Abstract
Background
Improving continuity is challenging in residency training practices. Studies have shown that empanelment enables high-performing primary care and is foundational to improve accountability and continuity.
Objective
An empanelment process was created in a large, urban, residency training practice as an effective approach to enhancing continuity among residents and their patients.
Methods
In 2016, we formed an empanelment committee that included stakeholders from the department of medicine, the internal medicine residency program, and hospital and IT leadership. This committee set goal panel sizes, selected an empanelment algorithm, determined which patients needed re-empanelment, and facilitated medical record integration. Empanelment was followed and reassessed quarterly for 2 years. We measured anticipated visit demand using visits in the prior year and continuity using the continuity for physician formula.
Results
Of 18 495 active patients in July 2016, 8411 (45%) were assigned a new PCP in the empanelment process. At baseline, panel sizes and expected visit demand were highly variable among residents (from 40 to 107 and 120 to 480, respectively). Empanelment led to more equivalent panel sizes and expected visit demand across same year residents (eg, PGY-3: 80–100 and 320–440, respectively). Continuity for all PCPs in the practice improved from 63% before empanelment to over 80% after empanelment, and improved from 55% to 72% for individual residents.
Conclusions
In a large and complex practice environment, we were able to empanel resident clinic patients to improve continuity and maintain it over 2 years.
What was known and gap
Internal medicine residents spend less time in the outpatient than inpatient setting, making continuity of care challenging.
What is new
An empanelment committee made up of key stakeholders set goals for panel sizes, selected an empanelment algorithm, determined which patients needed re-empanelment, and facilitated medical record integration.
Limitations
Program was implemented at a single practice site with 2 specific electronic health records, which may limit generalizability; level of continuity achieved was only studied for 2 years.
Bottom line
An empanelment committee helped improve continuity of care in a large and complex practice environment.
Introduction
Because internal medicine residents spend far less time in the outpatient setting than the inpatient setting, and faculty preceptors often balance supervisory roles with other academic work, there are myriad challenges to access and continuity in resident training practices. Residency training practices often serve patients from underserved communities with multiple comorbidities and psychosocial needs that increase the complexity of care. One approach to facilitating responsibility for patients and coordinating practice teams is to empanel resident practices. Empanelment is the act of assigning individual patients to individual primary care providers (PCPs) or care teams, which facilitates clear accountability for any given patient's care.1 Without accountability and responsibility for each patient in the practice (both on the trainee and preceptor levels), sustained improvement in continuity and quality metrics may prove impossible.
Continuity has been an ongoing challenge in primary care, especially in practices with many residents. Prior studies have suggested that empanelment may lead to improved continuity and quality,1–4 and that it enables high-performing primary care practices to facilitate accountability for quality, population health management, and access.5,6 In a recent review that examined interventions to improve continuity in resident practices, interventions were related to advanced access scheduling and innovative residency schedule changes.2 The authors proposed that requiring resident patient empanelment would be an effective approach to enhancing continuity.2 To our knowledge, there is little published data about the effects of empanelment on resident continuity.
Methods
Internal Medicine Associates (IMA) is a large, urban, hospital-based, internal medicine residency clinic practice. Each year, 131 residents see patients precepted by a group of 18 core attending physicians. The practice also includes 16 part-time attending physicians and nurse practitioners (NPs) who provide direct patient care. IMA serves approximately 17 000 patients per year, largely from the East Harlem neighborhood of Manhattan, a population with a relatively high burden of medical and psychosocial comorbidities and poor health outcomes.
Before 2016, residents rotated through their outpatient blocks at irregular intervals. Since 2016, they began an 8+2 rotation (8 weeks of inpatient followed by 2 weeks of outpatient) at regular intervals and moved to a 6+2 schedule in 2017. During the ambulatory 2-week block, interns see continuity patients in 5 half-day sessions per week, while residents have 4 half-day and 1 urgent care sessions per week. Because residents are on outpatient rotations for 2-week blocks, they are teamed with other residents who can cover patients while they are on inpatient blocks. For example, if resident A is on ambulatory block 1, resident B is on block 2, resident C on block 3, and resident D on block 4, these residents form a team and cover for one another throughout their inpatient and outpatient cycles. Each half-day clinical session has 4, 6, and 7 follow-up patient slots available for postgraduate year 1 (PGY-1), PGY-2, and PGY-3 residents, respectively, ideally used for that resident's patients or patients on his or her team.
Our empanelment committee convened in 2016 and included leadership from our division of general internal medicine, department of medicine, residency program, faculty and residents from IMA, ambulatory medical director, director of education, associate director of quality, data analysts, and process engineers. This required an ongoing time investment, but no cost other than information technology (IT) effort. This committee reviewed baseline data and made decisions related to the implementation plan (Box).
box Decisions Reviewed by Stakeholders/Empanelment Committee.
Setting goal panel size for residents (PGY-1, PGY-2, PGY-3), NPs, and faculty
Whether and how to incorporate a patient complexity measurement
Time frame for defining patients considered “active” in clinic
Whether to include patients with only “urgent” or “walk-in” visits
Developing and refining an empanelment algorithm to determine attribution
Selecting the process to determine which patients were currently assigned to their “correct” PCP and which needed to be re-empaneled
Integrating the empanelment process into our electronic health record
Abbreviations: PGY, postgraduate year; NP, nurse practitioner; PCP, primary care physician.
We measured access to care using the amount of time to the third next available or return appointment. Continuity was measured using the continuity for physician formula (PHY), which is the number of appointments a PCP has with his or her patients over the total number of patients seen.7 For residents, we measured PHY specifically for each resident as well as for each resident team (the number of appointments a resident team has with their own patients).
Empanelment Algorithm
Defining “Active” Patients and Inclusion of Patients With Only Urgent Visits:
Professional societies who advocate for empanelment have not agreed via consensus on the definition of “active” patients.8,9 Our committee agreed to define patients as active if they had one or more new or follow-up visits in the last 18 months.
Determining if PCP Is Correct and Planning for Empanelment:
Looking back to the prior 18 months of data, we used a slightly modified version of the 4-cut method to assign a PCP to all patients (Table).3 Patients who would have been assigned to residents who graduated were assigned to new residents based on the panel size and visit capacity of each PCP.
Table.
Empanelment 4-Cut Methodology3
| Cut | Description | Assigned PCP |
| 1 | Patients only seen by 1 PCP | Assigned to that PCP |
| 2 | Patients seen by multiple PCPs, but 1 PCP the majority of the time | Assigned to the majority PCP |
| 3 | Patients who have seen 2 or more PCPs equally (no majority PCP) | Assigned to the most recent nonurgent visit PCP |
| 4 | Patients who have seen multiple PCPs | Assigned to the last PCP seen or discussed by practice |
Abbreviation: PCP, primary care physician.
Patient Complexity Measurement:
Published empanelment models have used different ways to incorporate complexity, including age, gender, diagnoses, and cost.10 We were somewhat limited by available data and wanted to maintain access to care, even for patients who need many visits with their PCPs; therefore, we chose to use the number of visits each patient had during the preceding year as a proxy for complexity and for predicted access requirement. We termed this “expected visit requirement” (figures 1 and 2). There is no current accepted measure for patient complexity, and though we recognized that this is a crude approximation for risk, we chose it based on feasibility in a short time frame and to reflect the access any given patient might need on a PCP's panel in the coming year. For new patients, the visit demand for the upcoming year was assumed to be the practice average of 3.2 visits.
Figure 1.
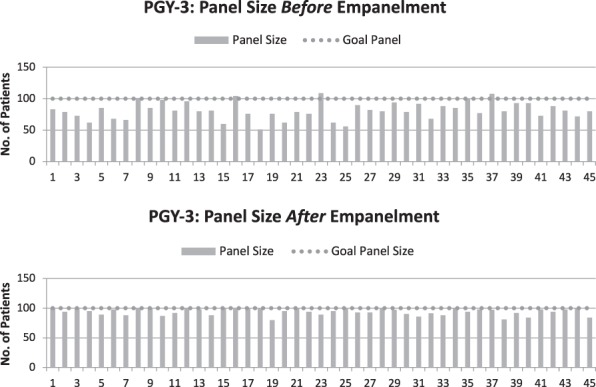
Postgraduate Year 3 (PGY-3) Panel Size Variability Before and After Empanelment
Figure 2.
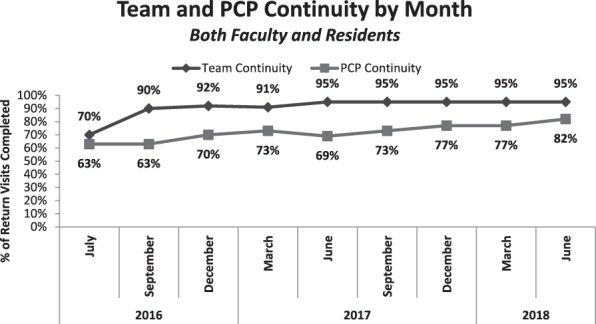
Team and Primary Care Physician (PCP) Continuity
Goal Panel Sizes:
When assigning patients to PCPs, we considered yearly visit capacity—the total visits a PCP can accommodate per year based on their clinical time and number of patients seen. Their panel size goal is the total number of patients a panel can accommodate per year. Aligning PCP panel size, visit demand, and visit capacity is essential, as mismatch can lead to unbalanced workload and limited access to over-paneled PCPs.10 Goal panel sizes were determined using the 4-cut method.3
 |
Integrating with EHR:
We worked closely with an electronic health record (EHR) system (Epic) and a scheduling system (Cerner). We built a process to ensure that PCP data in Epic would automatically flow to Cerner every week to maintain empanelment. Panel size variability and expected visit requirement across all residents were compared pre- and postintervention.
This project was declared institutional board review exempt as per institutional policy.
Results
At the time of initial empanelment in July 2016, there were 18 495 active patients (seen in the last 18 months) in IMA. Resident continuity using the PHY was 55%, and for the practice (residents, attendings, and nurse practitioners) was 63%, at baseline.
A total of 3374 patients (18%) had either no PCP or a PCP no longer working in IMA listed in the EHR, and therefore needed to be re-empaneled. Another 5037 (27%) were also re-empaneled after the 4-cut method revealed a more appropriate PCP.
Goal panel sizes were calculated for residents and faculty. Practice days per year were calculated for residents by multiplying the number of sessions in continuity practice per week (5 for PGY-1s, 4 for PGY-2s/PGY-3s) by the 10 weeks of outpatient they complete each year (50 for PGY-1s and 40 for PGY-2s/PGY-3s). This number was then multiplied by the number of return slots per session, and then divided by the average number of visits per patient. The calculated ideal panel size varied between residency years and between residents and faculty due to variations in template and slot availability. For example:
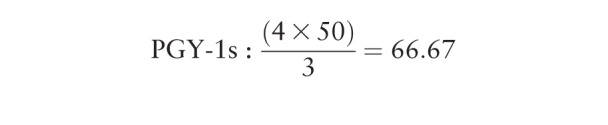 |
Using these calculations, the committee decided to set panel size goals at approximately 65–75 for PGY-1s, 75–85 for PGY-2s, and 90–100 for PGY-3s. Using the same process with faculty templates, the panel size goal for faculty was set at approximately 150 patients per half-day session patient care per week.
Before empanelment, panel sizes and expected visit demand were highly variable even among residents, and were not aligned to the direct patient care availabilities of residents, faculty, or NPs. Upon completion of empanelment, the 27% of patients who had never been seen by their PCP had a different PCP assigned using the 4-cut methodology (Table). The 18% of patients who still didn't have a PCP after the 4-cut method because they had only been seen by PCPs who were no longer working in the practice were distributed among under-paneled residents with low expected visit volume. As a result, 8411 (45%) patients were assigned a new PCP in the empanelment process.
At baseline, panel sizes and expected visit demand were highly variable among residents, (from 40 to 107 and 120 to 480, respectively). Empanelment led to more equivalent panel sizes and expected visit demand across residents in the same year (PGY-3s: 80–100 and 320–440, respectively), with similar findings for PGY-1 and PGY-2 residents (P < .005). In the year preceding empanelment, resident continuity was 55%. After empanelment, we have seen the percentage of resident continuity visits completed consistently improving, reaching 72% by June 2018 for individual resident PCP and 95% for resident team (figure 2). For all PCPs in IMA (faculty, NPs, residents), continuity improved from 65% before empanelment to over 80%.
We estimate approximately 15 hours of committee and individual stakeholder time was needed for this intervention. Physician and administrator time was a 1-hour weekly meeting for 12 weeks, and then leaders worked with IT for another 3 hours to set up processes and automation for ongoing changes and monitoring.
Discussion
Through stakeholder-developed algorithms for resident continuity clinic patient panel size, expected visit demand, and resident team coverage, we markedly increased and maintained patient continuity over 2 years for both residents and PCPs in a large internal medicine clinic. We also reduced variation in resident panel size.
Studies have shown that continuity improves patient outcomes and PCP satisfaction.11,12 Other studies have described attempts to increase resident continuity that focus on schedule and template changes, changes in the EHR, and changes to the residency schedule overall, though many of these increased resident continuity to 50%–65%, slightly less than we have achieved with our combination of empanelment, teaming, and schedule changes.2 As continuity increased, residents' satisfaction in the IMA clinic improved, with the outpatient rotation becoming the most popular element of the training program. This may have been multifactorial as other educational interventions were made during this time frame.
Because we carried out this process at 1 practice site and with 2 specific EHRs, this may reduce the generalizability and replicability of our process. Continuity was followed for just 2 years; therefore, we do not know how much additional time and effort may be required to maintain a defined panel of resident patients. Also, because resident teams were used along with individual residents to calculate continuity, this may not translate into improved patient perceptions of continuity.
IMA continues to regularly monitor and report on visit continuity using a patient-centered formula for residents, resident teams, faculty, and NPs. We plan for interteam patient transitions when residents graduate to ensure all patients are re-empaneled to active or new residents with low panel sizes or low expected visit demand. We plan to measure whether improvements in continuity led to increased quality in our resident clinic.
Conclusion
Through stakeholder-developed algorithms for resident continuity clinic patient panel size, expected visit demand, and resident team coverage, we re-empaneled 45% of our clinic population and increased resident PCP continuity from 55% to 72%, and resident team continuity from 70% to 95%, over 2 years.
References
- 1.Safety Net Medical Home Initiative. Empanelment: Establishing Patient-Provider Relationships. May 2013. http://www.safetynetmedicalhome.org/sites/default/files/Executive-Summary-Empanelment.pdf Accessed February 28, 2019.
- 2.Walker J, Payne B, Clemans-Taylor B, Snyder ED. Continuity of care in resident outpatient clinics: a scoping review of the literature. J Grad Med Educ. 2018;10(1):16–25. doi: 10.4300/JGME-D-17-00256.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christiansen E, Hampton MD, Sullivan M. Patient empanelment: a strategy to improve continuity and quality of patient care. J Am Assoc Nurse Pract. 2016;28(8):423–428. doi: 10.1002/2327-6924.12341. [DOI] [PubMed] [Google Scholar]
- 4.Mainous AG, III, Koopman RJ, Gill JM, Baker R, Pearson WS. Relationship betweeen continuity of care and diabetes control: evidence from the third National Health and Nutrition Examination Survey. Am J Public Health. 2004;94(1):66–70. doi: 10.2105/ajph.94.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573–576. doi: 10.1370/afm.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grumbach K, Olayiwola JN. Patient empanelment: the importance of understanding who is at home in the medical home. J Am Board Fam Med. 2015;28(2):170–172. doi: 10.3122/jabfm.2015.02.150011. [DOI] [PubMed] [Google Scholar]
- 7.Darden PM, Ector W, Moran C, Quattlebaum TG. Comparison of continuity in a resident versus private practice. Pediatrics. 2001;108(6):1263–1268. doi: 10.1542/peds.108.6.1263. [DOI] [PubMed] [Google Scholar]
- 8.Grumbach K. Patient Empanelment. HSAG webinar. July 2016. https://www.hsag.com/en/events/2016/july-20162/patient-empanelment Accessed February 28, 2019.
- 9.Patient Centered Primary Care Institute. Empanelment: What Do You Do After Every Patient Has An Assigned Care Team? March 18, 2014. http://www.pcpci.org/sites/default/files/webinar-related/PCPCI_Empanelment_03-17-14.pdf Accessed February 28, 2019.
- 10.Rajkomar A, Yim JW, Grumbach K, Parekh A. Weighting primary care patient panel size: a novel electronic health record derived measure using machine learning. JMIR Med Inform. 2016;4(4):e29. doi: 10.2196/medinform.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saultz JW, Albedaiwi W. Interpersonal continuity of care and patient satisfaction: a critical review. Ann Fam Med. 2004;2(5):445–451. doi: 10.1370/afm.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saultz JW, Lochner J. Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med. 2005;3(2):159–166. doi: 10.1370/afm.285. [DOI] [PMC free article] [PubMed] [Google Scholar]


