Abstract
Resveratrol has been shown to have antioxidant and anti-proliferative properties in multiple cancer types. Here we demonstrate that H460 lung cancer cells are more susceptible to resveratrol treatment in comparison to human bronchial epithelial Beas-2B cells. Resveratrol decreases cell viability and proliferation, and induces significant apoptosis in H460 cells. The apoptosis observed was accompanied by an increase in hydrogen peroxide (H2O2) production, Bid, PARP and caspase 8 activation, and downregulation of pEGFR, pAkt, c-FLIP and NFkB protein expression. Furthermore, treatment with H2O2 scavenger catalase significantly inhibited resveratrol-induced c-FLIP downregulation, caspase-8 activation and apoptosis. Overexpression of c-FLIP in H460 cells (FLIP cells) resulted in the inhibition of resveratrol-induced H2O2 production, and a significant increase in resveratrol-induced apoptosis in comparison to H460 cells. In FLIP cells, catalase treatment did not rescue cells from a decrease in cell viability and apoptosis induction by resveratrol as compared to H460 cells. Resveratrol treatment also led to VEGF downregulation in FLIP cells. Furthermore, inhibition of pEGFR or pAkt using erlotinib and LY294002 respectively, enhanced the negative effect of resveratrol on FLIP cell viability and apoptosis. The reverse was observed when FLIP cells were supplemented with EGF, or transfected with WT-AKT plasmid; resulting in a 20% decrease in resveratrol-induced apoptosis. In addition, transfection with WT-AKT plasmid resulted in the inhibition of pro-apoptotic protein activation, and c-FLIP and pAkt downregulation. Overall, resveratrol induced apoptosis in H460 lung cancer cells by specifically targeting pAkt and c-FLIP dowregulation by proteasomal degradation in a EGFR-dependent manner.
Keywords: Lung cancer, Resveratrol, Apoptosis, c-FLIP, Akt, Hydrogen peroxide
INTRODUCTION
Apoptosis is a critical physiological mechanism for all forms of organism development and homeostasis [1]. Apoptotic cell death occurs through the highly regulated intrinsic or extrinsic pathways that initiate cell death by triggering DNA fragmentation, membrane blebbing, and chromatin condensation [2]. The intrinsic pathway is initiated by cytochrome c release and other pro-apoptotic factors from the mictochondria into the cytosol, which forms the apoptosome protein complex that initiates caspase-9 activation. Caspase-9 in turn is responsible for activation of other downstream caspases and apoptosis [3–6]. The oncogene Bcl-2 inhibits apoptosis from the mitochondria by regulating mitochondrial permeability and thus cytochrome c release [7].
The extrinsic pathway is primarily triggered through formation of a death-inducing signaling complex (DISC) that is composed of caspase-8 and the death receptor, FADD (Fas-Associated protein with Death Domain); whose recruitment to the DISC results in the activation of additional effector caspases downstream [8–10]. Death ligands binding to their respective receptors can also trigger an increase in oxidative stress. This oxidative stress can then lead to an increase in reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and superoxide [11, 12] production. Oxidative stress can become unbalanced when an internal or external stress stimulus is significant or prolonged, leading to the upregulation and accumulation of ROS within cells. This accumulation precedes oxidative damage and can result in cell death [13, 14].
Epidermal growth factor receptor (EGFR) is an oncogenic member of the tyrosine kinase cell surface receptor family. The EGF ligand binds to the EGFR triggering receptor phosphorylation that in turn stimulates multiple downstream pathways that function in a host of cellular processes including cell proliferation and migration [15]. One of the major downstream pathways activated by EGFR phosphorylation is the PI3K/Akt pathway. The PI3K/Akt signal transduction pathway plays a vital role in several cellular functions including cell differentiation, proliferation, and angiogenesis in response to varying extracellular signals that determine whether Akt functions in a pro- or anti-apoptotic role [16]. Akt is shown to respond to Fas ligand (FasL) stimulation of the Fas death receptor, leading to a functional role in extrinsic pathway apoptosis [17]. Akt is also known to regulate the expression of pro- and anti-apoptotic proteins such as caspase-9, cytochrome c, and Cellular FLICE (FADD-like interleukin-1 beta-converting enzyme) inhibitory protein (c-FLIP) [12, 18]. Several reports suggest that the PI3K/Akt signal transduction pathway functions in angiogenesis by targeting vascular endothelial growth factor (VEGF) expression through activation of the hypoxia-inducible factor-1 (HIF-1) transcription factor; a known regulator of VEGF gene expression [19–20].
c-FLIP is majorly described as an anti-apoptotic protein due to its inactive C-terminal domain that enables it to bind and inhibit the activation and release of caspase-8 from the DISC [21–23]. However there are reports detailing a proapoptotic role for c-FLIP. 13 spliced variants of c-FLIP have thus far been identified; three of which are expressed cellularly as protein isoforms: a 26 kDa short form (c-FLIPS), the 24 kDa form of c-FLIP (c-FLIPR), and the 55 kDa long form (c-FLIPL) [24–25]. The short and long forms of c-FLIP are the most common forms detected in human cells. The long form of c-FLIP (c-FLIPL) has been shown to function as an activator of caspase-8 at the Fas (CD95) death receptor [26–27]. Overexpression of c-FLIPL is reported to upregulate and activate several pro-survival pathways, including NF-kB, ERK, JNK, Wnt, and Akt proteins, thereby enhancing their anti-apoptotic function and promoting cell survival [25, 28–29].
Resveratrol is a naturally-occurring plant-based compound that is ubiquitous in mulberries, blueberries and peanuts, but is most commonly found in grapes [30]. Studies have repeatedly shown that resveratrol has anti-proliferative and pro-apoptotic effects in various cancer cell types both in vitro and in vivo [31–33]. In this study, we investigated the potential effects of resveratrol on lung cells. We found that H460 lung cancer cells are more susceptible to the cytotoxic effects of resveratrol in comparison to non-tumorigenic Beas-2B lung cells. Resveratrol-mediated cytotoxic effects involved an increase in H2O2 production and the activation of caspase 8. Furthermore, we identify that resveratrol targets H460 lung cancer cells for apoptosis by specifically targeting the c-FLIP protein and its downstream effector pAkt for degradation in an EGFR-dependent manner.
MATERIALS AND METHODS
Chemicals and Reagents
Resveratrol, Catalase, N-acetyl-L-cysteine (NAC), Thiazolyl Blue Tetrazolium Bromide (MTT), N-acetyl cysteine, Lactacystin, 6-anilinoquinoline-5,8-quinone (LY294002) were obtained from Sigma-Aldrich (St Louis, MO). Epidermal growth factor (EGF) was purchased from BD Biosciences (San Jose, CA) and Erlotinib (Erl) was obtained from VWR International (Radnor, PA). Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP) was purchased from Calbiochem (La Jolla, cA). Hoechst 33342, dichlorofluo-rescein (DCF) diacetate, and dihydroethidium (DHE) were from Molecular Probes (Eugene, OR). Human VEGF ELISA kit was purchased from Thermo Fisher (Waltham, MA). Antibodies against Caspase-8, pEGFR, EGFR, Bcl-2, Bid, NFkB, PARP, pAkt, Akt, and secondary antibodies (mouse and rabbit) were obtained from Cell Signaling Technology (Danvers, MA). Antibodies for GAPDH and c-FLIP (FLIPS/L) were obtained from Santa Cruz Biotechnologies (Dallas, TX), and the β-actin antibody was obtained from Sigma-Aldrich (St. Louis, MO). Lipofectamine 2000 was from Invitrogen (Carlsbad, CA).
Cell Culture
The human lung epithelial cancer cell line NCI-H460, and human bronchial epithelial Beas-2B cells were obtained from the American Type Culture Collection (Manassas, VA). NCI-H460 cells were cultured in RPMI-1640 medium (Hy-Clone) containing 5% FBS, 2 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin in a 5% CO2 environment at 37°C. Cells were trypsinized using a 0.05% trypsin solution (HyClone). Beas-2B cells were cultured in Dul-becco’s Modified Eagle medium (Thermo Scientific) supplemented with 5% FBS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin in a 5% CO2 environment at 37°C.
Plasmids and Stable Transfection
Stable NCI-H460 transfectants of glutathione peroxidase (GPx), c-FLIP, and control pCMV plasmid were generated by culturing H460 cells as previously described [34]. Western Blot analysis was used to verify stable transfectants, which were passaged and stored just as NC-H460 cells. The GPx, FLIP, and CMV H460 stable cells were a generous gift from the lab of Dr. Yon Rojanasakul (Department of Pharmaceutical and Pharmacological Sciences, West Virginia University, Morgantown, West Virginia). Wild-type (WT)-AKT and control pcDNA3 plasmids were used for Akt transfection experiments.
Caspase Assay
Caspase activity was determined by fluorometric assay as previously described [35]. Fluorometric quantification was done using a Gen 5 2.0 All-In-One Microplate Reader (BioTek Instruments Inc.).
Apoptosis Assay
Apoptosis was determined by Hoechst 33342 DNA fragmentation assay as previously described [12, 36]. Image analysis was done using a EVOS All-in-one digital inverted fluorescence microscope with software and ImageJ software (Java image processing, NIH).
Reactive Oxygen Species (ROS) Detection
Cellular ROS production was determined using DHE and DCF-DA fluorescent probes as previously described [12, 37]. Fluorescence intensity was analyzed using an All-In-One Microplate Reader (BioTek Instruments Inc.) at the respective excitation/emission wavelengths.
Cell Proliferation Assay
Cells proliferation was analyzed using CyQUANT® dye-binding solution (Invitrogen) according to the manufacturer’s protocol. This assay is a highly sensitive fluorescence-based assay that determines the number of healthy, stable cultured cells [38]. The fluorescence intensity of each sample was measured at the excitation and emission wavelengths of 485 and 535 nm, respectively.
MTT Assay
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay was performed in 96-well plates as previously described [39]. Absorbance was measured at 570 nm.
Enzyme-linked Immunosorbent Assay (ELISA)
After treatments, cell supernatants were collected and analyzed for VEGF protein levels using a Human VEGFA ELISA kit (Thermo Scientific) per the manufacturer’s protocol. Optical density was determined on an All-In-One Microplate Reader (BioTek Instruments Inc.) at 450 nm.
Western Blotting
After specific treatments, cells were lysed and prepared for Western blotting as previously described [39]. Equal amount of proteins per sample (20 μg) were resolved on 10% SDS-PAGE before quantification by imaging densitometry using ImageJ (NIH, Image analysis using Java) digitizing software. Mean densitometry data from independent experiments were normalized to the control.
Statistical Analysis
The data represent means (±S.E.M) from three or more independent experiments. Statistical analysis was performed by Student’s t test at a significance level of p < 0.05.
RESULTS
Resveratrol Induces Apoptosis in Lung Cancer Cells Through ROS
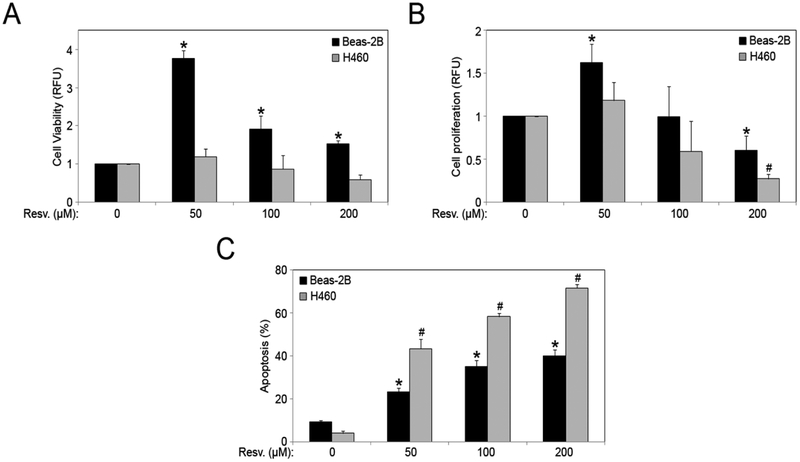
The effect of resveratrol on cell viability and proliferation in non-tumorigenic lung epithelial Beas-2B and lung cancer H460 cell-lines was analyzed. Beas-2B cells are more viable as compared to H460 cells in response to resveratrol treatment (Fig. 1A). After an initial increase in cell proliferation with a lower dose of 50 μM, resveratrol significantly inhibited cell proliferation in a dose-dependent manner in both cell lines (100 and 200 μM), (Fig. 1B). Next, Beas-2B and H460 cells were treated with varying doses of resveratrol (0–200 μM), and Hoechst 33342 assay was used to determine apoptosis. There was a dose-dependent increase over control in cell apoptosis with resveratrol treatment (Fig. 1C), and the effect was significantly more pronounced in H460 cells. The cytotoxic effects of resveratrol were more prominent in H460 lung cancer cells as compared to the non-tumorigenic Beas-2B cells.
Fig. (1).
Resveratrol induces apoptosis in lung cells. (A) Beas-2B and H460 cells were treated with resveratrol (0–200 μM) for 24 hours and cell viability was assessed by MTT assay. (B) Beas-2B and H460 cells were treated with resveratrol (0–200 μM) for 48 hours and cell proliferation was assessed by CyQUANT® assay. (C) Beas-2B and H460 cells were treated with resveratrol (0–200 μM) for 12 hours and analyzed for apoptosis by Hoechst 33342 assay. Graphs represent relative fluorescence intensity over untreated control. Plots are mean ± S.E.M (n = 3). *, p < 0.05 versus Beas-2B non-treated control. #, p < 0.05 versus H460 non-treated control.
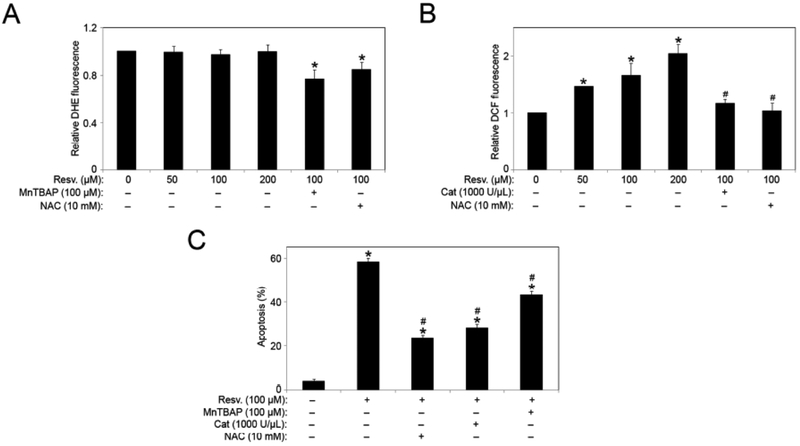
Next we set out to determine whether the apoptosis-inducing effect of resveratrol is associated with ROS production. We measured the increase in cellular ROS in response to resveratrol exposure in H460 cells using specific fluorescent probes for hydrogen peroxide (H2O2) and superoxide. Resveratrol had minimal effects on superoxide production as indicated by DHE fluorescence, but induced H2O2 production in a dose-dependent manner (Figs. 2, A and B). We also utilized ROS modulators in combination with resveratrol to confirm the effect of resveratrol on H2O2 and superoxide levels. Co-treatment with either the general antioxidant N-acetyl cysteine (NAC) or superoxide dismutase mimetic MnTBAP (superoxide scavenger) significantly inhibited resveratrol-induced DHE signal. Similarly, resveratrol-induced increase in DCF signal was significantly inhibited by co-treatment with NAC or catalase (H2O2 scavenger). H460 cells were treated with resveratrol in the presence or absence of various ROS modulators, including NAC, catalase, and MnTBAP; and apoptosis was analyzed. All the antioxidants we tested significantly reduced the apoptosis induced by resveratrol, with NAC and catalase having more pronounced effects (Fig. 2C).
Fig. (2).
Resveratrol-induced ROS mediates the apoptotic response. (A) Subconfluent (90%) monolayers of H460 cells were treated with varying doses of resveratrol in the presence and absence of NAC (10 mM) and MnTBAP (100 μM) for 1 hour. Superoxide levels were analyzed by spectrofluorometric measurement of DHE fluorescence. (B) Subconfluent (90%) monolayers of H460 cells were treated with varying doses of resveratrol in the presence and absence of NAC (10 mM) and Catalase 1000 (U/μl), for 1 hour. Hydrogen peroxide levels were analyzed by spectrofluorometric measurement of DCF fluorescence. (C) Cells were pretreated with MnTBAP (100 μM), Catalase (1000 U/μl) or NAC) (10 mM) for 1 hour followed by resveratrol (100 μM) treatment for 12 hours and analyzed for apoptosis by Hoechst 33342 assay. Graphs represent relative fluorescence intensity over untreated control. Data are mean ± S.E.M (n≤4). *, p < 0.05 versus non-treated control. #, p < 0.05 versus resveratrol (100 μM) treatment.
Effect of Resveratrol and ROS Modulators on Apoptosis Regulatory Proteins
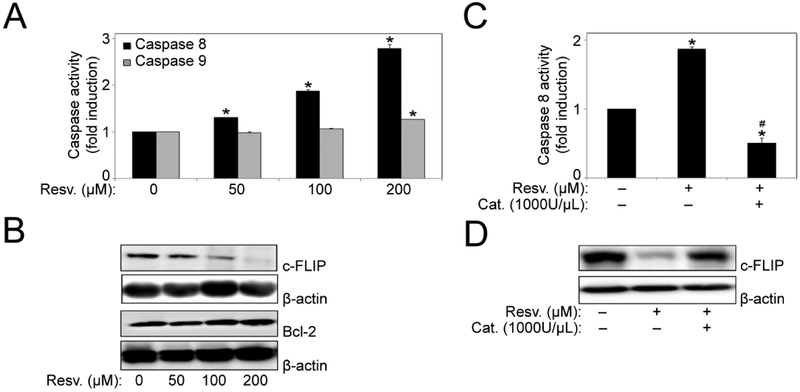
To provide mechanistic insight into resveratrol-induced apoptosis, we investigated the involvement of apoptotic pathways. H460 cells were treated with resveratrol and analyzed for caspase-8 and caspase-9 activation by Caspase Activity assay. Resveratrol significantly induced caspase-8 activation in a dose-dependent manner in H460 cells, while a minor increase in caspase-9 activation was also observed (Fig. 3A). To further confirm the involvement of the apoptotic pathways, we probed for Bcl-2 and c-FLIP; key anti-apoptotic proteins of the mitochondrial and death receptor pathway, respectively, in response to resveratrol treatment. Resveratrol down-regulated c-FLIP in a dose-dependent manner but had minimal effect on the expression levels of Bcl-2 protein (Fig. 3B). Furthermore, to assess the involvement of H2O2, cells were treated with resveratrol in the presence or absence of the H2O2 scavenger catalase and its effect on caspase-8 activation and c-FLIP expression was determined. Pretreatment with catalase significantly blocked resveratrol-induced caspase-8 activation, and also reversed the resveratrol-mediated downregulation of c-FLIP expression (Fig. 3, C and D). This indicated that resveratrol is inducing apoptosis in H460 cells by triggering H2O2 generation leading to caspase-8 activation and downregulation of c-FLIP protein through the death receptor pathway.
Fig. (3).
Resveratrol mediated regulation of apoptosis regulatory proteins. (A) H460 cells were treated with resveratrol (0–200 μM) for 12 hours and cell lysates were analyzed for caspase-8 and −9 levels using Caspase activity assay. Plots show relative fluorescence intensity over untreated control. (B) H460 cells were treated with resveratrol (0–200 μM) for 12 hours. Cell lysates were collected and analyzed for c-FLIP and Bcl-2 protein expression by Western blotting. All blots were reprobed with β-actin antibody for loading control. Representative blots from three or more independent experiments are shown. (C) Cells were treated with resveratrol for 12 hours in the presence or absence of Catalase 1000 (U/μl) and caspase-8 activity was assessed by Spectrofluorometry. (D) Cells were treated with resveratrol for 12 hours in the presence or absence of Catalase 1000 (U/μl). Cell lysates were collected and analyzed for c-FLIP protein expression by Western blotting. All blots were reprobed with β-actin antibody for loading control. Representative blots from three or more independent experiments are shown. Data are mean ± S.E.M (n=3). *, p < 0.05 versus non-treated control. #, p < 0.05 versus resveratrol (100 μM) treatment.
c-FLIP Plays a Critical Role in Resveratrol-Induced Apoptosis by Modulating ROS
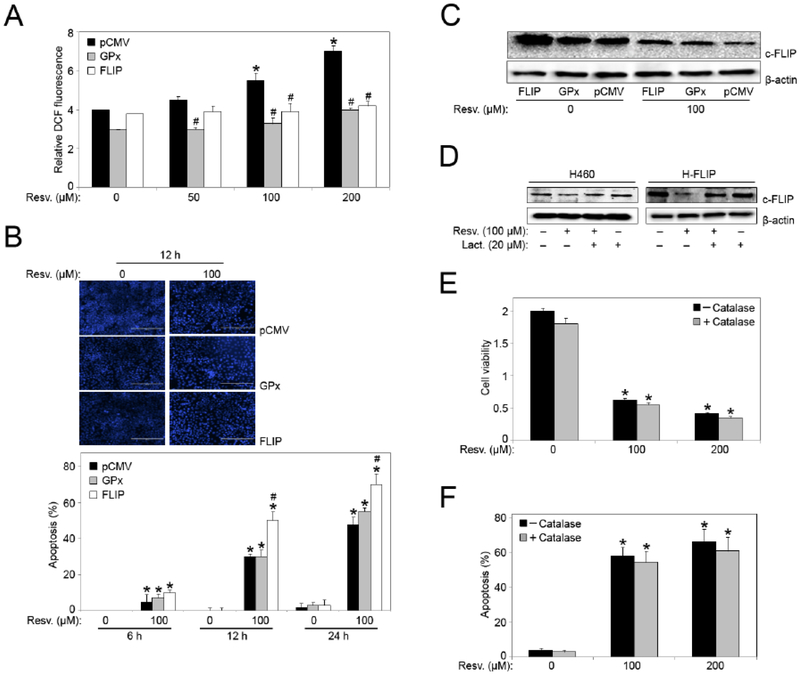
To confirm the role of H2O2 and c-FLIP in resveratrol-induced apoptosis, H460 cells overexpressing either glutathione peroxidase (GPx) (antioxidant enzyme that reduces H2O2 to water), c-FLIP (FLIP) or vector-transfected control plasmid (pCMV) were treated with varying doses of resveratrol (0–200 μM) and H2O2 generation was measured. Fig. (4A) shows that overexpression of c-FLIP significantly inhibited resveratrol-induced H2O2 generation in FLIP cells, similar to GPx cells, versus vector-transfected control. This indicated a direct link between c-FLIP and ROS, and therefore an antioxidant role for c-FLIP in resveratrol-induced apoptosis previously seen in H460 cells (Figs. 1 and 2). Next, we analyzed apoptosis in response to resveratrol treatment in the stable cell-lines by Hoechst 33342 assay. Fig. (4B) shows that overexpression of GPx resulted in less cell death from resveratrol-induced apoptosis, similar to control plasmid (pCMV), in comparison to FLIP cells where apoptosis was significantly enhanced. Furthermore, even though FLIP cells showed decreased H2O2 production, they were more susceptible to apoptosis in response to resveratrol at both the 12 and 24 hour time points (Fig. 4B). The increase in resveratrol-induced apoptosis in FLIP cells indicates that there are other mechanisms involved in the regulation of c-FLIP during resveratrol-mediated apoptosis.
Fig. (4).
Effects of GPx and c-FLIP overexpression on resveratrol-induced apoptosis and ROS generation. (A) Hydrogen peroxide levels were analyzed in FLIP-, GPx- and mock-transfected (pCMV) H460 cells by Spectrofluorometric measurement of DCF fluorescence after 1 hour treatment with resveratrol. *, p < 0.05 versus non-treated control. #, p < 0.05 versus pCMV transfected cells. (B) FLIP-, GPx- and mock-transfected (pCMV) H460 cells were treated with resveratrol (0–200 μM) for 6, 12, and 24 hour time points and analyzed for apoptosis by Hoechst 33342 assay. Representative fluorescence micrographs of stained cells treated for 12 hours. *, p < 0.05 versus non-treated control. #, p < 0.05 versus pCMV and GPx transfected cells. Data are mean ± S.E.M (n≤4). (C) FLIP-, GPx- and mock-transfected (pCMV) H460 cells were treated with resveratrol (100 μM) for 12 hours and analyzed for c-FLIP protein expression by Western blotting. All blots were reprobed with β-actin antibody for loading control. Representative blots from three or more independent experiments is shown. (D) H460 and FLIP cells were pre-treated with lactacystin (proteasome inhibitor) for 1 hour before treatment with resveratrol (100 μM) for 12 hours and analyzed for c-FLIP protein expression by Western blotting. All blots were reprobed with β-actin antibody for loading control. Representative blots from three or more independent experiments is shown. (E) FLIP cells were treated with resveratrol (0–200 μM) for 24 hours in the presence or absence of Catalase 1000 (U/μl) and cell viability was assessed by MTT assay. (F) FLIP cells were treated with resveratrol (0–200 μM) for 12 hours in the presence or absence of Catalase 1000 (U/μl) and analyzed for apoptosis by Hoechst 33342 assay. Graphs represent relative fluorescence intensity over untreated control. Data are mean ± S.E.M (n≤4). *, p < 0.05 versus non-treated control.
Analysis of c-FLIP expression in GPx and FLIP cells further showed that resveratrol significantly downregulated c-FLIP expression in these cells as compared to control treatment as well as vector-transfected control cells (Fig. 4C). Although catalase reversed the effect of resveratrol on FLIP protein in H460 cells, overexpression of GPx led to downregulation of FLIP in response to resveratrol treatment. Additionally, since resveratrol significantly downregulated c-FLIP expression in FLIP cells, it is possible that resveratrol may specifically target c-FLIP protein in H460 cells. To determine the mechanism that facilitates resveratrol-induced c-FLIP downregulation, we analyzed proteasomal degradation in H460 and FLIP cells using the proteasome inhibitor lactacystin. Pretreatment with lactacystin inhibited resveratrol-induced downregulation of c-FLIP protein expression in both H460 and FLIP cells; returning it to baseline (control) levels (Fig. 4D). Pre-treatment of FLIP cells with catalase did not rescue cells from a significant reduction in cell viability, and almost sixty percent increase in resveratrol-induced apoptosis was observed with catalase pretreatment (Figs. 4, E and F). Since we also observed low H2O2 levels in FLIP cells; this data indicates that although H2O2 plays an important role in H460 cells, overexpression of c-FLIP protein results in H2O2 reduction and reverses the protective effect of catalase previously seen on H460 cells in Fig. (2).
Resveratrol Induces Activation of Pro-Apoptotic Proteins and pAkt Downregulation in FLIP Cells
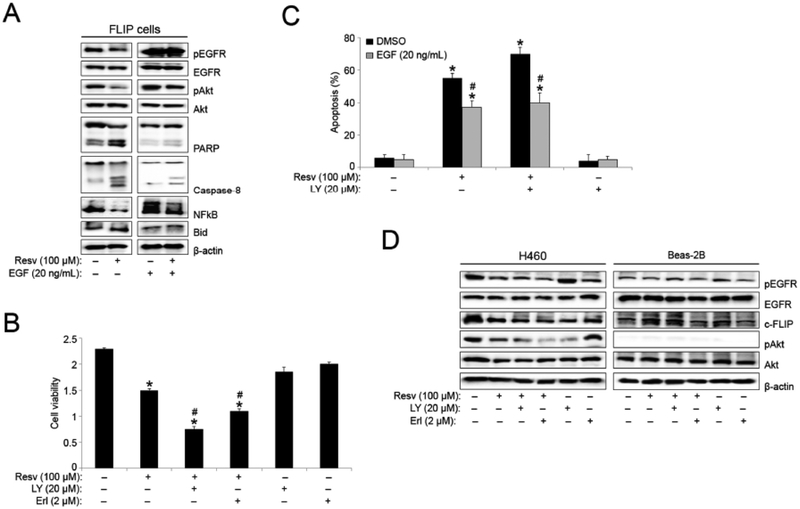
To further identify protein targets involved in resveratrol-induced apoptosis in FLIP cells, we analyzed the expression levels of other critical pro- and anti-apoptotic proteins. Resveratrol treatment significantly downregulated pAkt and NFkB expression, and increased cleavage and activation of the pro-apoptotic proteins; caspase-8, PARP, and Bid in FLIP cells, as compared to control (Fig. 5A). To further assess the significance of pAkt modulation in resveratrol-induced apoptosis, we also probed for pEGFR and EGFR. Resveratrol induced the downregulation of pEGFR, a direct upstream modulator of Akt phosphorylation. To determine the importance of pEGFR to resveratrol-induced H460 lung cancer cell apoptosis, FLIP cells were serum starved for 24 hours before being supplemented with EGF. Western blot analysis as above indicated a clear decrease in pAkt downregulation. EGF supplementation also resulted in a significant decrease in PARP, Bid, and caspase 8 activation as previously observed with resveratrol treatment.
Fig. (5).
Resveratrol-induced EGFR downregulation modulates pAkt expression in FLIP cells. (A) FLIP cells were serum starved for 24 hours before serum free medium was supplemented with DMSO (vehicle) or EGF (20 ng/mL). The FLIP cells were then treated with resveratrol (100 μM) for 12 hours and analyzed for pEGFR, EGFR, pAkt, Akt, PARP, caspase-8, NFkB, and Bid protein expression by Western blotting. All blots were reprobed with β-actin antibody for loading control. Representative blots from three or more independent experiments are shown. (B) FLIP cells were pretreated with LY294002 (20 μM) and Erlotinib (2 μM) for 1 hour followed by resveratrol (100 μM) treatment for 12 hours and analyzed for cell viability by MTT assay. (C) FLIP cells were serum starved for 24 hours before serum free medium was supplemented with DMSO (vehicle) or EGF (20 ng/mL). The FLIP cells were then pretreated with LY294002 (20 μM) for 1 hour followed by resveratrol (100 μM) treatment for 12 hours and analyzed for apoptosis by Hoechst 33342 assay. (D) H460 and Beas-2B cells were pretreated with LY294002 (20 μM) and Erlotinib (2 μM) for 1 hour followed by resveratrol (100 μM) treatment for 12 hours and analyzed for pEGFR, EGFR, c-FLIP, pAkt, and Akt protein expression by Western blotting. All blots were reprobed with β-actin antibody for loading control. Representative blots from three or more independent experiments are shown. Graphs represent relative fluorescence intensity over untreated control. Data are mean ± S.E.M (n≤4). *, p < 0.05 versus non-treated control. #, p < 0.05 versus resveratrol (100 μM) treatment.
To determine the effect of pEGFR and pAkt modulation on resveratrol-induced effects in FLIP cells, we pre-treated cells with the PI3K/Akt inhibitor and the pEGFR inhibitor LY294002 and erlotinib, respectively, along with resveratrol. Both LY294002 and erlotinib enhanced the negative effect of resveratrol on FLIP cell viability (Fig. 5B). FLIP cells supplemented with EGF were pre-treated with LY294002 prior to resveratrol treatment. Inhibition of pAkt enhanced resveratrol-induced apoptosis, while EGF supplementation rescued FLIP cells from resveratrol and LY294002-induced apoptosis (>20% decrease in apoptosis) (Fig. 5C). The data indicates that resveratrol mediates apoptosis in H460 cells through the downregulation of pEGFR, which in turn negatively regulates the anti-apoptotic proteins; c-FLIP and pAkt. To determine if this downregulation is responsible for the enhanced inhibition of cell viability and proliferation, along with increased apoptosis we observed in H460 cells as compared to Beas-2B cells, we analyzed the expression of pEGFR, c-FLIP and pAkt in both cell lines after LY294002 and erlotinib pre-treatment followed by resveratrol treatment. LY294002 pre-treatment significantly enhanced resveratrol-induced c-FLIP and pAkt downregulation in H460 cells, while the expression of both proteins was not affected by resveratrol and/or LY294002 treatment in Beas-2B cells (Fig. 5D). Most significantly, erlotinib pre-treatment resulted in further downregulation of pAkt and c-FLIP in conjunction with resveratrol in H460 cells, indicating a possible EGFR/Akt/c-FLIP pathway for resveratrol-induced apoptosis in lung cancer cells.
Akt Activation Inhibits Resveratrol-Induced Apoptosis
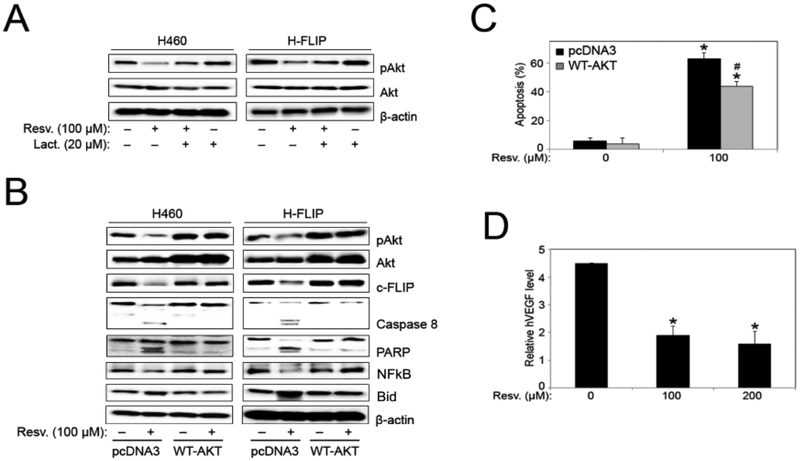
Pretreatment with lactacystin inhibited resveratrol-induced downregulation of pAkt protein expression in both H460 and FLIP cells, similar to the c-FLIP degradation observed in Figs. (4D and 6A). To further explore the involvement of Akt in c-FLIP expression in H460 cells, both H460 and FLIP cells were transfected with a wild type Akt plasmid (WT-AKT), and treated with resveratrol. Western blotting confirmed transfection as indicated by the overexpression of total Akt and pAkt protein in WT-AKT-transfected cells as compared to control pcDNA3 samples (Fig. 6B). While resveratrol induced marked activation of pro-apoptotic proteins (caspase-8, PARP, Bid), coupled with significant downregulation of NFkB, pAkt and c-FLIP in control pcDNA3- transfected samples; H460 and FLIP cells overexpressing Akt showed no significant modulation in protein expression in response to resveratrol treatment as compared to untreated control samples transfected with WT-AKT (Fig. 6B). However, comparing WT-AKT transfected cells with pcDNA3-transfected cells, Akt overexpression inhibited resveratrol-induced downregulation of NFkB, c-FLIP, and activation of Bid, caspase-8 and PARP proteins. Furthermore, transfection with WT-AKT protected cells from resveratrol-induced apoptosis, as assessed by Hoechst assay (Fig. 6C). Vascular endothelial growth factor (VEGF), a downstream effector directly regulated by Akt, was significantly inhibited in FLIP cells in response to resveratrol treatment as assessed by ELISA (Fig. 6D).
Fig. (6).
Akt overexpression inhibits resveratrol-induced apoptosis. (A) H460 and FLIP cells were pre-treated with lactacystin (proteasome inhibitor) for 1 hour before treatment with resveratrol (100 μM) for 12 hours and analyzed for pAkt and Akt protein expression by Western blotting. All blots were reprobed with β-actin antibody for loading control. Representative blots from three or more independent experiments are shown. (B) H460 and FLIP cells were transfected with pcDNA3 control and WT-AKT plasmid followed by resveratrol (100 μM) treatment for 12 hours, and analyzed for pAkt, Akt, c-FLIP, caspase-8, PARP, NFkB, and Bid protein expression by Western blotting. All blots were reprobed with β-actin antibody for loading control. Representative blots from three or more independent experiments are shown. (C) FLIP cells were transfected with pcDNA3 control and WT-AKT plasmid followed by resveratrol (100 μM) treatment for 12 hours and analyzed for apoptosis by Hoechst 33342 assay. Graphs represent relative fluorescence intensity over untreated control. Data are mean ± S.E.M (n≤4). *, p < 0.05 versus non-treated control. #, p < 0.05 versus resveratrol (100 μM) treatment. (D) FLIP cells were treated with resveratrol (0–200 μM) for 48 hours and VEGF levels were assessed by ELISA. Graphs represent relative fluorescence intensity over untreated control. Data are mean ± S.E.M (n≤4). *, p < 0.05 versus non-treated control.
DISCUSSION
The effects of resveratrol depend upon the dose and duration of resveratrol exposure as well as the cell system being used [39–42]. Several studies in lung, ovarian, and prostate cancer models show that resveratrol induces apoptosis by inducing ROS production [43–45]. This study focuses on the effects of resveratrol on non-tumorigenic bronchial epithelial cells (Beas-2B) and H460 lung cancer cell line. Our data indicates that resveratrol has a more potent effect on H460 cells in comparison to Beas-2B cells, as observed in Fig. (1). This effect was evident on both cell viability and proliferation, while apoptosis induction was doubled in H460 cells treated with resveratrol in comparison to Beas-2B cells. Considering increased susceptibility and sensitivity of H460 cells to resveratrol treatment, we focused on the mechanism of resveratrol-induced cell death in H460 cells.
ROS plays a key role in several cellular processes including cell migration, survival, differentiation, and apoptosis [13–14]. During oxidative stress, unregulated ROS levels can lead to significant cellular damage and even carcinogenesis. However, excessive ROS production that is not scavenged typically results in cell death for both normal and cancer cells. Focusing on the resveratrol-induced apoptosis in H460 cells, we observed an increase in ROS production, specifically H2O2, in response to resveratrol treatment in H460 cells (Fig. 2). This was corroborated by pretreatment with the H2O2 scavenger catalase, which significantly protected H460 cells from resveratrol-induced apoptosis. Interestingly, this protective effect of catalase was lost in H460 cells overex-pressing FLIP protein as catalase pretreatment had no significant effect on the decreased cell viability and increase in apoptosis induced by resveratrol treatment (Fig. 4, E and F), as opposed to what we observed in resveratrol-treated H460 cells in Fig. (2).
A direct correlation between the expression level of c-FLIP and its cellular function as a pro- or anti-apoptotic protein has been reported. Reports indicate that low c-FLIP expression is typically associated with DISC promotion and caspase-8 activation, while high levels of endogenous FLIP is associated with the inhibition of apoptosis [26–27, 46–47]. High expression levels of c-FLIP have also been observed in several cancer forms (prostate, liver, colorectal, cervical, Burkitt’s lymphoma, non-Hodgkin’s lymphoma, among others) [24, 48–49]. The overexpression of c-FLIP in multiple malignancies indicates a possible role for c-FLIP in cancer progression and hence its importance as a therapeutic target in multiple cancer therapies. [24, 50–51]. The data in (Fig. 4) clearly shows that FLIP overexpression (FLIP cells) inhibits resveratrol-induced upregulation of H2O2. GPx cells also displayed resveratrol-induced H2O2 inhibition as expected. This inhibition of H2O2 production was confirmed in apoptosis analysis of resveratrol-treated GPx and FLIP cells. When GPx cells were treated with resveratrol, the results were similar to the effect of catalase pretreatment on H460 cells which resulted in a reduction in resveratrol-induced apoptosis (data not shown). These data clearly indicate that H2O2 plays a critical role in resveratrol-mediated apoptosis in H460 cells.
As detailed before, c-FLIP is generally labeled as an anti-apoptotic protein, but can function as both anti- and pro-apoptotic based on protein expression levels [52–54]. c-FLIP is significantly downregulated in response to resveratrol-treatment, and this effect is reversed when cells are pre-treated with catalase (Fig. 3). This indicates that there is a link between resveratrol-mediated H2O2 production and c-FLIP expression levels in H460 cell death. c-FLIP expression is tightly regulated within the cell through ubiquitination and proteasomal degradation [55–56]. Inhibition of proteasome with lactacystin rescued c-FLIP downregulation by resveratrol. Therefore, it is clear that resveratrol decreases c-FLIP levels through facilitating and promoting its proteasomal degradation. The proteolytic cleavage and self-activation from pro-caspase-8 to the active caspase-8 form is a critical step in apoptosis that is highly regulated by c-FLIP [21–23]. Resveratrol induces c-FLIP downregulation to facilitate apoptosis; a conclusion that is bolstered by an increase in the cleavage and activation of PARP, Bid, and caspase-8, three pro-apoptotic proteins, in FLIP cells (Fig. 5A). This data suggests that resveratrol is inducing apoptosis in H460 cells primarily through the death receptor extrinsic apoptotic pathway. c-FLIP inhibits the activation of caspase-8 at the DISC, which in turn prevents the downstream activation of effector caspases thereby inhibiting apoptosis. H2O2 seems to play a major role here in H460 cells as data shows catalase pretreatment reverses the activation of caspase-8 observed in resveratrol-treated H460 cells (Fig. 3).
The anti-cancer effects of resveratrol have been reported to involve PI3K/Akt signaling and c-FLIP downregulation [57]. Furthermore, the PI3K/Akt signaling pathway has been shown to modulate c-FLIP expression and protein stability within the cell [58–59]. Analysis of the PI3K/Akt signaling pathway here demonstrated that pAkt was significantly downregulated in resveratrol-treated FLIP cells (Fig. 5A). Importantly, the PI3K/Akt pathway inhibitor LY294002 enhanced the effect of resveratrol on cell viability and apoptosis in FLIP cells. LY294002 is a very potent and specific inhibitor of the phosphatidylinositol-3-kinases (PI3Ks), and has been shown to block PI3 kinase-dependent Akt phosphorylation and kinase activity [60–61]. In order to further elucidate the pathway that mediates resveratrol-induced apoptosis in lung cancer cells, we looked at cell surface receptors upstream of the PI3K/Akt pathway. Western blot analysis of EGFR showed significant protein downregulation that coordinates with the downstream downregulation of pAkt that we observed (Fig. 5). The EGFR tyrosine kinase family is heavily involved in stimulating downstream pathways that control cellular migration, proliferation, and even tumor invasion, migration, and metastasis [15]. EGFR is an oncogene due to the fact that its signaling dysregulation has been implicated in a significant percentage of tumors, specifically ones with epithelial origin [62]. EGF ligand activity has also been implicated in VEGF signaling thereby also functioning in angiogenesis regulation [63]. Resveratrol not only downregulated EGFR expression; but FLIP cells supplemented with EGF showed a clear reduction in resveratrol-induced apoptosis effects. These effects included a decrease in the downregulation of anti-apoptotic proteins (c-FLIP, pAkt, NFkB), and a clear inhibition of the activation of proapoptotic proteins (Bid, caspase 8, PARP) (Fig. 5). This data was corroborated by the 20% decrease in resveratrol-induced apoptosis in FLIP cells supplemented with EGF (Fig. 5C). Pre-treatment with the well-established EGF inhibitor erlotinib, as observed with LY294002, enhanced the negative effect of resveratrol on FLIP cell viability (Fig. 5B). NFkB is a potent transcription factor downstream of Akt that regulates anti-apoptotic gene transcription [64]; while Bid is a pro-apoptotic member of the Bcl-2 family of proteins. Several reports have indicated that caspase 8 and its substrate Bid can be activated in response to various apoptotic stimuli [65]. The expression of these two opposing proteins serves to further elucidate the mechanism by which resveratrol induces apoptosis in H460 cells. The data shown here in Fig.(5) identifies a mechanism for resveratrol-induced apoptosis of lung cancer cells that is dependent on pAkt and c-FLIP downregulation; a process mediated by EGFR activity.
Once we established pEGFR, c-FLIP and pAkt downregulation as major factors involved in resveratrol-induced apoptosis in H460 cells, we wanted to determine if resveratrol had any effect on the expression of these proteins in normal Beas-2B lung cells. Interestingly, resveratrol had minimal effects on the expression of all three proteins (pEGFR, c-FLIP, and pAkt) in Beas-2B cells in complete contrast to H460 cells (Fig. 5). pAkt was further downregulated when resveratrol-treated FLIP cells were pre-treated with erlotinib, further indicating the importance of EGFR signaling in resveratrol-induced apoptosis. The significance of pAkt was further established here as pre-treatment with LY294002 also resulted in further downregulation of c-FLIP in H460 cells, indicating a direct link between the function of both proteins in resveratrol-induced apoptosis in H460 cells. Proteasomal degradation inhibition also negated resveratrol-induced pAkt downregulation; similar to what we observed with c-FLIP in Figs. (4 and 6). Data from FLIP cells transfected with a WT-AKT plasmid showed that Akt overexpression can protect and rescue cells from resveratrol-induced apoptosis (Fig. 6). Akt overexpression in both H460 and FLIP cells resulted in the inhibition of resveratrol-induced apoptosis observed by both the downregulation of pro-apoptotic proteins (caspase 8, Bid, and PARP), and the inhibition of pAkt, c-FLIP, and NFkB downregulation. This was corroborated by a 20% reduction in resveratrol-induced apoptosis in WT-AKT transfected cells.
As detailed earlier, Akt can regulate angiogenesis by targeting expression of the downstream factor, VEGF, through the activation of the VEGF transcription factor HIF-1 [19–20, 35], and EGF signaling can also modulate VEGF expression. Resveratrol has been shown to significantly reduce VEGF secretion in A549 lung cancer cells, SKOV-3 and OVCAR-8 ovarian cancer cell lines through inhibition of the PI3K/Akt/mTOR signaling pathway [66–68]. In this study, we observed a clear dose-response reduction in VEGF levels in FLIP cells treated with resveratrol (Fig. 6); which correlated with the observed downregulation in pAkt levels in FLIP cells.
This study shows that endogenous c-FLIP in H460 lung cancer cells functions in an anti-apoptotic role. However, overexpression of c-FLIP in H460 cells (FLIP cells) results in enhanced resveratrol-induced apoptosis; a clear indication that c-FLIP expression levels determine the role it plays in resveratrol-mediated H460 cell apoptosis. Resveratrol also induces apoptosis in FLIP cells through the downregulation of pEGFR, and the downstream downregulation of pAkt and NFkB, in conjunction with the upregulation of the pro-apoptotic factors; Bid, PARP and caspase-8. Our data shows that supplementing FLIP cells with EGF can rescue cells from resveratrol-induced apoptosis, while inhibiting pEGF with erlotinib in conjunction with resveratrol treatment enhances resveratrol-induced negative effects on H460 cells. The data here also shows that overexpression of c-FLIP in conjunction with the inhibition of Akt enhances resveratrol-induced apoptosis in FLIP cells. c-FLIP overexpression (FLIP cells) inhibits the upregulation of H2O2 previously observed in H460 cells after resveratrol treatment, without affecting resveratrol-induced apoptosis. This data suggests that since c-FLIP overexpression results in its functioning in a pro-apoptotic role; upregulation of ROS is not needed to induce apoptosis. pAkt downregulation is coordinated with a dose-dependent decrease in VEGF expression, suggesting that pAkt is playing an anti-apoptotic role in FLIP cells, and may be regulating its downstream factor VEGF. Future investigation will determine if resveratrol treatment in conjunction with PI3K/Akt signaling inhibition can negatively affect angiogenesis in in vivo lung cancer models. As stated before, dysregulation of apoptotic cell death pathways is one of the leading causes of lung cancer progression. Cell death in cancer treatment, whether by radiation therapy or chemo-therapy, depends upon the induction of apoptotic pathways. Therefore, dysregulation of these cell death pathways represents a significant hindrance for curative therapy [69–70].
The present study provides evidence that H460 lung cancer cells are more susceptible to resveratrol-induced cell death in comparison to normal lung cells (Beas-2B), and this susceptibility is predicated on the downregulation of pEGFR signaling that stimulates the downstream downregulation of pAkt and c-FLIP. We have established an important mechanistic model for resveratrol-induced apoptosis in H460 cells that is mediated by ROS accumulation, caspase-8 activation, and the downregulation of c-FLIP and pAkt proteins by proteasomal degradation; which the data indicates is EGFR-dependent. This study also reveals that EGFR, c-FLIP and Akt protein expression manipulation within H460 cells has clear and pronounced effects on resveratrol-induced apoptosis. The new information highlighted in this study indicating a critical role for c-FLIP protein in lung cancer warrants further investigation and may be an important therapeutic target in lung cancer.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health [grants HL112630 and CA173069].
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Degterev A; Yuan J, Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol 2008, 9 (5), 378–390. [DOI] [PubMed] [Google Scholar]
- [2].Green DR, Means to an End: Apoptosis and Other Cell Death Mechanisms. Cold Spring Harbor Laboratory Press: New York,2011. [Google Scholar]
- [3].Vaux DL, Apoptogenic factors released from mitochondria. Biochim Biophys Acta 2011, 1813 (4), 546–550. [DOI] [PubMed] [Google Scholar]
- [4].Plati J; Bucur O; Khosravi-Far R, Apoptotic cell signaling in cancer progression and therapy. Integr Biol (Camb) 2011, 3 (4), 279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu X; Kim CN; Yang J; Jemmerson R; Wang X, Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 1996, 86 (1), 147–157. [DOI] [PubMed] [Google Scholar]
- [6].Yuan S; Topf M; Reubold TF; Eschenburg S; Akey CW, Changes in Apaf-1 conformation that drive apoptosome assembly. Biochemistry 2013, 52 (13), 2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Green DR; Reed JC, Mitochondria and apoptosis. Science 1998, 281 (5381), 1309–1312. [DOI] [PubMed] [Google Scholar]
- [8].Suda T; Takahashi T; Golstein P; Nagata S, Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993, 75 (6), 1169–1178. [DOI] [PubMed] [Google Scholar]
- [9].Debatin KM; Beltinger C; Bohler T; Fellenberg J; Friesen C; Fulda S; Herr I; Los M; Scheuerpflug C; Sieverts H; Stahnke K, Regulation of apoptosis through CD95 (APO-I/Fas) receptor-ligand interaction. Biochem Soc Trans 1997, 25 (2), 405–410. [DOI] [PubMed] [Google Scholar]
- [10].Xu G; Shi Y, Apoptosis signaling pathways and lymphocyte homeostasis. Cell Res 2007, 17 (9), 759–771. [DOI] [PubMed] [Google Scholar]
- [11].Medan D; Wang L; Toledo D; Lu B; Stehlik C; Jiang BH; Shi X; Rojanasakul Y, Regulation of Fas (CD95)-induced apoptotic and necrotic cell death by reactive oxygen species in macrophages. J Cell Physiol 2005, 203 (1), 78–84. [DOI] [PubMed] [Google Scholar]
- [12].Iyer AK; Azad N; Talbot S; Stehlik C; Lu B; Wang L; Rojanasakul Y, Antioxidant c-FLIP inhibits Fas ligand-induced NF-kappaB activation in a phosphatidylinositol 3-kinase/Akt-dependent manner. J Immunol 2011, 187 (6), 3256–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bae YS; Oh H; Rhee SG; Yoo YD, Regulation of reactive oxygen species generation in cell signaling. Mol Cells 2011, 32 (6), 491–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Valko M; Leibfritz D; Moncol J; Cronin MT; Mazur M; Telser J, Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007, 39 (1), 44–84. [DOI] [PubMed] [Google Scholar]
- [15].Normanno N; De Luca A; Bianco C; Strizzi L; Mancino M; Maiello MR; Carotenuto A; De Feo G; Caponigro F; Salomon DS, Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366 (1), 2–16. [DOI] [PubMed] [Google Scholar]
- [16].Cantley LC, The phosphoinositide 3-kinase pathway. Science 2002, 296 (5573), 1655–1657. [DOI] [PubMed] [Google Scholar]
- [17].Gulbins E; Hermisson M; Brenner B; Grassme HU; Linderkamp O; Dichgans J; Weller M; Lang F, Cellular stimulation via CD95 involves activation of phospho-inositide-3-kinase. Pflugers Arch 1998, 435 (4), 546–554. [DOI] [PubMed] [Google Scholar]
- [18].Suhara T; Mano T; Oliveira BE; Walsh K, Phosphatidylinositol 3-kinase/Akt signaling controls endothelial cell sensitivity to Fas-mediated apoptosis via regulation of FLICE-inhibitory protein (FLIP). Circ Res 2001, 89 (1), 13–19. [DOI] [PubMed] [Google Scholar]
- [19].Jiang BH; Jiang G; Zheng JZ; Lu Z; Hunter T; Vogt PK, Phosphatidylinositol 3-kinase signaling controls levels of hypoxiainducible factor 1. Cell Growth Differ 2001, 12 (7), 363–369. [PubMed] [Google Scholar]
- [20].Forsythe JA; Jiang BH; Iyer NV; Agani F; Leung SW; Koos RD; Semenza GL, Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996, 16 (9), 4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tsuchiya Y; Nakabayashi O; Nakano H, FLIP the Switch: Regulation of Apoptosis and Necroptosis by cFLIP. Int J Mol Sci 2015, 16 (12), 30321–30341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kataoka T, The caspase-8 modulator c-FLIP. Crit Rev Immunol 2005, 25 (1), 31–58. [DOI] [PubMed] [Google Scholar]
- [23].Peter ME, The flip side of FLIP. Biochem J 2004, 382 (Pt 2), e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Safa AR, Roles of c-FLIP in Apoptosis, Necroptosis, and Autophagy. J Carcinog Mutagen 2013, Suppl 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Safa AR, c-FLIP, a master anti-apoptotic regulator. Exp Oncol 2012, 34 (3), 176–184. [PMC free article] [PubMed] [Google Scholar]
- [26].Micheau O; Thome M; Schneider P; Holler N; Tschopp J; Nicholson DW; Briand C; Grutter MG, The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem 2002, 277 (47), 45162–45171. [DOI] [PubMed] [Google Scholar]
- [27].Chang DW; Xing Z; Pan Y; Algeciras-Schimnich A;Barnhart BC; Yaish-Ohad S; Peter ME; Yang X, c-FLIP(L)is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J 2002, 21 (14), 3704–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chang L; Kamata H; Solinas G; Luo JL; Maeda S;Venuprasad K; Liu YC; Karin M, The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell 2006, 124 (3), 601–613. [DOI] [PubMed] [Google Scholar]
- [29].Quintavalle C; Incoronato M; Puca L; Acunzo M; Zanca C; Romano G; Garofalo M; Iaboni M; Croce CM; Condorelli G, c-FLIPL enhances anti-apoptotic Akt functions by modulation of Gsk3beta activity. Cell Death Differ 2010, 17 (12), 1908–1916. [DOI] [PubMed] [Google Scholar]
- [30].Vidavalur R; Otani H; Singal PK; Maulik N, Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp Clin Cardiol 2006, 11 (3), 217–225. [PMC free article] [PubMed] [Google Scholar]
- [31].Yang T; Wang L; Zhu M; Zhang L; Yan L, Properties and molecular mechanisms of resveratrol: a review. Pharmazie 2015, 70 (8), 501–506. [PubMed] [Google Scholar]
- [32].Baur JA; Sinclair DA, Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 2006, 5 (6), 493–506. [DOI] [PubMed] [Google Scholar]
- [33].Brakenhielm E; Cao R; Cao Y, Suppression of angiogenesis, tumor growth, and wound healing by resveratrol, a natural compound in red wine and grapes. FASEB J 2001, 15 (10), 1798–1800. [DOI] [PubMed] [Google Scholar]
- [34].Kongkaneramit L; Sarisuta N; Azad N; Lu Y; Iyer AK;Wang L; Rojanasakul Y, Dependence of reactive oxygen species and FLICE inhibitory protein on lipofectamine-induced apoptosis in human lung epithelial cells. J Pharmacol Exp Ther 2008, 325 (3), 969–977. [DOI] [PubMed] [Google Scholar]
- [35].Iyer AK; Ramesh V; Castro CA; Kaushik V; Kulkarni YM; Wright CA; Venkatadri R; Rojanasakul Y; Azad N, Nitric oxide mediates bleomycin-induced angiogenesis and pulmonary fibrosis via regulation of VEGF. J Cell Biochem 2015, 116 (11), 2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Allen S; Sotos J; Sylte MJ; Czuprynski CJ, Use of Hoechst 33342 staining to detect apoptotic changes in bovine mononuclear phagocytes infected with Mycobacterium avium subsp. paratuberculosis. Clin Diagn Lab Immunol 2001, 8 (2), 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wojtala A; Bonora M; Malinska D; Pinton P; Duszynski J; Wieckowski MR, Methods to monitor ROS production by fluorescence microscopy and fluorometry. Methods Enzymol 2014, 542, 243–262. [DOI] [PubMed] [Google Scholar]
- [38].Jones LJ; Gray M; Yue ST; Haugland RP; Singer VL, Sensitive determination of cell number using the CyQUANT cell proliferation assay. J Immunol Methods 2001, 254 (1–2), 85–98. [DOI] [PubMed] [Google Scholar]
- [39].Venkatadri R; Muni T; Iyer AK; Yakisich JS; Azad N, Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis 2016, 7, e2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].van Ginkel PR; Sareen D; Subramanian L; Walker Q; Darjatmoko SR; Lindstrom MJ; Kulkarni A; Albert DM; Polans AS, Resveratrol inhibits tumor growth of human neuroblastoma and mediates apoptosis by directly targeting mitochondria. Clin Cancer Res 2007, 13 (17), 5162–5169. [DOI] [PubMed] [Google Scholar]
- [41].Hwang JT; Kwak DW; Lin SK; Kim HM; Kim YM; Park OJ, Resveratrol induces apoptosis in chemoresistant cancer cells via modulation of AMPK signaling pathway. Ann N Y Acad Sci 2007, 1095, 441–448. [DOI] [PubMed] [Google Scholar]
- [42].Soo E; Thakur S; Qu Z; Jambhrunkar S; Parekh HS; Popat A, Enhancing delivery and cytotoxicity of resveratrol through a dual nanoencapsulation approach. J Colloid Interface Sci 2016, 462, 368–374. [DOI] [PubMed] [Google Scholar]
- [43].Pais-Morales J; Betanzos A; Garcia-Rivera G; Chavez-Munguia B; Shibayama M; Orozco E, Resveratrol Induces Apoptosis-Like Death and Prevents In Vitro and In Vivo Virulence of Entamoeba histolytica. PLoS One 2016, 11 (1), e0146287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mikula-Pietrasik J; Sosinska P; Murias M; Wierzchowski M;Brewinska-Olchowik M; Piwocka K; Szpurek D; Ksiazek K,High Potency of a Novel Resveratrol Derivative, 3,3’,4,4’-Tetrahydroxy-trans-stilbene, against Ovarian Cancer Is Associated with an Oxidative Stress-Mediated Imbalance between DNA Damage Accumulation and Repair. Oxid Med Cell Longev 2015, 2015, 135691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lang F; Qin Z; Li F;Zhang H; Fang Z; Hao E, Apoptotic Cell Death Induced by Resveratrol Is Partially Mediated by the Autophagy Pathway in Human Ovarian Cancer Cells. PLoS One 2015, 10 (6), e0129196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Irmler M; Thome M; Hahne M; Schneider P; Hofmann K; Steiner V; Bodmer JL; Schroter M; Burns K; Mattmann C; Rimoldi D; French LE; Tschopp J, Inhibition of death receptor signals by cellular FLIP. Nature 1997, 388 (6638), 190–195. [DOI] [PubMed] [Google Scholar]
- [47].Krueger A; Baumann S; Krammer PH; Kirchhoff S, FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol 2001, 21 (24), 8247–8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ryu BK; Lee MG; Chi SG; Kim YW; Park JH, Increased expression of cFLIP(L) in colonic adenocarcinoma. J Pathol 2001, 194 (1), 15–19. [DOI] [PubMed] [Google Scholar]
- [49].Ullenhag GJ; Mukherjee A; Watson NF; Al-Attar AH; Scholefield JH; Durrant LG, Overexpression of FLIPL is an independent marker of poor prognosis in colorectal cancer patients. Clin Cancer Res 2007, 13 (17), 5070–5075. [DOI] [PubMed] [Google Scholar]
- [50].Dao P; Smith N; Scott-Algara D; Garbay C; Herbeuval JP; Chen H, Restoration of TRAIL-induced apoptosis in resistant human pancreatic cancer cells by a novel FAK inhibitor, PH11. Cancer Lett 2015, 360 (1), 48–59. [DOI] [PubMed] [Google Scholar]
- [51].Allen JE; Prabhu VV; Talekar M; van den Heuvel AP; Lim B; Dicker DT; Fritz JL; Beck A; El-Deiry WS, Genetic and Pharmacological Screens Converge in Identifying FLIP, BCL2, and IAP Proteins as Key Regulators of Sensitivity to the TRAIL-Inducing Anticancer Agent ONC201/TIC10. Cancer Res 2015, 75 (8), 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Golks A; Brenner D; Fritsch C; Krammer PH; Lavrik IN, c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem 2005, 280 (15), 14507–14513. [DOI] [PubMed] [Google Scholar]
- [53].Bangert A; Cristofanon S; Eckhardt I; Abhari BA; Kolodziej S; Hacker S; Vellanki SH; Lausen J; Debatin KM; Fulda S, Histone deacetylase inhibitors sensitize glioblastoma cells to TRAIL-induced apoptosis by c-myc-mediated downregulation of cFLIP. Oncogene 2012, 31 (44), 4677–4688. [DOI] [PubMed] [Google Scholar]
- [54].Zhang L; Zhang X; Barrisford GW; Olumi AF, Lexatumumab (TRAIL-receptor 2 mAb) induces expression of DR5 and promotes apoptosis in primary and metastatic renal cell carcinoma in a mouse orthotopic model. Cancer Lett 2007, 251 (1), 146–157. [DOI] [PubMed] [Google Scholar]
- [55].Shirley S; Micheau O, Targeting c-FLIP in cancer. Cancer Lett 2013, 332 (2), 141–150. [DOI] [PubMed] [Google Scholar]
- [56].Kim Y; Suh N; Sporn M; Reed JC, An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J Biol Chem 2002, 277 (25), 22320–22329. [DOI] [PubMed] [Google Scholar]
- [57].Zhang DQ; Sun P; Jin Q; Li X; Zhang Y; Zhang YJ; Wu YL; Nan JX; Lian LH, Resveratrol Regulates Activated Hepatic Stellate Cells by Modulating NF-kappaB and the PI3K/Akt Signaling Pathway. J Food Sci 2016, 81 (1), H240–245. [DOI] [PubMed] [Google Scholar]
- [58].Moriyama H; Yonehara S, Rapid up-regulation of c-FLIP expression by BCR signaling through the PI3K/Akt pathway inhibits simultaneously induced Fas-mediated apoptosis in murine B lymphocytes. Immunol Lett 2007, 109 (1), 36–46. [DOI] [PubMed] [Google Scholar]
- [59].Moumen A; Ieraci A; Patane S; Sole C; Comella JX; Dono R; Maina F, Met signals hepatocyte survival by preventing Fas-triggered FLIP degradation in a PI3k-Akt-dependent manner. Hepatology 2007, 45 (5), 1210–1217. [DOI] [PubMed] [Google Scholar]
- [60].Vlahos CJ; Matter WF; Brown RF; Traynor-Kaplan AE; Heyworth PG; Prossnitz ER; Ye RD; Marder P; Schelm JA; Rothfuss KJ; et al. , Investigation of neutrophil signal transduction using a specific inhibitor of phosphatidylinositol 3-kinase. J Immunol 1995, 154 (5), 2413–2422. [PubMed] [Google Scholar]
- [61].Vlahos CJ; Matter WF; Hui KY; Brown RF, A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 1994, 269 (7), 5241–5248. [PubMed] [Google Scholar]
- [62].Citri A; Yarden Y, EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 2006, 7 (7), 505–516. [DOI] [PubMed] [Google Scholar]
- [63].Larsen AK; Ouaret D; El Ouadrani K; Petitprez A, Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacol Ther 2011, 131 (1), 80–90. [DOI] [PubMed] [Google Scholar]
- [64].Perkins ND, Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 2007, 8 (1), 49–62. [DOI] [PubMed] [Google Scholar]
- [65].Tang D; Lahti JM; Kidd VJ, Caspase-8 activation and bid cleavage contribute to MCF7 cellular execution in a caspase-3-dependent manner during staurosporine-mediated apoptosis. J Biol Chem 2000, 275 (13), 9303–9307. [DOI] [PubMed] [Google Scholar]
- [66].Sahin E; Baycu C; Koparal AT; Burukoglu Donmez D; Bektur E, Resveratrol reduces IL-6 and VEGF secretion from co-cultured A549 lung cancer cells and adipose-derived mesenchymal stem cells. Tumour Biol 2015. [DOI] [PubMed] [Google Scholar]
- [67].Lee CS; Choi EY; Lee SC; Koh HJ; Lee JH; Chung JH, Resveratrol Inhibits Hypoxia-Induced Vascular Endothelial Growth Factor Expression and Pathological Neovascularization. Yonsei Med J 2015, 56 (6), 1678–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hogg SJ; Chitcholtan K; Hassan W; Sykes PH; Garrill A, Resveratrol, Acetyl-Resveratrol, and Polydatin Exhibit Antigrowth Activity against 3D Cell Aggregates of the SKOV-3 and OVCAR-8 Ovarian Cancer Cell Lines. Obstet Gynecol Int 2015, 2015, 279591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Abend M, Reasons to reconsider the significance of apoptosis for cancer therapy. Int J Radiat Biol 2003, 79 (12), 927–941. [DOI] [PubMed] [Google Scholar]
- [70].Melet A; Song K; Bucur O; Jagani Z; Grassian AR; Khosravi-Far R, Apoptotic pathways in tumor progression and therapy. Adv Exp Med Biol 2008, 615, 47–79. [DOI] [PubMed] [Google Scholar]