. doi: 10.1037/emo0000237. [DOI] [PubMed] [Google Scholar]
- 26.Carr L., Iacoboni M., Dubeau M.-C., Mazziotta J. C., Lenzi G. L. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrari P. F., Gerbella M., Coudé G., Rozzi S. Two different mirror neuron networks: the sensorimotor (hand) and limbic (face) pathways. Neuroscience. 2017;358:300–315. doi: 10.1016/j.neuroscience.2017.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leslie K. R., Johnson-Frey S. H., Grafton S. T. Functional imaging of face and hand imitation: towards a motor theory of empathy. NeuroImage. 2004;21(2):601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 29.Keysers C., Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Current Opinion in Neurobiology. 2009;19(6):666–671. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Gallese V. Intentional attunement: a neurophysiological perspective on social cognition and its disruption in autism. Brain Research. 2006;1079(1):15–24. doi: 10.1016/j.brainres.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 31.Iacoboni M. Imitation, empathy, and mirror neurons. Annual Review of Psychology. 2009;60(1):653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- 32.Rizzolatti G., Sinigaglia C. The mirror mechanism: a basic principle of brain function. Nature Reviews Neuroscience. 2016;17(12):757–765. doi: 10.1038/nrn.2016.135. [DOI] [PubMed] [Google Scholar]
- 33.De Stefani E., Innocenti A., De Marco D., Gentilucci M. Concatenation of observed grasp phases with observer’s distal movements: a behavioural and TMS study. PLoS One. 2013;8(11, article e81197) doi: 10.1371/journal.pone.0081197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iacoboni M. Understanding others: imitation, language, and empathy. In: Hurley S., Chater N., editors. Perspectives on Imitation: from Neuroscience to Social Science. Vol. 1. Cambridge, MA, USA: MIT Press; 2005. pp. 77–99. [Google Scholar]
- 35.Rizzolatti G., Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27(1):169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 36.Gallese V., Fadiga L., Fogassi L., Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 37.Rizzolatti G., Fadiga L., Gallese V., Fogassi L. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3(2):131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 38.Wicker B., Keysers C., Plailly J., Royet J. P., Gallese V., Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664. doi: 10.1016/S0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- 39.Ferrari P. F., Gallese V., Rizzolatti G., Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. The European Journal of Neuroscience. 2003;17(8):1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- 40.Di Cesare G., De Stefani E., Gentilucci M., De Marco D. Vitality forms expressed by others modulate our own motor response: a kinematic study. Frontiers in Human Neuroscience. 2017;11 doi: 10.3389/fnhum.2017.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldman A. I., Sripada C. S. Simulationist models of face-based emotion recognition. Cognition. 2005;94(3):193–213. doi: 10.1016/j.cognition.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Oberman L. M., Winkielman P., Ramachandran V. S. Face to face: blocking facial mimicry can selectively impair recognition of emotional expressions. Social Neuroscience. 2007;2(3-4):167–178. doi: 10.1080/17470910701391943. [DOI] [PubMed] [Google Scholar]
- 43.Stel M., van Knippenberg A. The role of facial mimicry in the recognition of affect. Psychological Science. 2008;19(10):984–985. doi: 10.1111/j.1467-9280.2008.02188.x. [DOI] [PubMed] [Google Scholar]
- 44.Trilla Gros I., Panasiti M. S., Chakrabarti B. The plasticity of the mirror system: how reward learning modulates cortical motor simulation of others. Neuropsychologia. 2015;70:255–262. doi: 10.1016/j.neuropsychologia.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallese V. The roots of empathy: the shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology. 2003;36(4):171–180. doi: 10.1159/000072786. [DOI] [PubMed] [Google Scholar]
- 46.Gallese V. The intentional attunement hypothesis the mirror neuron system and its role in interpersonal relations. In: Wermter S., Palm G., Elshaw M., editors. Biomimetic Neural Learning for Intelligent Robots: Intelligent Systems, Cognitive Robotics, and Neuroscience. Springer; 2005. pp. 19–30. [DOI] [Google Scholar]
- 47.Keysers C., Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends in Cognitive Sciences. 2007;11(5):194–196. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Niedenthal P. M. Embodying emotion. Science. 2007;316(5827):1002–1005. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- 49.Winkielman P., McIntosh D. N., Oberman L. Embodied and disembodied emotion processing: learning from and about typical and autistic individuals. Emotion Review. 2009;1(2):178–190. doi: 10.1177/1754073908100442. [DOI] [Google Scholar]
- 50.Niedenthal P. M., Mermillod M., Maringer M., Hess U. The Simulation of Smiles (SIMS) model: embodied simulation and the meaning of facial expression. The Behavioral and Brain Sciences. 2010;33(6):417–433. doi: 10.1017/s0140525x10000865. [DOI] [PubMed] [Google Scholar]
- 51.De Stefani E., De Marco D., Gentilucci M. The effects of meaning and emotional content of a sentence on the kinematics of a successive motor sequence mimiking the feeding of a conspecific. Frontiers in Psychology. 2016;7 doi: 10.3389/fpsyg.2016.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giannini A. J., Tamulonis D., Giannini M. C., Loiselle R. H., Spirtos G. Defective response to social cues in Möbius’ syndrome. The Journal of Nervous and Mental Disease. 1984;172(3):174–175. doi: 10.1097/00005053-198403000-00008. [DOI] [PubMed] [Google Scholar]
- 53.Calder A. J., Keane J., Cole J., Campbell R., Young A. W. Facial expression recognition by people with Möbius syndrome. Cognitive Neuropsychology. 2000;17(1-3):73–87. doi: 10.1080/026432900380490. [DOI] [PubMed] [Google Scholar]
- 54.Rives Bogart K., Matsumoto D. Facial mimicry is not necessary to recognize emotion: facial expression recognition by people with Moebius syndrome. Social Neuroscience. 2010;5(2):241–251. doi: 10.1080/17470910903395692. [DOI] [PubMed] [Google Scholar]
- 55.Bate S., Cook S. J., Mole J., Cole J. First report of generalized face processing difficulties in Möbius sequence. PLoS One. 2013;8(4, article e62656) doi: 10.1371/journal.pone.0062656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krueger J., Michael J. Gestural coupling and social cognition: Möbius syndrome as a case study. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bogart K. R., Tickle-Degnen L., Ambady N. Compensatory expressive behavior for facial paralysis: adaptation to congenital or acquired disability. Rehabilitation Psychology. 2012;57(1):43–51. doi: 10.1037/a0026904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ring E. F. J., Ammer K. Infrared thermal imaging in medicine. Physiological Measurement. 2012;33(3):R33–R46. doi: 10.1088/0967-3334/33/3/R33. [DOI] [PubMed] [Google Scholar]
- 59.Anbar M. Assessment of physiologic and pathologic radiative heat dissipation using dynamic infrared imaging. Annals of the New York Academy of Sciences. 2002;972(1):111–118. doi: 10.1111/j.1749-6632.2002.tb04560.x. [DOI] [PubMed] [Google Scholar]
- 60.Merla A., Romani G. L. Thermal signatures of emotional arousal: a functional infrared imaging study. 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2007; Lyon, France. pp. 247–249. [DOI] [PubMed] [Google Scholar]
- 61.Ioannou S., Ebisch S., Aureli T., et al. The autonomic signature of guilt in children: a thermal infrared imaging study. PLoS One. 2013;8(11, article e79440) doi: 10.1371/journal.pone.0079440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manini B., Cardone D., Ebisch S. J. H., Bafunno D., Aureli T., Merla A. Mom feels what her child feels: thermal signatures of vicarious autonomic response while watching children in a stressful situation. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merla A. Thermal expression of intersubjectivity offers new possibilities to human-machine and technologically mediated interactions. Frontiers in Psychology. 2014;5 doi: 10.3389/fpsyg.2014.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pavlidis I., Dowdall J., Sun N., Puri C., Fei J., Garbey M. Interacting with human physiology. Computer Vision and Image Understanding. 2007;108(1-2):150–170. doi: 10.1016/j.cviu.2006.11.018. [DOI] [Google Scholar]
- 65.Shastri D., Merla A., Tsiamyrtzis P., Pavlidis I. Imaging facial signs of neurophysiological responses. IEEE Transactions on Bio-Medical Engineering. 2009;56(2):477–484. doi: 10.1109/TBME.2008.2003265. [DOI] [PubMed] [Google Scholar]
- 66.Levine J. A., Pavlidis I., Cooper M. The face of fear. The Lancet. 2001;357(9270):p. 1757. doi: 10.1016/S0140-6736(00)04936-9. [DOI] [Google Scholar]
- 67.Nozawa A., Tacano M. Correlation analysis on alpha attenuation and nasal skin temperature. Journal of Statistical Mechanics: Theory and Experiment. 2009;2009(1, article P01007) doi: 10.1088/1742-5468/2009/01/p01007. [DOI] [Google Scholar]
- 68.Nakanishi R., Imai-Matsumura K. Facial skin temperature decreases in infants with joyful expression. Infant Behavior & Development. 2008;31(1):137–144. doi: 10.1016/j.infbeh.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Nakayama K., Goto S., Kuraoka K., Nakamura K. Decrease in nasal temperature of rhesus monkeys (Macaca mulatta) in negative emotional state. Physiology & Behavior. 2005;84(5):783–790. doi: 10.1016/j.physbeh.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Kuraoka K., Nakamura K. The use of nasal skin temperature measurements in studying emotion in macaque monkeys. Physiology & Behavior. 2011;102(3-4):347–355. doi: 10.1016/j.physbeh.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 71.Ebisch S. J., Aureli T., Bafunno D., Cardone D., Romani G. L., Merla A. Mother and child in synchrony: thermal facial imprints of autonomic contagion. Biological Psychology. 2012;89(1):123–129. doi: 10.1016/j.biopsycho.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 72.Pavlidis I., Tsiamyrtzis P., Shastri D., et al. Fast by nature - how stress patterns define human experience and performance in dexterous tasks. Scientific Reports. 2012;2(1) doi: 10.1038/srep00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kreibig S. D. Autonomic nervous system activity in emotion: a review. Biological Psychology. 2010;84(3):394–421. doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 74.Ioannou S., Morris P., Mercer H., Baker M., Gallese V., Reddy V. Proximity and gaze influences facial temperature: a thermal infrared imaging study. Frontiers in Psychology. 2014;5 doi: 10.3389/fpsyg.2014.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hahn A. C., Whitehead R. D., Albrecht M., Lefevre C. E., Perrett D. I. Hot or not? Thermal reactions to social contact. Biology Letters. 2012;8(5):864–867. doi: 10.1098/rsbl.2012.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ioannou S., Gallese V., Merla A. Thermal infrared imaging in psychophysiology: potentialities and limits. Psychophysiology. 2014;51(10):951–963. doi: 10.1111/psyp.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aureli T., Grazia A., Cardone D., Merla A. Behavioral and facial thermal variations in 3- to 4-month-old infants during the Still-Face Paradigm. Frontiers in Psychology. 2015;6 doi: 10.3389/fpsyg.2015.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pavlidis I., Levine J., Baukol P. Thermal image analysis for anxiety detection. Proceedings 2001 International Conference on Image Processing (Cat. No.01CH37205); 2001; Thessaloniki, Greece. pp. 315–318. [Google Scholar]
- 79.Ioannou S., Morris P., Terry S., Baker M., Gallese V., Reddy V. Sympathy crying: insights from infrared thermal imaging on a female sample. PLoS One. 2016;11(10, article e0162749) doi: 10.1371/journal.pone.0162749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Albanese O., Molina P. The Development of Emotion Comprehension and Its Assessment. The Italian Standardization of the Test of Emotion Comprehension (TEC) Milano, Italy: UNICOPLI; 2008. [Google Scholar]
- 81.Pons E., Harris P. Test of Emotion Comprehension: TEC. Oxford, England: Oxford University Press; 2000. [Google Scholar]
- 82.Nhan B. R., Chau T. Classifying affective states using thermal infrared imaging of the human face. IEEE Transactions on Bio-Medical Engineering. 2010;57(4):979–987. doi: 10.1109/TBME.2009.2035926. [DOI] [PubMed] [Google Scholar]
- 83.Roddie I. C. The role of vasoconstrictor and vasodilator nerves to skin and muscle in the regulation of the human circulation. Annals of The Royal College of Surgeons of England. 1963;32(3):180–193. [PMC free article] [PubMed] [Google Scholar]
- 84.Paolini D., Alparone F. R., Cardone D., van Beest I., Merla A. ‘The face of ostracism’: the impact of the social categorization on the thermal facial responses of the target and the observer. Acta Psychologica. 2016;163:65–73. doi: 10.1016/j.actpsy.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 85.Ardizzi M., Martini F., Umiltà M. A., Evangelista V., Ravera R., Gallese V. Impact of childhood maltreatment on the recognition of facial expressions of emotions. PLoS One. 2015;10(10, article e0141732) doi: 10.1371/journal.pone.0141732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ardizzi M., Umiltà M. A., Evangelista V., Di Liscia A., Ravera R., Gallese V. Less empathic and more reactive: the different impact of childhood maltreatment on facial mimicry and vagal regulation. PLoS One. 2016;11(9, article e0163853) doi: 10.1371/journal.pone.0163853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carr E. W., Winkielman P. When mirroring is both simple and “smart”: how mimicry can be embodied, adaptive, and non-representational. Frontiers in Human Neuroscience. 2014;8:p. 505. doi: 10.3389/fnhum.2014.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.LeDoux J. E. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23(1):155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 89.Wood A., Rychlowska M., Korb S., Niedenthal P. Fashioning the face: sensorimotor simulation contributes to facial expression recognition. Trends in Cognitive Sciences. 2016;20(3):227–240. doi: 10.1016/j.tics.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 90.Künecke J., Hildebrandt A., Recio G., Sommer W., Wilhelm O. Facial EMG responses to emotional expressions are related to emotion perception ability. PLoS One. 2014;9(1):p. e84053. doi: 10.1371/journal.pone.0084053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kolb B., Wilson B., Taylor L. Developmental changes in the recognition and comprehension of facial expression: implications for frontal lobe function. Brain and Cognition. 1992;20(1):74–84. doi: 10.1016/0278-2626(92)90062-Q. [DOI] [PubMed] [Google Scholar]
- 92.Camras L. A., Allison K. Children’s understanding of emotional facial expressions and verbal labels. Journal of Nonverbal Behavior. 1985;9(2):84–94. doi: 10.1007/BF00987140. [DOI] [Google Scholar]
- 93.Field T. M., Woodson R., Greenberg R., Cohen D. Discrimination and imitation of facial expression by neonates. Science. 1982;218(4568):179–181. doi: 10.1126/science.7123230. [DOI] [PubMed] [Google Scholar]
- 94.Oostenbroek J., Slaughter V., Nielsen M., Suddendorf T. Why the confusion around neonatal imitation? A review. Journal of Reproductive and Infant Psychology. 2013;31(4):328–341. doi: 10.1080/02646838.2013.832180. [DOI] [Google Scholar]
- 95.Oostenbroek J., Suddendorf T., Nielsen M., et al. Comprehensive longitudinal study challenges the existence of neonatal imitation in humans. Current Biology. 2016;26(10):1334–1338. doi: 10.1016/j.cub.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 96.Meltzoff A. N., Murray L., Simpson E., et al. Re-examination of Oostenbroek et al. (2016): evidence for neonatal imitation of tongue protrusion. Developmental Science. 2018;21(4, article e12609) doi: 10.1111/desc.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meltzoff A. N., Moore M. K. Imitation of facial and manual gestures by human neonates. Science. 1977;198(4312):75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- 98.Nagy E., Pilling K., Orvos H., Molnar P. Imitation of tongue protrusion in human neonates: specificity of the response in a large sample. Developmental Psychology. 2013;49(9):1628–1638. doi: 10.1037/a0031127. [DOI] [PubMed] [Google Scholar]
- 99.Simpson E. A., Murray L., Paukner A., Ferrari P. F. The mirror neuron system as revealed through neonatal imitation: presence from birth, predictive power and evidence of plasticity. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1644):p. 20130289. doi: 10.1098/rstb.2013.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ferrari P. F., Tramacere A., Simpson E. A., Iriki A. Mirror neurons through the lens of epigenetics. Trends in Cognitive Sciences. 2013;17(9):450–457. doi: 10.1016/j.tics.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tramacere A., Ferrari P. F. Faces in the mirror, from the neuroscience of mimicry to the emergence of mentalizing. Journal of Anthropological Sciences. 2016;94:113–126. doi: 10.4436/JASS.94037. [DOI] [PubMed] [Google Scholar]
- 102.Murray L., de Pascalis L., Bozicevic L., Hawkins L., Sclafani V., Ferrari P. F. The functional architecture of mother-infant communication, and the development of infant social expressiveness in the first two months. Scientific Reports. 2016;6(1):p. 39019. doi: 10.1038/srep39019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Meltzoff A. N., Moore M. K. Newborn infants imitate adult facial gestures. Child Development. 1983;54(3):702–709. doi: 10.2307/1130058. [DOI] [PubMed] [Google Scholar]
- 104.Marshall P. J., Meltzoff A. N. Neural mirroring systems: exploring the EEG μ rhythm in human infancy. Developmental Cognitive Neuroscience. 2011;1(2):110–123. doi: 10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rayson H., Bonaiuto J. J., Ferrari P. F., Murray L. Early maternal mirroring predicts infant motor system activation during facial expression observation. Scientific Reports. 2017;7(1, article 11738) doi: 10.1038/s41598-017-12097-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strobel L., Renner G. Quality of life and adjustment in children and adolescents with Moebius syndrome: evidence for specific impairments in social functioning. Research in Developmental Disabilities. 2016;53-54:178–188. doi: 10.1016/j.ridd.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 107.Hickok G. Do mirror neurons subserve action understanding? Neuroscience Letters. 2013;540:56–58. doi: 10.1016/j.neulet.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Csibra G. Mirror neurons and action observation. Is simulation involved? What Do Mirror Neurons Mean? Interdisciplines Web Forum. http://www.interdisciplines.org/mirror/papers/4.
- 109.Tramacere A., Pievani T., Ferrari P. F. Mirror neurons in the tree of life: mosaic evolution, plasticity and exaptation of sensorimotor matching responses. Biological Reviews of the Cambridge Philosophical Society. 2017;92(3):1819–1841. doi: 10.1111/brv.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hickok G. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. Journal of Cognitive Neuroscience. 2009;21(7):1229–1243. doi: 10.1162/jocn.2009.21189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Urgesi C., Candidi M., Avenanti A. Neuroanatomical substrates of action perception and understanding: an anatomic likelihood estimation meta-analysis of lesion-symptom mapping studies in brain injured patients. Frontiers in Human Neuroscience. 2014;8 doi: 10.3389/fnhum.2014.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Michael J., Sandberg K., Skewes J., et al. Continuous theta-burst stimulation demonstrates a causal role of premotor homunculus in action understanding. Psychological Science. 2014;25(4):963–972. doi: 10.1177/0956797613520608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carta A., Mora P., Neri A., Favilla S., Sadun A. A. Ophthalmologic and systemic features in Möbius syndrome an Italian case series. Ophthalmology. 2011;118(8):1518–1523. doi: 10.1016/j.ophtha.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 114.Ekman P. An argument for basic emotions. Cognition and Emotion. 1992;6(3-4):169–200. doi: 10.1080/02699939208411068. [DOI] [Google Scholar]
- 115.Russell J. A. Core affect and the psychological construction of emotion. Psychological Review. 2003;110(1):145–172. doi: 10.1037/0033-295X.110.1.145. [DOI] [PubMed] [Google Scholar]
- 116.Gross A. L., Ballif B. Children’s understanding of emotion from facial expressions and situations: a review. Developmental Review. 1991;11(4):368–398. doi: 10.1016/0273-2297(91)90019-K. [DOI] [Google Scholar]
- 117.Parker A. E., Mathis E. T., Kupersmidt J. B. How is this child feeling? Preschool-aged children’s ability to recognize emotion in faces and body poses. Early Education and Development. 2013;24(2):188–211. doi: 10.1080/10409289.2012.657536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.