Abstract
Plasma lipoproteins are essential vehicles of lipid distribution for cellular energy and structural requirements as well as for excretion of lipid excess. Imbalances in lipoprotein metabolism are known to contribute to metabolic diseases ranging from vascular inflammation and atherosclerosis to obesity and diabetes. The lipid and protein cargo carried by lipoprotein subclasses have long been the focus of studies exploring the contribution of plasma lipoproteins in health and in metabolic disorders. More recent studies have revealed the presence of non-coding RNA as a new form of cargo carried by plasma lipoproteins. Lipoprotein-associated microRNAs have been identified to distribute differentially among plasma lipoprotein subclasses and contribute to cellular signaling. These findings highlight plasma lipoprotein-associated RNA as a potential source of biological signaling and warrants a renewed interest in the study of plasma lipoprotein biology. This chapter describes principles and methods based on density ultracentrifugation and size exclusion chromatography for the isolation of plasma lipoproteins as a source of extracellular RNA.
Keywords: Lipoprotein, sequential density ultracentrifugation, FPLC chromatography, HDL, VLDL, LDL, extracellular RNA
1. Introduction
Plasma lipoproteins have long been recognized for their essential role in distributing both energy-rich and structural lipids, as well as vitamins and micronutrients to cells through-out the mammalian organism (1). Select lipoprotein classes, including chylomicrons and very-low density lipoproteins (VLDL) that are secreted by the intestine and liver respectively, serve as the major mediators of lipid and micronutrient distribution. Through catabolic and lipolytic processes that occur in the circulation and the endothelial lining of organs and tissues, such lipoproteins are converted to remnants including low-density lipoproteins (LDL) that are cleared by the liver (1). An accumulation of remnant lipoproteins in plasma, caused by metabolic defects including through mutations in the low density lipoprotein receptor, have been identified as an important source of systemic and vascular inflammation and atherosclerosis (2). An important cause of such lipoprotein-induced inflammation and cardiovascular disease has been linked to their uncontrolled absorption by monocytes in the circulation and macrophages in the vessel wall (3). In contrast, high density lipoproteins (HDL) that are derived primarily through the lipolytic remodeling of VLDL and chylomicrons, have been shown to participate in the removal of cholesterol and lipid deposits from lesional macrophages which they deliver to the liver for excretion into bile in a process referred to a reverse cholesterol transport (4). Such “cholesterol-efflux” property of HDL has long been touted as a primary mode of action in preventing atherosclerosis buildup and cardiovascular disease (5).
Beyond its role in mediating the cellular release of cholesterol, HDL is recognized to prevent cardiovascular diseases by exerting numerous anti-inflammatory properties (6). Among these include the capacity for HDL to carry bio-active protein cargo such as the anti-oxidant enzyme paraoxonase that prevents the oxidation of lipids in lipoproteins including LDL and thereby the propensity for foam cell formation in the vessel wall (6). HDL is also recognized to carry bioactive lipid cargo such as sphingosine-1-phosphate that provides a panoply of protective signaling properties in the cardiovascular and immune systems (7, 8). Recent findings have introduced non-coding RNA including microRNA as yet a new class of nucleic acid cargo carried by HDL and other plasma lipoproteins (9). Interestingly, the levels of select microRNA in both HDL and LDL have been shown to alter during conditions of metabolic excess including hyperlipidemia (9). Moreover, HDL-associated micro-RNAs have been shown to participate in cellular signaling within the cardiovascular system (10). Such exciting findings have spurred a renewed interest in the isolation and study of plasma lipoproteins both as biomarkers for disease states and mediators of RNA bio-distribution and intercellular communication.
A robust and widely used method to isolate plasma lipoproteins, first reported in 1955 by Richard Havel and colleagues while at the National Institutes of Health in Bethesda (11), is based on sequential density ultracentrifugation (SD-UC) of plasma that is adjusted to well-defined densities using potassium bromide salt. Another procedure commonly used to separate plasma lipoproteins makes use of size exclusion chromatography, often with a fast-protein liquid chromatography (FPLC) system combined with columns filled with high-resolution stationary phases. While more rapid and convenient than sequential density ultracentrifugation, FPLC separation of plasma lipoproteins is used most often as an analytical approach for the assessment of plasma lipoprotein profiles in experimental murine models of lipoprotein metabolism (12). Affinity chromatography using columns linked to monoclonal antibodies specific to select apolipoproteins provides yet another approach to selectively isolate plasma lipoprotein subclasses (13). However, the use of low pH required to elute lipoproteins retained by the immuno-affinity column poses unknown risk for the loss of nucleic acids from the purified lipoprotein. Thus, sequential density ultracentrifugation remains by far the gold standard method for the isolation of well-defined lipoprotein classes in sufficient quantities to allow for subsequent in vitro or in vivo studies of lipoprotein function and RNA composition. Moreover, the availability of smaller desktop-sized ultracentrifuges and rotor systems allow for the isolation of lipoproteins from as little as 2 mL of plasma which facilitates the study of lipoprotein-biology in genetically engineered mouse models of human diseases (14). This chapter describes principles and methods for isolation and characterization of lipoprotein classes from blood plasma.
2. Materials
2.1. Sequential gradient ultracentrifugation
Potassium bromide (KBr) (Sigma).
Optima MAX-XP tabletop ultracentrifuge (Beckman Coulter)
TLA-100.3 rotor (Beckman Coulter)
Polypropylene Quick-Seal 3.5 mL tubes (Beckman Coulter)
CentriTube slicer (Beckman Coulter)
1 mL slip-tip disposable syringe (BD Biosciences)
BD PrecisionGlide needle, 21 G x 1.5 inch (BD Biosciences)
Optima XPN 90 floor-mode ultracentrifuge (Beckman Coulter)
Type 50.2 Ti rotor (Beckman Coulter)
Polypropylene 39 mL Quick-Seal tubes (Beckman Coulter)
Phosphate buffered saline, pH 7.4 with 1 mM EDTA
2.2. FPLC chromatography
Isocratic HPLC pump with a manual Rheodyne sample injector (Fisher Scientific)
Programmable 96-well fraction collector (Fisher Scientific)
Superose 6, 10/300 GL FPLC size exclusion column (GE Healthcare)
2.3. SDS-PAGE and coomassie blue detection
PROTEAN II xi cell (Bio-Rad)
30% Acrylamide/Bis solution (Bio-Rad)
Ammonium persulfate (APS) (Bio-Rad)
TEMED (Bio-Rad)
ImageQuant LAS 4000
2.4. Lipoprotein cholesterol analysis
Wako cholesterol E assay (Wako Diagnostics)
Control serum I (Wako Diagnostics)
Control serum II (Wako Diagnostics)
Microplate spectrophotometer (Thermo Fisher Scientific or any other brand)
2.5. Lipoprotein RNA analysis
mirVana™ PARIS™ RNA and Native Protein Purification Kit (Thermo Fisher Scientific)
Sodium acetate, 3M, PH5.2 (Amresco LLC)
Ethanol, molecular biology grade (Sigma)
Agilent 2100 bioanalyzer instrument
Agilent RNA 6000 pico kit
Agilent small RNA kit
3. Methods
3.1. Isolation of very-low and low-density lipoprotein (VLDL/LDL) fraction from plasma
Collect blood from a human subject or from mice into EDTA coated tubes according to institutional guidelines. When bleeding mice for non-terminal experiments, one may typically obtain no more than 250 μL of blood from each mouse. (See Note 1)
Spin the blood for 10 min at 2,000 x g at 4°C to remove erythrocytes and white cells.
Platelets must next be removed by spinning the plasma for 30 min at 15,000 x g.
Non-fasted plasma can sometimes produce a milky fat-rich layer of chylomicrons that can be removed by gentle pipetting if this class of lipoprotein is not of interest (See Note 2). Subsequently, gently pipet the clear platelet- and chylomicron-free supernatant.
Pool all the plasma into a fresh 15-mL tube kept on ice and record the exact volume.
Weigh out the needed amount of KBr (See Note 3) required to produce a density of 1.063 g/mL using the formula: plasma (mL) X 0.0834 = KBr (g). Add the KBr to the plasma and let the tube sit on ice then mix slowly using a wide bore pipet to avoid causing excessive shear stress in the solution.
Add 0.834 g KBr into 10 mL PBS to make d=1.063 g/mL KBr/PBS solution.
Transfer the plasma to ultracentrifuge tubes using 3 mL syringes.
Add d=1.063 g/mL KBr/PBS solution to fill the tube (total volume ~3.5 mL).
Balance the tubes; make sure that the weight difference between two tubes is within 0.1g to avoid causing an imbalance in the rotor.
Seal the tubes with a pre-heated Beckman Tube Sealer.
Place the sealed ultracentrifuge tubes into a pre-chilled rotor (4°C).
Place the rotor in the pre-chilled ultracentrifuge (4°C), centrifuge at 100,000 x g.
After 24 hours, stop the ultracentrifuge and gently take the tubes out of the rotor while avoiding disturbing the separated layers of lipoproteins.
Set up a Beckman Tube Slicer; rinse the blade and the holder with ddH2O and allow it to dry.
Put in the blade and tighten the screw to secure the blade.
Place a tube into the tube holder and lower the holder arm using the bottom dial to secure the tube in place.
Adjust the cutting position in relation to the blade to cut approximately the top 1/5 of the tube (See Note 4) that will contain the VLDL/LDL (See Note 5).
Cut the tube in one movement of the slicer mechanism.
Insert a 21 gauge needle into the top portion of the tube above the blade in order to allow air entry during the subsequent aspiration step.
Insert a second needle attached to a 1 mL syringe immediately above the blade.
Gently aspirate the lipoprotein fraction by drawing on the syringe plunger.
Carefully disassemble the Tube Slicer instrument to allow for the removal of the top portion of the tube (See Note 6).
Slide the blade away from the tube and collect the infranatant in the bottom 4/5 portion of the tube that will contain the HDL.
3.2. Isolation of HDL fraction
Precisely record the volume extracted from the bottom portion of the centrifuge tube. Weigh out the required amount of KBr to produce a density of 1.21 g/mL in the plasma using the formula provided in Table 1: d=1.063 plasma (mL) X 0.235 = KBr (g). Add the KBr to the plasma and place the tube on ice. Mix slowly using a wide bore pipet.
Prepare d=1.21 g/mL KBr/PBS by adding 3.15 g of KBr to 10 mL PBS according to Table 1.
Transfer the plasma to an ultracentrifuge tube using 3 mL syringes.
Add sufficient amount of cold d=1.21 g/mL KBr/PBS solution to fill the tube.
Balance the tubes; make sure that the difference between 2 tubes is within 0.1g.
Seal the tube with a Beckman Tube Sealer.
Place the ultracentrifuge tubes into a pre-chilled rotor (4°C).
Place the rotor in the pre-chilled ultracentrifuge (4°C), centrifuge at 100,000 x g for 24 hours.
Remove the tubes while being careful not to disturb the lipoprotein layers.
Set up Beckman tube slicer as above making sure to cut at the 1/5 top portion of the tube.
Cut the tubes in one movement.
Aspirate the HDL fraction using a 1 mL syringe as detailed above, rinse the top of centrifuge tube.
Discard the infranatant.
Table 1.
Conversion factors for density adjustment among plasma, PBS, VLDL, LDL and HDL using Solid KBr
| Initial density (g/mL) | Final density (g/mL) | Conversion Factor | |
|---|---|---|---|
| Plasma >> VLDL/LDL | 1.006 | 1.063 | 0.0834 |
| PBS >> VLDL/LDL | 1.006 | 1.063 | 0.0834 |
| VLDL/LDL >> HDL | 1.063 | 1.21 | 0.235 |
| PBS >> HDL | 1.006 | 1.21 | 0.315 |
| Plasma >> VLDL | 1.006 | 1.019 | 0.0185 |
| VLDL >> LDL | 1.019 | 1.063 | 0.0629 |
3.3. Dialysis
Label dialysis cassette and make a dot in the corner that will be used for injection.
Equilibrate cassette 10 min in 2 L cold PBS.
Inject lipoprotein fraction into the cassette while taking care not to perforate the membrane.
Vacuum aspirate the residual air to ensure an efficient dialysis.
Dialyze in a cold room against PBS for 2 h and change the buffer using fresh PBS; subsequently, after a second 2 h period of dialysis, dialyze against fresh PBS overnight.
Remove the lipoprotein fractions from the dialysis cassettes using a 21 gauge needle and 1 mL syringe, and store on ice or in a refrigerator.
3.4. Sub-fractionation of SD-UC−isolated plasma lipoproteins by FPLC
Lipoprotein classes isolated by SD-UC can be further resolved by using size exclusion FPLC-chromatography using a Superose 6, 10/300 GL column from GE Health Care. A 200 μL volume of freshly isolated HDL or VLDL/LDL lipoprotein fraction is loaded into a 200 μL loop fixed onto a manual Rheodyne injector system that is in line with an isocratic HPLC pump set at a flow rate of 0.4 mL/minute using PBS-EDTA as a mobile phase.
Upon injecting the loaded lipoproteins into the system, immediately start the fraction collector that is set to collect for 90 seconds for a total volume of 500 μL into a 96-well collection plate.
Maintain collection for at least 60 minutes after which the system is ready for a second injection.
Clean and store column according to guidelines provided by the manufacturer.
Determine the lipoprotein cholesterol distribution in the fractions using the Cholesterol E detection kit described below.
3.5. Determination of cholesterol levels in lipoprotein fractions
Prepare a standard curve provided in the Cholesterol E kit
Prepare a 100 fold dilution of plasma samples and lipoprotein fractions using ddH2O; vortex and keep on ice
Add 100 μL diluted plasma, lipoprotein fractions and standards in triplicate to appropriate wells of a 96-well plate
Add 150 μL cholesterol E buffer to appropriate wells in a 96-well plate;
Shake plate using a plate mixer and incubate at 37 °C for 5–15 min;
Read absorbance using a plate reader set at 690 nm and integrate data.
3.6. Apolipoprotein detection by SDS-PAGE
Mix a 100 μL volume of each of the lipoprotein fractions with Laemmli buffer. Heat the mixed samples at 95 °C for 5 minutes.
Using a gradient maker system, prepare 5%−12% gradient polyacrylamide gels containing SDS as a denaturant (See Note 7).
Load the gels with a molecular-weight marker spanning 10 kDa to 250 kDa along with the denatured lipoprotein samples and resolve the proteins by electrophoresis.
Visualization of resolved proteins in the PAGE is achieved by incubating the gel with Coomassie blue stain for 2 hrs.
Subsequently, the gels are de-stained in 5% methanol and 7.5% glacial acetic acid solution (See Note 8) and images are captured using an ImageQuant LAS 4000.
3.7. Lipoprotein RNA extraction using mirVana PARIS kit
Add 400 μL VLDL/LDL or HDL samples in 2 mL tubes
Add 400 μL pre-warmed 2X Denaturing Solution. Immediately mix thoroughly.
Add 800 μL Acid-Phenol:Chloroform, be sure to withdraw the bottom phase.
Vortex for 30–60 sec to mix.
Centrifuge for 5 min at 14,000 x g at room temp to separate the mixture into aqueous and organic phases.
Carefully remove the aqueous (upper) phase without disturbing the lower phase or the interphase, and transfer it to a fresh tube.
Add 1.25 volumes of 100% ethanol to the aqueous phase and mix thoroughly.
For each sample, place a filter cartridge into one of the collection tubes.
Pipet the lysate/ethanol mixture onto the filter cartridge. Apply a maximum of 700 μL to a filter cartridge at a time.
Centrifuge at 10,000 x g for ~30 sec.
Discard the flow-through, and repeat until all of the lysate/ethanol mixture is through the filter. Save the collection tube for the washing steps.
Apply 700 μL miRNA Wash Solution 1 to the Filter Cartridge and centrifuge at 10,000 x g for ~15 sec, discard the flow-through from the collection tube, and replace the filter cartridge into the same collection tube.
Apply 500 μL Wash Solution 2/3 and draw it through the Filter Cartridge as in the previous step.
Repeat with a second 500 μL of Wash Solution 2/3.
After discarding the flow-through from the last wash, replace the filter cartridge in the same collection tube and spin the assembly for 1 min at to remove residual fluid from the filter.
Transfer the filter cartridge into a fresh collection tube. Apply 50 μL of preheated (95°C) nuclease-free water to the center of the filter, and close the cap. Centrifuge for ~30 sec to recover the RNA.
Collect the eluate and store it at –80°C.
3.8. RNA concentration by ethanol precipitation
-
1
Combine all eluates from the same lipoprotein sample and add nuclease-free water to reach a final volume of 400 μL
-
2
Add 50 μL 3 M PH 5.2 Sodium acetate and –20°C pre-chilled 100% ethanol (975 μL)
-
3
Incubate 30 min or overnight at –20°C
-
4
Centrifuge at 15,000 × g on a benchtop microcentrifuge at 30 minutes at 4°C.
-
5
Remove and discard the supernatant. Leave the pellet intact.
-
6
Wash the pellet with 500 μl 70% ethanol at room temperature.
-
7
Centrifuge at 15,000 × g for 5 minutes.
-
8
Remove and discard the supernatant. Leave the pellet intact.
-
9
With the lid open, place the tube in a 37°C heat block until the pellet is dry
-
10
Add 10 μL nuclease-free water to dissolve the RNA pellet
3.9. Lipoprotein total RNA characterization
-
11
Using an Agilent 2100 Bioanalyzer system, prepare a Gel-Dye mix by mixing 1 μL RNA dye concentrate with 65 μL filtered gel, vortex solution well and spin the tube at 13,000 x g for 10 min at room temperature.
-
12
Load 9 μL Gel-Dye mix to appropriate well and prime the RNA Pico chip
-
13
Load 9 μL Gel-Dye mix and the RNA conditioning solution to appropriate well
-
14
Pipette 5 μL of RNA marker in the well for ladder and all 11 sample wells.
-
15
Pipette 1 μL of the heat denatured ladder into the ladder well;
-
16
Pipette 1 μL of sample in each of the 11 sample wells
-
17
Vortex for 1 min at 2400 rpm in the IKA station;
-
18
Run the chip in the Agilent 2100 Bioanalyzer within 5 min.
3.10. Lipoprotein small RNA characterization
Prepare the Gel-Dye mixture by combining the RNA dye concentrate with filtered gel, vortex the solution well and spin the tube at 13,000 x g for 10 min at room temperature.
Load 9 μL Gel-Dye mix to appropriate well and prime the small RNA chip
Load 9 μL Gel-Dye mix and the RNA conditioning solution to appropriate wells
Pipette 5 μL of RNA marker in the well for ladder and all 11 sample wells.
Pipette 1 μL of the heat denatured ladder into the ladder well;
Pipette 1 μL of sample in each of the 11 sample wells
Vortex for 1 min at 2400 rpm in the IKA station;
Run the chip in the Agilent 2100 Bioanalyzer within 5 min.
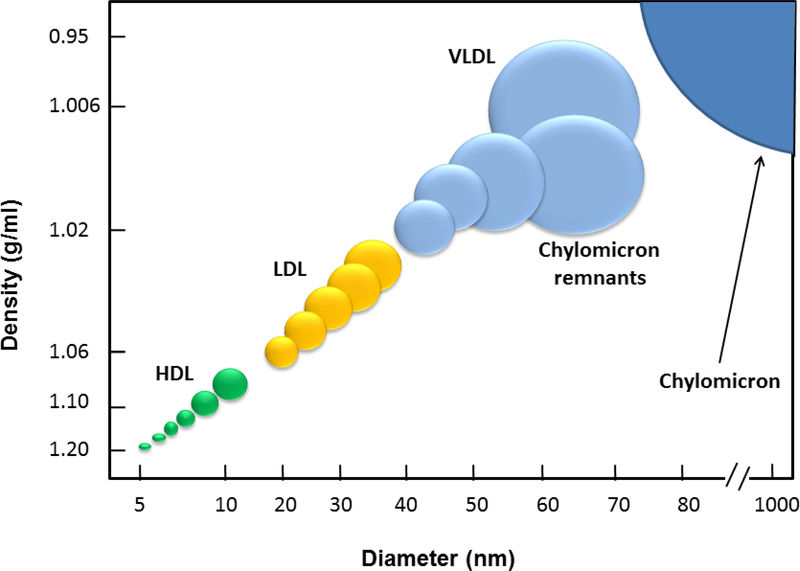
Figure 1. Relationship between the densities and size among plasma lipoproteins.
VLDL, very low-density lipoprotein; LDL, low-density lipoprotein; HDL, high-density lipoprotein
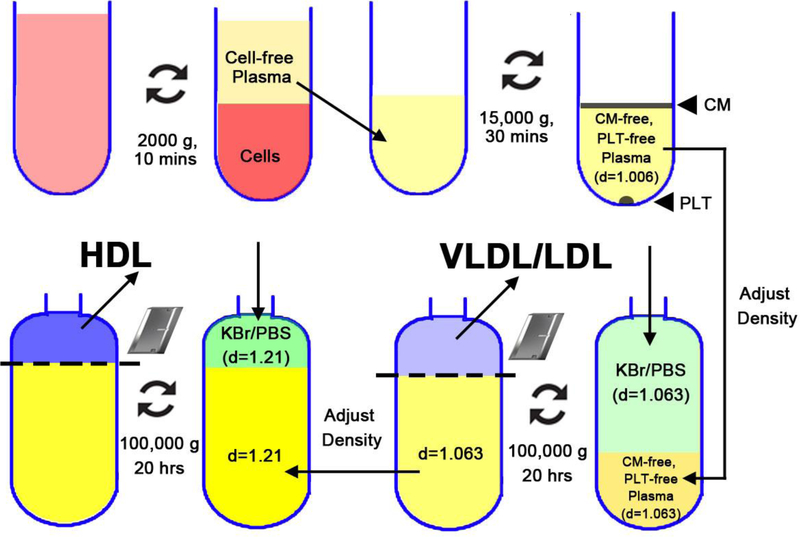
Figure 2. Experimental workflow of sequential density Ultracentrifugation (SD-UC) for lipoprotein fractionation.
(1) Collect and pool blood and centrifuge 2,000 x g for 10 min to pellet blood cells; (2) Recover cell-free plasma and centrifuge 15,000 x g for 30 min to pellet platelets (PLT) and float chylomicrons (CM); (3) Collect PLT-free, CM-free plasma and add KBr to adjust its density to 1.063 g/mL; transfer it into a ultracentrifugation tube and fill the tube with saline that has been adjusted to the same density; seal the tubes and centrifuge 100,000 x g for 20 hours to float the VLDL and LDL; (4) Cut the top of the tube to recover VLDL/LDL and add KBr to adjust the density of the remaining liquid from 1.063 g/mL to 1.21 g/mL; transfer it to a fresh ultracentrifugation tube and fill the tube with saline (density=1.21 g/mL); seal the tubes and centrifuge 100,000 x g for 18 hours to float the HDL fraction; (5) cut the top of the tube to collect HDL.
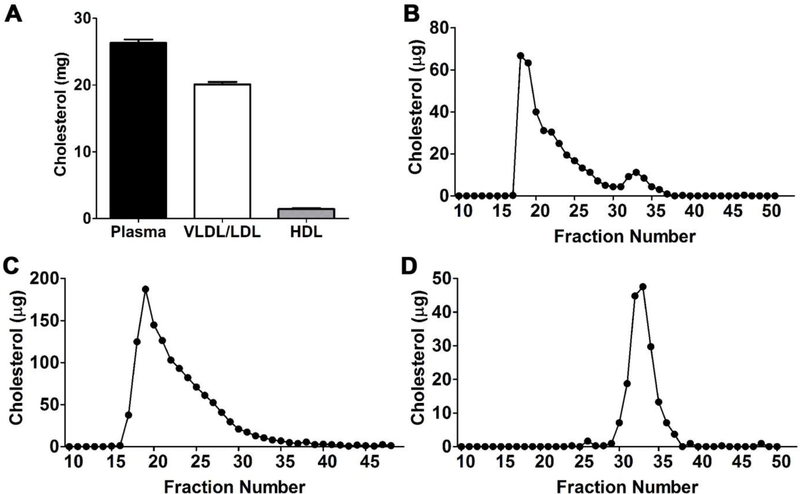
Figure 3. Cholesterol profiles of total plasma and plasma lipoproteins separated by SD-UC and FPLC.
VLDL/LDL and HDL were separated from plasma (6 mL) by sequential density ultracentrifugation. (A) The cholesterol content before and after isolation were determined; (B) Cholesterol profiles of total FPLC-fractionated plasma (200 μL), VLDL/LDL (100 μL) and HDL (75 μL).
Figure 4. Apolipoprotein distribution of total plasma and plasma lipoproteins separated by SD-UC and FPLC.
VLDL/LDL and HDL were separated from plasma (6 mL) by sequential density ultracentrifugation. (A) SDS-PAGE analysis of total plasma (3 μL), VLDL/LDL (10 μL) and HDL (10 μL). (B, C and D) SDS-PAGE analysis of FPLC fractions of 200 μL plasma (B), 100 μL VLDL/LDL (C) and 75 μL HDL (D). For each fraction, 90 μL sample was loaded. P: plasma; V/L: VLDL/LDL; H: HDL.
Figure 5. Lipoprotein RNA Characterization by Agilent Bioanalyzer.
Representative images of Agilent RNA 6000 Pico chip (A, B) and Small RNA chip (C, D) assays of total RNA isolated from plasma VLDL/LDL and HDL fractions isolated by sequential density ultracentrifugation.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health; 5U19CA179512 & 1U01HL126493 Extracellular RNA Communication Consortium Common Fund, and HL133575 to (RLR) which was administered by the Northern California Institute for Research and Education. The work was performed at the Veterans Affairs Medical Center, San Francisco, California. We thank Phat Duong and Allen Chung for excellent technical assistance with SDS-PAGE.
Footnotes
Based on our experience in isolating RNA from plasma lipoprotein subclasses for subsequent biochemical and sequencing analysis, we highly recommend using a minimum volume of 5 mL of plasma. Indeed, our preparations of lipoprotein classes obtained from such a volume of fresh or frozen plasma has led to successful unbiased RNA sequencing experiments (Dr. David Erle, UCSF, unpublished data). However, unpublished data from our group also suggests that freezing plasma could alter the abundance of microRNA species in select lipoprotein classes. Thus one should take caution when designing a study aimed at examining RNA species with archived frozen plasma samples.
A volume of 5 mL of plasma can be conveniently divided into two 3.5 mL centrifuge tubes to allow for rotor balancing during centrifugation in a tabletop ultracentrifuge. Alternatively, the entire 5 mL volume or even a larger volume of plasma can be loaded into large ultracentrifugation tubes (e.g. 39 mL Quick-Seal tubes) and a corresponding floor model ultracentrifuge / rotor system (e.g. Optima XPN 90/ 50.2 Ti). Although 5 mL of plasma can be readily obtained from a human blood donor, it will be more challenging to derive from mice. Therefore when planning to isolate RNA from mouse lipoproteins it is advised that a minimum of 10 to 15 mice be used for a terminal bleeding. Larger cohorts of mice should be used in situations when the mice are to be maintained for further study as only smaller volumes of blood can be obtained under such circumstances.
Because of their high catabolic rate we recommend to avoid the isolation of chylomicrons together with the more stable VLDL/LDL fraction. The very high levels of neutral lipid associated with chylomicrons, especially in non-fasted plasma specimens, could also pose unknown problems and even a bias in RNA extraction/recovery that could be caused by excessive amounts of neutral lipid and foreign RNA possibly derived from dietary sources (15). We have found that gently pipetting any white layer that forms at the top of the plasma that is spun at 13,000 x g for the removal of platelets, generally results in the elimination of the chylomicron fraction. However, ultracentrifugation of virgin plasma overnight at 100,000 x g will ensure the flotation of all chylomicrons to the top of the tube where they can be cut away from other lipoprotein subclasses and subsequently discarded or used as a source of RNA if so desired.
Potassium bromide salt (KBr) is highly hygroscopic and should be kept in a dry and preferably desiccant environment to prevent its hydration that would compromise its use to produce accurate density solutions. Because of this issue, it is recommended to make use of a densitometer to verify the density of the KBr-plasma samples as well as the KBr/PBS standard solutions.
The decision to cut the top 1/5 of the centrifuge tube after an overnight centrifugation of plasma is based on our experience in determining that this site is generally free of lipoproteins. The desired lipoproteins of lower density are known to float and accumulate in the top of the tube, while the lipoproteins of higher density sink to a lower portion of the tube.
Should there be a desire to separate VLDL from LDL, the density of the first centrifugation should be adjusted to d=1.019 g/mL that will allow VLDL to float and LDL will sediment along with HDL in the infranatant. Subsequently, LDL can be separated from HDL by adjusting the infranatant to a density of 1.063 g/mL. Table 1 presents the factors required to calculate the adjustments of densities using KBr.
We have observed that lipoproteins will float to the very top of the tubes. To avoid possible losses of lipoproteins that may collects on the inside of the cap wall, it is important to rinse the top of the cut centrifuge tube using a portion of the liquid fraction that has been aspirated from the tube with a syringe.
The use of 5–12% gradient gels is essential in order to resolve the full spectrum of proteins associated with lipoproteins. The molecular weight of apoB-100 and apoB-48, two important structural components of low density lipoproteins and chylomicron remnants, are approximately 550 kDa and 210 kDa respectively and will resolve in the upper 25% of the gradient gel. Conversely, the size of apoE and apoA1 and all other apolipoproteins is less than 50 kDa and will resolve in the lower 50% of the gradient gel as illustrated in Figure 4.
De-staining of a Coomassie blue gel can be assisted by introducing a few pieces of KimWipe paper that will absorb the blue dye released from the gel and will aid in clarifying the protein-negative portions of the gel.
5. References
- 1.Havel RJ, and Kane JP 2001. Introduction: Structure and metabolism of plasma lipoproteins In The Metabolic and Molecular Bases of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, and Vogelstein B, editors. New York: McGraw-Hill; 2705–2716. [Google Scholar]
- 2.Mahley RW, Huang Y, and Rall SC Jr. 1999. Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia): Questions, quandaries, and paradoxes. J. Lipid Res 40:1933–1949. [PubMed] [Google Scholar]
- 3.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, and Pittet MJ 2007. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, and Wang N 2008. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab 7:365–375. [DOI] [PubMed] [Google Scholar]
- 5.Murphy AJ, Westerterp M, Yvan-Charvet L, and Tall AR 2012. Anti-atherogenic mechanisms of high density lipoprotein: Effects on myeloid cells. Biochim Biophys Acta 1821:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, and Fogelman AM 2004. Antiinflammatory properties of HDL. Circ Res 95:764–772. [DOI] [PubMed] [Google Scholar]
- 7.Nofer J-R, van der Giet M, Tölle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Gödecke A, Ishii I, Kleuser B, et al. 2004. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Invest 113:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao R, Hoover HE, Honbo N, Kalinowski M, Alano CC, Karliner JS, and Raffai R 2010. High-density lipoprotein determines adult mouse cardiomyocyte fate after hypoxia-reoxygenation through lipoprotein-associated sphingosine 1-phosphate. Am J Physiol Heart Circ Physiol 298:H1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, and Remaley AT 2011. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabet F, Vickers KC, Cuesta Torres LF, Wiese CB, Shoucri BM, Lambert G, Catherinet C, Prado-Lourenco L, Levin MG, Thacker S, et al. 2014. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat Commun 5:3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havel RJ, Eder HA, and Bragdon JH 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest 34:1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudreault N, Kumar N, Posada JM, Stephens KB, Reyes de Mochel NS, Eberle D, Olivas VR, Kim RY, Harms MJ, Johnson S, et al. 2012. ApoE suppresses atherosclerosis by reducing lipid accumulation in circulating monocytes and the expression of inflammatory molecules on monocytes and vascular endothelium. Arterioscler Thromb Vasc Biol 32:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raffaï R, Maurice R, Weisgraber K, Innerarity T, Wang X, MacKenzie R, Hirama T, Watson D, Rassart E, and Milne R 1995. Molecular characterization of two monoclonal antibodies specific for the LDL receptor-binding site of human apolipoprotein E. J. Lipid Res 36:1905–1918. [PubMed] [Google Scholar]
- 14.Eberle D, Luk FS, Kim RY, Olivas VR, Kumar N, Posada JM, Li K, Gaudreault N, Rapp JH, and Raffai RL 2013. Inducible Apoe Gene Repair in Hypomorphic ApoE Mice Deficient in the Low-Density Lipoprotein Receptor Promotes Atheroma Stabilization with a Human-Like Lipoprotein Profile. Arterioscler Thromb Vasc Biol 33:1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baier SR, Nguyen C, Xie F, Wood JR, and Zempleni J 2014. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J Nutr 144:1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]