Abstract
Objective:
Cancer and Aging: Reflections for Elders (CARE) is a novel, telephone-delivered intervention designed to alleviate distress in older cancer patients. This pilot randomized controlled trial tested the feasibility and initial efficacy of CARE, drawing from age-appropriate developmental themes and well-established coping theory.
Method:
Eligible patients were ≥70 years old; ≥6 months post-diagnosis of lung,prostate, breast, lymphoma, or gynecological cancer; on active cancer treatment or within 6 months of ending cancer treatment; and had elevated scores on the Distress Thermometer (≥4) or Hospital Anxiety and Depression Scale (≥6). Participants completed five sessions of psychotherapy over 7 weeks with assessments at study entry, post-intervention, and 2 months post-intervention. Primary outcomes were feasibility and initial efficacy on anxiety and depression; secondary outcomes included demoralization, coping, loneliness, and spiritual well-being.
Results:
Fifty-nine participants were randomized to either the CARE arm (n = 31) or the enhanced Social Work Control arm (n = 28). The intervention was feasible and tolerable, meeting a priori criteria for rates of eligibility, acceptance, retention, assessment, and treatment fidelity. Upon completion of the intervention, participants in the CARE arm demonstrated lower mean depression scores (d = 0.58 [CI: 0.04–1.12],P = 0.01) and trended towards increased coping-planning (d = 0.30 [CI: −0.83 to 0.24], P = 0.18). Promising trends in anxiety (d = 0.41 [CI: −0.17 to 0.98], P = 0.10) emerged at 2 months post-intervention; effects for coping-planning dissipated.
Conclusion:
These pilot data suggest the CARE intervention is feasibly delivered, potentially impacts important psychosocial variables, and is accessible for older, frail patients with cancer. Future research will evaluate this intervention on a larger scale.
Keywords: anxiety, aging, cancer, counseling, depression, intervention, geriatric, oncology
1. INTRODUCTION
In 2016, nearly 62% of almost 16 million cancer survivors in the United States were aged 65 or older. By 2040, an estimated 73% of 26 million cancer survivors will be 65 or older.1,2 These are primarily older patients with breast, prostate, and colon cancer, for whom cancer treatment continues for long periods, analogous to a chronic disease.1 Thus, many older adults must cope with the demands of both aging and cancer for long periods of continuous treatment and monitoring.
Patients of all ages struggle to cope with the diagnosis and treatment of cancer. In a large study of adult cancer patients of all ages, 35% experienced a significant level of distress; the rate was even higher for cancer sites associated with poorer prognosis.2 Older people tend to cope better with illness and loss than younger individuals.2–6 However, the presence of aging-related physical concerns, comorbidity, and symptom burden can overwhelm coping abilities, leading to increased distress, anxiety, and depression.7,8 Additionally, comorbid medical illness is often a key feature of geriatric mood disorders, and older adults with cancer have some of the highest rates of completed suicide.9 Unfortunately, older adults with anxiety or depression are likely to remain undiagnosed and thus often go untreated.9–11 But even if diagnosed correctly, clinicians cannot assure that mental health intervention can or will be feasible, effective, and safe. Thus, a compelling rationale for the current study was to introduce and test a pilot intervention to assist older patients in coping with aging and cancer.
Interventions for older adults with chronic illness have found that while antidepressant pharmacotherapy is effective to address depressive symptoms, most older adults prefer psychotherapeutic or psychoeducational interventions over medication.10–12 While there is a growing psychotherapy evidence base for older adults broadly,13,14 we identified only two studies focusing on older patients with cancer. In one, a multidisciplinary team offered treatment to older adults with cancer, including physical therapy, cognitive behavioral training, symptom education, spiritual guidance, and a 200-page manual.15 The second study examined a subset of data from the IMPACT study.16 This study tested the use of a depression care manager who followed patients for 12 months under the supervision of both a primary care physician and psychiatrist; the authors reported a reduction in depressive symptoms at 6 and 12 months.16 These are sound interventions with positive outcomes; however, these were relatively small studies, lacked a theoretically driven intervention, and were not specifically tailored to the developmental issues related to aging or the needs of older adults.
The theoretical framework for the Cancer and Aging: Reflections for Elders (CARE) intervention is based on the integration of two well-established models in the psychosocial literature: the coping paradigm of Folkman17–19 and the developmental stages of life as outlined by Erikson20–22 and expanded on by Vaillant.23 Folkman’s model proposes that people deal with significant distress by using “meaning schemas” or basic beliefs about the world as rational assumptions for what happens to them. When confronted with a life-threatening event such as cancer, these assumptions can be shattered. An individual must develop a new meaning schema that integrates the new catastrophic event with an acceptable and tolerable “present.” The process of reappraisal, in which an individual revises the meaning of events in ways that are more consistent with their new situation, is a critical element of adaptive coping, and we identify it as a core mechanism of the CARE intervention.17 The theoretical foundation of the CARE model integrates Folkman’s concept of reappraisal within the context of Erikson’s theory of development in the later stages of life. Erikson’s 8th stage of life is based on the developmental tasks of establishing comfort with one’s lived life (i.e., ego integrity) or the alternative—unsuccessful achievements, which result in despair (i.e., regrets and despondency). “Ego integrity” is achieved by putting one’s life into a tolerable perspective, and by developing a sense of generativity by giving back to younger generations. According to the theory, older adults who do not achieve ego integrity are more likely to experience despair and disdain and have fewer resources for coping with stressors like cancer.20–22 Thus, the CARE intervention is founded on joining Folkman’s reappraisal to Erikson’s life stages with the goal of helping older adults to better cope with the demands of illness and aging to achieve peace and acceptance of their life stage. This includes helping patients problem solve ways to become more socially connected and engage in helpful coping.
The primary aim of this pilot RCT was to test the feasibility, toler- ability, and acceptability of CARE by examining the rates of eligibility, acceptance, and adherence. We hypothesized that CARE would be feasible, tolerable, and acceptable. An exploratory aim was to examine the preliminary effects of CARE on measures of psychological well-being compared with a control group that received an enhanced social work intervention.
2. METHOD
2.1. Study design
Participants were recruited from two Northeastern Comprehensive Cancer Centers, Joan Karnell Cancer Center at the Pennsylvania Hospital and Memorial Sloan Kettering Cancer Center (MSK). This study was approved by the MSK (protocol # 09–116) and Joan Karnell Cancer Center (protocol # 814950) Institutional Review Boards. This trial was registered at the US National Institutes of Health (ClinicalTrials.gov) #NCT00984321.
2.2. Participants
Patients eligible for the pilot intervention were (1) 70 years of age or older; (2) ≥6 months post-diagnosis for breast, prostate, lung, lymphoma, or gynecological cancer and on active cancer treatment (or within 6 months of active treatment); (3) English-speaking; (4) score of ≥4 on the Distress Thermometer,24 or a score of ≥6 on the Hospital Anxiety and Depression Scale (HADS25–27); (5) ≥60 on Karnofsky Performance Rating (KPR28); and (6) ≤11 on the Blessed Orientation Memory Concentration (BOMC29) test.
2.3. Procedure
There was a two-step screening process for this study. The first step was a review of the medical charts of prospective participants to screen for initial eligibility (ie, age, cancer diagnosis, and cancer treatment) and, if initial eligibility criteria were met, then these patients were contacted by letter and follow-up phone calls. Interested participants went through the second step of the screening process for eligibility by completing the HADS and Distress Thermometer, KPR, and the BOMC over the phone. Patients who met the distress criteria then provided verbal consent over the phone and were enrolled to the study. In following the Code of Federal Regulations Title 45, Part 46, Subpart A, a signed consent was not required for this study. This study was considered “minimal risk” by our IRB, and written consent was not required. No participants were ruled out for KPR or BOMC. Participants were randomized (with equal probability) to either the CARE intervention or the Enhanced Social Work Control (ESWC). The randomization was stratified by cancer type (breast, prostate, lung, lymphoma, and gynecological) and gender by the MSK Office of Clinical Research Protocol Participation Registration system.
Participants were administered a battery of questionnaires at study entry (at the time of enrollment and within 2 weeks prior to starting the intervention), post-intervention (within 1 month of completing the last intervention session), and at 4-month post-study entry. The questionnaires were completed over the phone with a research assistant who was blinded to group assignment. Other research assistants who were not blinded had minimal contact with the participants and were responsible for consenting participants, screening for distress, and coordinating the participants’ initial session.
2.3.1. Intervention
The CARE intervention integrated two theoretical models: Folkman’s cognitive model of coping and Erikson’s developmental model of psychosocial tasks associated with the later stages of life (Figure 1). Based on the theoretical framework described above, CARE was developed to encompass several therapeutic approaches to help patients reappraise their situation in the context of achieving ego integrity. Therapeutic elements such as information-giving and information- receiving, discussion of concerns, problem solving, coping skills training, expression of emotion, and social support, are all structured to help patients put past regrets into a tolerable perspective, and to consider how to give back to younger generations.
FIGURE 1.
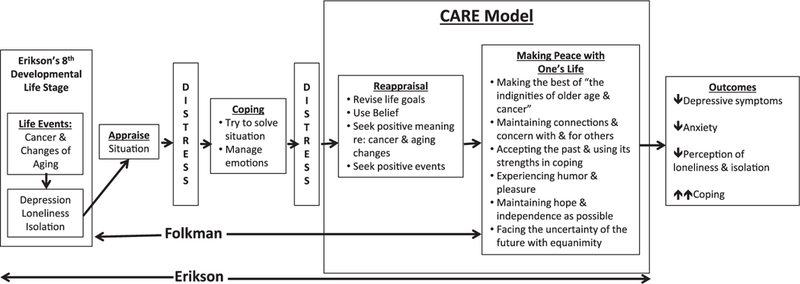
CARE model. Adapted from the original work of Folkman17–19 and Erikson20,21
Expert panel
The structure and themes of CARE were originally presented to an expert panel of older adults with cancer. Over the course of seven meetings, the participants gave feedback on the relative salience and utility of intervention content. After the manual was revised with their feedback, the intervention was tested with a second group of older patients in a group format. They, too, provided feedback and suggestions for modification.
CARE structure
The CARE intervention consisted of five sessions* (45 min; Table 1) that took place over approximately 7 weeks. All sessions were facilitated by a master’s or doctoral-level trained mental health professional (four interventionists total). Each session followed a similar pattern: (1) reviewing the session topic; (2) defining challenges associated with the topic; (3) exploring “guided questions” surrounding the topic; and (4) assessing ways to reframe stressful emotions, thoughts, or behaviors; identifying past coping skills or problem solving related to the challenges. Homework assignments were distributed at the end of each session.
TABLE 1.
CARE intervention session topics, themes, and outline
| Session | Topic | Theme | Outline |
|---|---|---|---|
| 1 | Cancer story and overview | Learn about the patient’s cancer story and introduce patient to the session themes and process. Developmental task: life review | 1. Introductions: therapist and patient 2. Overview (treatment goals, logistics) 3. Overview of five sessions (review member handbook) 4. Explore patient’s experiences of cancer and aging 5. Wrap-up (review session 1, overview of session 2, and homework ideas) |
| 2 | Coping with cancer and aging | Dealing with the combined challenges of illness and aging. Developmental tasks: concern of life in the face of death; struggle to accept the inalterability of the past and unknowable future | 1. Brief review of session 1 and review of homework ideas (if completed) 2. Introduce session 2 topics: -Topic A: coping with the losses and benefits of cancer and aging -Topic B: fears and concerns about the future 3. Define problems/challenges re: topics A and B 4. Explore guided questions re: topics A and B 5. Assess ways of reappraisal 6. Wrap-up (review session 2, introduce session 3 and homework ideas) |
| 3 | Loneliness and the stigma of cancer and aging | Loneliness and reduced social circles in later years. Developmental task: social connections; link between the past and the present | 1. Brief review of session 2 and review homework ideas (if completed) 2. Introduce session 3 topic: -Loneliness, cancer, and aging 3. Define problems/challenges re: session topic 4. Explore guided questions 5. Wrap-up (review session 3, introduce session 4 and homework ideas) |
| 4 | Making peace with one’s life | Coming to terms with “who I am now.” Developmental task: making peace with one’s life as a unified whole; achieve a sense of wisdom through life’s experience and lessons; “keeper of meaning” | 1. Brief review of session 3 and homework ideas (if completed) 2. Introduce session 4 topics -Topic A: making peace with one’s life -Topic B: acquiring wisdom 3. Define problems/challenges re: topics A and B 4. Explore guided questions re: topics A and B 5. Wrap-up (review session 4, introduce session 5 and homework ideas) |
| 5 | Reflection and review | Summarize and review treatment themes: developmental task: social connections; link between the past and the present; making peace with one’s life as a unified whole; achieve a sense of wisdom through life’s experience and lessons; “ keeper of meaning” | 1. Explore patient’s thoughts/feelings re: termination 2. Brief review of sessions 4 and 5 topics 3. Patient’s feedback re: general intervention (ie, what has it been like for you to participate in this process? Do you have a better understanding of ways to cope with and reappraise difficult situations re: aging and cancer? If so, how? Have there been any changes in the way you view your life?) 4. Reflection on overall experience. Express appreciation for participation and say goodbyes. |
ESWC structure
The control condition (ESWC), which is currently considered standard of care, consisted of an initial telephone session with one of several social workers specializing in geriatrics (six interventionists total) and four subsequent phone calls (spaced at similar time interval as the CARE arm sessions, ranging from 15 to 30 min) from the social worker in order to control for the number of sessions held with participants in the CARE arm. Phone session content after the initial social work assessment was focused on checking in regarding patient progress on goals and plans set in the initial assessment and general supportive psychotherapy. Participants in the ESWC who completed the study were given the option to participate in the intervention arm if interested.
The CARE arm received in-depth training: (1) in-person review of the manual with study investigators, (2) observation of several cases, and (3) conducting 1 to 2 “mock” cases with study investigators. CARE therapists received weekly, hour-long ongoing supervision throughout the course of the study. For ESWC, there was weekly supervision facilitated by the primary social worker at MSK.
2.3.2. Outcomes
We adopted Leon, Davis, and Kraemer’s (2011) criteria for demonstrating feasibility in a pilot study. As such, the primary outcome of this pilot study was the feasibility of the CARE intervention, which was determined by rates of eligibility (percent screened for participation who were eligible), acceptance (proportion of those who screen eligible who enroll),† retention (attrition and number of sessions completed by study arm), assessment process (proportion of planned assessments that are completed by participants), and treatment fidelity (percentage of session content covered across sessions by study therapists).
Our benchmark for retention and assessment is 80% such that if 80% of participants complete all sessions and assessments, then study feasibility will be demonstrated.
We estimated statistical power in the following binomial hypotheses: We assumed a null hypothesis that the population completion rate is 0.60 and an alternative hypothesis that the population completion rate is 0.85. If we observe 48 or more completers out of 60 to be enrolled, we will have a 92% power to reject the null hypothesis of 0.60 at a two-sided type I error rate of approximately 0.0002.
Research assistants rated fidelity utilizing a standardized adherence checklist form to evaluate sessions for a subset (60%) of participants in each arm (see supplemental materials for sample checklist). Treatment fidelity scores range from 0 to 100% based on the number of manualized session topics that were covered in the session; fidelity of 80 to 100% will be considered high fidelity.30 The study also examined the impact of CARE on several psychosocial variables. HADS25 was the primary outcome measure of anxiety and depression, with higher scores indicating more severe symptoms.25,31,32 Secondary outcomes included measures of demoralization (Demoralization Scale; higher scores indicating worse symptoms33), coping (3 out of 15 subscales from the COPE; higher scores indicating better coping34), spiritual well-being (FACIT Spiritual Well-Being Scale; higher scores indicating better well-being35,36), and loneliness (UCLA Loneliness Scale-Short Form; higher scores indicating worse symptoms37).
2.3.3. Statistical analyses
Analyses were conducted to examine differences in two primary psychosocial outcomes, depressive symptoms and anxiety, as well as the secondary psychosocial measures over time by group membership. Given the pilot nature of the data, the study was not powered to determine significant differences between the intervention and control groups but was powered to determine feasibility and initial efficacy. As such, both significance levels and effect sizes (Cohen’s d) are reported (d = 0.2, small; d = 0.5, medium; and d = 0.8, large effect). ANCOVA was used to identify differences in the psychosocial variables by arm with group assignment and scores at study entry predicting the 2- and 4-month scores.
3. RESULTS
3.1. Participants
Sixty-eight participants consented to the study and of those, 59 were randomized (CARE n = 31; ESWC n = 28). Participants were recruited between September 2009 and September 2013 when target enrollment was achieved; follow-up assessments were completed in February 2014. The sample was approximately evenly split by gender (53% female; n = 33) with a mean age of 76 (SD = 4). Participants were primarily Caucasian (90%; n = 56) and well-educated (81% had a college degree; n = 50). Half of the participants were married (52%; n = 32), 18% were divorced (n = 11) or single (n = 11), and 13% were widowed (n = 8).
3.2. Primary outcomes
3.2.1. Feasibility
There were no adverse events reported throughout the course of the trial. In total, 541 patients met initial eligibility criteria and were contacted about the study. Of those, 118 (22%) agreed to be screened for distress and participate in the study. In total, 68 patients (13% of those who met initial eligibility criteria and 58% of those who agreed to be screened) met distress eligibility and were offered participation in the study. All 68 individuals were consented and enrolled, reflecting a high acceptance rate among those who met both stages of eligibility.
Ultimately, 59 participants were randomized (Figure S2). Of the 31 participants who started the CARE intervention, three participants did not complete all sessions (89.3%). In the ESWC arm, 28 participants initiated the treatment and 1 did not complete all sessions (96.4%). Fifty-five participants provided follow-up data at the 2-month time point (post-intervention) across the intervention (n = 28) and control arms (n = 27), and 48 provided follow-up data at 4-month assessment point (n = 25, n = 23, respectively). Thus, 81.4% (48 out of 59) of participants completed the full number of sessions and the final study assessment, indicating adequate retention and assessment per our a priori cut-off of 80% as a benchmark for feasibility. Study attrition rate was not different by group at time 1 or time 2. Treatment fidelity was 88.5% or higher for all sessions (88.5–94.6%), again exceeding our threshold of 80% as an indication of high fidelity.
3.2.2. Psychosocial outcomes
At the 2-month assessment (Table 2), the CARE arm reported lower mean total HADS scores compared with the ESWC arm (d = 0.46 [CI: –0.07 to 0.99], P = 0.02) and lower mean HADS depression scores (d = 0.58 [CI:0.04–1.12], P = 0.01), with no differences related to anxiety (d = 0.15 [CI: –0.38 to 0.68], P = 0.44). The CARE arm, compared with ESWC, demonstrated promising trends, with small effects for reduced loneliness (d = 0.19 [CI: –0.34 to 0.72], P = 0.21), disheartenment (d = 0.28 [CI: –0.25 to 0.08], P = 0.12), and sense of failure (d = 0.26 [CI: –0.27 to 0.79], P = 0.19), increased meaning (d = 0.22 [CI: –0.75 to 0.31], P = 0.27), and improved coping-planning (d = 0.30 [CI: –0.83 to 0.24], P = 0.18), although these differences did not reach statistical significance. Participants in the ESWC arm trended towards larger increases in active coping (d = –0.28 [CI: –0.25 to 0.81], P = .26).
TABLE 2.
Two- And 4-month outcomes for psychosocial variables
| Study Entry |
2 months (Intervention Completion] |
4 months (2 months Post-Intervention) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ESWC M (SD) | CARE M (SD) | ESWC M (SD) | CARE M (SD) | P | Effect da (Cl) | ESWC M (SD) | CARE M (SD) | P | Effect d (Cl) | |
| HADS total | 11.62 (5.79) | 11.13 (5.80) | 12.07 (6.22) | 9.36 (5.51) | 0.02 | 0.46 (−0.07 to 0.99) | 12.33 (6.35) | 9.95 (5.08) | 0.09 | 0.42 (−0.16 to 0.99) |
| HADS-D | 6.00 (4.23) | 5.21 (3.34) | 6.58 (4.17) | 4.37 (3.39) | 0.01 | 0.58 (0.04 to 1.12) | 6.00 (3.80) | 5.06 (2.91) | 0.20 | 0.28 (−0.30 to 0.85) |
| HADS-A | 5.62 (3.13) | 5.91 (3.63) | 5.48 (3.34) | 5.00 (3.19) | 0.44 | 0.15 (−0.38 to 0.68) | 6.32 (3.99) | 4.92 (2.90) | 0.10 | 0.41 (−0.17 to 0.98) |
| Demoralization total | 26.51 (15.36) | 23.84 (14.98) | 25.64 (14.97) | 23.17 (15.33) | 0.32 | 0.16 (−0.37 to 0.69) | 24.96 (14.15) | 22.13 (14.94) | 0.29 | 0.19 (−0.38 to 0.77) |
| Dysphoria | 6.14 (4.13) | 6.41 (3.60) | 6.32 (3.91) | 6.59 (3.86) | 0.67 | −0.07 (−0.64 to 0.50) | 5.78 (3.07) | 6.55 (3.84) | 0.26 | −0.22 (−0.79 to 0.35) |
| Disheartenment | 8.24 (5.26) | 7.91 (4.91) | 8.54 (5.45) | 7.08 (4.88) | 0.12 | 0.28 (−0.25 to 0.08) | 8.25 (4.56) | 6.67 (4.93) | 0.09 | 0.33 (−0.25 to 0.91) |
| Helplessness | 3.86 (4.08) | 3.34 (2.99) | 3.53 (3.43) | 3.10 (3.24) | 0.56 | 0.13 (−0.40 to 0.66) | 3.20 (2.87) | 2.75 (2.75) | 0.51 | 0.16 (−0.41 to 0.73) |
| Sense of failure | 4.31 (3.14) | 3.56 (2.38) | 4.29 (3.70) | 3.47 (2.48) | 0.19 | 0.26 (−0.27 to 0.79) | 4.17 (3.66) | 3.45 (2.81) | 0.26 | 0.22 (−0.35 to 0.80) |
| Loss of meaning | 3.97 (4.31) | 2.62 (3.38) | 3.11 (4.31) | 2.86 (3.25) | 0.75 | 0.07 (−0.46 to 0.59) | 3.52 (3.49) | 2.74 (3.06) | 0.22 | 0.24 (−0.34 to 0.81) |
| COPE-active | 13.00 (2.35) | 13.34 (2.44) | 13.94 (1.96) | 13.34 (2.33) | 0.26 | −0.28 (−0.25 to 0.81) | 14.30 (2.07) | 13.29 (2.53) | 0.10 | −0.43 (−0.15 to 1.01) |
| COPE-behavioral disengagement | 7.07 (3.44) | 6.22 (2.52) | 6.89 (3.00) | 6.46 (2.43) | 0.47 | −0.16 (−0.37 to 0.69) | 6.01 (2.85) | 6.62 (1.76) | 0.30 | −0.26 (−0.31 to 0.84) |
| COPE-planning | 13.52 (2.91) | 13.16 (2.94) | 14.46 (2.16) | 13.71 (2.84) | 0.18 | 0.30 (−0.83 to 0.24) | 14.27 (2.18) | 14.36 (2.75) | 0.88 | 0.04 (−0.61 to 0.54) |
| FACIT-total | 31.92 (10.06) | 30.78 (10.00) | 30.40 (10.53) | 32.70 (10.54) | 0.24 | 0.22 (−0.75 to 0.31) | 30.73 (10.30) | 33.38 (8.96) | 0.17 | 0.28 (−0.85 to 0.30) |
| FACIT-meaning | 23.31 (6.90) | 22.91 (6.28) | 22.30 (7.22) | 23.82 (6.78) | 0.27 | 0.22 (−0.75 to 0.31) | 22.00 (6.75) | 24.97 (5.78) | 0.04 | 0.48 (−1.06 to 0.11) |
| FACIT-faith | 8.62 (5.56) | 7.88 (4.82) | 8.10 (5.34) | 8.94 (5.12) | 0.33 | 0.16 (−0.69 to 0.37) | 8.70 (5.58) | 8.50 (4.83) | 0.81 | −0.04 (−0.53 to 0.61) |
| UCLA loneliness | 14.93 (6.13) | 16.06 (5.19) | 15.90 (5.94) | 14.91 (4.53) | 0.21 | 0.19 (−0.34 to 0.72) | 15.88 (5.98) | 14.93 (4.89) | 0.31 | 0.18 (−0.40 to 0.75) |
Notes. Means presented in Table 2 for the 2- and 4-month assessments are estimated marginal means (generated by ANCOVA, reflecting adjustment for scores at study entry).
Positive effect sizes indicate that the CARE group demonstrated improvements trending towards greater magnitude than those in ESWC; negative effect sizes indicate that ESWC participants demonstrated improvements trending towards greater magnitude than those in ESWC.
At 4 months (2 months without either intervention, Table 2), the CARE group continued to trend towards making gains on main outcome variables. The effect on total HADS scores remained consistent (d = 0.42 [CI: –0.16 to 0.99], P = 0.09). The CARE arm trended towards reporting lower anxiety scores compared with ESWC (d = 0.41 [CI: –0.25 to 0.81], P = 0.10), while the observed differences in HADS depression scores lessened (d = 0.28 [CI: –0.30 to 0.85], P = 0.20). Effect sizes (d) also increased for disheart- enment (d = 0.33 [CI: –0.25 to 0.91], P = 0.09) and meaning (d = 0.48 [CI: –1.06 to 0.11], P = 0.04), indicating greater improvement for participants in the CARE arm across constructs. The effects on coping-planning did not persist (d = 0.04 [CI: –0.61 to 0.54], P = 0.88), and those in the ESWC arm maintained their increases in active coping (d = –0.43 [CI: –0.15 to 1.01], P = .10) relative to those in the CARE arm.
4. DISCUSSION
To date, there are no psychotherapeutic interventions specifically designed to address the emotional distress of older cancer patients. Interventions studied thus far in this population are designed for the general population and then retrospectively tested in the elderly with cancer. CARE is the only telephone-facilitated psychotherapy intervention specifically designed for older cancer patients with the input of older people with cancer. The primary aim of this pilot study, which was to demonstrate the feasibility of the CARE intervention, was achieved.
We calculated the acceptance rate in a number of different ways and project that 30% of those distressed older cancer patients agreed to participate in the study. This 30% needs to be viewed in the context that our recruitment was conducted by sending an initial invitation letter and then following up with a phone call to invite participation in the study. We would have expected a higher recruitment rate if this was done in person in the cancer clinics. However, we decided to recruit by phone to demonstrate that this recruitment could be accomplished without in-person contact. We also propose that the potential lower accrual rate achieved via telephone recruitment may be a reasonable trade-off for this population in terms of accessing homebound or more physically frail older adults who are not regularly attending outpatient appointments and may otherwise be overlooked for intervention participation. In future iterations of the study, we may test varying recruitment methods and solicit qualitative feedback from older patients about preferences for approaches.
The feasibility of the intervention was supported by the results, which indicate that all of the patients we contacted who we confirmed were experiencing moderate distress were interested in the intervention. In developing this intervention, our expert panel emphasized the importance of feasibility in a successful intervention, highlighting ease of session scheduling (e.g., telephone sessions compared with having to travel for appointments) and content of sessions. The pilot data demonstrate the benefits of this flexible, telephone-based approach as evidenced by the low attrition rate in the CARE arm and the fact that most participants completed all sessions. Our intervention also needed to be adaptable in multiple settings where there may be no access to psychiatrists or psychologists. It was therefore delivered by those with master’s level counseling degrees and proved possible in this pilot study. Future larger RCTs will also include a range of clinicians. The CARE intervention is also manualized to help deliver consistency among various therapists. These aims were achieved as the study utilized several different therapists, all of whom achieved high levels of treatment fidelity when rated by independent coders.
The second goal of the study was to examine the preliminary effects of the intervention. The results of this randomized trial comparing CARE to the ESWC intervention (matched for number of sessions) were encouraging. CARE demonstrated promising trends in psychosocial outcomes relative to the ESWC, as CARE participants reported reduced depression, anxiety, and demoralization. In psycho-social interventions, it is common for effects to decrease following the end of the intervention. While this occurred with HADS depression scores, the effect remained consistent for total HADS scores and strengthened for HADS Anxiety, disheartenment, and meaning scores. A future, larger scale study will potentially include booster sessions in order to enhance and maintain the skills achieved during the intervention. Moreover, we will examine the CARE treatment content relative to ESWC in order to try to understand why the ESWC arm reported larger improvements in active and behavioral disengagement coping skills. It is also important to note that the effects that were observed emerged when compared against an effective and proven control group that was run by experienced social workers in psycho-oncology. In sum, these preliminary results indicate that the CARE intervention has significant promise and warrants a larger scale RCT.
4.1. Clinical implications
Cancer and Aging: Reflections for Elders is a novel intervention and hypothesized to be effective for several key reasons. First, it involved feedback and ongoing discussion with older patients with cancer from the very initial development phases. This iterative process of elicitation from an expert panel of older adults with cancer speaks to the external validity of the themes and content of the sessions. With this solid foundation, it is also the first psychotherapy intervention to incorporate the developmental issues distinctive to older adults and the combined issues of aging and cancer. Finally, it was developed and tested through the telephone to reach older patients with cancer who have difficulty getting out of the house, navigating inclement weather or transportation, or who are geographically isolated. Thus, CARE has tremendous potential to reach those who are most in need of intervention.
4.2. Study limitations
Despite the promising findings related to feasibility and preliminary effects on psychological well-being identified here, as with any pilot study, there are some limitations to interpretability. First, our sample was predominately White and college-educated. Thus, the generaliz- ability of the effects and of patient satisfaction with the intervention may be limited. Future larger trials will include a more racially, ethnically, and educationally diverse sample in order to tailor the CARE intervention as necessary. Similarly, the homogeneity of the sample suggests that there may have been a selection bias in who opted to participate in the study, so future larger scale studies should make concerted efforts to recruit patients with a range of functional statuses in order to demonstrate acceptability and outcomes in patients with greater functional impairment. Additionally, we could not reliably compare those participants who completed the original 7 session format to the later 5 session format; in the next trial participants will all receive the standard session number. Although all participants completed pre-randomization and all subsequent study measures over the phone, we did not explicitly screen for hearing impairments, which will be important in future larger studies to ensure adequate session engagement. Finally, there were several relevant variables not captured in the data collected in this study, such as measures of successful aging and wisdom, which would allow analyses to be conducted on the hypothesized change mechanism of the CARE intervention; these constructs will be assessed in the next phase of intervention testing. Further research in a larger study should potentially modify the intervention to include brief monthly booster calls to help maintain the effects of the intervention over time.
5. CONCLUSIONS
These pilot data suggest the CARE intervention is feasibly delivered by a range of clinicians and provides a positive signal that the CARE intervention impacts important psychosocial variables for older patients with cancer. This novel treatment is unique in that it draws from age appropriate developmental themes in combination with well-established coping theory to deliver a targeted intervention for older patients in the oncology setting. Additionally, it has the potential to be highly accessible to frail older adults who may not be able to attend in-person sessions with regular frequency. Thus, by developing an intervention from the bottom up rather than retrofitting existing psychotherapy to this specific population, we demonstrate that it is possible to better serve the needs of our older patients.
Supplementary Material
ACKNOWLEDGEMENTS
Jimmie Holland died on December 24, 2017. She lived almost 90 years. Her impact on the human side of cancer care will last for generations. The journal you are reading and the article before you were brainstorms of this pioneer of Psycho-Oncology. Cancer and Aging: Reflections for Elders (CARE): A Pilot Randomized Controlled Trial of a Psychotherapeutic Intervention for Older Adults with Cancer was Jimmie’s last completed project, and this article most likely represents her last publication in the journal which she began. Yet it is but one of her many efforts that engaged her heart and soul. Jimmie aged gracefully with good humor, working until the very end. She shared those values with her patients, her colleagues, her friends, and of course her family and left her rich legacy for those she will never know who have cancer.
Funding for this study was provided by the Silbermann Foundation, Muriel Duenewald Lloyd Inspiration Fund, the National Cancer Institute (T32CA009461–34 and P30 CA08748–48), and the CALGB Foundation.
Funding information
Silbermann Foundation; CALGB Foundation; Muriel Duenewald Lloyd Inspiration Fund; National Cancer Institute, Grant/Award Numbers: P30 CA08748–48 and T32CA009461–34
Footnotes
ENDNOTES
The original study protocol included seven sessions; after the first 11 participants completed the interventions (CARE = 7; ESWC = 4), the number of sessions was reduced to 5. This decision was made after discussion by study investigators, as it became clear that the CARE intervention and ESWC content could be consolidated into five sessions, thus minimizing participant time commitment while still covering all content in sufficient detail.
The acceptance rate (the percent of patients who agreed to take part in the study) can easily be calculated. However, many of those who refused to take part in the study may not have been distressed and would not actually have qualified to take part in the study. In order to obtain an estimate of the proportion of distressed individuals who we were able to engage in the CARE intervention study, we implemented a brief substudy for those who refused to participate (see Figure S1).
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28. [DOI] [PubMed] [Google Scholar]
- 3.Blank TO et al. How do men “make sense” of their prostate cancer?: age and treatment factors. Gerontologist. 2003;43(1):342–343. [Google Scholar]
- 4.Blank TO, Bellizzi KM. After prostate cancer: predictors of well-being among long-term prostate cancer survivors. Cancer. 2006;106(10): 2128–2135. [DOI] [PubMed] [Google Scholar]
- 5.Eton DT, Lepore SJ. Prostate cancer and health-related quality of life: a review of the literature. Psychooncology. 2002;11(4):307–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IOM. In: Hewitt S MG, Stovall E, eds. From Cancer Patient to Cancer Survivor: Lost inTransition. Washington: The National Academies Press; 2007:18–35. [Google Scholar]
- 7.Kurtz M et al. Physical functioning and depression among older persons with cancer. Cancer Pract. 2001;9(1):11–18. [DOI] [PubMed] [Google Scholar]
- 8.Blazer. Epidemiology of late life depression In: Schneider LS RC, Lebowitz BE, et al. , eds. Diagnosis and Treatment of Depression in Late Life. Washington: American Psychiatric Press; 1994:9–19. [Google Scholar]
- 9.Erlangsen A, Stenager E, Conwell Y. Physical diseases as predictors of suicide in older adults: a nationwide, register-based cohort study. Soc Psychiatry Psychiatr Epidemiol. 2015;50(9):1427–1439. [DOI] [PubMed] [Google Scholar]
- 10.Scogin F, McElreath L. Efficacy of psychosocial treatments for geriatric depression: a quantitative review. J Consult Clin Psychol. 1994;62(1):69–74. [DOI] [PubMed] [Google Scholar]
- 11.Unützer J, Katon W, Callahan CM, et al. Depression treatment in a sample of 1,801 depressed older adults in primary care. J Am Geriatr Soc. 2003;51(4):505–514. [DOI] [PubMed] [Google Scholar]
- 12.Lin EHB, Katon W, von Korff M, et al. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA. 2003;290(18):2428–2429. [DOI] [PubMed] [Google Scholar]
- 13.Cuijpers P, Karyotaki E, Pot AM, Park M, Reynolds CF III. Managing depression in older age: psychological interventions. Maturitas. 2014;79(2):160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renn BN, Arean PA. Psychosocial treatment options for major depressive disorder in older adults. Curr Treat Options Psychiatry. 2017;4(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapid M, Rummans TA, Brown PD, et al. Improving the quality of life of geriatric cancer patients with a structured multidisciplinary intervention: a randomized controlled trial. Palliat Support Care. 2007;5(2):107–114. [DOI] [PubMed] [Google Scholar]
- 16.Fann JR, Fan MY, Unutzer J. Improving primary care for older adults with cancer and depression. J Gen Intern Med. 2009;24(Suppl 2): S417–S424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folkman S Positive psychological states and coping with severe distress. Soc Sci Med. 1997;45(8):1207–1221. [DOI] [PubMed] [Google Scholar]
- 18.Folkman S, Lazarus RS, Dunkel-Schetter C, DeLongis A, Gruen RJ. The dynamics of a stressful encounter: cognitive appraisal, coping, and encounter outcomes. J Pers Soc Psychol. 1986;50(5):992–1003. [DOI] [PubMed] [Google Scholar]
- 19.Folkman S, Lazarus RS, Pimley S, Novacek J. Age differences in stress and coping processes. Psychol Aging. 1986;2(2):171–184. [DOI] [PubMed] [Google Scholar]
- 20.Erikson E Childhood and Society. London: Vintage Books; 1950. [Google Scholar]
- 21.Erikson E, Erikson J, Kivnick H. Vital Involvement in Old Age. New York: W.W. Norton & Company, Inc; 1986. [Google Scholar]
- 22.Erikson JM. The Life Cycle Completed: Erik H. Erikson. New York: W.W. Norton & Company, Inc; 1997. [Google Scholar]
- 23.Vaillant G Aging Well. New York: Little Brown and Company; 2002. [Google Scholar]
- 24.Roth AJ, Kornblith AB, Batel-Copel L, Peabody E, Scher HI, Holland JC. Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer. 1998;82(10):1904–1908. [DOI] [PubMed] [Google Scholar]
- 25.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 26.Saracino RM, Weinberger MI, Roth AJ, Hurria A, Nelson CJ. Assessing depression in a geriatric cancer population. Psychooncology. 2017;26(10):1484–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer S, Kuhnt S, Götze H, et al. Hospital anxiety and depression scale cutoff scores for cancer patients in acute care. Br J Cancer. 2009;100(6):908–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky performance status scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53(9):2002–2007. [DOI] [PubMed] [Google Scholar]
- 29.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140(6):734–739. [DOI] [PubMed] [Google Scholar]
- 30.Borrelli B The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent. 2011;71(s1): S52–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibbotson T, Maguire P, Selby P, Priestman T, Wallace L. Screening for anxiety and depression in cancer patients: the effects of disease and treatment. Eur J Cancer. 1994;30A(1):37–40. [DOI] [PubMed] [Google Scholar]
- 32.Spinhoven P, van der Does AJ. Somatization and somatosensory amplification in psychiatric outpatients: an explorative study. Compr Psychiatry. 1997;38(2):93–97. [DOI] [PubMed] [Google Scholar]
- 33.Kissane DW, Wein S, Love A, Lee XQ, Kee PL, Clarke DM. The demoralization scale: a report of its development and preliminary validation. J Palliat Care. 2004;20(4):269–276. [PubMed] [Google Scholar]
- 34.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56(2):267–283. [DOI] [PubMed] [Google Scholar]
- 35.Brady Marianne J et al. A case for including spirituality in quality of life measurement in oncology. Psychooncology. 1999;8(5):417–428. [DOI] [PubMed] [Google Scholar]
- 36.Peterman AH, Fitchett G, and Cella DF, Modeling the relationship between quality of life dimensions and an overall sense of well-being, in Third World Congress of Psycho-Oncology 1996: New York, NY. [Google Scholar]
- 37.Hays RD, DiMatteo MR. A short-form measure of loneliness. J Pers Assess. 1987;51(1):69–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


