Abstract
Ultrasound-mediated transdermal delivery is a promising parenteral administration method for large-molecule or unstable medications. This study evaluated skin health and systemic delivery when administering enfuvirtide, an injectable antiretroviral medication, over a one-month period in a porcine model using a low-frequency cymbal transducer. Three groups received twice-daily treatments: 1) enfuvirtide injection control (n=12); 2) saline ultrasound control (n=6); and 3) enfuvirtide ultrasound treatment (n=13). Ultrasound parameters: 30-minute exposure, 90 mW/cm2, 24-26 kHz, and 15% duty cycle. No statistical difference in transepidermal water loss, a measure of skin health and function, was seen between ultrasound-treated and control skin sites for either saline (p=0.50) or enfuvirtide (p=0.29) groups. Average trough plasma concentrations of enfuvirtide were 0.6±0.2 μg/mL and 2.8±0.8 μg/mL for ultrasound and injection, respectively. Safety and efficacy results indicate chronic, low-frequency ultrasound exposure can be a practical means for transdermal delivery of medications such as enfuvirtide.
Keywords: ultrasound, transdermal, enfuvirtide, transducer, delivery, skin, transepidermal water loss, cymbal, chronic, large-molecule
Introduction
Pain is a nearly universal collateral effect associated with needle injections of medications for chronic conditions and diseases.(Mcmurtry et al., 2016; Parker, 2016) In patients with human immunodeficiency virus (HIV), pain can be compounded by injection site reactions (ISRs) reported to occur when using the prescribed medication enfuvirtide (T-20, marketed as Fuzeon®).(Loutfy et al., 2007) T-20 is an injectable peptide used as a ‘salvage’ drug to treat HIV-1, once HIV-1 has become resistant to other treatments, by acting to inhibit fusion and entry of the virus into CD4 cells (T-cells).(Wild et al., 1993; Kilby et al., 1998) Because T-20 is an entry inhibitor, it works outside the cell, blocking HIV-1 entry into a host cell. Rather than utilizing the typical encoded viral enzymes utilized by other antiretroviral medications, it boasts the potential benefit of not causing the characteristic multi-drug resistance pathways that arise in HIV patients due to mutations in encoding genes.(Zhang et al., 2002) However, other mechanisms of drug resistance remain possible, and nearly perfect regiment compliance must be maintained to arrest these mechanisms and sustain low viral transcription levels. Due to the peptide chain’s instability and clearance rate from the body, T-20 is prescribed as a twice daily injection (90 mg per 1 mL) to maintain the required therapeutic drug concentrations; however, adherence is a significant challenge due to the ISRs.(Kilby et al., 1998; Usa, 2012) During Phase III clinical trials, 98% of patients reported at least one mild or moderately painful ISR during the first week, and longer-term cutaneous reactions ranged from transient acute patterns to sclerodermalike lesions.(Borras-Blasco et al., 2008) Despite the potential consequences of missed doses, one clinical trial reported the rate of adherence (defined as administration of ≥95% of prescribed doses) at merely 58%.(Wright et al., 2008)
Ultrasound-mediated transdermal drug delivery represents a painless and non-invasive drug delivery method, with the potential to entirely replace subcutaneous injections. Additionally, transdermal drug delivery provides promise in reducing or eliminating ISRs by eliminating the irritation from repeatedly piercing the skin and the resultant pressure created under the skin from the drug bolus. Though the exact mechanism for enhanced skin permeabilization is not fully characterized, researchers in prior studies have postulated that the lipid membranes of the stratum corneum (outermost layer of the epidermis) in the skin are disrupted through cavitation, convection and thermal effects, and the generated defects are of a size that allows permeation of large molecules. (Wu et al., 1998; Polat et al., 2011) Previous in vivo studies have demonstrated the successful ultrasound-mediated transdermal delivery of compounds substantially larger than the typical passive diffusion limit through the stratum corneum of the skin (Molecular weight: ~500 Da) - including erythropoietin (48 kDa) and insulin (5.8 kDa).(Mitragotri et al., 1996; Boucaud et al., 2002; Park et al., 2007; Park et al., 2008; Smith, 2008) T-20 has a molecular weight of 4,492 Da, which is approximately 80% that of insulin.
Both high- (above 1 MHz) and low-frequency (< 100 kHz) ultrasound systems have been previously evaluated with acoustic intensities ranging from 0-15 W/cm2 of which a good overview of devices is given by Smith. (Smith, 2008) It has been suggested that low-intensity (≤100 mW/cm2) and low-frequency ultrasound does not adversely affect tissue integrity when used as a chronic treatment, in part since: 1) the size of cavitation bubbles in the coupling medium are larger than the stratum corneum thickness; and 2) stable cavitation is primarily induced instead of the more destructive transient cavitation.(Smith, 2008; American Institute of Ultrasound in Medicine, 2015) In support of this, several systems have demonstrated that cutaneous application of ultrasound below 50 kHz enhances wound healing in vivo over multiple treatment sessions, which has vast implications for medical care.(Samuels et al., 2013; Maan et al., 2014) Moreover, low-frequency ultrasound has also been shown to enhance transdermal drug transport by up to 1,000 times that of high-frequency ultrasound.(Mitragotri et al., 1996) Though many studies have focused on acute and short-term effects, the authors are not aware of long-term in vivo studies in which skin integrity was evaluated during transdermal drug delivery.
In this project we evaluated the long-term (29-day) effect of twice daily, low-intensity ultrasound-mediated transdermal treatment with saline or T-20 in a live porcine model, serving as a human skin analog. Visual skin inspection, transepidermal water loss (TEWL), and histological tissue analysis were used for skin health assessment. Circulating plasma concentrations of T-20 were also determined via liquid chromatography/electrospray ionization mass spectrometry (LC-MS/MS) to compare bioavailability of transdermal and subcutaneous injection treatments.
Materials and Methods
Ultrasound System
Ultrasound Transducer Active Patch
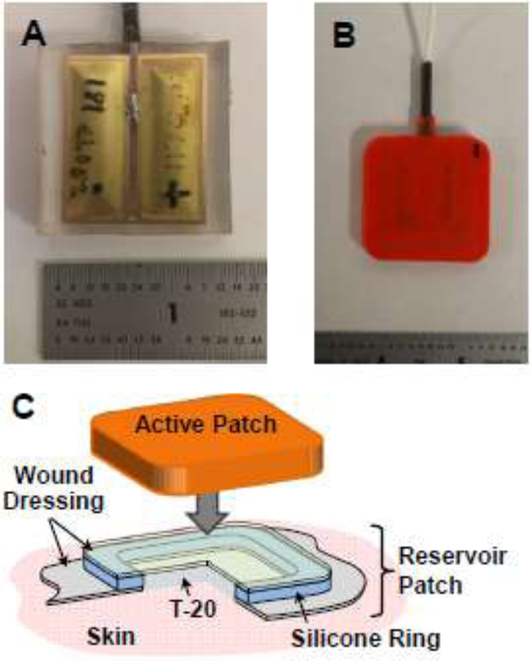
The detailed design and construction of low-frequency ultrasound projectors incorporating an array of cymbal transducers have been described in detail elsewhere.(Newnham et al., 1994; Maione et al., 2002; Luis et al., 2007) A rectangular cymbal geometry (13 mm × 32 mm) replaced round cymbals (Fig. 1A, 1B) to increase efficiency by bringing the length-extensional resonance of the lead zirconate-titanate (PZT) Navy Type I ceramic (42 kHz) closer to the end cap flexural resonance (24-26 kHz).(Luis et al., 2007) For the array, two rectangular transducers were connected in parallel, which also provided a larger area of vibration (i.e., cymbal cap area) within the same footprint relative to four round cymbals.
Figure 1. Ultrasound Active Patch.
Photographs of a potted Active Patch transducer with two rectangular cymbals operating in parallel with (A) clear encapsulant to show orientation and (B) opaque encapsulant used in study. (C) Illustration of the Reservoir Patch in cross-section; ultrasound gel is placed between the Active Patch and upper wound dressing layer in the Medication Patch, and T-20 is in direct contact with the skin.
The array was driven by a custom control board (electronics) designed to generate sinusoidal tone bursts at a 15% duty cycle and 1 Hz repetition rate. The control board incorporated an inductor and capacitor tuning circuit to match the drive electronics and array impedances and tune the resonance frequency (as denoted by zero ohms reactance). The control board measured the tuned array resonance frequency in real-time and dynamically adjusted the frequency of each tone burst to match. Electrical efficiency was maintained by accounting for small shifts in resonance frequency due to factors such as environmental or body (skin) temperature changes or self-heating. Variations in frequency with testing and experiments was generally within 2 kHz (24-26 kHz). The system operated from a 12 VDC external power supply.
Ultrasound Transducer Reservoir Patch
The Reservoir Patch (Fig. 1C) was assembled from a clear commercial wound dressing (Mepore®, Mölnlycke, Sweden) and a cast silicone ring. The ring was adhered to the dressing and served as the drug reservoir. The dressing area within the well was removed to allow the reconstituted T-20 to lie directly against the skin during testing. Additional dressing layer material was adhered to the top of the silicone ring to close the reservoir well. Two 25-Gauge needles were placed through the Reservoir Patch silicone ring wall prior to application on the subject; one needle served as the dosing channel while the other was utilized to remove any air bubbles within the well. Both needles were removed following successful delivery of the T-20 (or saline), prior to applying the ultrasound transducer (Active Patch, Fig. 1B, 1C) over the drug-filled Reservoir Patch.
In vitro Ultrasound Exposimetry
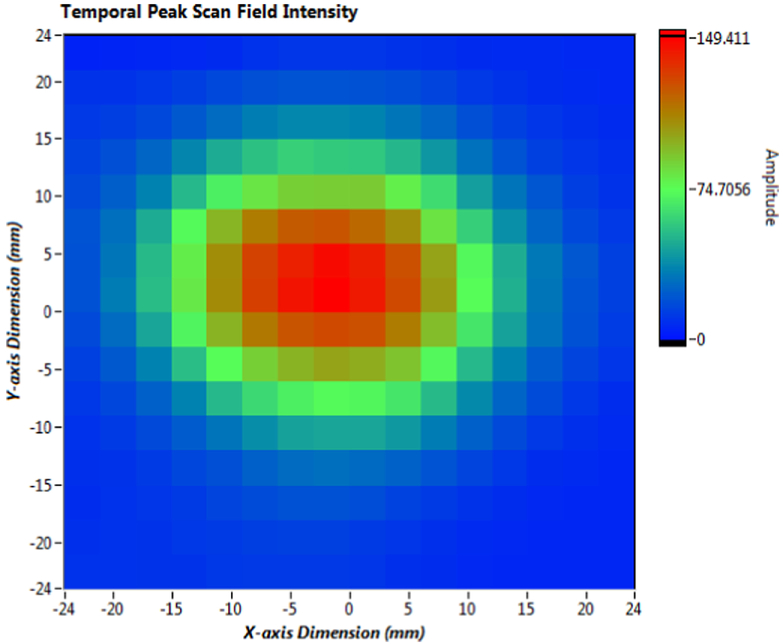
The acoustic intensity of the transducer was determined according to guidelines established by the American Institute of Ultrasound in Medicine.(American Institute of Ultrasound in Medicine, 2004) The pressure field from the transducer was measured in a water tank (60 cm × 60 cm × 130 cm) with a calibrated miniature hydrophone (Model TC4013, Teledyne Reson, Inc., Camarillo, CA) and integrated preamplifier. The hydrophone was automatically positioned using a 3-axis positioning system (Velmex Inc., East Bloomfield, NY) under computer control. The array front face was immersed in the tank and measured with the acoustic axis in the vertical plane. Measurements were taken 2 mm from the array face with a lateral spatial sampling of 3 mm over a scanning area of 48 mm × 48 mm. The lateral −3 dB beam width, as shown in the representative acoustic intensity plot, is approximately 1 cm at a 2 mm distance (Fig. 2). The signals were acquired and digitized using a 200 MHz oscilloscope (National Instruments, Austin, TX). From the digitized signal, the pulse-intensity integral and frequency spectrum were calculated, followed by calculation of temporal peak intensity.(Medicine, 2004) The position of the spatial peak-temporal peak intensity (Isptp) was determined within the scanning area, and the hydrophone was fixed at that point. The Isptp was then measured every 5 seconds over a 30-minute duration (n=360), and the mean and standard deviation of Isptp were calculated as 92.6±0.8 mW/cm2.
Figure 2. Acoustic intensity plot.
The measured lateral −3 dB beam width, as shown in the representative acoustic intensity plot, is approximately 1 cm at a distance of 2 mm.
In vitro HIV Fusion Assay
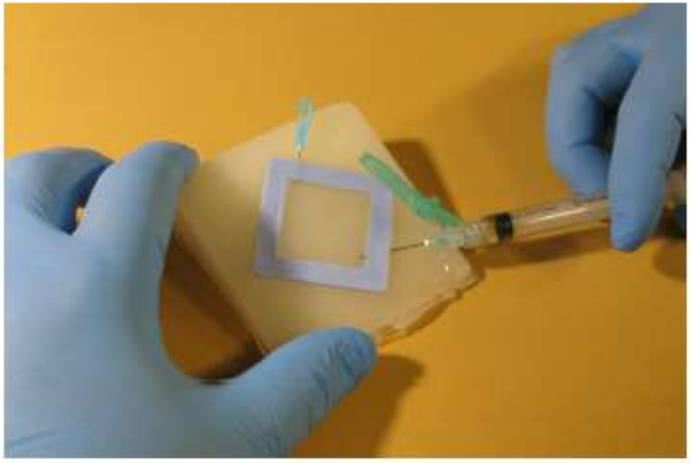
To verify that T-20’s exposure to ultrasound did not cause significant catabolic or conformational changes (and subsequent suppression of inhibitory capacity), T-20 activity following insonification was evaluated in vitro. Using the PhenoSense™ Entry Assay (Monogram Biosciences, South San Francisco, CA), a recombinant virus assay that measures the susceptibility of live HIV-1 strains to inhibitors of virus-host cell membrane fusion, the lack of catabolic or conformational changes was confirmed. Supplies and Ultrasound Transducer Active Patches (Active Patches) were delivered to Monogram Biosciences, who performed the insonification and in vitro assays according to standard operating procedures and written test protocols. Lyophilized T-20 (90 mg, Genentech, South San Francisco, CA) was reconstituted within the original vial as per the manufacturer’s instructions. The T-20 for the Control group was not subjected to any additional processing before the assays. For each test in the Active and Sham groups, the reconstituted T-20 was immediately injected into an Ultrasound Transducer Reservoir Patch (Reservoir) that was adhered to a skin tissue analog (Fig. 3), followed by application and placement of acoustic coupling gel and an Active Patch, respectively. The skin tissue analog was composed of polyvinyl chloride plastisol,(Bohndiek et al., 2013; Jeong et al., 2017) cast into triangular wedges to minimize direct acoustic reflections from the back surface. The reconstituted T-20 remained in the Reservoir for thirty (30) minutes: the Active Patch was activated for the full duration during Active group testing, and remained off for the duration during Sham group testing. For both the Sham and Active groups, T-20 was removed from the Reservoir Patch and then used in the Entry Assay. The Entry Assay was completed with three separate HIV-1 strains, JRCSF, HXB2, and 92HT594, in triplicate assays per strain. The IC50 and fold change for each strain were quantified. Each HIV-1 strain was analyzed separately. Data was checked for normal distribution (Shapiro-Wilk normality test) and equal variances (Bartlett test of homogeneity of variances) and subsequently analyzed by a One-way analysis of variance, followed by a Tukey’s multiple comparison test (IC-50/92HT595, Fold Change/92HT595, Fold Change/HXB2, and Fold Change/JRCSF) or alternatively analyzed by a Kruskal-Wallis test followed by a Dunn’s multiple comparison for data sets violating ANOVA assumptions (IC-50/JRCSF and IC-50/HXB2).
Figure 3. In vitro bench skin analog.
The skin tissue analog composed of polyvinyl chloride plastisol, cast into triangular wedges to minimize direct acoustic reflections from the back surface. One needle is used to load the reconstituted drug into the Reservoir Patch, while the second needle allows air to exit. Both needles are removed prior to placement of the Active Patch on the drug reservoir.
Animal Experiments
Animals
All procedures described herein were reviewed and approved by The Pennsylvania State University Institutional Animal Care and Use Committee and were performed in adherence with the “Principles of Laboratory Animal Care” (NIH publication #85-23, revised in 1985). The study population was comprised of 31 Yorkshire pigs (3-4 months of age, both genders, 43-59 kg on Day 1 of study) obtained from The Pennsylvania State University Swine Center (University Park, PA). Subjects were individually housed in a temperature- and humidity-controlled room (21 ± 3°C; 55 ± 15%) and exposed to a 12:12-hr light:dark cycle (lights on at 07:00) with multiple runs within the room (one animal per run). Water was available ad libitum throughout the study and feed (Penn State Swine Center) was administered twice daily. All subjects received their designated treatment for 29 days with euthanasia performed on Day 30.
Subjects were designated to one of three experimental treatment groups: the Saline Group (denoted SG, n=6) received twice-daily ultrasound exposure with saline administration (no T-20); the Active Group (denoted AG, n=13) received twice-daily ultrasound exposure with transdermal T-20 administration (90 mg/treatment); the Injection Group (denoted IG, n=12) received twice-daily subcutaneous injections of T-20 (90 mg/treatment). The AG and IG were each subdivided into two sub-groups (n=6 for each sub-group): bioavailability (b) and chronic (c). Thirteen (13) more blood collections (denoted by “B” on Table 1) were completed for the bioavailability groups (bIG and bAG) relative to the chronic groups (cIG and cAG) to evaluate pharmacokinetics over a 24-hour period, but were otherwise identical in protocol. Subjects underwent all treatments in a conscious state and were allowed free movement within their run or transport cart for the duration of all treatments.
Table 1.
Study experimental design. Timing of TEWL measurements (T) and Blood draws (B) are presented over the thirty (30) day study.
| Day of Experiment | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 25 | 26 | 27 | 28 | 29 | 30 | ||||||||||
| Saline (SG) | T | T | T | T | T | T | |||||||||||||||||||||
| T-20 AG + IG |
Bio | B B T |
B B | B | B B T |
B | B T |
B | B | BBB | B T |
B | T | B | … | T | B | B T |
|||||||||
| Chronic | T | T | T | B | T | B | B | T | B | T | B | B T |
|||||||||||||||
A passive control group, transdermal T-20 administration using no ultrasound, was not used in the study since it was not expected to get meaningful results. From a general standpoint, the large molecule size is expected to prohibit significant diffusion through the stratum corneum. More specifically, the partition coefficient (Log P), or measure of transport and partitioning of a drug through the skin layers and into tissue as based on its lipophilic and hydrophilic phases, is −14.7 for T-20 whereas ideal transdermal transport requires Log P between +2.0 and +3.0, with transport specifically into the stratum corneum increasing above +3.0. (Ham e Buckheit, 2015) This means that although transport from within the skin to the circulation is very high, the initial passive diffusion of T-20 into the stratum corneum is basically negligible.
T-20 In vivo Delivery
Lyophilized T-20 was obtained as one-month (60 vial) kits from a commercial medical vendor and reconstituted as per the manufacturer’s instructions (Genentech, South San Francisco, CA). Each T-20 vial was diluted with 1.1 mL sterile water to formulate a 90 mg/1 mL dose.(Usa, 2012) For AG subjects, reconstituted T-20 was further diluted to 1.5 mL (final volume) with sterile water to completely fill the Reservoir Patch well.
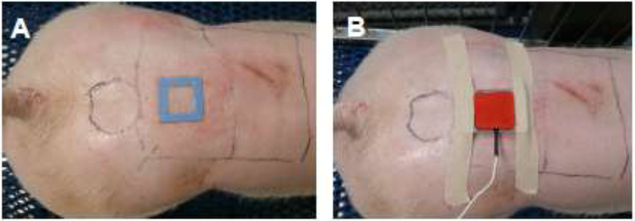
For AG and SG subjects, twice-daily treatments were administered on alternating morning and evening treatment sites. For each treatment, a Reservoir Patch was adhered to each subject, either 1.5 mL of reconstituted T-20 or saline, respectively, was injected into the Reservoir Patch well, where it would make direct contact with the skin, and the loading needles were removed (Fig. 4A). An aluminum ring was placed around the reservoir and served to align the Reservoir Patch with the Active Patch transducer. After application of a thin layer of ultrasound coupling gel, the Active Patch transducer was secured over the Reservoir Patch (Fig. 4B). Ultrasound treatments consisted of applying the activated ultrasound transducer (Active Patch) for 30 minutes. At the conclusion of the active treatment duration, the Active Patch transducer and aluminum ring were removed, leaving the drug-filled or saline-filled reservoir patch in place until the next treatment, allowing for maximum diffusion of the drug to diffuse across the skin. For each subsequent treatment period, the previous Reservoir Patch was removed and a new Reservoir Patch was placed onto the subject (on the alternating site) and filled with either a new dose of T-20 or saline. For IG subjects, subcutaneous injections of reconstituted T-20 (90 mg/1 mL) were administered twice-daily via 20G needles in the shoulder region of each subject. Morning and evening injections were given on alternating sides when possible.
Figure 4. Placement of the Reservoir Patch (A) and Active Patch (B) on a subject.
(A) Following active treatment (30 minutes), the passive drug-containing Reservoir Patch remained on the Subject for a twelve-hour treatment period. (B) Ultrasound patch (Active Patch) lying on top of the Reservoir Patch, following addition of reconstituted drug. The Active Patch was aligned with the reservoir using a rounded metal alignment band held during active treatment using medical adhesive bandage. The Control Sites for TEWL measurements are represented with a circle, and the general treatment areas (Patch Site) are represented with rectangles.
TEWL Measurements
Transepidermal water loss (TEWL) is a well-accepted indicator to assess skin barrier function; the non-invasive measurement correlates the rate of water evaporation from the skin to skin integrity (health).(Seidenari et al., 1996; Grove et al., 1999; Kolli e Banga, 2008; Montaser-Kouhsari et al., 2014) A lower TEWL measurement signifies more intact skin with greater moisture retention. TEWL was used to regularly monitor skin health in all subjects in the SG and AG groups. TEWL data was collected using a calibrated wireless Tewameter® TM 300 probe (CK Electronics, Cologne, Germany) with 1 Hz sampling linked to a laptop computer. Each recorded TEWL data point was taken at a minimum of 20 seconds of measurement as a 10-sample running average after readings were stable for at least 10 seconds. For the SG (n=6) and cAG (n=7) groups, TEWL data was recorded every 5 days starting on Day 1, with additional recordings on Day 2 and prior to euthanasia on Day 30 (denoted by “T” on Table 1). For the bAG (n=6) group, TEWL data was recorded every 5 days starting on Day 1 and prior to euthanasia, with additional recordings at the 12-hour, Day 2, and Day 3-time points (denoted by “T” on Table 1). For each measurement, the TM 300 was placed directly onto the Control Site and over the location where the Active/Reservoir Patch resided (Patch Site). The Control Site was represented with a circle, and the general treatment areas (Patch Site) were represented with rectangles (Fig. 4). TEWL values were compared and analyzed by Unpaired Student’s T-test. Room temperature, humidity, and outside temperature were recorded for each treatment for the duration of all experimental periods. All three variables subsequently underwent a correlation analysis to investigate if a potential correlation with TEWL measurements existed.
Blood Sampling and Processing
Blood samples (≥ 25 μL) were collected from the marginal ear veins of all Subjects treated with T-20 (transdermal [AG] or injected [IG]) over the 30-day protocols. Thirteen (13) more collections were completed for the bIG and bAG groups relative to the cIG and cAG groups (denoted by “B” on Table 1). Subjects were not restrained during blood sampling; food treats were used to facilitate collections. All blood samples were collected into EDTA-coated capillary vials and plasma separated by centrifugation (3,000 × g for 5 min at room temperature). Plasma samples were frozen and stored at −80°C until subsequent LC-MS/MS analysis was performed (University of Alabama at Birmingham, Birmingham, AL). Low-protein binding pipette tips (MidSci, St. Louis, MI) and microcentrifuge storage vials (Eppendorf, Hamburg, Germany) were used to minimize possible peptide adhesion.
Day 30 and Histological Analysis
At the end of each experimental period, Subjects were initially sedated with ketamine hydrochloride/sodium xylazine (10-12 mg/kg and 1-2 mg/kg, intramuscular injection) and then euthanized via Euthasol injection (pentobarbitol) into either the vena cava or cannulated ear vein. Tissue (skin) samples (5 cm × 5 cm) from the Patch Sites and Control Sites were subsequently collected for histopathology. Samples were fixed by immersion overnight in 4% formalin at room temperature. Samples were subsequently washed, dehydrated, cleared, and embedded in paraffin using an automated tissue processor. Tissue sections (5 μm) were cut using a rotary microtome, and serial sections were mounted (The Pennsylvania State University, Animal Diagnostic Laboratory, University Park, PA). Tissue samples were stained using hematoxylin and eosin,(Lillie, 1976) and blindly evaluated for differences between treatments groups by the clinical veterinarian (JWD).
LC-MS/MS Assay and Pharmacokinetics
T-20 Internal Standard
A stable isotopically labelled internal standard (IntSt) for T-20 plasma quantification, d60-enfuvirtide, was synthesized by D. El Atmioui and H. Hilkmann (The Netherlands Cancer Institute, Amsterdam, The Netherlands). Using a previously developed technique for T-20 IntSt synthesis, the six leucine residues in enfuvirtide were replaced by d10-leucine residues.(Van Den Broek et al., 2006) Sufficient IntSt was synthesized for all analysis to be completed.
LC-MS/MS Assay
T-20 plasma concentrations were quantitatively assessed with liquid chromatography/electrospray ionization mass spectrometry (LC-MS/MS) adapted from techniques previously developed from van den Broek et al. (Van Den Broek et al., 2006; Bennetto-Hood et al., 2007) Stock and working solutions were prepared in methanol with a hydroxylamine-acetic acid buffer (pH 6.75) (93:7, vol/vol). Experimental plasma samples were prepared through solid-phase extraction, and chromatographic separation was performed isocratically on a reversed-phase Waters Symmetry C18 column. Acquisition was performed in selected ion monitoring mode for the protonated molecular ions [M+4H]4+ with precursor/product transitions of 1123→1082 m/z for Enfuvirtide (T-20) and 1138→1097 m/z for IntSt. Analytical T-20 concentration ranges from 0.1 to 10.0 μg/mL were quantified using a 50 μL plasma sample, and the ratios of the analyte versus IntSt (MW: 5,552 Da) were determined. Standard curves were developed using linear regression analysis with a mean coefficient of determination (R2) of 0.9969.
Results
HIV Fusion Assay
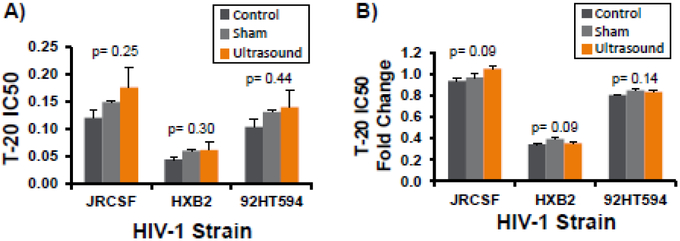
The assay measures the half maximal inhibitory concentration (IC50), which represents a quantitative measure of the effectiveness of a substance to inhibit a specific biological or biochemical function. For each of the three separate HIV-1 strains examined, the mean IC50 for all samples was below 0.2 (Fig. 5A) and did not significantly differ due to treatment group (Control: Dark Gray Bars, Sham Treatment: Light Gray Bars, and Active Group: Orange Bars). Additionally, the T-20 IC50 Fold Change was also analyzed, and no statistical differences were detected due to treatment for any of the cells lines examined (Fig. 5B; p > 0.05).
Figure 5. Quantification of T20’s anti-viral capability following insonification tested in vitro.
The half maximal inhibitory concentration (IC50, A) and IC50 Fold change (B) of T-20 following insonification tested in three separate HIV-1 strains. Treatments: Control (Dark Gray), Mock Ultrasound Exposure (Light Gray Bars) and 30-minute insonification (Orange Bars). Each bar represents triplicate wells/HIV-1 strain. No statistical differences were detected in any of the three strains, p >0.05, Two-way analysis of variance or Kruskal-Wallis Test, Error bars= standard error of the mean.
TEWL results
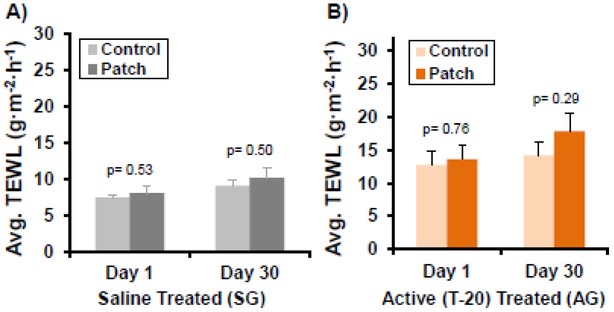
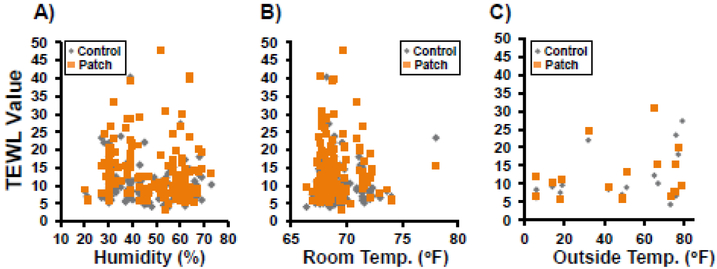
Over the 30-day experimental period, there were no distinguishable differences between Patch Site and Control Site TEWL measurements for both the SG and AG groups (Fig. 6). Fluctuations in ambient humidity, room temperature or outside temperature did not bias TEWL measurements (Fig. 7). All humidity levels measured below 30% (Fig. 7A) were from the Operating Room (Day 1) during acclimation before moving to the study room where humidity was controlled. Variations in measured values between Subjects and between days are believed to be due to several reasons, including variations in pressure from the metal alignment band, and damage to the Active Patches/Reservoirs as the Subjects scratched and moved in the runs at off-times. The latter resulted in inadequate coverage of the treatment site over the full 12-hour period. Varying degrees of skin reactions due to the adhesive bandages were also detected (Fig. 8) in five (5) of the Subjects (SG, AG).
Figure 6. TEWL measurements between Patch and Control Sites for Subjects treated with (A) Saline or (B) T-20.
No statistically significant differences were detected over the 29 days of treatments, suggesting the ultrasound did not adversely affect skin integrity or health. Student’s T-test, statistical significance of mean difference p<0.05. Sham Group (SG): n=6 per treatments, Active Group (AG): n=12-13 per treatments, Error bars= standard error of the mean.
Figure 7. TEWL measurement values relative to multiple environmental parameters.
No correlation (R2≤0.01) was seen between Transepidermal Water Loss (TEWL) (Patch Sites [Orange] and Control Sites [Gray] measurement and A) room humidity (%) or B) room temperature (°F). A very weak correlation was seen between TEWL and C) outside temperature at animal transport on Day 1 (R2≤0.18).
Figure 8. Representative example of adhesive induced rash.
Adhesive used to attach the Active Patch over the Reservoir Patch twice-daily for 29 days caused a rash (arrow), this was the case in both saline and T-20 treated Subjects (Subject 11, T-20 Active Patch at Day 2, Top panel and Day 26, Lower Panel. Morning and Evening treatment locations are designated.
T-20 Delivery and Pharmacokinetics
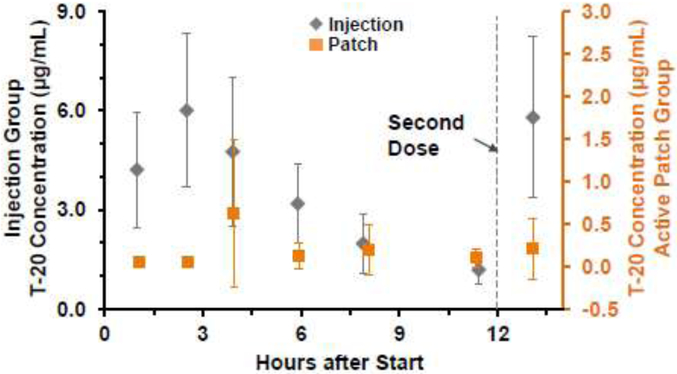
The lowest plasma concentration of T-20 reached before the next dose (Ctrough) for the Active Patch groups bAG and cAG was 0.6±0.2 μg/mL over the duration of the study (Fig. 9, Orange Triangles). This was approximately 22% of the Ctrough of 2.8±0.8 μg/mL measured in the subcutaneous injection groups bIG and cIG (Fig. 9, Gray Diamonds). As a reference, typical human plasma Ctrough concentrations are reported as 3.3±1.6 μg/mL. (Usa, 2012)
Figure 9. Plasma concentrations of T-20 treated Subjects.
Plasma was quantified every three days (following six treatments) prior to the subsequent T-20 treatment. Ultrasound-mediated T-20 Patch (Patch- Orange Triangles) corresponds to approximately 21% of the bioavailability of the quantified subcutaneous injection samples (Injection- Gray Diamonds). Injection Group (IG): n=9-12 per time point, Active Group (AG): n=11-13 per treatments, Error bars= standard deviation.
Data from initial treatment (Day 1) suggests a longer time to maximum concentration observed (Tmax) and lower maximum/peak serum concentration (Cmax) following transdermal delivery compared to IG Subjects (Fig. 10), possibly due to different absorption characteristics as the drug diffuses first from the Reservoir Patch to the dermis and then into the capillaries. However, the lower overall concentrations following the first treatment resulted in several measurements below quantifiable levels (BQL= <0.1 μg/mL). Rather than omitting measurements below BQL, a BQL/2 value was used for calculations since this has shown less bias in estimation.(Bergstrand e Karlsson, 2009) This did not enable full pharmacokinetic evaluation of the transdermal delivery method, and only pharmacokinetics of IG Subjects was determined.
Figure 10. Bioavailability profile of plasma T-20 in Subjects following initial T-20 treatment.
The Active Patch (Orange Squares) data suggests a longer Tmax and more uniform bioavailability for T-20 compared to the Injections (Gray Diamonds) treated Subjects. Injection Group (IG): n=5 per treatments, Active Group (AG): n=4-5 per treatments, Error bars= standard deviation.
The initial concentration-time profile of IG T-20 is similar to the profile detected in humans, though the time of peak was noticeably shorter (Tmax Subjects = 2.5 hours; Tmax humans = 6.9 hours (median; range of 3 to 12 hours)(Zhang et al., 2002; Usa, 2012). There was relatively good agreement between the plasma half-life (t½) for the porcine model (3.97) versus that from the literature (3.80),(Zhang et al., 2002) and the only truly distinct difference was seen in the Tmax (Table 2).
Table 2.
T-20 pharmacokinetics in the porcine model (injection) as compared to humans. Data are means, with coefficients of variation in parentheses. Human data reproduced from Zhang et al., 2002.
| Porcine | Human* | |
|---|---|---|
| Cmax (μg/mL) | 6.31 (30%) | 4.59 (32%) |
| tmax (h) | 2.50 (9%) | 6.92 (36%) |
| AUC∞ (μg·h/mL) | 43.3 (34%) | 55.8 (22%) |
| t½ (h) | 3.97 (27%) | 3.80 (15%) |
| CI (L/h) | 2.31 (39%) | 1.68 (22%) |
Histology
Blinded analysis of the skin samples from the three groups revealed a range of tissue damage and inflammation. Parameters that demonstrated more than 25% occurrence between treatment groups are bolded for emphasis (Table 3/Table 4). Representative examples of tissue damage and inflammation are depicted (Fig. 11).
Table 3.
Histological analysis occurrence rate.
| No Significant lesions |
Inflammation or Inflammatory Infiltrates |
Lymphocytes | Eosinophils | Neutrophils | Monocytes | Langhans Giant cells | Macrophages | Hemmorhage | Ulceration | Necrosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T-20 Injection n=12 | 2 | 9 | 1 | 7 | 0 | 0 | 7 | 3 | 2 | 1 | 3 |
| 16.7% | 75% | 8.3% | 58.3% | 0% | 0% | 58.3% | 25% | 16.7% | 8.3% | 25% | |
| Saline Active n=6 | 3 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 50% | 33.3% | 0% | 16.7% | 0% | 16.7% | 0% | 0% | 0% | 0% | 0% | |
| T-20 Active n=13 | 7 | 5 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 1 | 0 |
| 53.8% | 38.5% | 0% | 0% | 15.4% | 0% | 7.7% | 0% | 0% | 7.7% | 0% |
Table 4.
Skin irritation occurrence rate.
| None | Similar to Eczema |
Minor | Major | Major+ Necrosis |
|
|---|---|---|---|---|---|
| T-20 Injection | 2 | 1 | 3 | 3 | 3 |
| Saline Active | 3 | 3 | |||
| T-20 Active | 7 | 1 | 4 | 1 |
Figure 11. Representative histological images of T20 and subcutaneous injection sites in pigs (hematoxylin and eosin stain).
T-20 Patch: No significant lesions were observed in the epidermis, dermis or subcutaneous tissue (A-D). T-20 Injection: No significant lesions were observed in the epidermis or dermis (E, F, J, K). Extensive deep subcutaneous granulomatous inflammation with many Langhans giant cells, perivascular lymphoid infiltrates, occasional foci with eosinophilic infiltrates and subcutaneous hemorrhage. In some areas macrophages surround amorphous eosinophilic material (G, H, I, L, M, N). Blinded histological analysis completed (JWD). EPI= epidermis, DER= dermis, SUB= subcutaneous. Magnification objective= 2X and 4X, scale bar= 1 mm, 10X and 20X, scale bar= 200 μm, and 40X, scale bar = 100 μm.
IG Subjects (subcutaneousT-20 injection group):
Nine (9) of twelve (12) Subjects (75%) exhibited deep subcutaneous granulomatous inflammation of variable severity, ranging from mild to severe. Small cutaneous ulcerations were observed in sections from one (1) Subject (8.3%) and no significant lesions were observed in sections from the remaining two (2) Subjects (16.7%).
SG Subjects (Saline-Ultrasound treated group):
Two (2) of the six (6) Subjects (33.3%) exhibited mild focal dermal perivascular inflammation. One (1) Subject exhibited dense cellular capillary beds in the dermis (monocytes and granulocytes). No significant lesions were observed in sections from three (3) Subjects (50%).
AG Subjects (T-20 Ultrasound Treatment group):
Five (5) of thirteen (13) Subjects (30.8%) exhibited inflammatory lesions affecting the epidermis and/or superficial dermis. These lesions, localized to a portion of the section, were characterized by dermal edema, inflammatory infiltrates, elongation of rete ridges at the dermo-epidermal junction and epidermal ulceration with superficial epidermal crusts. One (1) subject exhibited a few Langhans Giant cells in one small area of the superficial dermis (7.7%), while no significant lesions were observed in sections from seven (7) Subjects (53.8%). Analysis was completed on a single stained section from each Subject; the possibility exists that other small lesions may not have been represented in the section examined.
Discussion
The primary goal of this work was to evaluate the long-term effects and safety of a low-frequency, low-power, ultrasound-mediated transdermal delivery device for the injectable HIV medication enfuvirtide (T-20) when used in vivo in an animal model comparable to humans. Moreover, the long-term effects of continuous low-frequency, low-power ultrasound have not previously been evaluated. The secondary study goal was to demonstrate successful delivery of T-20 into the circulation and compare pharmacokinetic parameters with that of the currently approved injection regimen.
Typically in purely passive delivery of small molecules (< 500 Daltons), only a small portion of total drug content is absorbed through the skin; in lidocaine patches (Lidoderm®) only 3% of the total drug content is absorbed. (Paudel et al., 2010) Compared to current passive drug delivery approaches, Active Ultrasound Patches successfully provided delivery of a large “impermeable” molecule that is significantly higher than passive delivery of small permeable molecules. The quantified plasma T-20 levels demonstrated that ultrasound enhancement could not only overcome the size-dependent molecular barrier of the stratum corneum, but it could provide more complete delivery of a large molecule than is achieved with small molecules using passive delivery. Though the ultrasound-mediated transdermal delivery of T-20 did not provide maximum or sustained levels of T-20 equal to that detected via injection, nevertheless, the plasma levels of approximately 21% that of the injection demonstrates the benefit of transdermal delivery. This was also achieved without any modification to the T-20 (e.g., pH adjustment) or optimization of Active Patch parameters to enhance transdermal delivery. The work was performed using T-20 that was not modified for use as a transdermal agent, which demonstrates both a ‘worst-case’ scenario for drug delivery, but is also limited in determining what the ‘best-case’ scenario may be for delivery, or determining the contribution of multiple factors on skin sensitization, such as non-ideal pH of the solution (for transdermal application) relative to the T-20 itself. However, other formularies are beyond the scope of this work.
Concerns regarding the ultrasound technology directly affecting the drug to be delivered or the skin quality were directly tested. The authors successfully demonstrated that in vitro ultrasound exposure of T-20 did not cause significant catabolic or conformational changes (and subsequent suppression of inhibitory capacity) as determined using the PhenoSense™ Entry Assay. Additionally, negligible differences in skin quality between Control and Patch Sites (ultrasound-treated areas) were seen over a 30-day period, demonstrating that low-frequency, low-power ultrasound is a viable alternative for chronic delivery of medications. Based on the TEWL measurements, treatment using the low-frequency, low-power ultrasound alone (with saline- SG) does not significantly affect skin quality as compared with a control.
As verified with the histological analysis, mild inflammation is present after using the Active patch for an extended duration of one month, though this is consistent with other chronic conditions, i.e., eczema. It is currently unclear whether this minimal inflammation is due to the ultrasound treatment or due to irritation from adhesives application and removal and continuous coverage (occlusion). Based on the types of lesions observed, five general skin damage categories were made by the authors to simplify damage assessment, depending on the type and quantity of cells found to be present, or the presence of edema or necrosis (Table IV).
The authors hypothesize that the T-20 medication produces a localized sensitization that exacerbates the irritation from the adhesives, though this effect is less severe than the effects of subcutaneous bolus injection. A small effect in skin quality that was attributable to T-20 was seen, but it appeared less severe than samples from Injection Subjects. By comparing the T-20 treatment groups, the Injection group had a mere 16.7% of its Subjects exhibit no significant lesions, while this was 53.8% in the T-20 Active Group (Table 3). Of interest, the presence of inflammation or infiltration of eosinophils, Langhans Giant Cells, macrophages and necrosis were all reduced by greater than 25% in the T-20 Active group compared to the T-20 Injection Group.
It is acknowledged that this study is preliminary, and a more exhaustive study is needed for improved statistics. Subsequent studies are necessary to increase the effective plasma minimum concentration to that near subcutaneous injection. A larger patch dosage may be needed, similar in methodology to passive patches currently used for small molecules such as nicotine. The plasma T-20 concentrations quantified following delivery using ultrasound-mediated transdermal methods limited the ability to measure pharmacokinetic parameters. Conclusions are limited to one general skin type/thickness, and although the study demonstrates that transdermal T-20 delivery can be achieved, broad conclusions cannot be made regarding delivery rate or pharmacokinetics for many skin types or treatment areas on the body.
Acknowledgements
This work was supported through the National Institute of Allergy and Infectious Diseases/National Institutes of Health Program 2R44AI080335. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases/National Institutes of Health. The authors would like to thank Zachary Krieger, Jason Showers, Jenna Greaser, and Melissa Welker for help with animal handling and sample collection, and Chantelle Tuggle for mass spectrometry assay testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AMERICAN INSTITUTE OF ULTRASOUND IN MEDICINE, Acoustic output measurement standard for diagnostic ultrasound equipment, 2004. [Google Scholar]
- AMERICAN INSTITUTE OF ULTRASOUND IN MEDICINE, Statement on mammalian biological effects of ultrasound in vivo, 2015. [Google Scholar]
- BENNETTO-HOOD C; LONG M; ACOSTA E Development of a sensitive and specific liquid chromatography/mass spectrometry method for the determination of tenofovir in human plasma. Rapid Commun Mass Spectrom, 2007, v. 21, n. 13, p. 2087–94. [DOI] [PubMed] [Google Scholar]
- BERGSTRAND M; KARLSSON M Handling data below the limit of quantification in mixed effect models. AAPS Journal, 2009, v. 11, n. 2, p. 371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOHNDIEK SE; BODAPATI SANDHYA; VAN DE SOMPEL DOMINIQUE; KOTHAPALLI SRI-RAJASEKHAR; GAMBHIR SANJIVS Development and application of stable phantoms for the evaluation of photoacoustic imaging instruments. PLOS ONE, 2013, v. 8, n. 9, p. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORRAS-BLASCO J NAVARRO-RUIZ A; BORRAS C; CASTERA E Adverse cutaneous reactions associated with the newest antiretroviral drugs in patients with human immunodeficiency virus infection. Journal of Antimicrobial Chemotherapy, 2008, v. 62, n. 5, p. 879–888. ISSN 0305–7453. [DOI] [PubMed] [Google Scholar]
- BOUCAUD A ; GARRIGUE MA; MACHET L; VAILLANT L; PATAT F Effect of sonication parameters on transdermal delivery of insulin to hairless rats. J Controlled Release, 2002, v. 81, p. 113–119. [DOI] [PubMed] [Google Scholar]
- GROVE GL GROVE MARYJO; ZERWECK CHARLES; PIERCE ELIZABETH. Comparative metrology of the evaporimeter and the DermaLab® TEWL probe. Skin Research and Technology, 1999, v. 5, n. 1, p. 1–8, ISSN 1600–0846. [Google Scholar]
- HAM AS; BUCKHEIT RW Jr. Current and emerging formulation strategies for the effective transdermal delivery of HIV inhibitors. Ther Deliv, 2015, v. 6, n. 2, p. 217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEONG E-J SONG HYUN-WOO; LEE YONG-JAE; PARK SUJUN; YIM MIJUNG; LEE SUSUNG; KIM BONGKYU. Fabrication and characterization of PVCP human breast tissue-mimicking phantom for photoacoustic imaging. BioChip Journal, 2017, v. 11, n. 1, p. 67–75. [Google Scholar]
- KILBY JM; HOPKINS S; VENETTA TM; DIMASSIMO B; CLOUD GA; LEE JY; ALLDREDGE L; HUNTER E; LAMBERT D; BOLOGNESI D; MATHEWS T; JOHNSON MR; NOWAK MA; SHAW GM; SAAG MS Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nature Medicine, 1998, v. 4, n. 11, p. 1302–1307. ISSN 1078–8956. [DOI] [PubMed] [Google Scholar]
- KOLLI C; BANGA A Characterization of Solid Maltose Microneedles and their Use for Transdermal Delivery. Pharmaceutical Research, 2008, v. 25, n. 1, p. 104–113. ISSN 0724–8741. [DOI] [PubMed] [Google Scholar]
- LILLIE R Histopathologic Technic and Practical Histochemistry. 1976, 4 New York: McGraw-Hill. ISBN 0070378622. [Google Scholar]
- LOUTFY MR; HARRIS M; RABOUD JM; ANTONIOU T; KOVACS C; SHEN S; DUFRESNE S; SMAILL F; ROULEAU D; RACHLIS A; GOUGH K; LALONDE R; TSOUKAS C; TROTTIER B; WALMSLEY S; MONTANER J A large prospective study assessing injection site reactions, quality of life and preference in patients using the Biojector((R)) vs standard needles for enfuvirtide administration. Hiv Medicine, 2007, v. 8, n. 7, p. 427–432. ISSN 1464–2662. [DOI] [PubMed] [Google Scholar]
- LUIS J PARK EJ; MEYER RJ; SMITH NB. Rectangular cymbal arrays for improved ultrasonic transdermal insulin delivery. J Acoust Soc Am, 2007, v. 122, n. 4, p. 2022–30. ISSN 1520–8524. [DOI] [PubMed] [Google Scholar]
- MAIONE E SHUNG KK; MEYER RJ; HUGHES JW; NEWNHAM RE; SMITH NB. Transducer design for a portable ultrasound enhanced transdermal drug delivery system. IEEE Trans Ultrason Ferroelectr Freq Contr, 2002, v. 49, p. 1430–6. [DOI] [PubMed] [Google Scholar]
- MAAN Z; JANUSZYK M; RENNERT RC; DUSCHER D; RODRIGUES M; FUJIWARA T; WHITMORE NHA; HU MS; LONGAKER MT; GURTNER GC.. Noncontact, low-frequency ultrasound therapy enhances neovascularization and wound healing in diabetic mice. Plast Reconstr Surg, 2014, v. 134, n. 3, p. 402e–411e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCMURTRY C TADDIO A; NOEL M; ANTONY MM; CHAMBERS CT; ASMUNDSON GJG; RIDDELL RP; SHAH V; MACDONALD NE; ROGERS J; BUCCI LM; MOUSMANIS P; LANG E; HALPERIN S; BOWLES S; HALPERT C; IPP M; RIEDER MJ; ROBSON K; ULERYK E; BLEEKER EV; DUBEY V; HANRAHAN A; LOCKETT D; SCOTT J. Exposure-based interventions for the management of individuals with high levels of needle fear across the lifespan: a clinical practice guideline and call for further research. Cogn Behav Ther, 2016, v. 45, n. 3, p. 217–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITRAGOTRI S; BLANKSCHTEIN D; LANGER R Transdermal drug delivery using low-frequency sonophoresis. Pharm Res, 1996, v. 13, n. 3, p. 411–20. ISSN 0724–8741 (Print)0724–8741. [DOI] [PubMed] [Google Scholar]
- MONTASER-KOUHSARI L; ZARTAB H; FANIAN F; NOORIAN N; SADR B; NASSIRI-KASHANI M; FIROOZ A Comparison of intradermal injection with iontophoresis of abobotulinum toxin A for the treatment of primary axillary hyperhidrosis: a randomized, controlled trial. J Dermatolog Treat, 2014, v. 25, n. 4, p. 337–41. ISSN 0954–6634. [DOI] [PubMed] [Google Scholar]
- NEWNHAM RE; XU QC; YOSHIKAWA S Metal-electroactive ceramic composite actuators. (USPTO), U. S. P. A. T. O. USA: The Pennsylvania Research Corporation; 1994. [Google Scholar]
- PARK E; DODDS J; SMITH N Dose comparison of ultrasonic transdermal insulin delivery to subcutaneous insulin injection. Int J Nanomedicine, 2008, v. 3, n. 3, p. 335–41, 2008. ISSN 1176–9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK E; WERNER J; SMITH N Ultrasound mediated transdermal insulin delivery in pigs using a lightweight transducer. Pharmaceutical Research, 2007, v. 24, n. 7, p. 1396–401. [DOI] [PubMed] [Google Scholar]
- PARKER J Four steps to eliminate or reduce pain in children caused by needles (part 1). Pain Management, 2016. [DOI] [PubMed] [Google Scholar]
- PAUDEL K MILEWSKI M; Swadley CL; Brogden NK; Ghosh P; Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv, 2010, v. 1, n. 1, p. 109–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLAT B; HART DOUGLAS; LANGER ROBERT; BLANKSCHTEIN DANIEL. Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release, 2011, v. 152, n. 3, p. 330–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMUELS J; WEINGARTEN MS; MARGOLIS DJ; ZUBKOV L; SUNNY Y; BAWIEC CR; CONOVER D; LEWIN PA. Low-frequency (<100 kHz), low-intensity (<100 mW/cm2) ultrasound to treat venous ulcers: a human study and in vitro experiments. J Acoust Soc Am, 2013, v. 134, n. 2, p. 1541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIDENARI S; BELLETTI B; PELLACANI G Time course of skin changes induced by short-term occlusion with water: evaluation by TEWL, capacitance, and B-scanning echography. Skin Research and Technology, 1996, v. 2, n. 1. [DOI] [PubMed] [Google Scholar]
- SMITH N Applications of ultrasonic skin permeation in transdermal drug delivery. Expert Opin Drug Deliv, 2008, v. 5, n. 10, p. 1107–20. ISSN 1742–5247. [DOI] [PubMed] [Google Scholar]
- USA, G. Full prescribing information-Fuzeon. TRIMERIS, I; 2012. [Google Scholar]
- VAN DEN BROEK I SPARIDANS RW; HUITEM ADR; SCHELLENS JHM; BEIJNEN JH Development and validation of a quantitative assay for the measurement of two HIV-fusion inhibitors, enfuvirtide and tifuvirtide, and one metabolite of enfuvirtide (M-20) in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatog B, 2006, v. 837, p. 49–58. [DOI] [PubMed] [Google Scholar]
- WILD C; GREENWELL T; MATTHEWS T A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res Hum Retroviruses, 1993, v. 9, p. 1051–3. [DOI] [PubMed] [Google Scholar]
- WRIGHT D RODRIGUEZ A; GODOFSKY E; WALMSLEY S; LABRIOLA-TOMPKINS E; DONATACCI L; SHIKHMAN A; TUCKER E; CHIU YY; CHUNG J ; ROWELL L; DEMASI R ; GRAHAM N; SALGO M. Efficacy and safety of 48 weeks of enfuvirtide 180 mg once-daily dosing versus 90 mg twice-daily dosing in HIV-infected patients. Hiv Clinical Trials, 2008, v. 9, n. 2, p. 73–82. ISSN 1528–4336. [DOI] [PubMed] [Google Scholar]
- WU J CHAPPELOW J; YANG J; WEIMANN L. Defects generated in human stratum corneum specimens by ultrasound. Ultrasound Med Biol, 1998, v. 24, p. 705–10. [DOI] [PubMed] [Google Scholar]
- ZHANG X NIEFORTH K; LANG JM; ROUZIER-PANIS R; REYNES J; DORR A; KOLIS S; STILES MR; KINCHELOW T; PATEL IH Pharmacokinetics of plasma enfuvirtide after subcutaneous administration to patients with human immunodeficiency virus: Inverse Gaussian density absorption and 2-compartment disposition. Clin Pharmacol Ther, 2002, v. 72, n. 1, p. 10–9, Jul 2002. ISSN 0009–9236. [DOI] [PubMed] [Google Scholar]