Abstract
The term Fetal Alcohol Spectrum Disorder (FASD) describes all the deleterious consequences of prenatal alcohol exposure. Impaired social behavior is a common symptom of FASD. The zebrafish has emerged as a powerful model organism with which to examine the effects of embryonic alcohol exposure on social behavior due to an innate strong behavior, called shoaling. The relative transparency of the embryo also makes zebrafish powerful for cellular analyses, such as characterizing neural circuitry. However, as zebrafish develop, pigmentation begins to obscure the brain and other tissues. Due to mutations disrupting pigmentation, the casper zebrafish strain remains relatively transparent throughout adulthood, potentially permitting researchers to image neural circuits in vivo, via epifluorescence, confocal and light sheet microscopy. Currently, however the behavioral profile of casper zebrafish post embryonic alcohol exposure has not been completed. We report that exposure to 1% alcohol from either 6 to 24, or 24 to 26 hours postfertilization reduces the social behavior of adult casper zebrafish. Our findings set the stage for the use of this important zebrafish resource in studies of FASD.
INTRODUCTION
Fetal alcohol spectrum disorder (FASD) describes all the negative outcomes of prenatal alcohol (ethanol, ethyl alcohol) exposure [1]. These birth defects can be structural in nature and often consist of abnormalities across behavioral and/or cognitive domains. FASD symptoms include impaired attention, sociability, learning and memory [2,3]. People with FASD have trouble with understanding social cues [4]. They also have difficulty initiating and maintaining reciprocal friendships [5]. Furthermore, higher rates of problems with the law, unsuitable sexual behavior, depression and suicide are related to prenatal alcohol exposure [6]. The prevalence of FASD ranges between 2 to 5 % across regions of the world, including the US, Italy and South Africa [7,8]. For perspective, in the U.S., Autism Spectrum Disorder has an estimated prevalence ranging between 1.4 and 1.5% [9], indicating that alcohol may be the leading cause of birth defects. Due to its prevalence and the burden it places on individuals, it is critical that the causes of FASD be unearthed.
Animal models have been important in our understanding of the causes of FASD and multiple symptoms of FASD have been examined using zebrafish [10]. For example, growth abnormalities and craniofacial defects have been reported in zebrafish following embryonic alcohol exposure [11–13]. Defects in learning and memory have been reported in adult zebrafish with embryonic alcohol exposure [12,14]. Embryonic alcohol exposure in wild-type strains, such as AB and Tubingen (TU) has been shown to impair shoaling [15,16], a social grouping forming behavior [17] in adults. While much work in zebrafish has focused on the mechanisms of how ethanol causes structural defects, little is known about the origins of these behavioral defects.
In vivo visualization of neural circuits will help in deciphering the roots of the behavioral defects caused by embryonic alcohol exposure. Recently, transgenic tools such as genetically encode calcium indicators that are useful for monitoring neural activity have been used in young (larval) zebrafish [18–21]. However, due to normal opacification of skin and subdermal structures as zebrafish age their transparency decreases making similar analyses in adult neurons difficult. White et al [22] crossed the nacre strain, which completely lacks melanocytes, and the roy strain, that has sparse melanocytes and translucent skin, to extend zebrafish transparency into adulthood. They named their new double mutant casper [22].
The casper strain was primarily developed for cancer research, but its transparency will make it ideal to understand how embryonic alcohol exposure alters neurocircuitry. Strain-dependent behavioral differences have been reported in zebrafish [23]. Furthermore, to date only one study has examined the behavior of casper zebrafish [24]. Therefore, we decided to examine the effect embryonic alcohol exposure had on social behavior of adult caper zebrafish. We exposed eggs to 1% ethanol to from either 6 to 24 hours postfertilization (hpf) or 24 to 26 hpf.
We found that alcohol induced social defects in both groups of fish. Our results from the group exposed for only 2 hours, demonstrate that casper zebrafish are similarly sensitive to ethanol-induced behavioral defects as compared to wild-type fish [24–27]. Surprisingly, fish exposed for the longer duration, did not produce a more severe deficit.
METHODS
Zebrafish (Danio rerio) care and use
All embryos were raised and cared for using established IACUC protocols [28] approved by the University of Texas at Austin. Eggs from an incross of Tg [elavl3:CaMPARI(W391F+V398L)]jf9; casper zebrafish were exposed to 1% ethanol diluted in embryo medium from either 6 to 24 hours postfertilization (hpf) or 24 to 26 hpf. As our focus in the current paper is on the casper phenotype, we refer to these fish as casper throughout the text for simplicity. Behavioral testing was conducted after sexual maturity at 5 months of age.
Behavioral Apparatus
Adult social arenas consisted of a 37-l tank (50 × 25 × 30 cm, L × W × H) with 1.4-l ZT140 Aquaneering tanks placed outside along the width of the tank (See Fig. 1). During the trial one of the two tanks held 4 conspecific fish (2 males and 2 females), the stimulus side, while the other tank only contained water. The outer wall of these tanks was coated with white corrugated plastic to be consistent with the experimental tank and to block any other potential cues. White opaque barriers were placed between the 1.4-l tanks and 37-l tank. The experimental tank was illuminated by a 15 W fluorescent light-tube placed directly above it. The back side and bottom of the 37-l tank were coated with white corrugated plastic sheets to increase the contrast, reduce glare and reflections for video- tracking analysis. Two identical arenas were used in parallel. The behaviors of the experimental fish were recorded and analyzed in real-time using Ethovision.
Figure 1. Schematic representations of behavioral apparatus.
Apparatus used to assay social behavior in adult zebrafish. Each color marks a different zone, labeled 1 through 10. Every zone was 5 × 25 × 30 cm (L × W × H).
Behavioral Test Procedure
We placed adult experimental zebrafish into the test tank singly, and 30 seconds later we started a 20-minute long recording session. During the recording session, the subject was first presented with a 10-minute habituation period, followed by a 10-minute stimulus period during which test subjects could visualize a shoal of zebrafish in an adjacent tank. The side of the tank nearest the shoal alternated randomly across experimental subjects.
Quantification of Behavior and Statistical Analysis
Data were analyzed using SPSS (version 23) for the Mac. We quantified social preference by measuring the distance between the test fish and the side of the tank adjacent to the live shoal (average distance from stimulus in cm).
A mixed model ANOVA was used to investigate the effect of interval (20, 1-minute intervals, the repeated measure factor), alcohol treatment (3 conditions; between-subject variable) and their interaction had on the mean distance to the live shoal (Fig. 2). According to Mauchly’s test, the assumption of sphericity for the mean distance from stimulus variable, was violated [χ2(189) = 364.25, p < 0.001] so the degrees of freedom were corrected [ε = 0.40] using Greenhouse-Geisser estimates of sphericity.
Figure 2. Embryonic ethanol exposure blunts the shoaling response in adult zebrafish.
Average distance between the adult experimental fish and the live shoal plotted for 1-minute intervals of the 20-minute behavioral session. Mean ± SEM are shown. Control (n =12); 1% EtOH, 6 to 24 hpf (n=13) and 1.0% EtOH, 24 to 26 hpf (n=11). The horizontal bar above the x axis, from 10 to 20 minutes, depicts timeline during which the live shoal is visible to the experimental subjects. The alcohol concentration is shown above the graphs.
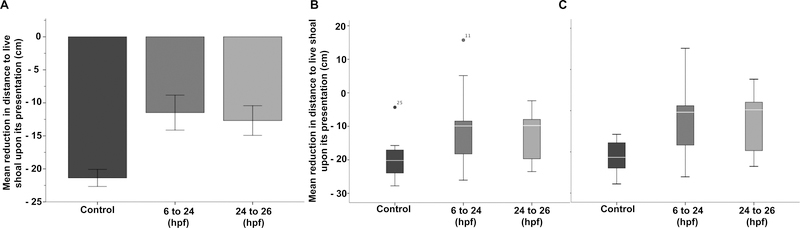
Since multiple comparison post hoc tests such as Tukey’s HSD post hoc are inappropriate for the analysis of a repeated measure, we calculated mean reduction in distance to stimulus. A one-way ANOVA followed by Tukey’s HSD post hoc test was used to examine the effect embryonic alcohol exposure had on the average reduction in the distance to stimulus (Fig. 3A). We also used this measure to determine outliers (Fig 3B & C) in our populations.
Figure 3. Alcohol treated fish are significantly further way from the live shoal.
(A) Bars represent the difference between the distance fish where from the live shoal before and after the live shoal is visible. Larger negative values suggest a stronger response to the conspecifics. Mean ± SEM are shown. (B) Box-plots represent the average reduction in distance to the live shoal upon its presentation. Note two outliers were identified using the SPSS. (C) Data reanalyzed after subjects 11 and 25 were removed. Mean ± SEM are shown. Sample size are as follows: Control (n =12); 1% EtOH, 6 to 24 hpf (n=13) and 1.0% EtOH, 24 to 26 hpf (n=11).
We used a mixed ANOVA to investigate the effect of zone (10, repeated measure factor), alcohol treatment (3 conditions; between-subject variable) and their interaction on the percentage of time spent across zones during stimulus presentation (Fig 4A). Again, sphericity was violated [ χ2(44) = 874.80 p < 0.001] according to Mauchly’s test, and the degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity [ε = 0.16]. We also quantified the amount of time fish spent in the Zone 1, the closest zone to the live shoal. A one-way ANOVA followed by Tukey’s HSD post hoc test was used to examine amount of time fish spent in the Zone 1 (Fig 4B).
Figure 4. Embryonic ethanol exposure alters the duration of time spent in zone 1 only when the live shoal is visible.
(A) Bars represent the time spent in all 10 zones during stimulus presentation. (B) Bars represent the time spent in Zone 1 during the stimulus presentation. Zone 1 is the zone closest to the live shoal, while zone 10- is the furthest away from the live shoal. Note the significant difference in the amount of time fish control fish spend in Zone. (C) Bars represent the average percentage of time spent in all zones during habituation, the first 10-minutes. (D) Bars represent the time spent in Zone 1 during habituation. Mean ± SEM are shown. Sample size are as follows: Control (n =12); 1% EtOH, 6 to 24 hpf (n=13) and 1.0% EtOH, 24 to 26 hpf (n=11). 1 compared to alcohol treated fish.
To determine whether alcohol exposure impacted locomotor activity, we examined the time fish spent in various zones during habituation (Fig 4C). A mixed ANOVA was used to investigate the effect of zone, alcohol treatment and their interaction on the percentage of time spent across zones. Again, sphericity was violated [ χ2(44) = 509.78, p < 0.001] and the degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity [ε = 0.28]. We also quantified the amount of time fish spent in the Zone 1, the closest zone to the live shoal, during habituation (Fig 4D). A one-way ANOVA was used to determine whether embryonic ethanol exposure altered the amount of fish spent in the Zone 1 during habituation.
We also sought to determine whether alcohol impaired the response to the visual presentation of the stimulus. Therefore, we examined the amount of time (latency) it took test subjects to reach zone closest to the stimulus (Zone 1), upon stimulus presentation via a one-way ANOVA (Fig 5).
Figure 5. Embryonic alcohol exposure does not impair the initial response to a social stimulus in adult zebrafish.
Bars represent the time fish take to reach Zone 1. The time is measured from start of the trial, thus stimulus exposure is at 600 seconds. Mean ± SEM are shown. Sample size are as follows: Control (n =12); 1% EtOH, 6 to 24 hpf (n=13) and 1.0% EtOH, 24 to 26 hpf (n=11) Note fish from all groups reach the zone closest to the live shoal (Zone 1) within a similar timeframe.
Statistical analysis of adult fish behavior demonstrated no significant Sex differences [F(1, 28) = 0.25, p = 0.63], Sex X Interval interaction [F (7.48, 209.47) = 0.68, p = 0.70], Sex X Alcohol treatment interaction [F(2, 28) = 0.68, p = 0.51], or Sex X Interval X Alcohol treatment interaction [F(14.96, 2090.47) = 1.09, p = 0.37]. As a result, data are pooled for sex in subsequent analyses.
RESULTS
Data obtained during the complete 20-minute behavioral test are displayed in Fig. 2. During habituation test fish cannot see the shoal and fish from all groups swim back and forth across the length of the tank. This results in an average distance approximating the middle of the tank (Fig. 2A & B). Once the live shoal is visible fish from all groups rapidly move towards the stimulus. However, the ethanol exposed groups do not move as close to the shoal and fail to maintain proximity (Fig. 2). We found a statistically significant interaction between interval and alcohol [F(14.96, 209.47) = 2.11, p = 0.01]. The main effect of alcohol treatment was not significant [F(2, 28) = 2.82, p = 0.077]. These findings are consistent with a blunted shoaling response in fish that were exposed to alcohol.
As an additional measure of the shoaling response, we tested if the duration of time spent in zones across the test chamber differed among groups. During habituation the distribution of duration in each zone was similar across groups, with fish spending more time in the zones nearest either end of the tank (Fig. 4C). We used a mixed ANOVA to analyze this measure. Consistent with the preference for either end of the tank, duration in each zone was significantly different [F(2.48, 77.00) = 2.92, p = 0.05] over the course of the habituation. Important for the current study, there was no main effect of alcohol treatment or an interaction effect of duration and alcohol treatment ([F(2, 31) = 1.14, p = 0.33], [F(4.96, 77.00) = 0.53), p = 0.75], respectively). This suggests that ethanol exposure doesn’t disrupt the overall swimming behavior of zebrafish prior to shoal presentation. Additionally, we examined the percentage of time fish spent in zone 1, the zone closest to the live shoal, during habituation. There was no significant effect ([F(2,31) = 1.04), p = 0.37]) (Fig. 4D).
To quantify the shoaling response, we subtracted the average distance during stimulus presentation from the average distance during habituation. Embryonic alcohol exposure significantly affects the reduction in distance towards the live shoal [F (2,331) = 6.09, p = 0.006)] (Fig 3A.). In this analysis, a larger negative value indicates a more robust response to the live shoal. Tukey’s HSD post hoc analysis demonstrated that control fish (M = −21.37, S.D. +/− 4.29) had a significantly stronger response to the live shoal than fish exposed to 1% alcohol from 6 to 24 hours postfertilization (hpf) (M = −11.48, S.D. +/− 9.18, p = 0.008) or from 24 to 26 hpf (M = −12.68, S.D. +/− 7.44, p = 0.02). The 18 hour ethanol treatment had no significant effect on social behavior relative to the 2 hour treatment.
Once the shoal becomes present, zebrafish appear to rapidly approach the stimulus (See Figs. 2 & 3). We characterized the effect of ethanol on this behavior by determining the latency to reach the zone closest to the shoal (Fig 5). Here we used a one-way ANOVA to analyze the data. There was no difference in the length of time test fish took to enter Zone 1 following the shoal presentation [F(2, 31) = 1.04, p = 0.37]. These findings suggest that neither the initial response to nor the ability to respond to the shoal was altered by alcohol exposure.
Once the shoal is present the duration of time becomes highly skewed to the side of the tank adjacent to the shoal, especially in untreated fish (Fig. 4A). We used mixed ANOVA to test for differences in these data. As during habituation, duration significantly varied across zones [F(1.45, 44.99) = 124.36, p < 0.001]. The main effect of alcohol treatment was non-significant [F(2,31) = 1.13, p = 0.34], importantly, there was a significant interaction between alcohol treatment and duration [F (2.09, 44.99) = 7.38, p < 0.001]. These data demonstrate that embryonic alcohol exposure alters the percentage of time spent across the zones of the testing arena.
We analyzed the zone nearest to the shoal, zone 1, (Fig 4B) to characterize the robustness of the shoaling response. Using a one-way ANOVA we found significant differences in the amount of time fish spent in zone 1 across groups [F(2,31) = 8.99, p = 0.001] (Fig. 5A). Tukey’s HSD post hoc analysis confirmed that when the shoal is visible control fish spend almost twice the amount of time (M = 83.07, S.D. +/− 18.53) in zone 1, compared to fish treated with 1% alcohol from 6 to 24 hpf (M = 43.98, S.D. +/− 27.24, p = 0.001) or 24 to 26 hpf (M = 51.07, S.D. +/− 22.94, p = 0.001). Here again, a longer ethanol exposure did not appear to cause greater disruption in shoaling behavior.
DISCUSSION
The aim of this study was of characterize the social behavioral phenotype of adult casper mutant zebrafish, which had been exposed to alcohol early during development. Previous research has demonstrated that embryonic alcohol exposure impairs social behavior in AB, and TU wild-type zebrafish [15,16]. For example, exposure to 1% ethanol from 24 to 26 hour postfertilization (hpf) decreases the shoaling response of AB zebrafish [15,25–27]. Our results, from exposing eggs from casper mutant zebrafish to 1 % ethanol from 24 to 26 hpf, matched our own findings using the AB strain (Fernandes et al., in revision 2018) as well as work from others [15,25–27].
We define a shoaling response as the decrease in distance of the test subject towards the group of zebrafish and the maintenance of close proximity to the group. When a single zebrafish witnesses a group of other zebrafish, it approaches and remains close to the group [29] regardless of whether the group is made of live fish or computer animations [30]. Our statistical analysis demonstrated that distance from the stimulus varied across treatment groups. Given that shoaling by our definition requires a reduction in distance as well as the maintenance of close proximity to the shoal, are alcohol treated fish not shoaling because they do not approach the shoal or do not stay nearby?
To begin to answer this question first we measured the reduction in distance towards the live shoal upon stimulus presentation. We found the initial response to the stimulus to be unimpaired by alcohol, that is, fish across all groups took approximately the same amount of time to enter the Zone 1, the zone closest to the live fish (Fig. 5), suggesting that alcohol did not impair either vision or locomotor function. Further supporting that locomotor function is unaffected by alcohol is the finding that during habituation there was no differences between treated and untreated fish in the distance moved. Our finding is consistent with previous results that show that similar alcohol exposure does not impair locomotor function in zebrafish [15,25–27].
Next, we examined the proximity and the maintenance of proximity to the live shoal. Interestingly, even though ethanol-exposed fish spend the most time in zone 1 during stimulus presentation (Fig. 4B), this amount of time is only approximately half the time control fish spend in the same zone. Surprisingly, we did not find a more severe behavioral deficit in fish that were treated with 1% ethanol from 6 to 24 hpf compared to those treated with 1% from 24 to 26 hpf. The nature of this ethanol-insensitive social response is unknown. Recent unpublished data from our lab, found that an increased concentration of ethanol from 1 to 1.5% between 24 and 26 hpf also did not cause increasingly severe social deficits (Fernandes et al., accepted 2018). Thus it is possible that 1% ethanol maximally disrupts neural networks associated with social behavior, such as the social behavioral network [31].
The social behavioral network consists of six reciprocally connected neural areas that are important sites of either activation or regulation of social behavior [32]. The SBN is highly conserved network and has been characterized in other fish [31]. Our results demonstrate that a 1% dose from 24 to 26 hpf, which has been shown to cause behavioral deficits in wild-type zebrafish causes similar deficits in the zebrafish mutant casper. Given the optical transparency of casper we believe that this mutant will help in determining the effect embryonic alcohol exposure has on the neurocircuitry potentially involved in the social behavioral network and thus in social behavior.
Acknowledgments
Funding
Funding to support this research was provided by the National Institutes of Health (NIH) /National Institute on Alcohol Abuse (NIAAA) [R01AA023426; U01AA021651] to J.K.E.
References
- [1].Stratton KR, Howe CJ, Battaglia FC, eds., Fetal alcohol syndrome: diagnosis, epidemiology, prevention, and treatment, National Academy Press, Washington, D.C, 1996. [Google Scholar]
- [2].Kodituwakku PW, Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review, Neurosci Biobehav Rev. 31 (2007) 192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- [3].Rasmussen C, Becker M, McLennan J, Urichuk L, Andrew G, An evaluation of social skills in children with and without prenatal alcohol exposure, Child Care Health Dev. 37 (2011) 711–718. doi: 10.1111/j.1365-2214.2010.01152.x. [DOI] [PubMed] [Google Scholar]
- [4].Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF, Fetal alcohol syndrome in adolescents and adults, JAMA, 265 (1991) 1961–1967. doi: 10.1001/jama.1991.03460150065025. [DOI] [PubMed] [Google Scholar]
- [5].Roebuck TM, Mattson SN, Riley EP, Behavioral and psychosocial profiles of alcohol-exposed children, Alcohol. Clin. Exp. Res. 23 (1999) 1070–1076. doi: 10.1111/j.1530-0277.1999.tb04227.x. [DOI] [PubMed] [Google Scholar]
- [6].Kelly SJ, Day N, Streissguth AP, Effects of prenatal alcohol exposure on social behavior in humans and other species, Neurotoxicol and Teratol. 22 (2000) 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, et al. , Prevalence and characteristics of fetal alcohol spectrum disorders, Pediatrics. 134 (2014) 855–866. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, et al. , Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies, Dev Disabil Res Rev. 15 (2009) 176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- [9].Christensen DL, Baio J, Braun KVN, Bilder D, Charles J, Constantino JN, et al. , Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012, MMWR Surveill Summ. 65 (2016) 1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fernandes Y, Buckley DM, Eberhart JK, Diving into the world of alcohol teratogenesis: A review of zebrafish models of fetal alcohol spectrum disorders, Biochem Cell Biol. 15 (2017) 514. doi: 10.1139/bcb-2017-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bilotta J, Barnett JA, Hancock L, Saszik S, Ethanol exposure alters zebrafish development: A novel model of fetal alcohol syndrome, Neurotoxicol Teratol. 26 (2004) 737–743. doi: 10.1016/j.ntt.2004.06.011. [DOI] [PubMed] [Google Scholar]
- [12].Carvan MJ III, Loucks E, Weber DN, Williams FE, Ethanol effects on the developing zebrafish: neurobehavior and skeletal morphogenesis, Neurotoxicol and Teratol. 26 (2004) 757–768. doi: 10.1016/j.ntt.2004.06.016. [DOI] [PubMed] [Google Scholar]
- [13].McCarthy N, Wetherill L, Lovely CB, Swartz ME, Foroud TM, Eberhart JK, Pdgfra protects against ethanol-induced craniofacial defects in a zebrafish model of FASD, Development. 140 (2013) 3254–3265. doi: 10.1242/dev.094938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fernandes Y, Tran S, Abraham E, Gerlai R, Embryonic alcohol exposure impairs associative learning performance in adult zebrafish, Behav. Brain Res. 265 (2014) 181–187. doi: 10.1016/j.bbr.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fernandes Y, Gerlai R, Long-Term Behavioral Changes in Response to Early Developmental Exposure to Ethanol in Zebrafish, Alcohol. Clin. Exp. Res. 33 (2009) 601–609. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Parker MO, Annan LV, Kanellopoulos AH, Brock AJ, Combe FJ, Baiamonte M, et al. , The utility of zebrafish to study the mechanisms by which ethanol affects social behavior and anxiety during early brain development, Prog Neuro-Psychopharmacol. 55 (2014) 94–100. doi: 10.1016/j.pnpbp.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pitcher TJ, Heuristic definitions of fish shoaling behaviour, Animal Behaviour. 31 (1983) 611–613. [Google Scholar]
- [18].Kawashima T, Kitamura K, Suzuki K, Nonaka M, Kamijo S, Takemoto-Kimura S, et al. , Functional labeling of neurons and their projections using the synthetic activity– dependent promoter E-SARE, Nat Meth. 10 (2013) 889–895. doi: 10.1038/nmeth.2559. [DOI] [PubMed] [Google Scholar]
- [19].Muto A, Kawakami K, Imaging functional neural circuits in zebrafish with a new GCaMP and the Gal4FF-UAS system, Commun Integr Biol. 4 (2011) 566–568. doi: 10.4161/cib.4.5.15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Muto A, Ohkura M, Abe G, Nakai J, Kawakami K, Real-Time Visualization of Neuronal Activity during Perception, Current Biology. 23 (2013) 307–311. doi: 10.1016/j.cub.2012.12.040. [DOI] [PubMed] [Google Scholar]
- [21].Fosque BF, Sun Y, Dana H, Yang C-T, Ohyama T, Tadross MR, et al. , Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators, Science. 347 (2015) 755–760. doi: 10.1126/science.1260922. [DOI] [PubMed] [Google Scholar]
- [22].White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, et al. , Transparent adult zebrafish as a tool for in vivo transplantation analysis, Cell Stem Cell. 2 (2008) 183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vignet C, Bégout M-L, Péan S, Lyphout L, Leguay D, Cousin X, Systematic Screening of Behavioral Responses in Two Zebrafish Strains, Zebrafish. 10 (2013) 365–375. doi: 10.1089/zeb.2013.0871. [DOI] [PubMed] [Google Scholar]
- [24].Parker MO, Brock AJ, Millington ME, Brennan CH, Behavioral Phenotyping of CasperMutant and 1-Pheny-2-Thiourea Treated Adult Zebrafish, Zebrafish. 10 (2013) 466–471. doi: 10.1089/zeb.2013.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fernandes Y, Rampersad M, Gerlai R, Impairment of social behaviour persists two years after embryonic alcohol exposure in zebrafish: A model of fetal alcohol spectrum disorders, Behav. Brain Res. 292 (2015) 102–108. doi: 10.1016/j.bbr.2015.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fernandes Y, Rampersad M, Gerlai R, Embryonic alcohol exposure impairs the dopaminergic system and social behavioral responses in adult zebrafish, Int. J. Neuropsychopharmacol. 18 (2015). doi: 10.1093/ijnp/pyu089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Buske C, Gerlai R, Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish, Neurotoxicol and Teratol. 33 (2011) 698–707. doi: 10.1016/j.ntt.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Westerfield M, The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio) University of Oregon Press, Eugene, 1993. [Google Scholar]
- [29].Fernandes Y, Rampersad M, Jia J, Gerlai R, The effect of the number and size of animated conspecific images on shoaling responses of zebrafish, Pharmacology Biochemistry and Behavior. 139 Pt B (2015) 94–102. doi: 10.1016/j.pbb.2015.01.011. [DOI] [PubMed] [Google Scholar]
- [30].Qin M, Wong A, Seguin D, Gerlai R, Induction of Social Behavior in Zebrafish: Live Versus Computer Animated Fish as Stimuli, Zebrafish. 11 (2014) 185–197. doi: 10.1089/zeb.2013.0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].O’Connell LA, Hofmann HA, The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis, J. Comp. Neurol. 519 (2011) 3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- [32].Newman SW, The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network, Annals of the New York Academy of Sciences. 877 (1999) 242–257. [DOI] [PubMed] [Google Scholar]