Abstract
Introduction:
A critical challenge in oncology is interpreting clinical trial results to inform clinical decision making. Clinical trials typically focus on overall survival (OS) and progression-free survival (PFS) as primary endpoints, which do not reflect early signs of meaningful patient benefit or harm. Cancer symptom response (CSR) can provide information about early treatment response, and studies show that CSR predicts long-term health outcomes.
Areas covered:
CSR requires careful consideration of its measurement and interpretation to facilitate integration into clinical practice. We describe considerations for the evaluation, analysis, and interpretation of CSR in clinical trials. To illustrate the potential clinical value of CSR, we performed a retrospective analysis of a three-arm randomized cooperative-group clinical trial.
Expert commentary:
Evaluation of CSR provides a meaningful assessment of early cancer treatment effects. It can act as an early signal of disease progression and death and thus can identify which patients with stable disease will have a more favorable prognosis. Future research will include development of methods for more accurate assessment of CSR, reduction of the number of symptoms used as signals for disease progression or survival by tumor type, and statistical methods that effectively correct for missing data and informative censoring.
Keywords: cancer symptom response, clinical trial endpoints, quality of life
1. Introduction
One of the critical challenges in oncology today is the interpretation of clinical trial results to inform clinical care and decision making. Oncology clinical trials typically rely on overall survival (OS) and, increasingly, progression-free survival (PFS; i.e., time to disease progression) as endpoints by which to evaluate treatment effectiveness. OS is the longstanding gold standard endpoint by which oncology clinical trials are evaluated. OS is recognized for being a clearly defined objective, and the clinical relevance of treatment-related OS benefits are obvious [1]. However, appropriate interpretation of OS can be complicated by many factors; oncology clinical trials often require large patient samples and a long follow-up period in order to demonstrate statistical significance of OS benefits. In addition, it is common for cancer patients to receive multiple sequential treatments after disease progression, confounding the effects of the initial (studied) cancer treatment on OS [2]. Finally, focusing on OS can make it difficult to understand the patient experience of treatment-related toxicities and/or disease related symptoms during the earlier phases of treatment, which often overlap (e.g., fatigue and pain could be caused by cancer itself or cancer treatments).
Noting these limitations researchers have increasingly used PFS as an alternative endpoint in oncology clinical trials. The movement from OS to PFS has sparked debate among experts regarding the appropriateness of PFS as a primary endpoint. Those in support of this movement point to both clinical and practical advantages of PFS compared to OS, such as less influence of multiple therapies, greater sensitivity among patients for whom OS is expected to be prolonged (e.g., early-stage disease), and shorter assessment timelines that can lead to faster approval of new treatments [3]. Critics of the movement from OS to PFS have raised concerns, including lack of evidence linking PFS to OS, uncertain quality of life benefit associated with PFS, and the risk of PFS measurement bias in practice [4].
Additional concerns related to both OS and PFS as endpoints in oncology clinical trials are the toxicities of the treatments being investigated and symptoms related to cancer itself. Treatments that are capable of producing complete tumor responses can also produce life-limiting fatigue, nausea/vomiting, pain, diarrhea, distress, and other symptoms. In addition, cancer itself can cause symptoms including fatigue and pain, among others. These toxicities and symptoms can limit the range of appropriate patients within a target population and, ultimately, their ability to remain on treatment [5–7]. Thus, additional endpoints are necessary to determine the implications of OS and PFS beyond the benefits of increased survival time, with or without evidence of progressive disease. That is, even if a treatment demonstrates a clear OS or PFS benefit, how is it tolerated by patients? This is especially relevant as long-term oral therapies are being used more and more frequently.
To this end, cancer symptom response (CSR) has evolved as a clinical outcome assessment in oncology clinical trials, and it is recognized as an accepted primary efficacy endpoint for regulatory approval of cancer treatment [8]. CSR is a complementary yet distinct endpoint from OS and PFS, and it requires careful consideration of its measurement and interpretation. Notably, cancer-related symptoms are experienced and interpreted directly by patients; thus, they are best assessed by patients themselves. Instruments to assess cancer-related symptoms, often referred to as patient-reported outcomes (PROs), have been included in many clinical trials [9]. In order to ensure that symptom assessments by patients produce valid and reproducible results, various symptom PROs have been developed and validated for a range of tumor types (e.g., lung, colorectal, renal, bone, and prostate).
CSR can signal early, otherwise undetectable disease activity [10,11]. Therefore, this endpoint may provide an earlier and more immediate overall assessment of patients’ treatment response, which addresses a critical weakness of both OS and PFS endpoints. Further, a growing body of evidence indicates that CSR is predictive of “standard” clinical outcomes including tumor response, disease progression, and survival [12,13]. These findings set CSR apart from other intermediate clinical trial endpoints.
Given the valuable information gained from assessing CSR, it is increasingly being incorporated as a primary endpoint in oncology clinical trials [9,14]. It is important to understand how to appropriately translate these findings into clinical practice. In this review, we describe important considerations for the evaluation, analysis, and interpretation of CSR in clinical trials, including potential measurement issues. We end with a discussion of integrating CSR assessment into clinical practice.
2. Evaluation of Cancer Symptom Response
The key points of this section, Section 3 (Analysis of Cancer Symptom Response), and Section 4 (Potential Measurement Issues) are summarized in Table 1.
Table 1.
Considerations for the evaluation, analysis, and potential measurement issues of cancer symptom response (CSR) in oncology clinical trials.
| Topic | Summary |
|---|---|
| Evaluation of CSR in oncology clinical trials |
• CSR assessments should include questionnaires developed and validated to
address disease-specific symptoms. • Optimal CSR assessment depends on numerous factors (e.g., the progressive nature of the disease, time from initial diagnosis, number and type of comorbidities, age, gender). • The goal of CSR assessment may vary across studies and populations. • Patients must complete CSR assessments at least twice to assess progress over time. CSR is typically evaluated frequently in clinical trials to minimize unobserved gaps in treatment effects. • Timing of CSR assessments should be carefully considered and planned to best capture the information of interest (e.g., pre- and post-chemotherapy administration). |
| Analysis of CSR in oncology clinical trials |
• There are multiple methods for assessing cancer treatment impact on
CSR. • Time to symptomatic progression (TTSP) assesses the length of time that transpires before symptom severity worsens. • Change from baseline assesses change in symptom severity from baseline to a pre-specified time point, and is typically compared between randomized treatment groups. • Important difference is the point at which a meaningful difference or change in symptoms is noted, which is instrument and context-specific. • Responder analysis assesses the proportion of patients who achieve a pre-specified level of improvement in symptoms over time with randomized treatment. |
| Potential measurement issues |
• Oncology clinical trials prefer analyzing CSR via TTSP analyses using an
intent-to-treat (ITT) approach, which includes all randomized patients regardless of trial withdrawal or drop-out. • Informative censoring occurs when withdrawal rates differ between treatment groups. • Informative censoring threatens the validity of ITT analysis and should be minimized with study design and analysis techniques (e.g., mixed effects analyses, imputation of missing data). • Responses to symptom-related questionnaires may be influenced by drug tolerability issues. • If CSR is highly correlated with other measures of efficacy (e.g., survival, tumor response), this lends credibility to CSR data as evidence of efficacy. • If CSR only correlates with adverse event reporting, differences in CSR could be attributed to treatment safety. |
Temel and colleagues [15] eloquently highlighted the importance of comprehensive cancer symptom assessment and management for non-small-cell lung cancer (NSCLC) patients. Citing clinical experience, they comment, “Although we have made steady improvements in the survival rates of patients with advanced-stage lung cancer, the majority of patients still experience distress and suffering…. Dyspnea, cough, fatigue, anorexia/ cachexia, and pain are the most common symptoms…. Therefore, comprehensive care of patients with advanced-stage NSCLC must include therapies targeted at these difficult and distressing symptoms.” The importance of focus on symptom management is not limited to NSCLC; it extends to all cancers where tumor involvement disrupts normal physiological responses resulting in disruptive and debilitating symptoms. In Table 2, we provide the number of publications in PubMed that report on PROs in clinical trials since 2010 by cancer site; a PubMed search for clinical trials was conducted in March 2018 using search terms for a specified tumor type and quality of life and/or PROs. Given the use of multiple terms used to describe PROs in the literature, it is unlikely that this search was exhaustive. However, this table shows that assessment of PROs are a common and important addition to the clinical oncology literature.
Table 2.
Number of papers published in PubMed since 2010 reporting on patient reported outcomes (PROs) in oncology clinical trials.
| Tumor type | No. publications listed in PubMed |
|---|---|
| Bladder | 37 |
| Brain | 156 |
| Breast | 1575 |
| Cervical | 41 |
| Colorectal | 301 |
| Gastric | 108 |
| Head/Neck | 330 |
| Leukemia | 95 |
| Lung | 378 |
| Melanoma | 52 |
| Mesothelioma | 21 |
| Multiple myeloma | 49 |
| Myelodysplastic syndrome | 24 |
| Non-Hodgkin’s lymphoma | 48 |
| Ovarian | 113 |
| Pancreatic | 113 |
| Prostate | 432 |
| Renal | 51 |
| Skin, basal cell carcinoma | 2 |
| Soft tissue sarcoma | 21 |
| Thyroid | 17 |
| Uterine | 114 |
Note. PubMed search was conducted for clinical trials using search terms for the specified tumor type and PROs and/or quality of life. Articles were restricted from 2010 through the date of search, March 16, 2018.
2.1. Use of validated questionnaires
The field of clinical oncology has long accepted the need to improve patients’ quality of life and attend to cancer-related symptoms. Methods to ascertain CSR in clinical trials have been demonstrated in oncology and other clinical areas including respiratory (i.e., asthma and chronic obstructive pulmonary disease), auto-immune deficiency, connective tissue, and neurologic disorders (i.e., ALS) [16–22]. In all of these therapeutic areas, the accepted method for symptom assessment is the use of daily to weekly patient diaries or questionnaires developed and validated to address disease-specific symptoms. In oncology, symptom questionnaires have been developed that are either general to cancer or specific to a primary tumor site. Recently international, consensus-based guidelines for including PROs in clinical trials have been developed, and they provide recommendations for designing clinical trial protocols for which PROs are a primary or secondary outcome [23].
2.2. Assessment of cancer symptom response
Assessment of CSR to therapy may vary depending on multiple factors, including the progressive nature of the disease, time from initial diagnosis, number and type of comorbidities, age, and gender. For example, for tumor types with typically slower progression, improvement in symptoms may be highly informative of treatment impact. In more aggressive disease, maintaining symptom levels can be viewed as beneficial based on the expected natural history of further deterioration over a short time period with less effective intervention. Thus, the goal of cancer-related symptom assessment (e.g. symptom maintenance or improvement) may vary across studies and populations.
2.3. Time of assessment
In order to evaluate CSR, patients need to complete a PRO questionnaire twice, at minimum – typically at baseline/randomization and during the treatment phase of the trial. This allows for assessment of patient progress over time. In oncology clinical trials, CSR is typically evaluated frequently to minimize unobserved gaps in treatment effects that may bias results [8,24]. Randomization in a clinical trial provides the conditions necessary to demonstrate that symptom response is attributable to treatment assignment rather than differences between patients. Often after the conclusion of the randomized, blinded portion of the trial, patients are entered into an open-label phase where only the active agent of interest is administered in an unblinded fashion. During this phase, it is useful to administer PROs to assess the durability of a treatment effect on symptoms.
In addition to the frequency of assessments, the timing of PRO assessments is critical to the interpretation of results. For example, given the transient nature of some treatment toxicities, assessing PROs before a dose of chemotherapy could elicit different responses than assessing PROs after a dose of chemotherapy. Timing of PRO assessments should be carefully considered and planned in order to best capture the information of interest.
3. Analysis of Cancer Symptom Response
Once data are collected from PROs, there are multiple methods for assessing treatment impact on CSR with these data. These methods are now described.
3.1. Time to symptomatic progression
Time to symptomatic progression (TTSP) refers to the assessment of the length of time that transpires before symptom severity worsens. Such an approach is clinically relevant in advanced (incurable) disease settings, where symptoms are likely to eventually worsen, based on the natural history of the disease. It has appeal statistically as it lends itself to a time-to-event analytic approach including hazard ratios and Kaplan-Meier plots. TTSP assesses CSR in a similar manner to how OS and PFS are evaluated in clinical trials. The symptom event is typically set as a pre-specified worsening in patient-reported symptom score. When evaluating TTSP, a longer time to symptomatic progression in one treatment group compared to another indicates symptom benefit. Often, this analysis includes disease progression and toxicity-caused withdrawal as events in the analysis, essentially forming a composite endpoint of deterioration. In addition to achieving statistical significance, the symptom benefit should also be clinically meaningful, a degree of benefit ideally defined prior to the start of the clinical trial.
3.1.1. Examples of TTSP.
Results from a phase III randomized, double-blind, placebo-controlled clinical trial of sorafenib for the treatment of hepatocellular carcinoma (HCC) were published in 2007 [25]. In this trial, TTSP was a co-primary efficacy endpoint with OS, and patients completed a PRO instrument that is specific to HCC – the Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep) Symptom Index (FHSI-8) [26]. The FHSI-8 is an 8-item measure that approximates disease-specific health-related quality of life (HRQoL) for patients with HCC by assessing pain, fatigue, nausea, weight loss, and jaundice. In this study, the magnitude of symptomatic progression for the FHSI-8 was defined as a decrease of at least 4 points, which needed to be confirmed at the subsequent assessment. Prior psychometric studies of this instrument had already determined that a 4-point change represented a substantial change in symptom response [27]. In this trial, treatment with sorafenib was associated with a 44% improvement in OS compared to a placebo control, and there was no difference in TTSP between treatment arms. The researchers concluded that sorafenib had a clinically meaningful OS benefit relative to placebo. As there was no difference in TTSP between treatment groups, the researchers concluded that sorafenib was well tolerated by patients, thus supporting the survival benefit [25].
In another phase III randomized trial, treatment with cabozantinib (vs. everolimus) was associated with improvements in PFS, OS, and objective tumor response rate for patients with metastatic renal cell carcinoma [28,29]. Follow-up exploratory analyses of TTSP were conducted with the 19-item Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI-19) [30] and the EuroQol Group’s five-level questionnaires (EQ-5D-5L) [31]. The FKSI-19 assesses disease-related symptoms, treatment side effects, and well-being associated with kidney cancer, whereas the EQ-5D-5L assesses is a standardized measure of health status comprised of five functional domains (i.e., mobility, self-care, usual activities, pain and discomfort, and anxiety and depression). There were no differences over time for FKSI-19 or EQ-5D-5L scores between the cabozantinib and everolimus conditions [32]. However, TTSP was improved in the cabozantinib condition, particularly among patients with bone metastases, although this was not associated with a quality of life advantage on the FKSI-19 or the EQ-5D-5L.
Another recent study reported on the results from a multicenter, randomized, double-blind, placebo-controlled, phase III trial of everolimus for patients with advanced, non-functional, well-differentiated gastrointestinal or lung neuroendocrine tumors [33]. Everolimus has already been shown to improve PFS compared to placebo [34]. In secondary analyses, TTSP was assessed using the FACT-General (FACT-G) [35], which assesses four domains of overall HRQoL (i.e., physical, emotional, social/family, and functional well-being) in patients with cancer (not site specific). There were no difference in TTSP for patients treated with everolimus compared to placebo [33]. The authors concluded that everolimus improves PFS while preserving overall HRQoL for this specific patient population.
3.2. Change from baseline
An alternate method to assessing CSR is to calculate the change in total PRO scores from baseline to a pre-specified time point, and then compare these changes between randomized treatment groups.
3.2.1. Examples of change from baseline.
Pardo and colleagues [36] compared the impact of several primary treatments for localized prostate cancer on quality of life in patients who were not receiving adjuvant hormonal therapy. Changes in generic and prostate cancer-specific quality of life from pre-treatment to three years post-intervention were compared between treatment groups. Generic quality of life was assessed with the physical and mental quality of life components of the Short Form-36 Health Survey (SF-36) [37]. Prostate cancer-specific quality of life was assessed with the Expanded Prostate Cancer Index Composite (EPIC), which includes two urinary subscales (irrigative-obstructive and incontinence) and three summary scores (hormonal, sexual, and bowel) [38]. There were significant differences in type and longevity of adverse effects associated with each treatment group, leading the authors to conclude that these results could facilitate shared clinical decision making between patients and providers [36].
In a separate study, Fizazi and colleagues [39] used a combined analytic approach including TTSP and change from baseline to assess prostate cancer-specific HRQoL and pain in men with metastatic castration-resistant prostate cancer. The primary outcomes of this trial demonstrated the superiority of treatment with enzalutamide vs. placebo for overall survival [40]. In secondary analyses, prostate cancer-specific HRQoL was assessed with the FACT-Prostate (FACT-P) [41,42]. Similar to other FACT measures, the FACT-P assesses HRQoL across the domains of physical, emotional, social/family, and functional well-being in addition to items assessing prostate-related symptoms. In addition, pain was assessed with the Brief Pain Inventory-Short Form (BPI-SF) [43], which assesses pain severity and pain interference. Secondary analyses evaluated time to first skeletal-related event (e.g., bone metastasis), change in pain from baseline, time to pain progression (strongly associated with bone metastasis), change in HRQoL from baseline, and time to HRQoL deterioration. Results revealed that treatment with enzalutamide was superior than placebo across all outcomes [39].
A later trial built upon these results and assessed enzalutamide vs. another treatment, bicalutamide, for the treatment of men with asymptomatic or mildly symptomatic metastatic castration-resistant prostate cancer [44]. In secondary analyses of a phase II, randomized, double-blind trial, Heidenreich and colleagues used a combined analytic approach including TTSP and change from baseline to assess treatment effects on HRQoL and pain. Prostate cancer specific HRQoL was assessed with the FACT-P [41,42], and pain was assessed with the BPI-SF [43]. In addition, overall quality of life was assessed with the European Quality of Life 5-Domaine Scale (EQ-5D) [45] which is comprised of five functional domains (i.e., mobility, self-care, usual activities, pain and discomfort, and anxiety and depression). In this study, TTSP was longer with enzalutamide, as assessed with the FACT-P and EQ-5D. Although both treatment groups reported an increase in pain, there was no difference in time to pain progression on the BPI-SF. In addition, there was more favorable change from baseline on the FACT-P in the group treated with enzalutamide [44].
3.3. Identifying important differences in symptom assessments
Tumor response in oncology clinical trials is typically determined by RECIST or mRECIST criteria, which define tumor improvement, stabilization, or progression based on the size of cancerous lesions [46,47]. A 50% reduction in measurable tumor size without new lesion formation is considered a partial tumor response. Similarly, a 25% increase in size or new lesion formation is considered disease progression. Similar criteria can be assigned to CSR. The magnitude of improvement in a PRO typically required to determine a “response” is often referred to as the minimally important difference (MID) or minimal clinically important difference (MCID). We propose that the “minimal” modifier can be discarded, preferring to propose what is a well-supported “important difference” (ID) or “clinically important difference” (CID) for individual change, as well as for group comparison. Typically, the value for individual change is larger than that for group comparisons. The ID or CID is instrument specific, and even context-specific, rather than universal like mRECIST criteria. In other words, there will be a unique ID or CID criteria for a given measure used in a given application. The basis for proposing a trial-specific ID should be specified in advance, preferably with a commitment to test a range of ID values for robustness of effect.
3.3.1. ID assignment.
The ID is the point at which a meaningful difference or change in symptoms is noted. The estimated ID, including its lower bound (i.e., the MID) can be based on the relationship of the PRO measure to other accepted efficacy or HRQoL endpoints. As these endpoints serve as anchors from which to estimate important differences, use of this approach is often referred to as an anchor-based approach. Alternatively, the low end of a measure ID can be estimated based on the distribution of responses to the instrument seen in multiple relevant samples. This is often referred to as a distribution-based method. Before one specifies an instrument-specific ID, supportive evidence from several studies is optimal.
3.3.2. Examples of ID-based endpoints.
A recent exploratory analysis used pooled data from two trials comparing enzalutamide and a placebo-control for treating patients with either chemotherapy-naïve or post-chemotherapy metastatic castration-resistant prostate cancer [40,48,49]. The analysis explored associations of the FACT-P [41,42] – with OS and PFS. The ID for the FACT-P was defined as a 10-point change [50]. Results were presented as reduction of risk for OS and PFS based on ID increases, or improvements, in FACT-P scores [49].
Cella and colleagues [51] used a combined analytic approach including change from baseline and ID-based endpoints to compare the impact of nivolumab vs. everolimus on the quality of life of patients with advanced renal cell carcinoma in a phase III trial. Disease-specific symptoms in renal cancer were assessed with the FACT-Kidney Symptom Index-Disease Related Symptoms (FKSI-DRS) [52]. In this study, the ID criteria for the FKSI-DRS was pre-set as a 2-point difference from baseline. In addition, overall quality of life was assessed with the EQ-5D questionnaires [45]. Findings revealed more patients with clinically meaningful improvements in disease-specific symptoms since baseline in the group receiving treatment with nivolumab [51].
3.4. Responder analysis
A responder analysis refers to the proportion of patients who achieve a pre-specified level of improvement in cancer symptoms over time with randomized treatment. One example of a pre-established benchmark used for responder analyses in clinical trials is the proportion of patients who achieve the ID over time with randomized treatment. Another example is the proportion of patients who report improvement, maintenance, or worsening of symptoms over time with randomized treatment.
3.4.1. Example of responder analysis.
Smith and colleagues [53] reported a secondary responder analysis from a randomized, phase III trial of duloxetine compared to placebo for treating peripheral neuropathy caused by cancer chemotherapy. They specifically assessed the proportion of patients achieving pain reduction between groups. Pain reduction was defined as a 30% and 50% reduction in total score on the BPI-SF [43]. Results indicated that treatment with duloxetine was associated with greater reductions in pain compared to placebo [53].
4. Potential Measurement Issues
4.1. Informative censoring
In clinical trials, the preferred method for assessing treatment responses between groups is to conduct an intention-to-treat (ITT) analysis. This type of analysis includes all patients who were randomized to receive treatment in efficacy and safety assessments, regardless of participant withdrawal or drop-out from the trial. A threat to the validity of an ITT analysis is when withdrawal rates differ between treatment groups (i.e., more patients drop out in one treatment compared to the other). This results in the loss of critical data that is needed to adequately compare the effects of treatment arms.
Differential withdrawal between treatment groups during the course of an oncology clinical trial can occur because of between-group differences in disease progression and death. Both of these reasons for withdrawal, disease progression and death, contribute to efficacy endpoints in clinical trials. This can create a bias in symptom or quality of life results known as informative censoring. So, in a treatment where an inferior treatment produces greater risk for disease progression and death, it becomes very likely that patients in the clinically inferior arm of the trial will withdraw earlier and in higher numbers compared to those randomized to receive the more effective treatment. Differential withdrawal between treatment groups can also occur due to differences in toxicities associated with the treatments, again leading to differential withdrawal rates. Finally, surviving patients in any treatment arm (i.e., those that do not withdraw) typically have better health status at baseline than patients who withdrew earlier [54,55].
In cases such as these where informative censoring occurs, the effect will be to underestimate the CSR in one arm. Thus, assessment of cancer symptoms requires careful selection of study design and analysis techniques to help minimize this potential. Several statistical methods to correct for informative censoring have been explored and published [56–60]. These include mixed effects analyses that group patient responses by level of data completeness and give greater weight to more complete data (e.g., pattern mixture models), and statistical modeling to impute values for missing data that may include a mortality adjustment to account for missing data due to death (e.g., multiple imputation). There is no single recommended method for adjusting for missing data that cannot be ignored. It is important to review clinical trials results on CSR with this issue in mind, as well as methods that may have been used to account or adjust for the missingness.
4.2. Differentiating cancer symptom response and treatment-related adverse events
CSR is an informative endpoint to assess patient response to treatment. However, responses to symptom-related questionnaires may be influenced by drug tolerability issues. This can come about because patients are often asked about symptoms that can be related to both cancer and treatment, such as nausea and fatigue, and also is influenced by the timing of assessments since treatment-related symptoms can be transient. When patient responses are related to treatment toxicity, the effect is to overestimate CSR for the study arm associated with the lowest level of toxicity.
Because safety and efficacy data are collected simultaneously in randomized clinical trials, it is possible to assess the potential influence of drug-related toxicity on patients’ symptom response. If CSR is highly correlated with other measures of efficacy, such as survival or tumor response, this lends credibility to the CSR data as evidence of efficacy. Alternatively, if CSR correlates only with adverse event reporting, differences in symptom response may be attributed to treatment safety.
One example where drug-related toxicity may have influenced patient CSR is from a phase III trial comparing chemotherapy regimens in stage IC-IV ovarian cancer [61]. Results demonstrated a statistically significant difference in symptoms based on patient responses to the EORTC core questionnaire (QLQ-C30), which assesses overall quality of life of cancer patients [62]. However, there were no significant between-group difference in survival or tumor response rates [60]. Given this inconsistency, consideration of between-group differences in tolerability profile was required. Follow-up analyses found significantly more reported cases of neurotoxicity in the treatment group that failed to show an improvement in symptoms. Therefore, symptom scores obtained in the trial may not have shown a between-group difference in cancer symptoms; rather, they may have reflected the difference in side effects profiles of the two chemotherapy regimens.
Teasing apart cancer-related symptoms and CSR from treatment toxicities can be challenging, and is best achieved with a strong understanding of the side effect profiles of treatments. In addition, timing of assessments is critical for distinguishing between CSR and drug-related toxicity (e.g., assessment of symptoms before treatment administration or after).
5. Interpretation of Cancer Symptom Response
5.1. Cancer symptom response may be an early signal of treatment response
Researchers have used the evaluation of CSR to demonstrate the clinical efficacy of treatments in studies involving a number of cancers. Some recommend that CSR should be used to evaluate treatment effects in most cancers, particularly for patients with metastatic disease [63].
An example of a study that has linked CSR to treatment efficacy is an observational study conducted in patients with metastatic renal cell carcinoma. Wilson and colleagues [64] retrospectively compared TTSP by treatment with palliative radiotherapy. TTSP was defined as symptom recurrence or worsening, as reported by patients. After adjusting for disease and patient characteristics at baseline, they found statistically significant treatment differences in TTSP. Results demonstrated the clinical importance of administering interferon-alpha with radiotherapy in this patient group versus interferon-alpha alone, which was associated with a shorter TTSP [64].
Verma and colleagues [65] conducted a study among HER-2 positive advanced breast cancer patients who had previously been treated with trastuzumab and a taxane. They randomly assigned patients to receive either trastuzumab emtansine (T-DM1) or lapatinib plus capecitabine. The trial’s primary outcomes were PFS and OS, and the secondary outcomes were objective tumor response rate, treatment toxicity, and TTSP. Treatment with T-DM1 was associated with better outcomes along every outcome (i.e., improved PFS and OS, higher objective response rate, less treatment toxicity, and prolonged TTSP). Thus, T-DM1 was associated with more immediate markers of treatment efficacy (e.g., TTSP) as well as subsequent treatment response (e.g., PFS and OS), and T-DM1 was identified as a potentially therapeutic agent among HER-2 positive advanced breast cancer patients who experienced disease progression on prior treatment [65].
From a different perspective, lack of CSR can signal that a treatment is not effective. For example, Janne and colleagues [66] recently reported on a randomized clinical trial of selumetinib plus docetaxel versus docetaxel alone for patients with KRAS-mutant advanced non-small cell lung cancer (NSCLC). Results indicated that the addition of selumetinib did not improve outcomes (i.e., PFS, OS), was related to more frequent serious adverse events, and had no effect on TTSP or symptom improvement rates. Thus, by any measure, the addition of selumetinib to docetaxel was not more effective than docetaxel alone in this setting.
Evaluation of CSR has enabled researchers to assess not only the comparative benefits of treatments, but also the relative importance of symptom resolution in specific cancer populations. For example, in a randomized trial of NSCLC patients, single-agent gemcitabine provided better symptom response than cisplatin/venidesine doublet therapy [67] based on patient responses to the Lung Cancer Symptom Scale, which assesses quality of life for patients with lung cancer [68]. Symptom control was similar in older (≥ 65y) and younger patients, and only slightly better among patients with better performance status (Karnofsky status ≥ 80%) [67].
5.2. Cancer symptom response as an early signal for tumor response, disease progression, and mortality
Reviews of the literature show that CSR in clinical trials is highly predictive of stable disease, progressive disease, and death. A meta-analysis that included multiple cancer sites (e.g., lung, breast, prostate, and colorectal) found that, across studies, improvements in PROs were most strongly associated with complete or partial tumor response (followed by stable disease and progressive disease) [12]. Other reviews of various cancer sites have found that baseline PROs are relevant and independent prognostic factors for survival [54,55].
Comparable results were found in studies of specific tumor types. In a review specific to NSCLC, Heyes [69] cited 11 clinical trials where NSCLC symptom response (as measured by the FACT-Lung) predicted tumor response, including partial and complete response, stable disease, and progressive disease. Subsequent studies of NSCLC have reported similar results [70]. Other examples of studies that have found relationships between PROs and tumor response, disease progression, and/or mortality include samples of head and neck cancer [71], advanced colorectal cancer [72], and metastatic castration-resistant prostate cancer [73].
5.2.1. Example in practice.
To illustrate the potential clinical and prognostic value of TTSP in a longitudinal clinical trial, we performed a retrospective analysis of data from ECOG study 5592 [74]. This was a three-arm randomized clinical trial that compared cisplatin plus etoposide to two doublet chemotherapy arms that both contained paclitaxel and cisplatin for treatment of advanced NSCLC. We found that TTSP was highly predictive of other efficacy endpoints, including best overall response to treatment, time to clinical progression, and survival. As shown in Figure 1, patients who progressed early on CT scans also tended to show earlier symptomatic progression (p<0.07) (Figure 1a), and TTSP was significantly associated with shorter versus longer survival (p<0.05) (Figure 1b). Finally, TTSP was significantly associated with best clinical response, with responding patients reporting 1 to 2 months additional symptom-free time compared to patients with stable or progressive disease (p<0.01 and p<0.001, respectively) (Figure 1c).
Figure 1.
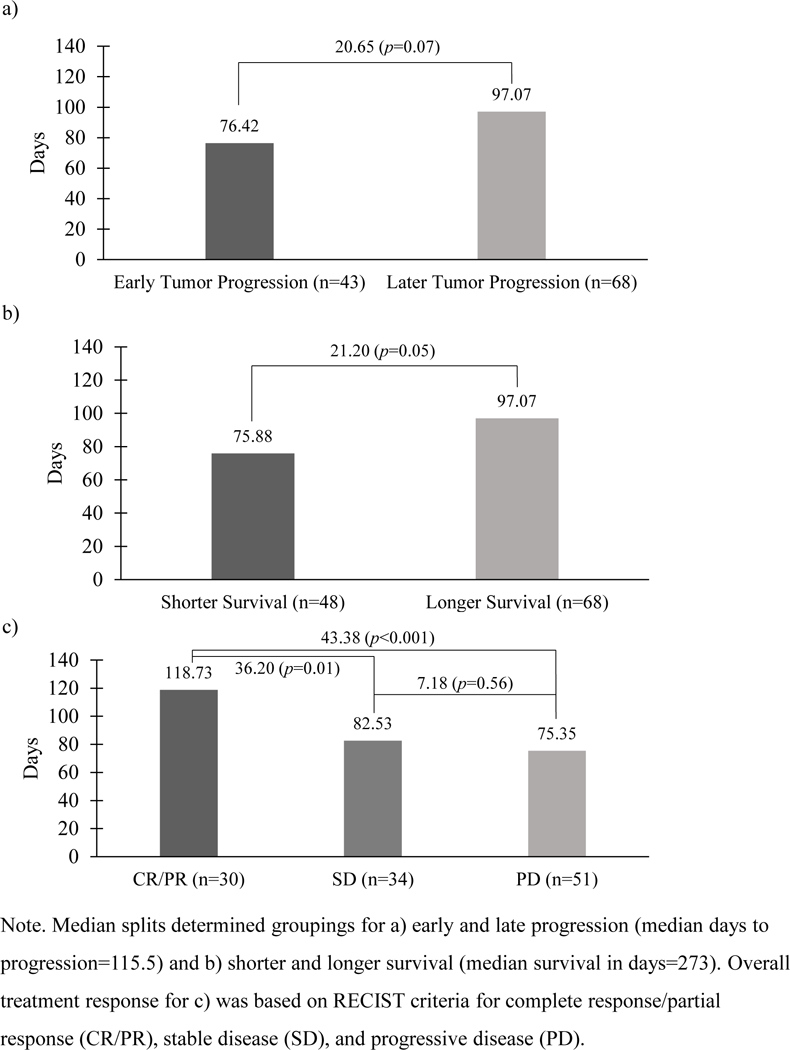
Mean days to cancer symptom progression based on a) time to tumor progression, b) survival time, and c) overall treatment response.
6. Assessment of Cancer Symptom Response in Clinical Practice
Given the predictive nature of CSR from clinical trials, symptom evaluation in clinical practice can assist clinicians in their evaluation of individual patients undergoing cancer treatment [75–77]. Indeed, studies have shown that routine assessment of cancer symptoms in the clinic can help clinicians better respond to individual patient needs, resulting in reduced symptoms over time, improved HRQoL, and improved OS.
An early Dutch study found that lack of a formal method for routinely collecting PRO information contributed to inadequate symptom management [78]. Since then, studies found that routine assessment of CSR can significantly improve discussions with patients, can facilitate early recognition and resolution of serious symptoms leading to improved symptom control, and leads to increased patient satisfaction [79]. Recently, a group from Memorial Sloan Kettering reported on a trial in which patients with metastatic cancers receiving cancer treatment were randomized to either 1) repeated electronic PRO assessments and integration of symptom reports into the patients’ electronic medical records, or 2) usual care (i.e., discussing cancer-related symptoms during clinical encounters and phone reporting of concerning symptoms between appointments, as initiated by the patient) [80,81]. At one year, patients in the electronic PRO condition reported greater improvement in HRQoL, had less admissions to the emergency room or hospital, and remained on chemotherapy longer compared to patients in the usual care condition [80]. At median seven-year follow-up, patients in the electronic PRO condition also had increased survival compared to the usual care condition [81]. It is possible that early responsiveness to patients’ concerning cancer-related symptoms resulted in more effective care during routine cancer treatment.
Based on results of these studies, symptom self-assessment by patients in the clinic is highly recommended [78]. Guidelines for implementing routine assessments of CSR into daily clinical care are available, such as those from the International Society for Quality of Life Research [82]. With greater use of electronic medical records in clinical care, there are now many electronic systems available for assessing cancer-related systems [83] as well as guidelines to assist with the integration of CSR assessments into electronic medical records [84]. Clinicians are advised to consider how results of routine CSR assessments can be directly utilized in patient discussions and treatment appraisals. Routine use of an instrument should be carefully discussed with staff. A process should be implemented that puts the least burden on staff and patient flow and permits for timely review of results. Finally, while there is no universally accepted symptom assessment tool for office-based use, several have been implemented and are available; careful selection of an appropriate instrument and methods for systematic review are highly recommended.
7. Conclusion
Evaluation of CSR in clinical trials provides a meaningful assessment of the early effects of cancer treatment, and the utility of CSR for comparing cancer treatments has been well documented. Studies show that CSR can act as an early signal of disease progression and death, and it can guide evaluates as to which patients with stable disease will have a more favorable prognosis. Finally, given the success of including cancer symptom PROs in clinical trials, clinicians should consider this type of ongoing evaluation in their clinical practice. Recommendations for assessing CSR in clinical practice include the use of short questionnaires provided to patients on an ongoing basis, and in a manner that is well integrated into routine practice.
8. Expert Commentary
Assessing CSR in oncology clinical trials can yield meaningful information related to early treatment response, and CSR also predicts long-term health outcomes (e.g., tumor response, PFS, and OS). Given the valuable information gained from assessing CSR in clinical trials, we sought to explore important considerations for the evaluation, analysis, and interpretation of CSR in clinical trials, including potential measurement issues. Notably, interpretation of improvement vs. maintenance of cancer-related symptoms may vary depending on a host of factors (e.g., time from initial cancer diagnosis, the progressive nature of disease, number and type of patient comorbidities, gender, and age). While there are a variety of ways to analyze CSR data, oncology trials generally favor time to symptomatic progression analyses, which assess the length of time that transpires before symptom severity worsens with an intent-to-treat (ITT) approach. ITT analyses are subject to validity threats, including the potential for informative censoring, and this must be carefully considered when interpreting clinical trial results.
Despite an increasing amount of data from HRQoL studies in oncology (both clinical trials and observational studies), clinical care in most settings continues to occur without formal collection or use of patient-reported symptoms. Routine assessments of CSR can assist clinicians in their evaluation of individual patients undergoing cancer treatment, and studies have demonstrated the benefits of incorporating routine evaluation of CSR into clinical practice. Clinicians should consider this type of ongoing evaluation in their clinical practice using short questionnaires provided to patients on an ongoing basis and in a manner that is well integrated into routine practice – possibly via integration into electronic medical records. Technological advances in the past decade related to the assessment of CSR have streamlined the process of administering CSRs to patients, storing patient data, and receiving and interpreting patient data.
Opportunities for growth in this field include continued assessment of CSR in clinical trials and refinement of assessments. Future research should focus on further discovery of methods for more accurate assessment of symptom response, reduction of the number of symptoms used as signals for disease progression or survival by tumor type, and statistical methods that effectively correct for missing data and informative censoring. Refinement in these areas will ultimately help to make patient-reported data more interpretable and therefore actionable and relevant to clinical practice; however current challenges to this goal are barriers to collecting high quality data and aligning these self-report data to clinical endpoints. We recommend that future research work toward identifying patient-centered thresholds for clinical relevance and meaningful change (improvement or worsening), and linking patient responses and changes to clinical events to deepen the opportunities for shared decision-making.
9. Five-Year View
This review summarized the importance of evaluating CSR in cancer clinical trials, considerations for the evaluation, analysis, and interpretation of CSR in clinical trials, and recommendations for incorporating routine assessment of CSR into clinical practice with individual patients. In the next five years, we expect to see greater application of advances in measurement science to CSR, such as the development and application of composite end points that include multiple components (e.g., tumor-related end points as well as patient-centered end points). The development of composite end points has the potential to simplify the process of assessing CRS, and could help patients, providers, and decision-makers better understand the total clinical benefit of an intervention beyond OS. We also hope that, in the next five years, there will be an uptake in the routine assessment of CSR within cancer clinical care settings using validated measures of patient-reported outcomes.
Key Issues.
A critical challenge in oncology is interpreting clinical trial results to inform clinical decision making. Traditional endpoints in oncology clinical trials are overall survival (OS) and progression-free survival (PFS). However, these endpoints do not account for early signs of meaningful patient benefit or harm.
Cancer symptom response (CSR) is an endpoint that can provide meaningful information about early treatment response. CSR is a measure of change in cancer symptoms, which is best reported by the patients themselves. Studies show that CSR predicts long-term health outcomes including tumor response, PFS, and OS.
Interpretation of CSR (e.g., improvement vs. maintenance of symptoms) may vary depending on a host of factors (e.g., the progressive nature of disease, time from initial cancer diagnosis, number and type of patient comorbidities, age, and gender). Oncology clinical trials generally prefer analyzing CSR via time to symptomatic progression analyses, which assesses the length of time that transpires before symptom severity worsens, with an intent-to-treat (ITT) approach. Threats to the validity of ITT analyses include the potential for informative censoring, and this must be carefully considered when interpreting clinical trial results.
CSR can assist clinicians in their evaluation of individual patients undergoing cancer treatment, and studies have demonstrated the benefits of incorporating routine evaluation of CSR into clinical practice. Clinicians should consider this type of ongoing evaluation in their clinical practice using short questionnaires provided to patients on an ongoing basis and in a manner that is well integrated into routine practice.
Acknowledgement:
Author LCB was funded by the NCI training grant T32 CA193193. The authors would like to acknowledge David Eton PhD for assistance in statistical analyses for Figure 1.
Funding details: None.
Footnotes
Financial and competing interest disclosure: All authors declare that they have no conflicts of interest.
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to the readers.
References
- 1.Driscoll JJ, Rixe O. Overall survival: still the gold standard: why overall survival remains the definitive end point in cancer clinical trials. Cancer J 2009;15(5):401–405. [DOI] [PubMed] [Google Scholar]
- 2.Lebwhol D, Kay A, Berg W, Baladi JF, et al. Progression-free survival: gaining on overall survival as a gold standard and accelerating drug development. Cancer J 2009;15(5):386–394. [DOI] [PubMed] [Google Scholar]
- 3.Korn RL, Crowley JJ. Overview: progression-free survival as an endpoint in clinical trials with solid tumors. Clin Cancer Res 2013;19(10):2607–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol 2012;20(10):1030–1033. [DOI] [PubMed] [Google Scholar]
- 5.Leonardi MC, Zampino MG, Luca F, et al. Pre-operative radiochemotherapy with raltitrexed for resectable locally-advanced rectal cancer: a phase II study. Anticancer Res 2006;26(3B):2419–2423. [PubMed] [Google Scholar]
- 6.Kobayashi M, Tsuburaya A, Nagata N, et al. A feasibility study of sequential paclitaxel and S-1 (PTX/S-1) chemotherapy as postoperative adjuvant chemotherapy for advanced gastric cancer. Gastric Cancer 2006;9(2):114–119. [DOI] [PubMed] [Google Scholar]
- 7.Saif MW, Mehra R. Incidence and management of bevacizumab-related toxicities in colorectal cancer. Expert Opin Drug Saf 2006;5(4):553–566. [DOI] [PubMed] [Google Scholar]
- 8.United States Food and Drug Administration (FDA). Guidance for Industry. Clinical Trial Endpoints for the Approved of Cancer Drugs and Biologics Washington DC: US Department of Health and Human Services; 2005. [Google Scholar]
- 9. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29 – 2018 Mar 02.
- 10.Cella D Quality of life considerations in patients with advanced lung cancer. Semin Oncol 2004;31(6 Suppl 11):16–20. [DOI] [PubMed] [Google Scholar]
- 11.Brown DJ, McMillan DC, Milroy R. The correlation between fatigue, physical function, the systemic inflammatory response, and psychological distress in patients with advanced lung cancer. Cancer 2005;103(2):377–382. [DOI] [PubMed] [Google Scholar]
- 12.Victorson D, Soni M, Cella D. Metaanalysis of the correlation between radiographic tumor response and patient-reported outcomes. Cancer 2006;106(3):494–504.*Meta-analysis of the association between tumor response and patient-reported outcomes, including symptom response and health-related quality of life.
- 13.Secord AA, Coleman RL, Havrilesky LJ, et al. Patient-reported outcomes as end points and outcome indicators in solid tumors. Nat Rev Clin Oncol 2015;12(6):358–370.*Review of the correlations of patient-reported outcomes with treatment response and survival.
- 14.Wilson MK, Karakasis K, Oza AM. Outcomes and endpoints in trials of cancer treatment: the past, present, and future. Lancet Oncol 2015;16(1):e32–42. [DOI] [PubMed] [Google Scholar]
- 15.Temel JS, Pirl WF, Lynch TJ. Comprehensive symptom management in patients with advanced-stage non-small-cell lung cancer. Clin Lung Cancer 2016;7(4):241–249. [DOI] [PubMed] [Google Scholar]
- 16.Chio A, Logroscino G, Hardiman O, et al. Prognostic factors in ALS: a critical review. Amyotrophic Lateral Sclerosis 2009:10(5–6):310–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren XS, Kazis LE, Lee A, et al. The role of generic and disease-specific measures of physical and role functioning in assessing patient outcomes: a longitudinal study. J Ambul Care Manage 2005;28(2):157–166. [DOI] [PubMed] [Google Scholar]
- 18.Yamada K, Ito H, Nakamura H, et al. Stroke patients’ evolving symptoms assessed by tractography. J Magn Reson Imaging 2004;20(6):923–929. [DOI] [PubMed] [Google Scholar]
- 19.Webb A, Norton M. Clinical assessment of symptom-focused health-related quality of life in HIV/AIDS. J Assoc Nurses AIDS Care 2004;15(2):67–78; quiz 79–81. [PubMed] [Google Scholar]
- 20.Astoro NW, Samsuridjal D, Djoerban Z, et al. Quality of life of HIV patients and influential factors. Acta Medica Indonesiana 2007;37(1):2–7. [PubMed] [Google Scholar]
- 21.Richards JM Jr., Hemstreet MP. Measures of life quality, role performance, and functional status in asthma research. Am J Resp Crit Care 2012;149(2 Pt 2):S31–S39. [DOI] [PubMed] [Google Scholar]
- 22.Habraken JM, van der Wal WM, ter Riet G, et al. Health-related quality of life and functional status in end-stage COPD: a longitudinal study. Eur Respir J 2011;37(2):280–288. [DOI] [PubMed] [Google Scholar]
- 23.Calvert M, Kyte D, Mercieca-Bebber R, et al. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA 2018;319(5):483–494.**Recommendations for items that should be included in clinical trial protocols in which patient-reported outcomes are a primary or secondary outcome.
- 24.Hollen PJ, Gralla RJ, Rittenberg CN. Quality of life as a clinical trial endpoint: determining the appropriate interval for repeated assessments in patients with advanced lung cancer. Support Care Cancer 2004;12(11):767–773. [DOI] [PubMed] [Google Scholar]
- 25.Llovet J, Ricci S, Mazzaferro V, et al. Randomized phase III trial of soragenib versus placebo in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2007;LBA1
- 26.Yount S, Cella D, Webster K, et al. Assessment of patient-reported clinical outcome in pancreatic and other hepatobiliary cancers: the FACT Hepatobiliary Symptom Index. J Pain Symptom Manage 2002;24(1):32–44. [DOI] [PubMed] [Google Scholar]
- 27.Steel JL, Eton DT, Cella D, et al. Clinically meaningful changes in health-related quality of life in patients diagnosed with hepatobiliary carcinoma. Ann Oncol 2006;17(2):304–312. [DOI] [PubMed] [Google Scholar]
- 28.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma. N Engl J Med 2015;373:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016:17;917–927. [DOI] [PubMed] [Google Scholar]
- 30.Rao D, Butt Z, Rosenbloom S, et al. A comparison of the Renal Cell Carcinoma-Symptom Index (RCC-SI) and the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI). J Pain Symptom Manage 2009;38:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cella D, Escudier B, Tannir NM, et al. Quality of life outcomes for cabosantinib versus everolimus in patients with metastatic renal cell carcinoma: METEOR phase III randomized trial. J Clin Oncol 2018;36:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavel ME, Singh S, Strosberg JR, et al. Health-related quality of life for everolimus versus placebo in patients with advanced, non-functional, well-differentiated gastrointestinal or lung neuroendocrine tumours (RADIANT-4): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1411–1422. [DOI] [PubMed] [Google Scholar]
- 34.Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a ransomised, placebo-controlled, phase 3 study. Lancet 2016;387:968–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 2003;1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pardo Y, Guedea F, Aguilo F, et al. Quality-of-life impact on primary treatments for localized prostate cancer in patients without hormonal treatment. J Clin Oncol 2010;28(31):4687–4696. [DOI] [PubMed] [Google Scholar]
- 37.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 2000;56:899–905. [DOI] [PubMed] [Google Scholar]
- 39.Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised phase 3 AFFIRM trial. Lancet Oncol 2014;15:1147–1156. [DOI] [PubMed] [Google Scholar]
- 40.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New Engl J Med 2012;367(13):1187–1197. [DOI] [PubMed] [Google Scholar]
- 41.Esper P, Mo F, Chodak G, et al. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Adult Urology 1997;50(6):920–928. [DOI] [PubMed] [Google Scholar]
- 42.Victorson DE, Beaumont JL, Rosenbloom SK, et al. Efficient assessment of the most important symptoms in advanced prostate cancer: the NCCN/FACT-P symptom index. Psycho-Oncol 2011;20(9):977–983. [DOI] [PubMed] [Google Scholar]
- 43.Cleeland CS & Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap 1994;23:129–138. [PubMed] [Google Scholar]
- 44.Heidenreich A, Chowdhury S, Klotz L, et al. Impact of enzalutamide compared with bicalutamide on quality of life in men with metastatic castration-resistant prostate cancer: additional analyses from the TERRAIN randomized clinical trial. Eur Urol 2017;71(4):534–542. [DOI] [PubMed] [Google Scholar]
- 45.Pickard AS, Neary MP, & Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007;5:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. JNCI-J Natl Cancer I 2000;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 47.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 48.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. New Engl J Med 2014;371(5):424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beer TM, Miller K, Tombal B, et al. The association between health-related quality-of-life scores and clinical outcomes in metastatic castration-resistant prostate cancer patients: exploratory analyses of AFFIRM and PREVAIL studies. Eur j Cancer 2017;87:21–29. [DOI] [PubMed] [Google Scholar]
- 50.Cella D, Nichol MB, Eton D, et al. Estimating clinically meaningful changes for the functional assessment of cancer therapy-prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 2009;12(1):124–129. [DOI] [PubMed] [Google Scholar]
- 51.Cella D, Grunwald V, Nathan P, et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial. Lancet Oncol 2016;17:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cella D, Young S, Brucker PS, et al. Development and validation of a scale to measure disease-related symptoms of kidney cancer. Value Health 2007;10:285–293. [DOI] [PubMed] [Google Scholar]
- 53.Smith EML, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy. JAMA-J Am Med Assoc 2013;309(13):1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gotay CC, Kawamoto CT, Bottomley A, et al. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol 2008;26(8):1355–1636. [DOI] [PubMed] [Google Scholar]
- 55.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol 2009;10(9):865–871.*Meta-analysis concluding that health-related quality of life predicts survival in patients with cancer.
- 56.Zwinderman AH. Statistical analysis of longitudinal quality of life data with missing measurements. Qual Life Res 1992;1(3):219–224. [DOI] [PubMed] [Google Scholar]
- 57.Hollen PJ, Gralla RJ, Cox C, et al. A dilemma in analysis: issues in the serial measurement of quality of life in patients with advanced lung cancer. Lung Cancer 1997;18(2):119–136. [DOI] [PubMed] [Google Scholar]
- 58.Morita S, Kobayaski K, Eguchi K, et al. Analysis of incomplete quality of life data in advanced stage cancer: a practical application of multiple imputation. Qual Life Res 2005;14(6):1533–1544. [DOI] [PubMed] [Google Scholar]
- 59.Fairclough D. Design and analysis of quality of life studies in clinical trials Boca Raton, FL: Chapman & Hall; 2002. [Google Scholar]
- 60.Fielding S, Fayers P, Ramsay C. Predicting missing quality of life data that were later recovered: an empirical comparison of approaches. Clin Trials 2010;7(4):333–342. [DOI] [PubMed] [Google Scholar]
- 61.Vasey PA, Jayson GC, Gordon A, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian cancer. JNCI-J Natl Cancer I 2004;96(22):1682–1691. [DOI] [PubMed] [Google Scholar]
- 62.Aaronson KN, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QOQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. JNCI 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 63.Smith I Goals of treatment for patients with metastatic breast cancer. Semin Oncol 2006;33(Suppl 2):2–5. [DOI] [PubMed] [Google Scholar]
- 64.Wilson D, Hiller L, Gray L, et al. The effect of biological effective dose on time to symptom progression in metastatic renal cell carcinoma. Clin Oncol 2003;15(7):400–407. [DOI] [PubMed] [Google Scholar]
- 65.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. New Engl J Med 2012;367(19):1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janne PA, van den Heuvel MM, Barlesi F, et al. Selumetinib plus docetaxel compared with docetaxel alone and progression-free survival in patients with KRAS-mutant advanced non-small cell lung cancer. JAMA 2017;317(18):1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vansteenkiste J, Vandebroek J, Nackaerts K, et al. Influence of cisplatin-use, age, performance status, and duration of chemptherapy on symptom control in advanced non-small cell lung cancer: detailed symptom analysis of a randomised study comparing cisplatin-vindesine to gemcitabine. Lung Cancer 2003;40(2):191–199. [DOI] [PubMed] [Google Scholar]
- 68.Hollen PJ, Gralla RJ, Kris MG, et al. Quality of life assessment in individuals with lung cancer: testing the lung cancer symptom scale (LCSS). Eur J Cancer 1993;29(Suppl 1):S51–S58. [DOI] [PubMed] [Google Scholar]
- 69.Heyes A Clinical trial experience with functional assessment of cancer therapy-lung in conventional and targeted non-small cell lung cancer therapy. Semin Oncol 2004;31(Suppl 9):16–22. [DOI] [PubMed] [Google Scholar]
- 70.Cella D, Eton D, Hensing TA, et al. Relationship between symptom change, objective tumor measurements, and performance status during chemptherapy for advanced lung cancer. Clin Lung Cancer 2008;9(1):51–58. [DOI] [PubMed] [Google Scholar]
- 71.Abendstein H, Nordgren M, Boysen M, et al. Quality of life and head and neck cancer: a 5 year prospective study. Laryngoscope 2005;115(12):2183–2192. [DOI] [PubMed] [Google Scholar]
- 72.Popov I, Jelic S, Radosavljevic D, et al. The role of stable disease in objective response assessment and its impact on survival in advanced colorectal cancer: is “stable disease” a homogenous response category? Neoplasma 1999;46(2):132–139. [PubMed] [Google Scholar]
- 73.Cella D, Traina S, Li T, et al. Relationship between patient-reported outcomes and clinical outcomes in metastatic castration-resistant prostate cancer: post hoc analysis of COU-AA-301 and COU-AA-302. Ann Oncol 2018;39:392–397. [DOI] [PubMed] [Google Scholar]
- 74.Bonomi P, Kim I, Fairclough D, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern cooperative Oncology Group Trial. J Clin Oncol 2000;18(3):623–631. [DOI] [PubMed] [Google Scholar]
- 75.Davis K, Cella D. Assessing quality of life in oncology clinical practice: a review of barriers and critical success factors. J Clin Outcomes Manag 2002;9:327–332. [Google Scholar]
- 76.Sloan JA, Frost MH, Berzon R, et al. The clinical significance of quality of life assessments in oncology: a summary for clinicians. Support Care Cancer 2006;14(10):988–998. [DOI] [PubMed] [Google Scholar]
- 77.Varricchio CG, Ferrans CE. Quality of life assessments in clinical practice. Semin Oncol Nurs 2012;26(1):12–17. [DOI] [PubMed] [Google Scholar]
- 78.Detmar SB, Aaronson NK, Wever LD, et al. How are you feeling? Who wants to know? Patients’ and oncologists’ preferences for discussing health-related quality-of-life issues. J Clin Oncol 2000;18(18):3295–3301.*Early study discussing the importance of addressing health-related quality of life in daily clinical practice.
- 79.Kotronoulas G, Kearney N, Maguire R, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service oucomes in cancer care? A systematic review of controlled trials. J Clin Oncol 2014;32(14):1480–1501. [DOI] [PubMed] [Google Scholar]
- 80.Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016;34(6):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318(2):197–198.**Large randomized controlled trial that showed monitoring of patient-reported outcomes during routine cancer treatment predicts longer survival compared to usual care.
- 82.Snyder CF, Aaronson NK, Choucair AK, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qual Life Res 2012;21(8):1305–1314.**User’s Guide for implementing patient-reported outcomes assessments in clinical practice.
- 83.Jensen RE, Snyder CF, Abernethy AP, et al. Review of electronic patient-reported outcomes systems used in cancer clinical care. J Oncol Pract 2014;10(4):e215–222.*Review of available electronic patient reported outcomes systems.
- 84.PCORI. Users’ Guide to Integrating Patient-Reported Outcomes in Electronic Health Records 2017. Retrieved from https://www.pcori.org/document/users-guide-integrating-patient-reported-outcomes-electronic-health-records.*Guide to integrating patient-reported outcomes into electronic health records for use in daily clinical practice.


