Abstract
In the current era of high consumption and increasing waste, many products that are believed to be unusable can find a new purpose in the market. For example, the grape peel waste resulting from the production of wine contains numerous bioactive compounds. In reality, grape peels are by-products of winemaking that can be conveniently reused in many different ways, including agronomic use and cosmetic industry applications. Moreover, the by-products can also be used in the energy field as biomass for the production of biogas or in food plants for the production of energy. In this article, to extract polyphenols, grape peels were processed via a cyclically pressurized extraction method known as rapid solid-liquid dynamic extraction (RSLDE), which does not require the use of any organic solvent or include heating or cooling processes that can cause the loss of substances of interest. To better understand the cyclically pressurized extraction process, a numerical simulation was performed to evaluate the exchange between the grape piece solid matrix and water during the extraction process. Furthermore, a finite element model was used to numerically determine the time-dependent concentration distribution at specific times.
Keywords: Nutrition, Food technology, Food analysis, Food science
1. Introduction
Polyphenols, particularly flavonoids, are ubiquitous in plant foods (fruits, vegetables, legumes, and some beverages) and are also widespread in many medicinal plants. Numerous epidemiological and experimental studies have suggested that polyphenols have multiple beneficial effects on human health [1, 2]. The biological activity of polyphenols is associated with their chemical and biochemical properties, including their ability to act as antioxidants, their antineoplastic effects, their ability to regulate gene expression in chronic degenerative diseases, and their anti-diabetic potential [3, 4, 5, 6]. However, the bioavailability of polyphenols varies, depending mainly on the food source and the forms of polyphenols that it contains [7]. The grapes used for wine production represent a good example of an agricultural product rich in nutritional value and health benefits due to their vitamins, minerals and antioxidants. In particular, the flavonoids in grapes are located mainly in the peels. Some types of flavonoids are also found in grape seeds and stalks, but they are generally absent in the flesh. Approximately 15% of the fruit, however, consists of the peel, and peels represent a wine-making process waste material that is reused in part for the production of brandy, ethanol, tartaric acid, dyes, and fertilizers. Today, the use of agro-food processing waste by-products represents a concrete action that addresses the problem of agricultural production sustainability and provides the growing food, nutraceutical, pharmaceutical and cosmetic industries with low-cost, high-quality raw materials that were destined for disposal for the production of natural ingredients. In particular, the target of some food industries is to develop snacks and drinks composed of grapes, which are known to contain high amounts of polyphenols. To extract these polyphenolic compounds from grapes, a type of liquid-liquid extraction that involves the use of organic solvents, such as ethanol, methanol, formic acid or acetone and water in different proportions, is usually used. However, this type of extraction can involve considerable health risks and can be damaging to the environment; consequently, extraction methods that reduce the use of organic solvents, including microwave extraction [8], supercritical fluid extraction (SFE) [9], and ultrasound-assisted extraction (UAE) [10, 11], have recently been developed. To reduce the environmental impact and create a greener extraction technique, this work investigated a green extraction process for polyphenols using the peels of Aglianico grapes, a prestigious variety of grape grown in Campania (Italy) that is particularly rich in nutrients and antioxidants; this process uses a cyclically pressurized extraction method known as rapid solid-liquid dynamic extraction (RSLDE) with a Naviglio extractor at room temperature [12]. Literature data show that conventional and non-conventional methods have been applied for the extraction of high-added value compounds from winery-processed materials. Non-conventional technologies represent a promising tool for recovering high-added value compounds from winery wastes and by-products. However, several parameters influence the choice of technology used to recover these compounds, such as the matrix being processed, selectivity, energy consumption, equipment cost, and value of the extract [13]. In particular, compared to other known techniques, RSLDE is an innovative solid-liquid extraction technology that allows rapid extraction of solid matrices containing extractable substances with an organic or inorganic solvent and with their mixtures. Most current methods aim to heat the extraction system to increase yield and accelerate extraction times, while RSLDE performs extraction at room temperature, using an increase in extraction liquid pressure on the solid matrix for extraction. Extracting at low temperatures is important because the use of low temperature avoids applying thermal stress on thermolabile substances. In this way, it is possible to determine the composition of the substances present in the different matrices without inducing changes in the active principles contained therein [12]. In addition, the process was modelled and studied using a finite element method.
2. Materials and methods
2.1. Plant material
This study was conducted using the Aglianico grape cultivar, which is a red grape cultivar that is widespread in Southern Italy and is renowned for the quality of its wines. It is grown in several Italian regions, but it is mainly cultivated in Campania from the renowned "Feudi di San Gregorio" vineyard in Sorbo Serpico, Avellino (Campania, Italy) (41°00′35.39″N/14°57′34.29″E). Four types of grape samples were examined: Group I: directly harvested grapes; Group II: peels and seeds from grapes that had been subjected to the crushing process and subsequently sent to a tank for maceration; Group III: crushed and dried grapes produced by Imepa (Southern Industry Drying Agricultural Products, Salerno, Campania, Italy); and Group IV: crushed, dried and shredded grapes produced by Imepa.
Grape samples (200 g) were extracted using a Naviglio extractor, series LAB, model EXNA0101 (500 cc). This method is a solid-liquid extraction technique based on the cyclic application of a variable pressure. The instrument uses pressure (approximately 8-10 bar) in a rapid and simplified manner to extract substances not chemically bound to a solid matrix in a liquid medium at room temperature using deionized water as the solvent.
2.2. Instrumentation and chemicals
The instruments used in this study included a UV-VIS spectrophotometer, model 1601, equipped with a 1-cm optical path cuvette (Shimadzu, Tokyo, Japan); a gas chromatograph equipped with a programmable split-splitless injector (PSS), a flame ionization detector (FID) (Auto System XL) (Perkin Elmer, Norwalk, CT, USA) and an Rtx-5 capillary column (l = 30 m, i.d. = 0.25 mm, and f.t. = 0.25 microns; Restek, Bellefonte, PA, USA) with a 5% diphenyl and 95% dimethylsilicone stationary phase; a gas chromatograph, model 17A (Shimadzu, Tokyo, Japan), equipped with a split-splitless injector and interfaced with a mass spectrometer, model QP-5000, with an SPB-5 capillary column (l = 60 m, i.d. = 0.25 mm, and f.t. = 0.25 micron; Supelco, Bellefonte, PA, USA); and a Karl Fisher titrator, model KF 2026 (Crison Instruments, Baar, Switzerland). All High Performance Liquid Chromatography (HPLC) grade reagents and solvents were purchased from Merck (Darmstadt, Germany). Before the extraction process, the dried and crushed grape samples were weighed with a Gibertini Europe 1700 analytical balance (Novate Milanese, Milan, Italy). The grape samples had a uniform moisture content of 18%.
2.3. Experimental tests
Shredded and dried Aglianico cultivar grape peels were supplied by Imepa and were prepared via the following steps: 1) receipt of frozen raw material; 2) defrosting in a cell with a maximum temperature (T) of 8 °C; 3) washing in sterile water with a T of <50 °C; 4) drying on looms in an air-steam exchange oven at a T between 40 °C and 70 °C for 2 hours at a relative humidity (RH) of <18% 5) slow grinding; and 6) packaging. Before packaging, the samples were also subjected to microbiological analysis that included a visual macroscopic analysis of 1000 g to assess anything indicating foreign bodies in the product, an evaluation of any visible formations suggestive of surface moulds or signs of entomic infestation, and a direct microscopic and parasitological analysis on 10 g of sediment diluted 1:10 to evaluate normal non-pathogenic microbial flora, rare fungi, and pests and their eggs. The notes, comments and corrections pertaining to the hazard analysis critical control point (HACCP) guidelines provided by Imepa stated that the product is hygienically healthy for the above parameters and that the fruit is completely free from any visible signs of biological contamination.
2.4. Folin-Ciocalteu method for total polyphenols
The total amount of phenolics was determined using the Folin-Ciocalteu method [14]. A calibration curve of gallic acid was prepared, and the results were determined using a regression equation from the calibration curve, with each measurement expressed as mg of gallic acid equivalents per gram of sample (GAE/g). In this method, 1 ml of each extract, diluted appropriately with deionized water to obtain an absorbance in the range of the prepared calibration curve, was mixed with 1 ml of 3-fold-diluted Folin-Ciocalteu phenol reagent. Two millilitres of 35% sodium carbonate solution was added to the mixture, which was then shaken thoroughly and diluted to 6 ml by adding 2 ml of water. The mixture was allowed to stand for 30 min, and the blue colour that formed was measured at 700 nm using a spectrophotometer. As the samples from Group IV had the highest concentrations of polyphenols, the assays were focused on these samples (Table 1).
Table 1.
Extractions performed and their results in terms of total polyphenols.
| Group | Grape quantity used | Extraction process time | Total polyphenols |
|---|---|---|---|
| 1 | 200 g grapes | 72 h | 264.8 ± 5.8 mg/L |
| 2 | 200 g peels + seeds | 48 h | 674.5 ± 9.4 mg/L |
| 3 | 200 g dried peels Imepa | 48 h | 4110.3 ± 20.8 mg/L |
| 4 | 200 g dried and chopped peels Imepa | 48 h | 9210.4 ± 45.8 mg/L |
(Mean value ± dxm = standard deviation of the mean).
2.5. Experimental tests
A 200-g sample of grapes (Group IV) was stored at 10 °C in a hermetically sealed plastic container to prevent changes in its moisture content. The dried grapes were mixed several times for 2 min. Prior to extraction, the moisture content of the grapes was determined using an oven method at 105 °C for 24 hours, and the moisture content was 18% w/w [15].
2.6. Determination of the size
The size of the solid matrix of grape pieces (Group IV), including its length, width and thickness, was evaluated using a digital calliper before and during the process of solid-liquid extraction. From these values, the sphericity and geometric mean diameter (GMD) of the solid matrix of the grape pieces were determined [16]. The values obtained were used in numerical simulations to construct a mathematical model of the process.
2.7. Determination of total volume
To measure the volume without changing the moisture content, the grape solid matrix (Group IV) was sealed with food-grade polyethylene, which was very suitable for the volume used in this study. The initial total volume (Vpt) was evaluated using a 1000-ml graduated cylinder with a water volume of 500 ml by dipping in it into the volume to be measured.
The accessory mass volume (Va) was evaluated and was subtracted from the Vpt value, providing the real volume of the considered sample (Vp), that is, Vp=(Vpt-Va). Subsequently, to evaluate the initial volume and weight, the extraction process was performed for grape piece samples withdrawn from Group IV, and a rapid solid-liquid dynamic extractor (RSLDE) was used. The volume and weight values were calculated again during the extraction phase.
2.8. Determination of initial concentration
Experiments were performed to evaluate solute extraction from the solid matrix for the considered extraction process. A solid matrix of grapes was chosen. The samples (Group IV) were stored at −20 °C in tightly sealed plastic containers to prevent changes in their moisture content, and they were mixed several times for 2 min. Before the extraction process, the concentration of the grape piece solid matrix content was determined [17].
2.9. Determination of the dry residue
A 10-ml volume of extract was filtered and added to a calibrated dish, and the extract volume was evaporated to dryness using an oven at 50 °C. This process was continued until the remaining liquid volume was negligible, and the oven temperature was then increased to 105 °C. The sample was placed in a desiccator and was subsequently cooled to 20 °C for weighing. This operation was repeated until a constant weight was attained. The weight of the dry matter was calculated by subtracting the tare weight. Each determination was repeated three times, and the measurement differences were lower than 7%; the average value of each sample was determined and reported. After determining their initial dry weight and volume, the samples were extracted.
2.10. Extraction process using a Naviglio extractor
Two hundred gram grape samples (Group IV) were subjected to a total of 30 programmed cycles of pressure (with a maximum pressure of 10 bars) applied to the liquid phase in contact with the grape pieces over a period of 2 hours. The number of hits in the dynamic phase (nd) was 12; the dynamic operative phase (td) and the static operative phase (ts) were performed for 2 min each. All of the experiments were performed in triplicate, and their averages were used for numerical modelling and analyses. Determinations of the weight and volume were performed as described below at the times reported in Table 1 for the included samples (see the Results And Discussion Section). The grape samples were then subjected to programmed cycles of pressure applied to the liquid phase in contact with the product, as described above, while maintaining the same solid–liquid ratio. All of the experiments were repeated three times, and the measurement differences were less than 5%; therefore, the averages were used for numerical modelling and analyses.
First phase. In the first phase of the procedure, using a Naviglio extractor (Atlas Filtri Ltd., Padua, Italy), the initially sampled raw material was split into three batches of 200 g each.
Second phase. In the second phase of the procedure, a 200-g sample of the raw material was removed from the Naviglio extractor at the end of the extraction process and transferred to a sterile bag that was labelled to indicate the Naviglio procedure, extraction ending time, initial time, final time, product, quality, batch and date of sampling [18, 19, 20, 21, 22].
2.11. Mass spectrometric analysis
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS) experiments were performed on a 4800 ABSciex MALDI time-of-flight/time-of-flight (TOF/TOF) tandem mass spectrometer equipped with a nitrogen laser (337 nm). Sample extract (0.5 μl) was mixed (1/1, v/v) with a 10 mg/ml solution of 2,5-dihydroxybenzoic acid in acetonitrile/water (90:10, v/v). Spectra were acquired using a mass (m/z) range of 100–4000 Da for polyphenol identification. Selected extracted samples were dissolved in methanol and subjected to the same MALDI-TOF/TOF analysis. The different species detected in the MALDI spectra were fragmented by MALDI-TOF/TOF and identified based on literature data MALDI MS/MS spectra [23]. The species identified in the different samples are summarized in Table 2 (see the Results And Discussion Section).
Table 2.
Compound names and molecular weights of polyphenols identified in the Group IV samples via MALDI-TOF/TOF analysis.
| Grape peels dried | |
|---|---|
| Compound | m/z |
| Delphinidin | 303.26 |
| Petunidin | 317.28 |
| Malvidin | 331.34 |
| Peonidin-3-O-Monoglucoside | 463.12 |
| Delphinidin-3-O-Monoglucoside | 465.12 |
| Petunidin-3-O-Monoglucoside | 479.13 |
| Malvidin-3-O-Monoglucoside | 493.16 |
| A Type Vitisin of Pn-3-Glc/Acetone derivate of Malvidin-3-O-Glucoside | 531 |
| Malvidin-3-O-(6-O-Acetyl)Monoglucoside | 535.17 |
| Peonidin-3-O(6-O-P-Coumaroyl)Monoglucoside | 609.21 |
| Delphinidin-3-O-(6-O-Acetyl)Monoglicoside/Cyanidin -3,5-O Diglucoside | 611 |
| Petunidin-3-O-(6-O-Acetyl)Monoglicoside/Peonidin-3,5-Diglucoside | 625.20 |
| Malvidin-3,5-O-Diglucoside | 639.22 |
| Malvidin-Petunidin | 647.18 |
| Mv-3-O-(6-O-Caffeoyl)Monoglucoside | 655.22 |
| Malvidin-Malvidin (Fragment) | 661.21 |
| A-Type Visitin of Pn-3-P-Coumaryl- Glucoside | 677.18 |
| Delphinidin-3(6-O-P-Coumaroyl)-5-O-Diglucoside | 773.24 |
| Delphinidin-3-O-(6-O-Ferulyl)-5-Odiglucoside | 803.26 |
| Malvidin-Peonidin-Glucoside (Fragment) | 823.28 |
| Malvidin-Cyanidin-Diglucoside | 941.32 |
| Malvidin- Peonidin- Diglucoside | 955.33 |
| Malvidin -Delphinidin-Diglucoside | 957.34 |
| Malvidin- Malvidin -Diglucoside | 985.36 |
| Malvidin- Peonidin- Glucoside-P-Coumaroyl | 1101.39 |
| Malvidin -Delphinidin -P Coumaroyl Glucoside | 1103.39 |
| Malvidin- Malvidin -P Coumaroyl Glucoside | 1131.42 |
2.12. Analytical approach
The diffusion process at a constant temperature follows Fick's second law. Considering axisymmetric diffusion, Fick's three-dimensional (3D) equation is:
| (1) |
where M is the concentration of the extract from the considered matrix at time t and D is the diffusion parameter. Crank (1979) [24] proposed a suitable solution for Eq. (1) that considers a material with a spherical geometry having radius value r, described by:
| (2) |
| (3) |
where M is the instantaneous content concentration at a specified time t. Thus:
| (4) |
where MR is the concentration ratio and Me and Mi are the equilibrium and initial concentrations of the solid matrix during the process, respectively. Often, only a finite number of parameters of Eq. (2) are utilized to evaluate the MR parameter. Frequently, researchers exclusively utilize the first two terms of Eq. (4).
In this paper, the MR values were evaluated at a determined time t and utilized as input in a MATLAB program (R2016b). The diffusion parameter D for the considered matrix was evaluated at every instant t was examined. The geometry of the grape matrix is approximately ellipsoidal, so the diffusion parameter in Eq. (1) had to be modified. Gaston et al. (2002) [25] determined a method to evaluate the De parameter in cases of ellipsoidal geometry utilizing the following expression:
| (5) |
where fe is the sphericity parameter for the ellipsoid. Subsequently, the diffusion parameter D must be replaced with the evaluated De parameter.
2.13. Finite element approach for extraction with the cyclically pressurized extraction process
To numerically simulate the extraction process, a mathematical simulation was set up using a COMSOL (Rel 5.1) program. Evaluating the exchange phenomena between the grape matrix and the liquid phase during the extraction process was possible with this program. A time-dependent concentration distribution as a function of time was determined by applying a finite element model.
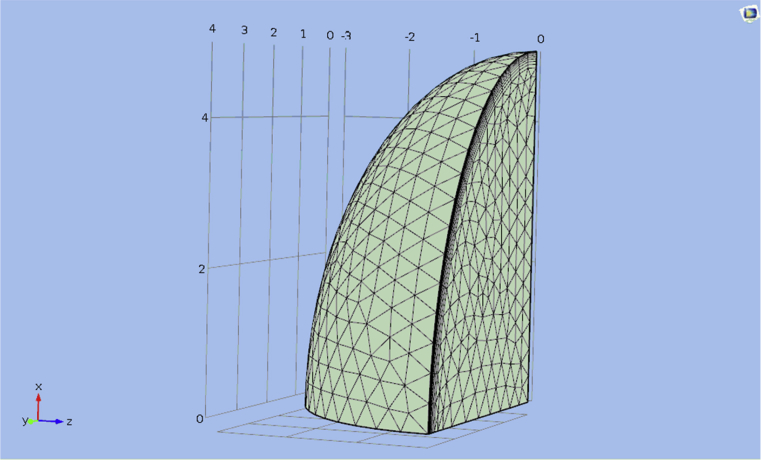
The shape of the grape matrix pieces was approximated as an ellipsoid. To model the grape matrix pieces, only a quarter of an ellipsoid was considered in 3D (Fig. 1). The COMSOL (Rel.5.1) program allowed the evaluation of the concentration parameter for each node at each time considered.
Fig. 1.
Finite element grid for a single grape piece.
A complete mesh consists of 10784 domain elements, 1406 boundary elements, and 103 edge elements with 8 layers near the external surface. The number of degrees of freedom was solved for 3341 (plus 1002 internal DOFs) with solution times of 8 s using an i7 processor at 3.3 GHz with 16 GB of RAM.
The diffusion parameter was evaluated by fitting Eq. (2), and the obtained data were input into the COMSOL program for computation of the concentration parameter for the considered nodes with a time range of 1 second. The total concentration of the grape solid matrix was also evaluated at 0.1, 2, 4, 10, 20, 32, and 48 hours by averaging the values of the considered nodes applied for the extraction process.
Moreover, the chemical and physical behaviour was defined by specifying the following parameters:
-
-
effective diffusion coefficient for a spheroidal geometry
-
-
initial essential oil content of the grape piece
-
-
equilibrium essential oil content of the grape piece; and
-
-
total time.
For a certain initial content concentration and for the proper diffusion coefficient of the material, the analysis evaluated the time-dependent concentration distribution at the nodes. An analogous approach has been performed to evaluate the temperature and moisture distribution during the cooling phase of the cryomaceration process for grapes [26]. For a finite element method, the calculated value of D is furnished in Eq. (1), and the MR parameters are computed for each node and for every instant t. The following hypotheses are considered when performing this approach for the considered matrix: (1) the diffusion parameter is independent of the concentration parameter; (2) the matrix is considered isothermal, and the heat exchange during the considered process is neglected; (3) the matrices are isotropic and homogeneous; and (4) the initial solute content of the grape matrix is uniformly distributed and constant inside the matrix.
3. Results and discussion
3.1. Extraction method
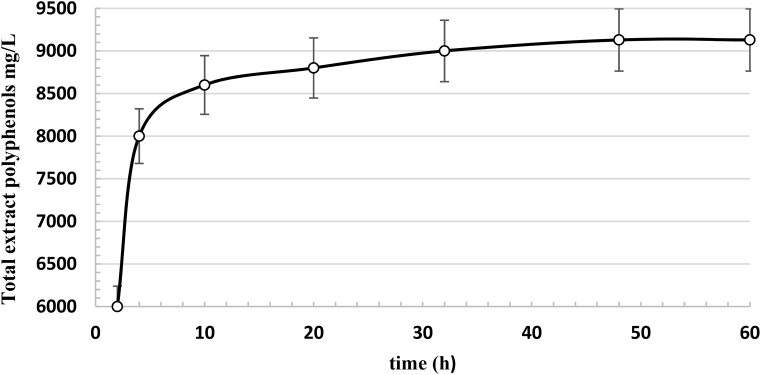
Table 1 shows the time period after which the extraction diagram was constant for each group and the corresponding value of the total extracted polyphenols. The kinetic curve for Group IV showed a higher content of extracted polyphenols than that observed for the other groups. Therefore, only the extraction model of Group IV was considered further, and only samples from this group were used to vary the geometry considered during the extraction process. Fig. 2 shows the extraction kinetics for Group IV during the cyclically pressurized process with a Naviglio extractor for polyphenol extraction. The trend of the rapid solid-liquid extraction kinetic dynamics was exponential, the initial extraction was very rapid, and the system reached a final equilibrium of polyphenols at approximately 48 hours, yielding a total extract of 9128 mg/L. The data used to evaluate the extraction kinetics diagram were computed in triplicate. Total polyphenol values were examined via a statistical study using multifactorial analysis of variance (ANOVA). The statistical importance of every parameter was evaluated utilizing the least significant differences (LSD) test. The data were statistically examined using Statgraphics Plus 5.1 software.
Fig. 2.
Graph of the polyphenol extraction kinetics during a cyclically pressurized extraction process using a Naviglio extractor with the Group IV samples.
The total polyphenol content was determined spectrophotometrically in accordance with the Association of Official Analytical Chemists (AOAC) method [15], which is based on the Folin-Ciocalteu method and used the relevant reagent: gallic acid was employed as the reference standard, and the results were expressed in mg of gallic acid equivalents per L of extract (GAE·L−1) (Fig. 2).
The compound name and MALDI-TOF/TOF product ions (m/z) for each identified polyphenol present in the Group IV extract are presented in Table 2.
3.2. Determination of the characteristic parameters
Table 3 shows the characteristic values of the parameters for the grape piece solid matrix in the Group IV grape samples as well as the diffusivity De calculated by Fick's law. The L1 length along the x-axis of the considered particle (mm) is the largest principal dimension. The L2 length along the y-axis of the considered particle (mm) is the second largest principal dimension, while the L3 length along the z-axis of the considered particle (mm) is the smallest principal dimension.
Table 3.
Some physical characteristics of the experimental mashed grape particles.
| Time (h) | L1 Length (mm) | L2 Width (mm) | L3Thickness (mm) | GMD = D (mm) | Sphericity ϕ | Sphericity factor – fe |
|---|---|---|---|---|---|---|
| 0 | 5.06 ± 0.92 | 4.22 ± 0.62 | 3.27 ± 0.31 | 3.71 | 0.9757 | 0.970839 |
| 2 | 5.01 ± 0.87 | 4.18 ± 0.43 | 3.23 ± 0.23 | 3.67 | 0.9754 | 0.970553 |
| 4 | 4.91 ± 0.78 | 4.09 ± 0.35 | 3.18 ± 0.08 | 3.60 | 0.9757 | 0.971199 |
| 10 | 4.86 ± 0.57 | 4.05 ± 0.63 | 3.15 ± 0.13 | 3.57 | 0.9758 | 0.971356 |
| 20 | 4.76 ± 0.71 | 4.01 ± 0.59 | 3.11 ± 0.05 | 3.53 | 0.9771 | 0.971997 |
| 32 | 4.71 ± 0.73 | 3.98 ± 0.34 | 3.08 ± 0.06 | 3.50 | 0.9775 | 0.972029 |
| 48 | 4.67 ± 0.72 | 3.93 ± 0.29 | 3.05 ± 0.04 | 3.46 | 0.9770 | 0.972019 |
Data are expressed as the mean ± SD. GMD: geometric mean diameter.
Moreover, the sphericity value ϕ, which is an index of the roundness of the grain, was also calculated using the method described by Jain and Bal (1997) [16].
For non-spherical particles, the sphericity was calculated as the ratio of the surface area of an equivalent sphere to the surface area of the grain [27]. The results for non-spherical particles are reported in Table 3.
However, we detected a decrease in the width and thickness of the grape matrix, and its sphericity parameter changed from 0.9757 to 0.9770. fe was computed, and it changed from 0.970 to 0.972.
This decrease in dimensions was due to the migration of solute content in the grape peel cell solid matrix, causing overall deflation. Ahromrit et al. (2006) provides a detailed discussion on dimensional changes during the extraction process [28].
By applying the expression of Fick's law using Eqs. (2) and (3), De was precisely calculated for each time period examined. For the polyphenol components, the De values ranged from 0.942 × 10−13 m2/s to 0.124 × 10−10 m2/s. These values were compared with those obtained in another study available in the literature, Madieta et al., (2008), in which De values ranging between D1 = 7 × 10−9 m2s−1 and D2 = 5 × 10−10 m2s−1 were used [29].
3.3. Chemical content
The results summarized in Table 1 show that the tests carried out using only deionized water with peels and seeds and no pulp yielded the highest polyphenol content at the maximum extraction time of 48 hours. After this time, the additional quantities obtained were negligible. Additionally, if a 48-hour process time is too long, it is possible to obtain an acceptable polyphenol content in the extract with shorter process times, causing a loss of approximately 30% compared to the 48-hour yield, as seen from the results reported in Table 1. Therefore, detecting a high rate of extraction using the experimental process was possible.
Food processing intended for conservation, transformation or extraction occupies an important place in various production processes. Therefore, green food processing represents a global strategy based on the discovery and design of processes to reduce energy and water consumption [30]. In particular, in the extraction sector, there has been a growing demand in recent years for new extraction techniques with reduced extraction times, reduced consumption of organic solvents and greater prevention of pollution, namely, so-called "green" methods. To improve the sensitivity and selectivity of analytical methods, ecological extraction techniques represent the sustainable alternative to the classic procedures of sample preparation used in the past. New extraction methods, such as UAE), microwave-assisted extraction (MAE), SFE and accelerated solvent extraction (ASE), are fast and efficient in the extraction of bioactive substances from different solid matrices [31]. These techniques can work at high temperatures and/or pressures, thereby greatly reducing extraction times. In the field of innovative techniques, a technology called rapid solid-liquid dynamic extraction (RSLDE) has been developed as a viable alternative to maceration. It is a "green" technique; in fact, compared to extraction with techniques such as SFE or ASE, it requires a minimum energy expenditure. It allows aqueous extraction to be performed in reduced times, which is impossible to achieve with maceration. The extraction is carried out using the formation of a negative pressure gradient from the inside to the outside of the solid matrix, so the solid-liquid extraction can be carried out at room temperature. Extracting at low temperatures is important because the use of low temperature avoids applying thermal stress on thermolabile substances.
On the other hand, the RSLDE, because of its innovative technology [12], allows the extraction of plants and vegetables in a much shorter time than is allowed by current technologies such as maceration, percolation, Soxhlet, microwaves, supercritical fluids, and ASE, avoiding the thermal stress to heat-sensitive substances. As a result, its application fields range from the production of herbal products to the beverage industry, the cosmetics industry, and the food industry, among others [18, 19, 20, 21, 22, 32, 33, 34, 35, 36, 37, 38]. In this study, the cyclically pressurized extraction process was found to significantly increase the extraction rate of the grape pieces, and the equilibrium conditions were thus attained in much shorter times than those required by other methods. The juice thus obtained must subsequently be subjected to a pasteurization process to make it stable and suitable for human consumption. Given the complexity of the food matrix in question, as well as the different variables that contribute to the modification of phenolic composition, the results obtained herein provide the first indications of the production of a grape-based beverage rich in polyphenols. In conclusion, the extraction and stabilization of grape polyphenols through the use of a Naviglio extractor provide an innovative approach for the use of grape pieces to develop new food ingredients. These results are of considerable importance because a diet rich in polyphenols provides significant protection against the development and progression of many chronic pathological conditions, including cancer, diabetes and cardiovascular problems. Furthermore, the intake of polyphenols, owing to their anti-radical action, contributes to the delay of ageing [39, 40, 41, 42, 43, 44].
3.4. Numerical analysis results
To perform a numerical simulation of the extraction process investigated in this study, a finite element model for grapes was used (Fig. 1).
The computed diffusion parameter was replaced in Eq. (1), and the equation was solved using the finite element method to evaluate the nodal flux-time diagrams for the polyphenols and their concentration distribution in the matrix at each instant considered. The data obtained for the predictions of the concentration values during the extraction process are shown in Fig. 3 a, b, c, and d.
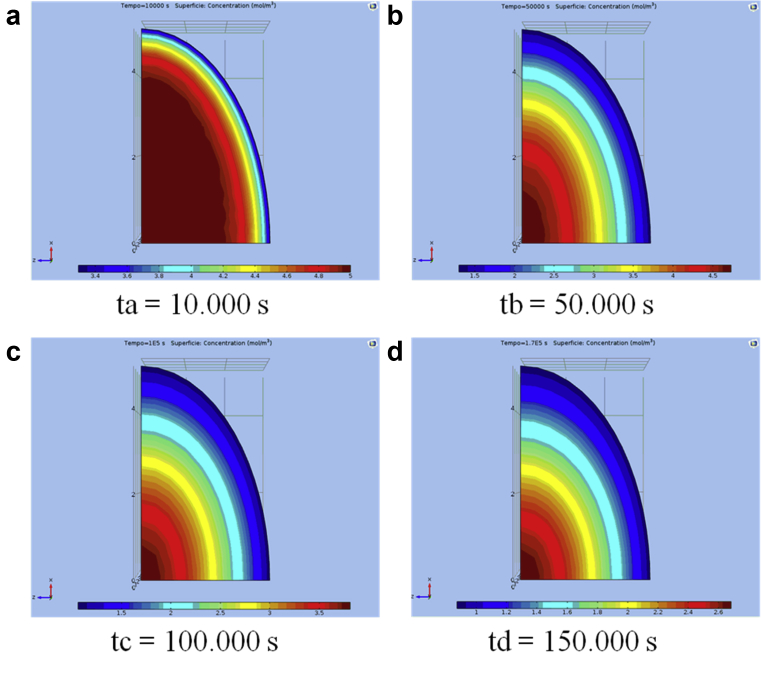
Fig. 3.
a, b, c, and d show the typical distribution of the solute concentration (diffusion) in the solid matrix of grape pieces during extraction as determined by a finite element analysis after t = 10 s, t = 50 s, t = 100 s, or t = 150 s, respectively, for the component polyphenols. The bar below each shape represents the content concentration (%) within a particle region at a given time.
Fig. 3 a, b, c, and d shows a gradual migration of the solute extract from the centre of the solid matrix to the surface. Theoretically, the finite element model, as shown in Fig. 3, indicates that even at the end of the extraction process, the concentration of the content throughout the solid matrix is not quite uniform. In Fig. 3 a, b, c, and d, we note that the extract solute gradient inside the matrix has high concentration values during the initial phase of the extraction and that these values decrease during the extraction process. At the end of the process, the concentration distribution is near constant for each node. These figures show the concentration at various points along a cross-section of a grape piece particle, from the surface to the centre of the solid matrix.
4. Conclusion
In recent years, interest has increased in specific compounds that can be obtained from the by-products of the wine industry. This interest, combined with the interest of the wine industry in reducing the impact of their products and reducing the volume and disposal costs of waste, has led to a greater interest in research on more efficient utilization of by-products rich in substances with high added value. In fact, in exhausted by-products of grapes, it is possible to find different bioactive compounds, including polyphenols. It is therefore important to develop methods that make it possible to use these by-products completely on a large scale and economically. The extraction of chemical compounds with a high biomass added value already represents an important market in the pharmaceutical, cosmetic, nutraceutical and agricultural sectors; thus, extraction processes can be defined as sustainable, and it is important to use energy efficient technologies based on environmentally friendly solvents. Based on the results obtained, RSLDE is an efficient procedure for extracting polyphenols, in addition to demonstrating the exhaustion of solid matrices containing extractable substances in a short time, at low operating temperatures (environmental or sub-environmental) and with reproducibility of extraction. These features ensure the production of quality extracts, and compared to other techniques currently in use, the process has a low environmental impact. In addition, to better understand the cyclically pressurized extraction process, it has been modelled and studied using the finite element method. For added value, the exhausted peels that remain after the extractive process can be dried and used as manure and/or animal feed.
Declarations
Author contribution statement
Monica Gallo, Daniele Naviglio: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Rosalba Giacco, Gabriele Riccardi, Delia Lungo: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Andrea Formato, Gaetano Formato: Performed the experiments; Analyzed and interpreted the data.
Angela Amoresano: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are very grateful to the "Feudi di San Gregorio" winery in Avellino, Italy, for providing the grapes for this study.
References
- 1.Watson R.R., Preedy V.R., Zibadi S., editors. Polyphenols: Prevention and Treatment of Human Disease. Academic press; 2018. https://books.google.it/ [Google Scholar]
- 2.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. [Google Scholar]
- 3.Kim Y., Keogh J., Clifton P. Polyphenols and glycemic control. Nutrients. 2016;8(1):17. doi: 10.3390/nu8010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niedzwiecki A., Roomi M., Kalinovsky T., Rath M. Anticancer efficacy of polyphenols and their combinations. Nutrients. 2016;8(9):552. doi: 10.3390/nu8090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao H., Ou J., Chen L., Zhang Y., Szkudelski T., Delmas D. Dietary polyphenols and type 2 diabetes: human study and clinical trial. Crit. Rev. Food Sci. Nutr. 2018:1–9. doi: 10.1080/10408398.2018.1492900. [DOI] [PubMed] [Google Scholar]
- 6.Santhakumar A.B., Battino M., Alvarez-Suarez J.M. Dietary polyphenols: structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018;113:49–65. doi: 10.1016/j.fct.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Brglez Mojzer E., Knez Hrnčič M., Škerget M., Knez Ž., Bren U. Polyphenols: extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules. 2016;21(7):901. doi: 10.3390/molecules21070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Álvarez A., Poejo J., Matias A.A., Duarte C.M., Cocero M.J., Mato R.B. Microwave pretreatment to improve extraction efficiency and polyphenol extract richness from grape pomace. Effect on antioxidant bioactivity. Food Bioprod. Process. 2017;106:162–170. [Google Scholar]
- 9.Da Porto C., Natolino A. Supercritical fluid extraction of polyphenols from grape seed (Vitis vinifera): study on process variables and kinetics. J. Supercrit. Fluids. 2017;130:239–245. [Google Scholar]
- 10.González-Centeno M.R., Comas-Serra F., Femenia A., Rosselló C., Simal S. Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (Vitis vinifera L.): experimental kinetics and modeling. Ultrason. Sonochem. 2015;22:506–514. doi: 10.1016/j.ultsonch.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Tomaz I., Huzanić N., Preiner D., Stupić D., Andabaka Ž., Maletić E. Polyphenols in Plants. Academic Press; 2019. Extraction methods of polyphenol from grapes: extractions of grape polyphenols; pp. 151–167. [Google Scholar]
- 12.Naviglio D. Naviglio’s principle and presentation of an innovative solid-liquid extraction technology: extractor Naviglio. Anal. Lett. 2003;36(8):1647–1659. [Google Scholar]
- 13.Barba F.J., Zhu Z., Koubaa M., Sant'Ana A.S., Orlien V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: a review. Trends Food Sci. Technol. 2016;49:96–109. [Google Scholar]
- 14.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 15.AOAC . twenty first ed. 2019. Official Methods of Analysis of Association of Official Analytical Chemists (AOAC) International.https://www.aoac.org [Google Scholar]
- 16.Jain R.K., Bal S. Properties of pearl millet. J. Agric. Eng. Res. 1997;66(2):85–91. [Google Scholar]
- 17.ASAE . American Society of Agricultural Engineers; 2003. Moisture Measurement – Unground Grain and Seeds.https://www.scirp.org Standard ASAE S352.2 FEB03. [Google Scholar]
- 18.Naviglio D., Formato A., Pucillo G.P., Gallo M. A cyclically pressurised soaking process for the hydration and aromatisation of cannellini beans. J. Food Eng. 2013;116(3):765–774. [Google Scholar]
- 19.Naviglio D., Ferrara L., Formato A., Gallo M. Efficiency of conventional extraction technique compared to rapid solid-liquid dynamic extraction (RSLDE) in the preparation of bitter liquors and elixirs. IOSR J. Pharm. 2014;4:14–22. https://www.researchgate.net/profile/Daniele_Naviglio/publication/262151185 [Google Scholar]
- 20.Naviglio D., Formato A., Gallo M. Comparison between 2 methods of solid–liquid extraction for the production of Cinchona calisaya elixir: an experimental kinetics and numerical modeling approach. J. Food Sci. 2014;79(9):E1704–E1712. doi: 10.1111/1750-3841.12563. [DOI] [PubMed] [Google Scholar]
- 21.Naviglio D., Montesano D., Gallo M. Laboratory production of lemon liqueur (Limoncello) by conventional maceration and a two-syringe system to illustrate rapid solid–liquid dynamic extraction. J. Chem. Educ. 2015;92(5):911–915. [Google Scholar]
- 22.Naviglio D., Formato A., Vitulano M., Cozzolino I., Ferrara L., Zanoelo E.F., Gallo M. Comparison between the kinetics of conventional maceration and a cyclic pressurization extraction process for the production of lemon liqueur using a numerical model. J. Food Process. Eng. 2017;40(2) [Google Scholar]
- 23.Flamini R., De Rosso M., Bavaresco L. Study of grape polyphenols by liquid chromatography-high-resolution mass spectrometry (UHPLC/QTOF) and suspect screening analysis. J. Anal. Methods Chem. 2015:10. doi: 10.1155/2015/350259. Article ID 350259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crank J. Oxford University Press; New York: 1979. The Mathematics of Diffusion. [Google Scholar]
- 25.Gaston A.L., Abalone R.M., Giner S.A. Wheat drying kinetics. Diffusivities for sphere and ellipsoid by finite elements. J. Food Eng. 2002;52(4):313–322. [Google Scholar]
- 26.Carillo M., Formato A., Fabiani A., Scaglione G., Pucillo G.P. An inertizing and cooling process for grapes cryomaceration. Electron. J. Biotechnol. 2011;14(6) [Google Scholar]
- 27.McCabe W.L., Smith J.C. third ed. McGraw Hill Book Company; Japan: 1984. Unit Operation of Chemical Engineering.https://scholar.google.it [Google Scholar]
- 28.Ahromrit A., Ledward D.A., Niranjan K. High pressure induced water uptake characteristics of Thai glutinous rice. J. Food Eng. 2006;72(3):225–233. [Google Scholar]
- 29.Madieta E., Zouid I., Siret R., Jourjon F. Excerpt from the Proceedings of the COMSOL Conference 2008 Hannover. 2008. Extraction of phenolic compounds from grape fruit. A comparison between a 3D FEM model and experimental results.https://www.comsol.pt/paper/download/37836/Madieta.pdf [Google Scholar]
- 30.Chemat F., Rombaut N., Meullemiestre A., Turk M., Perino S., Fabiano-Tixier A.S., Abert-Vian M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017;41:357–377. [Google Scholar]
- 31.Ameer K., Shahbaz H.M., Kwon J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: a review. Compr. Rev. Food Sci. Food Saf. 2017;16(2):295–315. doi: 10.1111/1541-4337.12253. [DOI] [PubMed] [Google Scholar]
- 32.Formato A., Gallo M., Ianniello D., Montesano D., Naviglio D. Supercritical fluid extraction of α- and β-acids from hops compared to cyclically pressurized solid-liquid extraction. J. Supercrit. Fluids. 2013;84:113–120. [Google Scholar]
- 33.Ferrara L., Naviglio D., Gallo M. Extraction of bioactive compounds of saffron (Crocus sativus L.) by ultrasound assisted extraction (UAE) and by rapid solid-liquid dynamic extraction (RSLDE) Eur. Sci. J. 2014;10(3) [Google Scholar]
- 34.Cozzolino I., Vitulano M., Conte E., D’Onofrio F., Aletta L., Ferrara L. Extraction and curcuminoids activity from the roots of Curcuma longa by RSLDE using the Naviglio extractor. Eur. Sci. J. 2016;12(10) [Google Scholar]
- 35.Gallo M., Conte E., Naviglio D. Analysis and comparison of the antioxidant component of Portulaca oleracea leaves obtained by different solid-liquid extraction techniques. Antioxidants. 2017;6(3):64. doi: 10.3390/antiox6030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallo M., Formato A., Ianniello D., Andolfi A., Conte E., Ciaravolo M. Supercritical fluid extraction of pyrethrins from pyrethrum flowers (Chrysanthemum cinerariifolium) compared to traditional maceration and cyclic pressurization extraction. J. Supercrit. Fluids. 2017;119:104–112. [Google Scholar]
- 37.Gallo M., Vitulano M., Andolfi A., DellaGreca M., Conte E., Ciaravolo M., Naviglio D. Rapid Solid-Liquid Dynamic Extraction (RSLDE): a new rapid and greener method for extracting two steviol glycosides (stevioside and rebaudioside A) from stevia leaves. Plant Foods Hum. Nutr. 2017;72(2):141–148. doi: 10.1007/s11130-017-0598-1. https://link.springer.com/article/10.1007/s11130-017-0598-1 [DOI] [PubMed] [Google Scholar]
- 38.Gallo M., Formato A., Formato G., Naviglio D. Comparison between two solid-liquid extraction methods for the recovery of steviol glycosides from dried stevia leaves applying a numerical approach. Processes. 2018;6(8):105. [Google Scholar]
- 39.Valdés L., Cuervo A., Salazar N., Ruas-Madiedo P., Gueimonde M., González S. The relationship between phenolic compounds from diet and microbiota: impact on human health. Food & Funct. 2015;6(8):2424–2439. doi: 10.1039/c5fo00322a. https://pubs.rsc.org/en/content/articlehtml/2015/fo/c5fo00322a [DOI] [PubMed] [Google Scholar]
- 40.Shahidi F., Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects–A review. J. Funct. Foods. 2015;18:820–897. [Google Scholar]
- 41.Xiao J.B., Hogger P. Dietary polyphenols and type 2 diabetes: current insights and future perspectives. Curr. Med. Chem. 2015;22(1):23–38. doi: 10.2174/0929867321666140706130807. https://www.ingentaconnect.com/content/ben/cmc/2015/00000022/00000001/art00005 [DOI] [PubMed] [Google Scholar]
- 42.Cherniack E.P. Molecular Basis of Nutrition and Aging. Academic Press; 2016. Polyphenols and aging; pp. 649–657. [Google Scholar]
- 43.Chiva-Blanch G., Badimon L. Effects of polyphenol intake on metabolic syndrome: current evidences from human trials. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/5812401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L., Miao M. Dietary polyphenols modulate starch digestion and glycaemic level: a review. Crit. Rev. Food Sci. Nutr. 2019:1–15. doi: 10.1080/10408398.2018.1544883. [DOI] [PubMed] [Google Scholar]