Abstract
The aim of this observational cross-sectional study with retrospective review of the data is to evaluate the efficacy of using technetium-99m-octreotide (Tc-99m-OCT) in imaging neuroendocrine tumors (NETs) in our tertiary care hospital. A total of 58 patients had Tc-99m-OCT were identified in our database, from January 2013 to December 2016. Forty-one patients (age range of 15–75 years) meet our inclusion criteria, namely histopathology proven NETs, Tc-99m-OCT scan, computed tomography (CT), or magnetic resonance imaging (MRI) done in our institute for correlation. Twenty-three patients had true positive Tc-99m-OCT scan. In addition to the primary tumors, the octreotide scan revealed metastasis in the lung, liver, and retroperitoneal lymph nodes. The smallest lesion detected on octreotide scan was a 4-mm pulmonary nodule that was missed on lung window CT scan. The Tc-99m-OCT had 17 true negative, one false negative, and no false positive. The CT and MRI scans had 18 true positive, 17 true negative, 5 false negative, and one false positive. The overall sensitivity, specificity, accuracy, positive, and negative predictive values of Tc-99m-OCT scan were 96%, 100%, 97%, 100%, and 94%, respectively. Whereas those of CT and MRI were 78%, 94%, 85%, 94%, and 77%, respectively. Our diagnostic accuracy of Tc-99m-OCT is high. We recommend that, in addition to the conventional radiological investigations, Tc-99m-OCT scan, or other somatostatin receptor imaging (SSR) is a mandate for better and accurate staging of patients with NETs.
Keywords: Gallium-68, indium-111, neuroendocrine tumors, octreotide, somatostatin, technetium-99m
INTRODUCTION
Neuroendocrine tumors (NETs) are the uncommon type of tumors arising from the epithelial tissue of the neuroendocrine system.[1] They can be either functioning or nonfunctioning type depending on the presence or absence of hormone and peptide secretions from the tumors. There are about 40–50 cases per million habitants which accounts for 0.5% of all adult cancers.[1] The NET form 2% to 4% of the adults with cancer of unknown primary.[2,3] Identifying the primary tumor in patients with NETs is crucial in the management of those patients even in the advanced stages with metastasis. Studies showed that resecting the primary tumor improves the quality of life and the overall survival in patients with NET even with metastatic disease.[4,5]
As the NETs have increased the density of somatostatin receptors (SSTRs), it was possible to visualize those tumors with radiolabeled somatostatin analogs. The imaging of NETs and other tumors with positive SSTRs started more than two decades back using (Indium-111-DTPA-[D-Phe1]-Octreotide) (In-111-OCT).[6] However, because the somatostatin analog is labeled with Indium-111, this enforced some limitations such as limited availability, high cost, higher radiation burden to the patient, and the overall suboptimal image quality.[7] One and a half-decade back Decristoforo et al. described the development of a Tc-99m-labeled somatostatin analog (Tyr3-octreotide [TOC] and hydrazinonicotinic acid [HYNIC]).[8] In their study, they found that Tc-99m-TOC had high tumor to background ratio and optimal imaging time, which was done as 1-day protocol as appose to the 2 days’ protocols (4 and 24 h imaging) for In-111-OCT. With the advent of positron emission tomography/computed tomography (PET/CT), more positron emitting radiotracers were used to image the NETs, those include fluorine-18-fluoro-2-deoxy-D-glucose followed by 6-fluoro-(18F)-L-3,4-dihydroxyphenylalanine.[9] However, Ga-68-DOTA-peptide PET/CT was found to be the most useful in imaging NETs including visualization, initial staging, and detection of relapse.[10] It is also used to select patients who are likely to benefit from molecular radiotherapy, i.e., peptide receptor radionuclide therapy (PRRT), which consists of administration of radiotherapeutic Yttrium-90 (Y-90) or Lutetium-177 (Lu-177) versions of the same peptides used for imaging of inoperable NET.[10] However, the latter is not readily available, hence most of the patients with NETs were scanned and operated based on the findings on multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI) only.
MATERIALS AND METHODS
Patients
We introduce Technetium-99m instead of Indium-111 labeled octreotide in the last quarter of 2012. In our database, there were 58 patients who had technetium-99m-octreotide (Tc-99m-OCT) scans done between January 2013 and December 2016. Forty-one patients meet our inclusion criteria; that were histopathology proven somatostatin positive tumors, biochemical evidence of somatostatin tumors, Technetium-99m labeled octreotide scan, CT, and MRI. They were 21 males and 20 females with age range of 15–75 years (mean age of 42.7 years) [Table 1]. The findings on the Tc-99m-OCT scans were correlated lesion by lesion to the available MDCT and MRI scans.
Table 1.
Demographic details of the patients with the histopathology of the lesions postsurgery
| n | Sex | Age | Histopathology |
|---|---|---|---|
| 1 | Male | 72 | Colon NET |
| 2 | Male | 36 | Poorly differentiated NET of duodenum |
| 3 | Male | 26 | Pancreatic insulinoma |
| 4 | Female | 28 | Paraganglioneuroma |
| 5 | Female | 31 | Medullary thyroid cancer |
| 6 | Male | 39 | Metastatic malignant NET with no known primary |
| 7 | Male | 30 | Pancreatic Insulinoma |
| 8 | Female | 28 | Paraganglioma |
| 9 | Female | 33 | Medullary thyroid cancer |
| 10 | Male | 41 | MEN1 with pancreatic NET |
| 11 | Female | 26 | MEN 1 with pancreatic islet cell tumors |
| 12 | Female | 63 | Endobronchial NET |
| 13 | Male | 41 | Gastric gastrinomas and pancreatic islet cell tumor |
| 14 | Male | 66 | Poorly differentiated gastric NET |
| 15 | Female | 47 | MEN 1 with multiple NETs in stomach, duodenum and pancreas |
| 16 | Male | 53 | NET of the lung |
| 17 | Male | 41 | Pheochromocytoma of pancreas |
| 18 | Female | 29 | Metastatic NET with no known primary |
| 19 | Female | 53 | Gastric NET |
| 20 | Female | 72 | Carcinoid tumor of ileum |
| 21 | Female | 58 | Carcinoid tumor of colon |
| 22 | Male | 33 | Endobronchial carcinoid tumor |
| 23 | Male | 60 | Metastatic NET |
| 24 | Male | 39 | Pancreatic NET |
| 25 | Male | 41 | Pancreatic insulinoma |
| 26 | Female | 26 | Paraganglioma |
| 27 | Male | 43 | Appendix NET |
| 28 | Female | 17 | Appendix carcinoid |
| 29 | Male | 31 | Appendix carcinoid |
| 30 | Female | 15 | Appendix NET |
| 31 | Female | 53 | Gastric carcinoid |
| 32 | Male | 59 | Mesenteric carcinoid |
| 33 | Female | 58 | Small bowel NET |
| 34 | Male | 12 | Left lobe bronchus carcinoid |
| 35 | Female | 72 | Small bowel NET |
| 36 | Male | 41 | Pancreatic NET |
| 37 | Female | 60 | Gastric NET |
| 38 | Male | 68 | Colon NET |
| 39 | Female | 17 | Lung carcinoid |
| 40 | Male | 43 | Appendix NET |
| 41 | Female | 25 | Appendix carcinoid |
NET: Neuroendocrine tumor; MEN: Multiple endocrine neoplasia
Patients preparations
The patient should be off somatostatin receptor blocking agents for 72 h before the study. Occasionally, patients will be studied on somatostatin receptor blocking agents to assess the degree of suppression of uptake in a known lesion. In addition, the patients are advised to be on a liquid diet for 2 days before the scan with a mild laxative to be taken the evening before. On the day of the procedure, the patient should fast until the end of the first scan but should be well hydrated. For breastfeeding mothers, they need to stop breastfeeding 12 h post-Tc-99m-OCT injection.[11]
Preparation technetium-99m-labeled octreotide
The Tc-99m-OCT is prepared as per the instructions attached to the product kit. The octreotide kit consists of two vials; vial no - 1 contains 20 μg of HYNIC-Tyr-Octreotide TFA and vial no - 2 contains 10 μg (EDDA) as a stabilizing reagent. The preparation starts by placing vial no - 1 in a suitable lead shielded container. This is followed by dissolving the content of vial no - 2 (EDDA) in 1 ml of saline and shaking it gently. Following that 0.5 ml from vial no - 2 (EDDA) is injected to vial no - 1 HYNIC-Tyr-(Octreotide). This step is followed by gentle shaking to dissolve the content of vial no - 1. 1ml of Tc-99m-sodium pertechnetate from the elution, which should be 2200 MBq (59.45 mCi), is injected to vial no - 1. The reconstituted vial no - 1 HYNIC-Tyr-(Octreotide) is then placed on the dry heater block which should is set at 80°. The vial should be boiled for 20 min using a breathing needle. The reconstituted vial is left to cool down at room temperature for 30 min. The radiopharmaceutical Tc-99m-HYNIC-Tyr-(Octreotide) should be used within 6 h.
Technetium-99m-octreotide scan
The dose used for adult patients is 740 MBq (20 mCi) Tc-99m-octreotide and the whole body, and static images are done at 2 and 4 h postradiotracer injection. The whole-body scan is performed using dual-head gamma camera, using image matrix of 256 × 1024 with speed of 8 cm/min and 5 min for each static image. The SPECT images are performed for the region of interest, as requested by the nuclear medicine physician, using 128 × 128 matrix, 1.45 zoom and 30 s per view for 32 frames.
This study was approved by the Ethics and Research Committee in our institution (MESRC-11/2015).
RESULTS
Out of the 41 patients who were included in this study, 23 showed single or multiple Tc-99m-OCT avid lesions. Most of our patients had either NET; some were known or with a strong family history of multiple neuroendocrine neoplasms (MEN) subtype I or II. Others had specific type of NET such as carcinoid tumors of bowel or bronchus, medullary thyroid cancer, islet cell tumors, and pheochromocytoma [Table 1].
The Tc-99m-OCT showed true positive results in 23 patients, whereas the CT and MRI showed true positive results in 18 patients. The Tc-99m-OCT showed one false-negative result in a patient with incidental finding of the left retroperitoneal mass, the histopathology of which was a well-differentiated paraganglioma. The CT and MRI, on the other hand, showed false-positive result in one patient with carcinoid tumor of the appendix, and few mesenteric lymph nodes and the patient was upstaged metastatic disease. The Tc-99m-OCT scan was performed to confirm the CT findings, but it showed no uptake in the mesenteric lymph nodes, on 6 months’ follow-up CT scan, the mesenteric lymph nodes resolved and the patient remained disease-free after 2 years of follow-up. Furthermore, the CT and MRI showed false-negative results in five patients, in which the TC-99m-OCT scan detected metastasis in the bones, lung and retroperitoneal lymph nodes, resulting in upstaging of those patients [Table 2].
Table 2.
Comparison between the technutium-99m-octreotide scan, computed tomography and magnetic resonance imaging scan results
| TP | TN | FP | FN | |
|---|---|---|---|---|
| OCT scan | 23 | 17 | - | 1 |
| CT and MRI | 18 | 17 | 1 | 5 |
OCT: Octeriotide; CT: Computed tomography; MRI: Magnetic imaging resonance; TP: True positive; TN: True negative, FP: False positive, FN: False negative
The octreotide scan SN, SP, PPV, and NPV were 96%, 100%, 100%, and 94%, respectively. On the other hand, the SN, SP, PPV, and NPV of the CT and MRI were 78%, 94%, 94%, and 77%, respectively. The Tc-99m-OCT showed significantly higher sensitivity and negative predictive value compared to those of CT and MRI. However, the specificity and positive predictive values of CT and MRI were not significantly lower than those of Tc-99m-OCT scan.
One of the patients in this cohort was a 47-year-old female who is known to have multiple endocrine neoplasia type 1 (MEN 1). She presented with severe diarrhea, and she was investigated for NET. The postcontract CT scan of the abdomen and pelvis revealed no abnormality apart for a small, 7 mm, benign-looking preduodenal lymph node. She then had Tc-99m-octreotide scan which revealed focal uptake in the preduodenal lymph node and intense uptake in the proximal small bowel [Figure 1]. For further confirmation, the patient was sent overseas for Gallium-68-DOTA scan which revealed intense, abnormal uptake in the stomach, duodenum, pancreas, and preduodenal lymph node. The patient underwent Whipple's procedure, and the histopathology report came as multiple gastrinomas in the stomach, duodenum, pancreas, and one preduodenal metastatic lymph node. In a second patient, who was a 72-year-old male with known NET of the colon and a single metastasis to the liver, the postcontrast CT scan of chest and abdomen showed the known metastatic lesion in the liver with a small, 9 mm, mesenteric lymph node of query significance. The Tc-99m-octreotide scan showed octreotide avid lesion in the liver, focal uptake in the mesenteric lymph node and a small, 4 mm, octreotide avid lung nodule that was overlooked in the CT scan as it was solitary and small, denoting additional metastatic foci and changing the surgical management in this patient [Figure 2]. Another patient who was 29-year-old female known as MEN1 and the postcontrast CT scan showed pancreatic lesion. The octreotide scan upstaged this patient by showing additional foci of octreotide avid lesions in the right breast and left distal femur in addition to the known lesion in the pancreas. Additional examples of octreotide scan upstaging the patients were in a 33-year-old female, known to have medullary thyroid cancer. Postcontrast CT chest that was done as part of the disease surveillance showed left-sided cervical lymph nodes only. However, the octreotide scan showed the uptake in the cervical lymph nodes in addition to focal uptake in the left clavicle. Reviewing the CT images revealed that there was a destructive lesion involving the distal aspect of the right clavicle, as this was at the tip of the field of view, it was overlooked on the CT scan.
Figure 1.
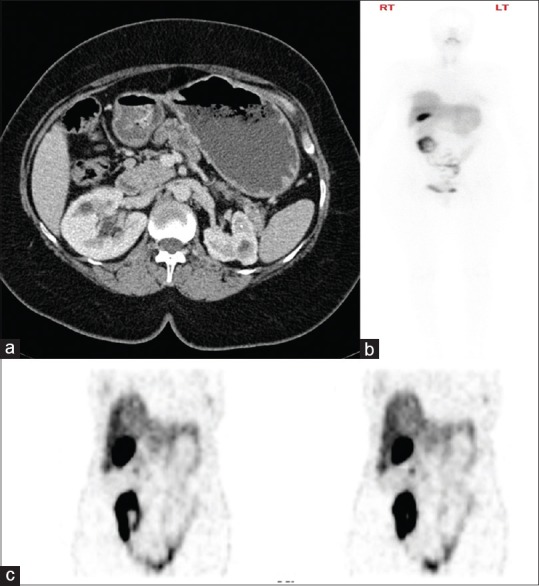
Tc-99m-OCT upstaged this patient with false-negative contrast-enhanced CT scan. Axial postcontrast CT scan image in (a) only found a 7 mm preduodenal lymph node, reported as benign looking (white arrow). The whole body of Tc-99m-OCT in (b) showed abnormal uptake in the small bowel (black arrow). Selected coronal images of the Tc-99m-OCT in (c) showed abnormal radiotracer uptake in the preduodenal lymph node (white arrow) and the proximal small bowel (black arrow). CT: Computed tomography; Tc-99m-OCT: Technetium-99m-octreotide
Figure 2.
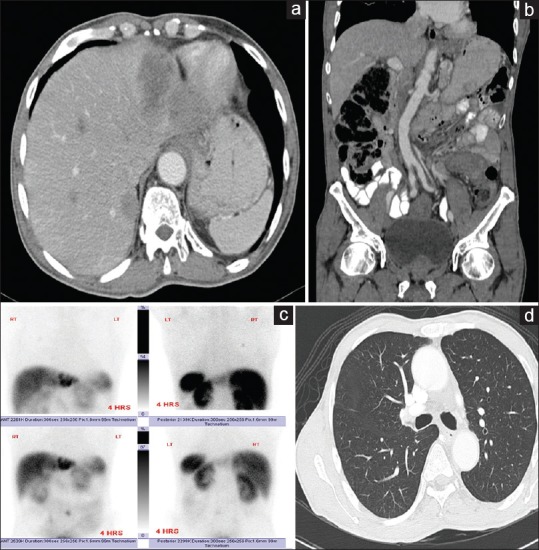
Tc-99m-OCT detected additional pulmonary nodule that was missed on CT scan. Axial CT scan image in (a) showed the metastatic lesion in the left liver lobe (white arrow). Coronal CT image in (b) showed the left retroperitoneal lymph node (white arrow). Static images of the Tc-99m-OCT in (c) showed the liver lesion (black arrow), the retroperitoneal lymph node (black arrowhead) and additional 4 mm pulmonary nodule that were missed on CT scan (white arrowhead). Axial lung window CT image in (d) showed the single pulmonary nodule (white arrow). CT: Computed tomography; Tc-99m-OCT: Technutium-99m-octeriotide
DISCUSSION
Somatostatin is a peptide hormone made of 14 amino acids; it is normally found in the cerebral cortex, hypothalamus, brain steam, gastrointestinal tract, and the pancreas.[6] To date, there are five known subtypes of somatostatin receptors (SSTR) that are expressed in various levels in NETs, SSTR 2 and 5 are the most common with their frequency of expression in the NET ranging from 70% to 90%.[12] Octreotide is the first somatostatin known analog; it is SST subtype 2 receptor agonists.[13,14] Somatostatin receptor (SSR) imaging is used for; gastrointestinal and brain tissues evaluation, NETs, lung cancer, meningioma, and lymphoma. In addition, it is used before PRRT.
For decades radiolabeled somatostatin analog, (DTPA-[D-Phe1]-Octreotide) – Indium-111 (In-111) was used in imaging NET.[6] In our institution, indium-111-octreotide scans were not performed for years mainly due to the high cost and availability. Patients with NETs were staged and operated-on based on the findings on CT and MRI only. In 2012, we started using Tc-99m instead of In-111 for the octreotide scan, and this solved the issues of availability as the octreotide is readily available as a cold kit in our department. It is also much cheaper than buying the In-111-labeled octreotide; and the overall image quality with Tc-99m is much better than those with In-111. In both Tc-99m and In-111-labeled octreotide, the spleen is the highest exposed organ (the critical organ). The estimated absorbed dose of the spleen with Tc-99m-OCT in mGy/MBq is 0.030[15] and that of In-111-OCT is 0.665.[16] The risk-weighted equivalent dose to the whole body or “effective dose” is measured in (mSv/MBq). The effective dose of Tc-99m-OCT is much less than that of In-111-OCT, 0.0056[15] and 0.117,[16] respectively [Table 3]. In addition, gamma Exposure rate at 1 cm from 1 mCi of Tc-99m is 720 mR/hr and that of In-111 is 3200 mR/h, i.e., the exposure of the workers form the In-111 is 4.4 times that of Tc-99m.
Table 3.
The adult estimated absorbed dose in mGy/MBq and the effective dose in mSv/MBq of Technetium-99m-Octreotide and Indium-111-Octreotide
| Organ | In-111-OCT (absorbed dose) |
Tc-99m-OCT (absorbed dose) |
|---|---|---|
| Spleen | 0.665 mGy/MBq | 0.030 mGy/MBq |
| Kidney | 0.488 mGy/MBq | 0.021 mGy/MBq |
| Liver | 0.109 mGy/MBq | 0.012 mGy/MBq |
| Urinary Bladder | 0.272 mGy/MBq | 0.014 mGy/MBq |
| Thyroid | 0.067 mGy/MBq | 0.004 mGy/MBq |
| Effective dose (mSv/MBq) | 0.117 | 0.0056 |
When comparing lesion by lesion detected by Tc-99m-OCT to those detected by MDCT and MRI, the octreotide study could detect all lesion except one, which was a well-differentiated paraganglioma. The sensitivity of octreotide in detecting NET was higher than that of MDCT and MRI (96% vs. 78%). The octreotide showed more lesions than MDCT and MRI which upstaged the patients and changed the management.
Reviewing the literature for Tc-99m-OCT, there were very few papers available, and the number of patients in this paper might be the largest mentioned in the literature so far. In the last decade, many centers shifted from conventional scintigraphy imaging for NET using In-111/Tc-99m labeled octreotide to PET/CT, particularly after the advent of Ga-68-DOTA peptides. Studies have shown the superiority of Ga-68-DOTA peptide PET/CT imaging over conventional SRS.[17] In a study that included 109 patients with gastroenteropancreatic NETs, Ga-68-DOTA-NOC PET/CT showed sensitivity and specificity of 78.3% and 92.5%, respectively, for primary tumor and 97.4% and 100% for metastases. It was better than a conventional imaging modality for the detection of both primary tumor (P < 0.001) and metastases (P < 0.0001). It changed the management strategy in 21 patients (19%) and supported management decisions in 32 patients (29%).[18]
Reasons for the preference of using Ga-68-DOTA include easy and economical synthesis of peptides which does not require a cyclotron, unlike In-111. Another important factor is the less imaging time (2 h for Ga-68-DOTA peptides, instead of the 4 h plus 24 h acquisition for In-111). When compared to the Tc-99m-OCT, both are imaged within 2 h, however, PET has higher spatial resolution compared to the single photon emission computed tomography (SPECT) (3-6 mm versus 10-15 mm). Although in our study, the smallest lesion detected was 4 mm lung nodule, the Tc-99m-OCT missed multiple small lesions in the stomach and pancreas that were detected by Ga-68-DOTA PET/CT scan. In addition, DOTA-peptides have higher and broader affinity for SSTRs compared to octreotide. Finally, tracer uptake quantification in the region of interest can be achieved using PET.[17]
CONCLUSION
In staging and follow-up patients with NETs, somatostatin receptor imaging (SSRT) is a mandate for better and accurate staging as well assessing the avidity of the primary tumor for future follow-up. In this study, we showed that the sensitivity and negative predictive values of Tc-99m-OCT scan is significantly higher than that of CT and MRI.
Using Tc-99m instead of In-111 had several advantages that include better availability, cheaper and higher quality images. This is in addition to less radiation exposure to both patients and nuclear medicine personnel. The introduction of PET imaging with 68Ga and its labeling with DOTA compounds has cleared the way for somatostatin receptor imaging with a viable PET agent, with all the advantages compared to single photon imaging. This has revolutionized the current and future clinical practice for NET.[19] The main limitation of the study is a retrospective data collection, and hence, all the patients included were known to have NETs, in addition, to the small sample size.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Plöckinger U, Rindi G, Arnold R, Eriksson B, Krenning EP, de Herder WW, et al. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS) Neuroendocrinology. 2004;80:394–424. doi: 10.1159/000085237. [DOI] [PubMed] [Google Scholar]
- 2.Kwekkeboom DJ, Krenning EP, Lebtahi R, Komminoth P, Kos-Kudła B, de Herder WW, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology. 2009;90:220–6. doi: 10.1159/000225951. [DOI] [PubMed] [Google Scholar]
- 3.Oberg K, Castellano D. Current knowledge on diagnosis and staging of neuroendocrine tumors. Cancer Metastasis Rev. 2011;30(Suppl 1):3–7. doi: 10.1007/s10555-011-9292-1. [DOI] [PubMed] [Google Scholar]
- 4.Rothenstein J, Cleary SP, Pond GR, Dale D, Gallinger S, Moore MJ, et al. Neuroendocrine tumors of the gastrointestinal tract: A decade of experience at the princess margaret hospital. Am J Clin Oncol. 2008;31:64–70. doi: 10.1097/COC.0b013e31807a2f49. [DOI] [PubMed] [Google Scholar]
- 5.Abbruzzese JL, Abbruzzese MC, Lenzi R, Hess KR, Raber MN. Analysis of a diagnostic strategy for patients with suspected tumors of unknown origin. J Clin Oncol. 1995;13:2094–103. doi: 10.1200/JCO.1995.13.8.2094. [DOI] [PubMed] [Google Scholar]
- 6.Sandler MP, Coleman RE, Patton JA, Th Wackers FJ, Gottschalk A. 4th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2003. Diagnostic Nuclear Medicine; p. 734. [Google Scholar]
- 7.Faintuch BL, Pereira NP, Faintuch S, Muramoto E, Silva CP. Lanreotide and octreotide complexed with technetium-99m: Labelling, stability and biodistribution studies. Braz J Pharm Sci. 2004;40:101–8. [Google Scholar]
- 8.Decristoforo C, Melendez-Alafort L, Sosabowski JK, Mather SJ. 99mTc-HYNIC-[Tyr3]-octreotide for imaging somatostatin-receptor-positive tumors: Preclinical evaluation and comparison with 111In-octreotide. J Nucl Med. 2000;41:1114–9. [PubMed] [Google Scholar]
- 9.Imperiale A, Rust E, Gabriel S, Detour J, Goichot B, Duclos B, et al. 18F-fluorodihydroxyphenylalanine PET/CT in patients with neuroendocrine tumors of unknown origin: Relation to tumor origin and differentiation. J Nucl Med. 2014;55:367–72. doi: 10.2967/jnumed.113.126896. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee SR, Pomper MG. Clinical applications of gallium-68. Appl Radiat Isot. 2013;76:2–13. doi: 10.1016/j.apradiso.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stabin MG, Breitz HB. Breast milk excretion of radiopharmaceuticals: Mechanisms, findings, and radiation dosimetry. J Nucl Med. 2000;41:863–73. [PubMed] [Google Scholar]
- 12.Papotti M, Bongiovanni M, Volante M, Allìa E, Landolfi S, Helboe L, et al. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002;440:461–75. doi: 10.1007/s00428-002-0609-x. [DOI] [PubMed] [Google Scholar]
- 13.Schmid HA, Schoeffter P. Functional activity of the multiligand analog SOM230 at human recombinant somatostatin receptor subtypes supports its usefulness in neuroendocrine tumors. Neuroendocrinology. 2004;80(Suppl 1):47–50. doi: 10.1159/000080741. [DOI] [PubMed] [Google Scholar]
- 14.Schmid HA. Pasireotide (SOM230): Development, mechanism of action and potential applications. Mol Cell Endocrinol. 2008;286:69–74. doi: 10.1016/j.mce.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Grimes J, Celler A, Birkenfeld B, Shcherbinin S, Listewnik MH, Piwowarska-Bilska H, et al. Patient-specific radiation dosimetry of 99mTc-HYNIC-tyr3-octreotide in neuroendocrine tumors. J Nucl Med. 2011;52:1474–81. doi: 10.2967/jnumed.111.088203. [DOI] [PubMed] [Google Scholar]
- 16.Krenning EP, Bakker WH, Kooij PP, Breeman WA, Oei HY, de Jong M, et al. Somatostatin receptor scintigraphy with indium-111-DTPA-D-phe-1-octreotide in man: Metabolism, dosimetry and comparison with iodine-123-tyr-3-octreotide. J Nucl Med. 1992;33:652–8. [PubMed] [Google Scholar]
- 17.Sharma P, Singh H, Bal C, Kumar R. PET/CT imaging of neuroendocrine tumors with (68)Gallium-labeled somatostatin analogues: An overview and single institutional experience from India. Indian J Nucl Med. 2014;29:2–12. doi: 10.4103/0972-3919.125760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naswa N, Sharma P, Kumar A, Nazar AH, Kumar R, Chumber S, et al. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: A prospective single-center study. AJR Am J Roentgenol. 2011;197:1221–8. doi: 10.2214/AJR.11.7298. [DOI] [PubMed] [Google Scholar]
- 19.Al-Nahhas A, Win Z, Szyszko T, Singh A, Nanni C, Fanti S, et al. Gallium-68 PET: A new frontier in receptor cancer imaging. Anticancer Res. 2007;27:4087–94. [PubMed] [Google Scholar]


