Abstract
Introduction:
We evaluated various morphological and molecular response criteria in metastatic castration-resistant prostate cancer (PCa) patient undergoing peptide receptor radioligand therapy (PRLT) with Lutetium177-prostate-specific membrane antigen (PSMA) by using Gallium 68-PSMA positron-emission tomography-computed tomography (Ga68-PSMA PET-CT).
Methods:
A total of 46 pre- and 8–12 weeks’ post-PRLT Ga68-PSMA PET-CT studies were reanalyzed (23 comparisons). Prostate-specific antigen drop of ≥50% and ≥25% increase was considered as partial response (PR) and progressive disease (PD), respectively, for biochemical response (BR) while change in-between was considered as stable disease (SD). Response evaluation criteria in solid tumors 1.1 (RECIST 1.1) and MD Anderson (MDA) criteria for morphological response while PET response criteria in solid tumors 1.0 (PERCIST 1.0) and European organization for research and treatment of cancer (EORTC) criteria for molecular response were used. Kappa coefficient was derived to see the level of agreement.
Results:
The proportion of PD, PR, and SD by BR and RECIST criteria was 9 (39.13%), 3 (13.04%), and 11 (47.83%) and 5 (21.74%), 2 (8.70%), and 16 (69.57%), respectively. The proportion of PD, PR, and SD was same by PERCIST and EORTC criteria and which were 8 (34.78%), 5 (21.74%), and 10 (43.48%). The proportion of PD, PR, and SD by MDA criteria was 1 (4.35%), 1 (4.35%), and 21 (91.30%), respectively. Poor agreement between BR and both morphological criteria while a statistically significant agreement with both molecular criteria seen.
Conclusion:
We concluded that molecular criteria performed better than morphological criteria in response assessment by Ga68-PSMA PET-CT in metastatic castration resistant PCa patients undergoing PRLT.
Keywords: European organization for research and treatment of cancer, gallium 68-prostate-specific membrane antigen positron-emission tomography-computed tomography, MDA, PET response criteria in solid tumors, response evaluation criteria in solid tumors 1.1, response assessment
INTRODUCTION
The proper evaluation of therapy response is pivotal in cancer treatment. Identifying nonresponders early help in avoiding unnecessary treatment and optimize their management.[1] Newer molecular-targeted therapies have opened a debate as to “which is the best criterion for treatment response?” Conventional response criteria might not depict the true picture due to different mechanisms of action of targeted therapies.[2] Although molecular response criteria have been proposed for this yet, these have not been adequately studied so far.[3,4] Peptide receptor radioligand therapy (PRLT) with Lutetium177-prostate-specific membrane antigen (Lu177-PSMA) is one of the examples of molecular therapies with promising results for end-stage metastatic castration resistant prostate cancer (mCRPC) patients.[5,6,7] In this article, we have compared various morphological and molecular response criteria in mCRPC patients treated with PRLT by Gallium 68-PSMA positron-emission tomography-computed tomography (Ga68-PSMA PET-CT).
METHODS
In our 190 Ga68-PSMA PET-CT studies referred for response evaluation under various treatments, 23 studies were done post-PRLT. Ga68-PSMA PET-CT was done within 2 weeks before PRLT and 8–12 weeks after PRLT. Standard protocol for in-house synthesis of Ga68-PSMA and PET-CT acquisition was used.[8,9] PSMA-11 was acquired from advanced biochemical compounds (ABx), and labeling was done in IQS fluidic labeling module (iTG) using 1.11 GBq iTG self-shielded Ga-68 generator. 2 MBq/kg body weight of labeled PSMA-11 was injected intravenously, and after 1 h, a full-body scan (vertex to mid-thigh) was acquired with a dedicated full-ring hybrid PET-CT system (Biograph TruePoint40 with LSO crystal from Siemens Healthcare at Rajiv Gandhi Cancer Institute and Research Centre, Delhi, India) with 4 min per bed position in three-dimensional mode. A noncontrast-enhanced CT scan (100 mAs and 120 kVp) was used for attenuation correction and anatomical interpretation. For PRLT, average 7.25 GBq (median 7.35, range 6.6–7.6) Lu177-PSMA-617 was injected with 1 L of normal saline infusion at 250 ml/h started 30 min before the injection of radiopharmaceutical.
Image interpretation
All PSMA PET-CT studies were reinterpreted independently by two nuclear medicine physicians and one radiologist without knowledge of biochemical and clinical findings. Increased PSMA uptake in comparison to background and not at the sites of known physiological biodistribution was taken as positive for disease. No size criteria were used for PET interpretation. Single-voxel maximum standard uptake value normalized to body weight (Maximum standardized uptake value [SUVmax]) was recorded. For CT, soft-tissue lesion other than the lymph node, ≥1 cm (cm) size in the longest axis was considered measurable, while for lymph node, it was ≥1 cm in the shortest axis. For better insight in bone lesions, the patients were categorized for number of lesions (1, 2–10, >10) and type of bone metastasis (only sclerotic, mixed, lytic, and marrow).
Response criteria
Pretreatment and 8–12 weeks’ post-PRLT prostate-specific antigen (PSA) were recorded for biochemical response (BR) analysis. A fall of ≥50% in PSA was considered as partial response (PR). A ≥25% increase and ≥2 ng/ml above the nadir was considered as progressive disease (PD).[10] Change in-between PR and PD (< −50% and < +25%) was considered as stable disease (SD).
Response evaluation criteria in solid tumors 1.1 (RECIST 1.1) were used for morphological response evaluation.[11] Target lesion was defined as ≥1-cm well-defined lesion for soft tissue in the longest axis while it was ≥1.5 cm in the shortest axis for lymph node. The largest sum of diameter (SoD) of five target lesions with maximum two lesions per organ was recorded. Sclerotic or lytic/sclerotic (mixed type) bone metastases were considered nonmeasurable (NM) lesions. ≥30% decrease in SoD was considered as PR, while ≥20% increase was considered as PD. Change in-between PR and PD (< −30% and < +20%) was considered as SD. In case of bone-only disease (NM disease), SD was considered for equivocal or no change.
PET response criteria in solid tumors 1.0 (PERCIST 1.0) were used with slight modification for PET response evaluation. Highest SUVmax was recorded for both PET studies irrespective of number of lesions. It might be two different lesions in comparison. A drop of ≥30% in highest SUVmax was considered as PR while a ≥30% increase was considered as PD. New PSMA avid lesion was also considered as PD. Change in-between PR and PD (< −30% and < +30%) was considered as SD. Disappearance of all the lesions with respect to adjacent background was considered as complete response (CR).
European organization for research and treatment of cancer (EORTC) criterion was also used for PET response evaluation. The highest SUVmax was recorded for both PET studies irrespective of number of lesions. It might be two different lesions in a comparison. A drop of ≥25% in the highest SUVmax was considered as PR while a ≥25% increase was considered as PD. New PSMA avid lesion was also considered as PD. Change in-between PR and PD (< −25% and < +25%) was considered as SD. Disappearance of all the lesions with respect to adjacent background was considered as CR.
Bone is the most common site of distant metastases in prostate cancer (PCa), and almost all patients with PCa develop bone metastasis during their disease course. Due to no clear method for the assessment of bone metastases in RECIST 1.1 except for lytic lesion with measurable soft-tissue component, we decided to analyze a criterion dedicated to bone metastases developed by The University of Texas MD Anderson Cancer Center (MDA criterion).[12] In this criterion, CR was considered as complete sclerotic fill-in of lytic lesion or normalization of bone density on CT. PR was the development of a sclerotic rim or partial sclerotic fill-in of lytic lesions. Interval visualization of lesions with sclerotic rim or new sclerotic lesions in the setting of other signs of response and absence of progressive bony disease was considered as flare and PR for the analysis. ≥50% decrease in measurable lesions or ≥50% subjective decrease in the size of ill-defined lesions on CT was also considered as PR. ≥25% increase in measurable lesions or ≥25% subjective increase in the size of ill-defined lesion or new bone lesion was considered as PD. Change in-between PR and PD (<−50% and < +25%) or equivocal change was considered as SD.
Statistical analysis
Mean ± standard deviation, medians, range (minimum to maximum), and interquartile range (IQR) were presented for quantitative data and absolute frequencies with percentage for categorical data. Pre- and post-PRLT changes in PSA, RECIST SoD, and SUVmax for individual data were tabulated. Estimated percentages of PD, PR, and SD based on the different criteria (BR, RECIST, PERCIST, EORTC, and MDA) were also tabulated. Cohen's Kappa coefficient (k) was derived to see the level of agreement between BR and other criteria. P < 0.05 was considered statistically significant. IBM New York, SPSS version 21 was used for the entire statistical analysis.
RESULTS
Baseline characteristics of the 23 studies were presented in Table 1. All patients had bone metastasis and mostly sclerotic type. No patient had pure lytic or marrow lesion. Liver metastasis was the most frequent site of visceral metastasis (4/23) and one patient also had PSMA avid pleural deposit. There was no patient with lymph node only metastasis in our study group.
Table 1.
Baseline characteristics of 23 studies
| Characteristics | Values |
|---|---|
| Age (years) | |
| Median | 75.5 |
| Range | 57-81 |
| Gleason | |
| Median | 7 |
| Range | 6-9 |
| Interval time between two PET-CT scans (months) | |
| Median | 2 |
| Range | 2-3 |
| Metastatic stage (M stage) (%) | |
| M1a (extrapelvic LN metastasis) | 0 |
| M1b (bone±LN metastasis) | 19 (82.61) |
| M1c (visceral±bone metastasis) | 4 (17.39) |
| Number of bone metastasis per patient | |
| 1 | 4 (17.39) |
| 2-10 | 0 |
| >10 | 19 (82.61) |
| Types of bone metastasis per patient | |
| Sclerotic | 21 (91.30) |
| Mixed | 2 (8.70) |
| Liver lesion | |
| Yes | 4 (17.39) |
| No | 19 (82.61) |
| Other visceral sites of metastasis | |
| Yes | 1 (4.35) |
| No | 22 (95.65) |
| Lymph node metastasis | |
| Yes | 15 (65.22) |
| No | 8 (34.78) |
PET-CT: Positron-emission tomography
Pre- and post-PRLT changes in PSA, RECIST SoD, and highest SUVmax for 23 studies are summarized in Table 2. Pre- and post-PRLT mean ± standard deviation, median, range (Min-Max), and IQR of PSA (ng/ml) were 113.87 ± 128.06, 66, 5.42–550, and 23.43–175.50 and 150.96 ± 166.25, 68, 5.42–552, and 25.35–217.55, respectively. Pre- and post-PRLT mean ± standard deviation, median, range (Min-Max), and IQR of RECIST SoD (cm) were 3.68 ± 2.49, 3.40, 1.60–12.00, and 2.00–4.20 and 4.66 ± 4.19, 3.50, 1.20–16.40, and 1.70–4.80, respectively. Pre- and post-PRLT mean ± standard deviation, median, range (Min-Max), and IQR of highest SUVmax were 35.69 ± 24.25, 28.70, 6.60–99.50, and 18.55–45.42 and 30.93 ± 19.57, 24.70, 12.90–92.60, and 17.75–35.35, respectively.
Table 2.
Pre- and post-peptide receptor radioligand therapy prostate-specific antigen, response evaluation criteria in solid tumors sum of diameter and maximum standard uptake values and responses by various criteria
| PSA | RECIST SoD | Highest SUVmax | Responses by various criteria | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-PRLT | Post-PRLT | Pre-PRLT | Post-PRLT | Pre-PRLT | Post-PRLT | BR | RECIST | PERCIST | EORTC | MDA |
| 37.1 | 445.0 | 4.7 | 10.0 | 6.6 | 19.4 | PD | PD | PD | PD | PD |
| 550.0 | 344.0 | 4.3 | 4.1 | 46.4 | 92.6 | SD | SD | PD | PD | SD |
| 344.0 | 194.0 | 4.1 | 7.5 | 92.6 | 25.7 | SD | PD | PD* | PD* | SD |
| 194.0 | 204.0 | 7.5 | 12.0 | 25.7 | 58.9 | SD | PD | PD | PD | SD |
| 204.0 | 552.0 | 12.0 | 16.4 | 58.9 | 43.8 | PD | PD | PD* | PD* | SD |
| 23.5 | 5.4 | 2.0 | 1.7 | 99.5 | 58.5 | PR | SD | PR | PR | SD |
| 5.4 | 8.9 | 1.7 | 1.7 | 58.5 | 58.7 | PD | SD | SD | SD | SD |
| 8.9 | 9.3 | 1.7 | 2.1 | 58.7 | 30.3 | SD | SD | PR | PR | SD |
| 9.3 | 222.1 | 2.1 | 4.8 | 30.3 | 13.7 | PD | PD | PD* | PD* | SD |
| 109.0 | 68.0 | 2.0 | 1.4 | 13.7 | 16.8 | SD | PR | SD | SD | SD |
| 179.0 | 45.6 | 1.8 | 1.6 | 38.0 | 23.2 | PR | SD | PR | PR | SD |
| 45.6 | 66.0 | 1.6 | 1.2 | 23.2 | 16.0 | PD | SD | PR | PR | SD |
| 66.0 | 165.0 | NM | NM | 16.0 | 18.2 | PD | SD | SD | SD | SD |
| 165.0 | 494.0 | NM | NM | 18.2 | 12.9 | PD | SD | SD | SD | SD |
| 119.0 | 70.0 | 4.3 | 4.0 | 29.7 | 24.7 | SD | SD | SD | SD | SD |
| 70.0 | 62.8 | 4.0 | 3.5 | 24.7 | 25.4 | SD | SD | SD | SD | SD |
| 62.8 | 42.7 | 3.5 | NM | 25.4 | 28.7 | SD | PR | SD | SD | SD |
| 42.7 | 31.2 | NT | NM | 28.7 | 22.7 | SD | SD | SD | SD | SD |
| 119.0 | 21.6 | 3.4 | 3.5 | 42.5 | 36.4 | PR | SD | SD | SD | SD |
| 21.6 | 23.0 | 3.5 | 3.5 | 36.4 | 17.6 | SD | SD | PR | PR | PR |
| 23.0 | 23.4 | 3.1 | 2.6 | 19.6 | 18.2 | SD | SD | SD | SD | SD |
| 23.4 | 95.2 | 2.6 | 2.3 | 18.2 | 32.2 | PD | SD | PD | PD | SD |
| 196.7 | 279.0 | NM | NM | 9.4 | 16.7 | PD | SD | PD | PD | SD |
PSA: Prostate-specific antigen, PRLT: Peptide receptor radioligand therapy, BR: Biochemical response, RECIST: Response evaluation criteria in solid tumors, PERCIST: Positron-emission tomography response evaluation criteria in solid tumors, EORTC: European organization for research and treatment of cancer, MDA: MD Anderson criteria, SoD: Sum of diameter, NM: Nonmeasurable, NT: Nontarget, PD: Progressive disease, *PD: Progressive disease due to new PSMA avid lesion despite decrease in highest SUVmax, PR: Progressive disease, SD: Stable disease, SUVmax: Maximum standard uptake value
The proportion of PD, PR, and SD by BR and RECIST criteria was 9 (39.13%), 3 (13.04%), 11 (47.83%) and 5 (21.74%), 2 (8.70%), 16 (69.57%), respectively. The proportion of PD, PR, and SD was same by PERCIST and EORTC criteria and which were 8 (34.78%), 5 (21.74%), and 10 (43.48%). The proportion of PD, PR, and SD by MDA criteria was 1 (4.35%), 1 (4.35%), and 21 (91.30%), respectively.
We calculated Cohen's Kappa coefficient to see the degree of agreement between BR criteria and other criteria for the proportion of PD, PR, and SD [Tables 3–6]. No patients in our group had CR by any criteria. There was poor agreement seen between BR and RECIST criteria, as well as with MDA criteria (P > 0.05). While a statistically significant (P < 0.05) and fair agreement was seen with BR and PERCIST criteria. Indeed, PERCIST and EORTC criteria showed a similar level of agreement with BR criteria.
Table 3.
Inter-rater agreement between biochemical response and response evaluation criteria in solid tumors criteria by deriving Cohen’s Kappa coefficient (k)
| Biochemical response | Total | P | κ | |||
|---|---|---|---|---|---|---|
| PD | PR | SD | ||||
| RECIST criteria | ||||||
| PD | 3 (13.04) | 0 (0.00) | 2 (8.70) | 5 (21.74) | 0.947 | 0.010 |
| PR | 0 (0.00) | 0 (0.00) | 2 (8.70) | 2 (8.70) | ||
| SD | 6 (26.09) | 3 (13.04) | 7 (30.43) | 16 (69.57) | ||
| Total | 9 (39.13) | 3 (13.04) | 11 (47.83) | 23 (100.00) | ||
RECIST: Response evaluation criteria in solid tumors, PD: Progressive disease, PR: Partial response, SD: Stable disease
Table 6.
Inter-rater agreement between biochemical response and MD Anderson criteria by deriving Cohen’s Kappa coefficient (k)
| Biochemical response | Total (%) | P | κ | |||
|---|---|---|---|---|---|---|
| PD (%) | PR (%) | SD (%) | ||||
| MDA criteria | ||||||
| PD | 1 (4.35) | 0 (0.00) | 0 (0.00) | 1 (4.35) | 0.698 | 0.035 |
| PR | 0 (0.00) | 0 (0.00) | 1 (4.35) | 1 (4.35) | ||
| SD | 8 (34.78) | 3 (13.04) | 10 (43.48) | 21 (91.30) | ||
| Total | 9 (39.13) | 3 (13.04) | 11 (47.83) | 23 (100.00) | ||
MDA: MD Anderson, PD: Progressive disease, PR: Partial response, SD: Stable disease
Table 4.
Inter-rater agreement between biochemical response and positron-emission tomography response criteria in solid tumors criteria by deriving Cohen’s Kappa coefficient (k)
| Biochemical response | Total (%) | P | κ | |||
|---|---|---|---|---|---|---|
| PD (%) | PR (%) | SD (%) | ||||
| PERCIST criteria | ||||||
| PD | 5 (21.74) | 0 (0.00) | 3 (13.04) | 8 (34.78) | 0.044 | 0.307 |
| PR | 1 (4.35) | 2 (8.70) | 2 (8.70) | 5 (21.74) | ||
| SD | 3 (13.04) | 1 (4.35) | 6 (26.09) | 10 (43.48) | ||
| Total | 9 (39.13) | 3 (13.04) | 11 (47.83) | 23 (100.00) | ||
PERCIST: Positron-emission tomography response criteria in solid tumors, PD: Progressive disease, PR: Partial response, SD: Stable disease
Table 5.
Inter-rater agreement between biochemical response and European organization for research and treatment of cancer criteria by deriving Cohen’s Kappa coefficient (k)
| Biochemical response | Total (%) | P | κ | |||
|---|---|---|---|---|---|---|
| PD (%) | PR (%) | SD (%) | ||||
| EORTC criteria | ||||||
| PD | 5 (21.74) | 0 (0.00) | 3 (13.04) | 8 (34.78) | 0.044 | 0.307 |
| PR | 1 (4.35) | 2 (8.70) | 2 (8.70) | 5 (21.74) | ||
| SD | 3 (13.04) | 1 (4.35) | 6 (26.09) | 10 (43.48) | ||
| Total | 9 (39.13) | 3 (13.04) | 11 (47.83) | 23 (100.00) | ||
EORTC: European organization for research and treatment of cancer, PD: Progressive disease, PR: Partial response, SD: Stable disease
DISCUSSION
PCa is the second common cancer in men and third most frequent cause of cancer-related death worldwide.[13] In general, the patients with early stage have good prognosis, while most with late-stage disease develop hormone and chemotherapy resistance during the course of their disease and will require new treatments.[14] Response assessment for newer therapies is crucial to avoid over treatment in nonresponder and therapy-related toxicity. Serum PSA is a tumor marker for PCa and is frequently used for response assessment in these setting. However, it fails to show the distribution of disease and posttreatment changes at disease sites.[15] Morphological criterion (RECIST 1.1) is recommended for the assessment of treatment response in guidelines despite its well-known limitations.[16] There are instances that disease sites appear stable by RECIST 1.1 criteria despite PSA response [Figure 1]. This is due to the fact that molecular changes appear before morphological changes and this has been one of the main limitations of these criteria.
Figure 1.
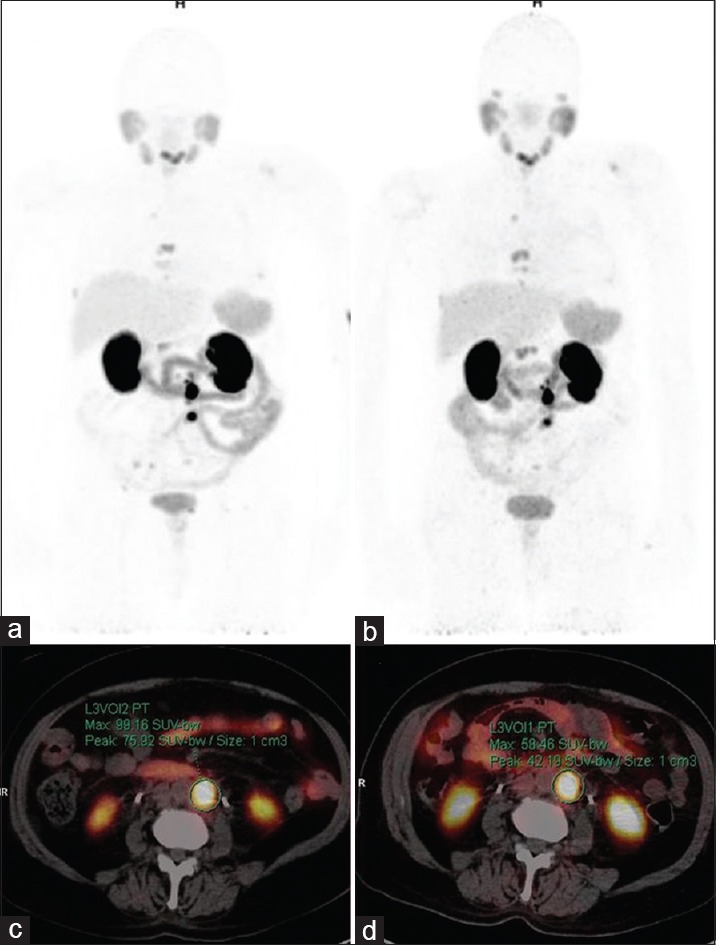
Gallium 68-prostate-specific membrane antigen positron-emission tomography-computed tomography maximum intensity projection (a and b) and axial images (c and d). 75-year-old metastatic castration-resistance prostate cancer patient post one cycles of Lutetium177-Prostate-specific membrane antigen showed prostate-specific antigen decrease from 23.5 to 5.4 ng/ml in 12 weeks. Baseline images (a and c) showed few prostate-specific membrane antigen avid lymph nodes and bone lesions (highest maximum standardized uptake value: 99.2). 12 weeks’ posttreatment images (b and d) showed few prostate-specific membrane antigen avid lymph nodes and bone lesions (highest maximum standardized uptake value: 58.5) with no change in size and number. Findings suggested stable disease by response evaluation criteria in solid tumors while partial response by positron-emission tomography response criteria in solid tumors and European organization for research and treatment of cancer
Bone metastasis contributes to major disease burden in PCa.[17] In most instances, bone metastases are sclerotic or mixed sclerotic/lytic while pure lytic lesions are rare.[18] Multiple bone metastases are a rule rather than the exception in end-stage PCa. In our group, all patients had bone metastasis, and 82.61% had multiple sites (>10) while 91.3% had sclerotic type. No patient had only lytic lesion. In RECIST criterion, no clear method for response assessment has been given for sclerotic or mixed lesions except for lytic lesion with measurable soft-tissue component. There might be instances that patients had bone-only disease and that would only be considered as NM by RECIST criteria [Figure 2]. In this study, 3/23 instances patient had NM disease and 1/23 instances patient had measurable but nontargetable lesion by RECIST. Hence, it will be hard to assess response by RECIST criteria in all end-stage PCa patients.
Figure 2.
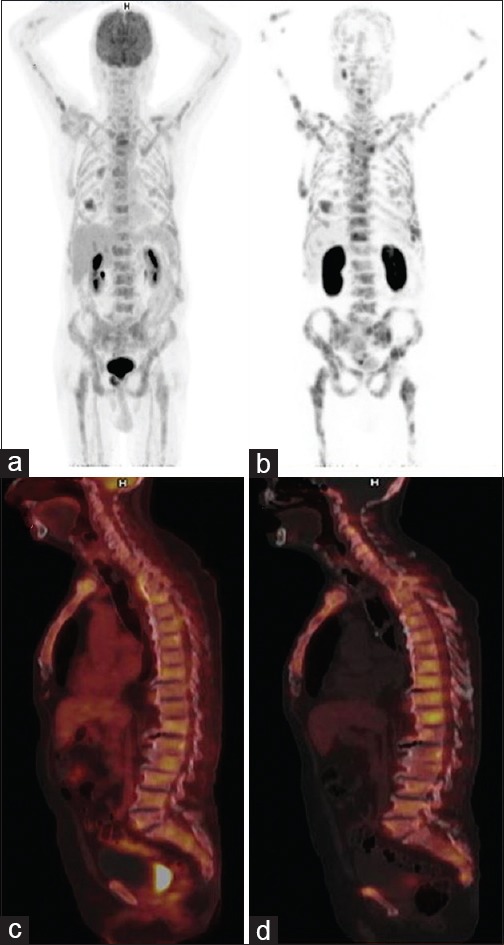
Gallium 68-prostate-specific membrane antigen positron-emission tomography-computed tomography maximum intensity projection (a and b) and sagittal (c and d) images. 81-year-old patient, post-Lutetium177-prostate-specific membrane antigen therapy showed prostate-specific antigen increase from 196.7 to 279.0 ng/ml. Baseline images (a and c) showed multiple sclerotic lesions with mild prostate-specific membrane antigen uptake in most (Highest Maximum standardized uptake value: 9.4). 12 weeks’ posttreatment images (b and d) showed multiple prostate-specific membrane antigen avid sclerotic bone lesions (Highest maximum standardized uptake value: 16.7). Findings suggested stable disease by response evaluation criteria in solid tumors and MDA while progressive disease by positron-emission tomography response criteria in solid tumors and European organization for research and treatment of cancer criteria
EORTC and PERCIST 1.0 are the two molecular response criteria developed to overcome the limitations of the morphological criteria. With growing research on fluorodeoxyglucose (FDG) PET-CT in cancer management, these molecular response criteria have shown promises in prognostication and for response assessment in comparison to standard morphological criteria.[19,20,21] One study comparing EORTC criteria to PERCIST for response evaluation by FDG PET/CT in metastatic colorectal cancer treated with irinotecan and cetuximab had showed similar response and good agreement (Kappa coefficient-0.76).[22] However, large randomized trials are lacking comparing these molecular criteria to morphological criteria. It is known that FDG PET-CT has shown poor results in PCa imaging due to its fatty acid metabolism favoritism.[23,24] Therefore, newer tracer targeting different metabolic pathways, for example, acetate, choline, anti-PSMA antibodies, PSMA inhibiting small molecules, etc., have been identified for PCa imaging.[25] PSMA is a transmembrane antigen overexpressed 100–1000 time in PCa cell as compared to normal and has shown positive correlation with grade and metastatic status.[26] Glu-NH-CO-NH-Lys-(Axe)-(Ga68 (HBED-CC]) (Ga68-PSMA-11) has shown high clinical value for lymph node staging[27] and for detecting local recurrence.[28,29] Limited data are available for its role in response evaluation to radiotherapy and chemotherapy in PCa.[30,31,32] Lu177-PSMA for PRLT is one of the promising investigational drugs for mCRPC. Recent studies have shown minimal toxicity and remarkable efficiency of PRLT in end-stage PCa patients.[5,6,7] However, there is a lack of literature comparing various morphological and molecular response criteria in this setting.
In our analysis, we have noticed that BR has poor agreement with RECIST while it was statistically significant and was in fair agreement with both molecular response criteria. In RECIST criteria, more patients showed SD (16/23) in post-PRLT scans. This was due to that 3/23 instances, lesions were NM and 1/23 instances, it was NT lesion and these remain stable in follow-up, while BR show PD in three out of four of these cases. In MDA criteria, SD contribution was further increased to 21/23. This was because most patients had multiple bone lesions (19/23) and mostly sclerotic type (21/23). Therefore, appreciating changes on CT images was poor as per the MDA criteria. On the other side, both molecular response criteria performed better, and the contribution of SD was almost similar to BR (10/23 vs. 11/23) in our study. In three instances, despite decrease in the highest SUVmax, there was new PSMA avid lesion, and hence, it was reported as PD as per molecular response criteria [Figure 3]. Indeed, BR criteria also showed PD in two of these cases. In 3/8 PD cases by molecular response criteria, BR criteria showed SD. In one case, it was due to new lesion despite reducing highest SUVmax. While in 3/9 PD cases by BR criteria, molecular response criteria showed SD. To understand these discrepancies, we realized, that we were analysing differentproperties of tumor cell using two different methods. In one, we were looking for changes in serum PSA, and in another, we were looking for changes in PSMA expression on tumor cell and these two properties might not go side by side. Hence, it will not be possible to have 100% concordances in these two criteria. The concordance between BR and RECIST criteria was seen in 10/23 cases, while between BR and PERCIST, it was in 13/23 cases.
Figure 3.
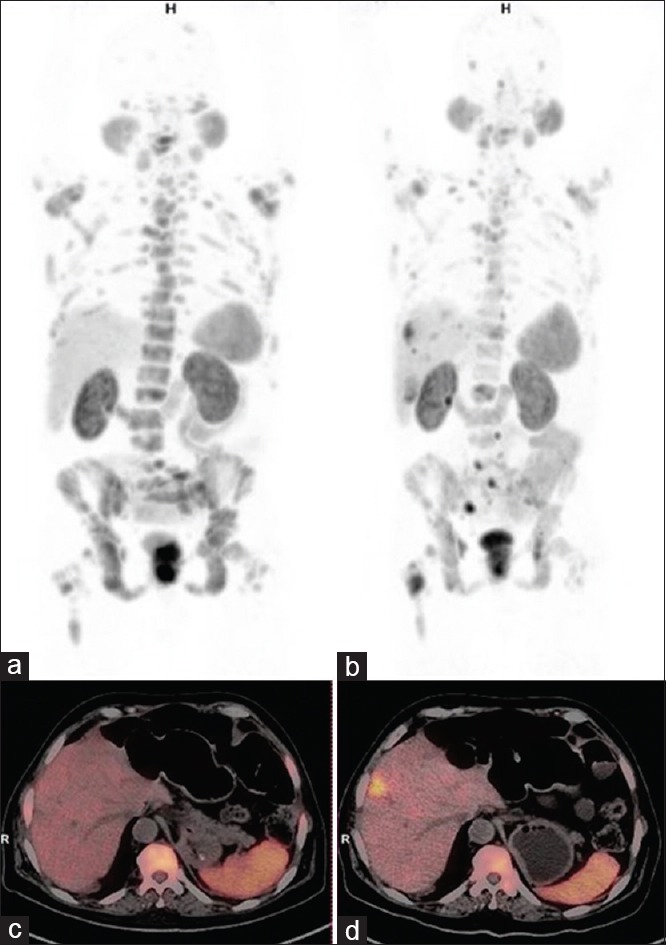
Gallium 68-prostate-specific membrane antigen positron-emission tomography-computed tomography maximum intensity projection (a and b) and axial images (c and d). 68-year-old metastatic castration-resistance prostate cancer patient post 1 cycles of Lutetium177-prostate-specific membrane antigen showed Prostate-specific antigen decrease from 344 to 194 ng/ml in 12 weeks. Baseline images (a and c) showed multiple prostate-specific membrane antigen avid sclerotic bone lesions (Highest maximum standardized uptake value: 92.6). 12 weeks’ posttreatment images (b and d) showed many prostate-specific membrane antigen avid bone lesions (Highest maximum standardized uptake value: 25.7) with new prostate-specific membrane antigen avid liver lesions. Findings suggested progressive disease by positron-emission tomography response criteria in solid tumors and European organization for research and treatment of cancer criteria due to new lesion despite decrease in highest maximum standardized uptake value
We found that there were few limitations of our study; first, the number of comparative studies was only 23. In PCa, bone is the predominant site of metastasis and hence morphological criteria tend to underestimate changes in the bone lesions due to known limitations. This was well reflected in our group of patients. In molecular response criteria, we have considered only one highest SUVmax per study for statistical analysis. There were instances where many lesions showed a decrease in SUVmax while one lesion showed increase in SUVmax and became more than the first study highest value. Hence, it was qualified as PD for response analysis. In other scenario, many lesions showed a decrease in SUVmax while some or one lesion showed increase in SUVmax but remained less than the first study highest value. Hence, it was qualified as PR or SD in overall response analysis. Therefore, we have realized that taking one highest value might not represent the real picture of molecular response in all sites. To overcome this confusion, in future, we have to take multiple lesions into the analysis. However, we have to find whether these target lesions with highest SUVmax will be based on the first PET study to see decrease in SUVmax or on second PET study to consider the smallest increase in SUVmax. A noncontrast CT for morphological delineation was not ideal; however, most of our patients were with lymph nodes and bone metastases.
CONCLUSION
Early detection of nonresponding disease will be helpful in deciding further treatment. Recommended morphological criterion has well-known limitations for response assessment, especially in bone-dominant disease. In this preliminary study, we conclude that both PERCIST and EORTC criteria are better than RECIST 1.1 in response assessment to PRLT in mCRPC patient using Ga68-PSMA PET-CT and in fair agreement with BR. Larger studies with more critical analysis of molecular response criteria are needed to recommend it in routine clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Curran SD, Muellner AU, Schwartz LH. Imaging response assessment in oncology. Cancer Imaging. 2006;6:S126–30. doi: 10.1102/1470-7330.2006.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnaghi C, Sclafani F, Basilico V, Doherty M. Response assessment in oncology: Limitations of anatomic response criteria in the era of tailored treatments. Q J Nucl Med Mol Imaging. 2011;55:589–602. [PubMed] [Google Scholar]
- 3.Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET study group. Eur J Cancer. 1999;35:1773–82. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 4.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–50S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. doi: 10.2967/jnumed.116.183194. [DOI] [PubMed] [Google Scholar]
- 6.Yadav MP, Ballal S, Tripathi M, Damle NA, Sahoo RK, Seth A, et al. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: Safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017;44:81–91. doi: 10.1007/s00259-016-3481-7. [DOI] [PubMed] [Google Scholar]
- 7.Bräuer A, Grubert LS, Roll W, Schrader AJ, Schäfers M, Bögemann M, et al. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1663–70. doi: 10.1007/s00259-017-3751-z. [DOI] [PubMed] [Google Scholar]
- 8.Amor-Coarasa A, Schoendorf M, Meckel M, Vallabhajosula S, Babich JW. Comprehensive quality control of the ITG 68Ge/68Ga generator and synthesis of 68Ga-DOTATOC and 68Ga-PSMA-HBED-CC for clinical imaging. J Nucl Med. 2016;57:1402–5. doi: 10.2967/jnumed.115.171249. [DOI] [PubMed] [Google Scholar]
- 9.Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1. 0. Eur J Nucl Med Mol Imaging. 2017;44:1014–24. doi: 10.1007/s00259-017-3670-z. [DOI] [PubMed] [Google Scholar]
- 10.Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–18. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1. 1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Costelloe CM, Chuang HH, Madewell JE, Ueno NT. Cancer response criteria and bone metastases: RECIST 1. 1, MDA and PERCIST. J Cancer. 2010;1:80–92. doi: 10.7150/jca.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends – An update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 14.Sridhar SS, Freedland SJ, Gleave ME, Higano C, Mulders P, Parker C, et al. Castration-resistant prostate cancer: From new pathophysiology to new treatment. Eur Urol. 2014;65:289–99. doi: 10.1016/j.eururo.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Emmenegger U, Ko YJ. PSA-based treatment response criteria in castration-resistant prostate cancer: Promises and limitations. Can Urol Assoc J. 2009;3:375–6. doi: 10.5489/cuaj.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino M, Jagannathan JP, Krajewski KM, O’Regan K, Hatabu H, Shapiro G, et al. Personalized tumor response assessment in the era of molecular medicine: Cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198:737–45. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin JK, Dayyani F, Gallick GE. Steps in prostate cancer progression that lead to bone metastasis. Int J Cancer. 2011;128:2545–61. doi: 10.1002/ijc.26024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta M, Choudhury PS, Goel HC, Rawal S, Talwar V. Is PSMA PET-CT better than bone scan? When and why. J Nucl Med Radiat Ther. 2017;8:342. [Google Scholar]
- 19.Sheikhbahaei S, Mena E, Yanamadala A, Reddy S, Solnes LB, Wachsmann J, et al. The value of FDG PET/CT in treatment response assessment, follow-up, and surveillance of lung cancer. AJR Am J Roentgenol. 2017;208:420–33. doi: 10.2214/AJR.16.16532. [DOI] [PubMed] [Google Scholar]
- 20.de Geus-Oei LF, Vriens D, van Laarhoven HW, van der Graaf WT, Oyen WJ. Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: A systematic review. J Nucl Med. 2009;50(Suppl 1):43S–54S. doi: 10.2967/jnumed.108.057224. [DOI] [PubMed] [Google Scholar]
- 21.Bailly C, Leforestier R, Campion L, Thebaud E, Moreau A, Kraeber-Bodere F, et al. Prognostic value of FDG-PET indices for the assessment of histological response to neoadjuvant chemotherapy and outcome in pediatric patients with Ewing sarcoma and osteosarcoma. PLoS One. 2017;12:e0183841. doi: 10.1371/journal.pone.0183841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skougaard K, Nielsen D, Jensen BV, Hendel HW. Comparison of EORTC criteria and PERCIST for PET/CT response evaluation of patients with metastatic colorectal cancer treated with irinotecan and cetuximab. J Nucl Med. 2013;54:1026–31. doi: 10.2967/jnumed.112.111757. [DOI] [PubMed] [Google Scholar]
- 23.von Mallek D, Backhaus B, Müller SC, Matthies A, Palmedo H, Jaeger U, et al. Technical limits of PET/CT with 18FDG in prostate cancer. Aktuelle Urol. 2006;37:218–21. doi: 10.1055/s-2006-932129. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9:230–4. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 25.Evans JD, Jethwa KR, Ost P, Williams S, Kwon ED, Lowe VJ, et al. Prostate cancer-specific PET radiotracers: A review on the clinical utility in recurrent disease. Pract Radiat Oncol. 2018;8:28–39. doi: 10.1016/j.prro.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5. [PubMed] [Google Scholar]
- 27.Gupta M, Choudhury PS, Hazarika D, Rawal S. A comparative study of 68Gallium-prostate specific membrane antigen positron emission tomography-computed tomography and magnetic resonance imaging for lymph node staging in high risk prostate cancer patients: An initial experience. World J Nucl Med. 2017;16:186–91. doi: 10.4103/1450-1147.207272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–74. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 29.Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: A systematic review and meta-analysis. Eur Urol. 2016;70:926–37. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Zschaeck S, Wust P, Beck M, Wlodarczyk W, Kaul D, Rogasch J, et al. Intermediate-term outcome after PSMA-PET guided high-dose radiotherapy of recurrent high-risk prostate cancer patients. Radiat Oncol. 2017;12:140. doi: 10.1186/s13014-017-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumann R, Koncz M, Luetzen U, Krause F, Dunst J. Oligometastases in prostate cancer: Metabolic response in follow-up PSMA-PET-CTs after hypofractionated IGRT. Strahlenther Onkol. 2018;194:318–24. doi: 10.1007/s00066-017-1239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seitz AK, Rauscher I, Haller B, Krönke M, Luther S, Heck MM, et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging. 2018;45:602–12. doi: 10.1007/s00259-017-3887-x. [DOI] [PubMed] [Google Scholar]


