Abstract
Objective
Total parotidectomy yields a large surgical defect that leads to both cosmetic and functional deficits which can be addressed with reconstruction. We evaluated the role of a pedicled submental flap for this reconstruction.
Methods
We reviewed all submental flap reconstructions that were performed for total parotidectomy defects between 2014 and 2016. Data regarding harvest technique, postoperative complications, flap survival, and adjuvant treatment details were recorded. Subjective information regarding retained volume after reconstruction was also obtained.
Results
During the time period, eight patients were identified and in all cases the patients underwent total parotidectomy with facial nerve sacrifice. All patients were discharged within 2 days of hospitalization with no complications or concerns regarding the viability of the flap. All but one patient had radiation therapy. Results with 9.9‐ to 37.5‐month follow‐up (mean 22.0 months) show limited volume loss without major contour defect or ear deformity in the follow‐up period.
Conclusions
Submental flap reconstruction is a feasible and reliable method for total parotidectomy defect. The inclusion of the mylohyoid muscle aids flap reliability and adds bulk. Inclusion of the dermis helps contour the overlying skin. The flap does not add morbidity or increased complexity intraoperatively or postoperatively.
Level of Evidence
4
Keywords: Submental flap, total parotidectomy, reconstruction, pedicled flap, volume restoration
INTRODUCTION
Large‐volume parotid tumors, both benign and malignant, can require a total parotidectomy with or without facial nerve preservation. Such procedures lead to a large soft tissue defect that has both short‐ and long‐term consequences. In the short term there is a large contour defect that is visually noticeable and distracting. Over time this soft tissue defect leads to rotation and inferior contracture of the ear. This worsens a patient's overall cosmetic appearance and can lead to patients withdrawing from or limiting social interactions as a result. Functionally, it can impair hearing rehabilitation, which is important in the older population as well as those undergoing radiation‐ and platinum‐based chemotherapy. Additionally, it can and affect the ability to wear glasses. In patients with malignancies, long‐term effects from adjuvant radiation will further exacerbate all of these effects and potentially cause additional soft tissue injury if there is not adequate vascularized tissue support.
The literature describes a variety of methods to help alleviate this defect and reconstruct these wounds. Soft tissue reconstruction of this defect has been attempted with avascular fat grafts,1, 2 pedicled flaps such as pectoralis major myocutaneous flap3 and supraclavicular flap,4 and free tissue transfer including radial forearm free flap, lateral arm flap,5 and anterolateral thigh flap.6, 7 The submental flaps has been used for a variety of head and neck reconstructive defects with excellent results. With our center's positive experience using these flaps, we began utilizing a de‐epithelialized submental flap for reconstruction of the parotid defect.
To our knowledge, this is the first paper reporting a series of patients that underwent reconstruction of a total parotidectomy defect with the use of this pedicled flap in a de‐epithelialized fashion.
MATERIALS AND METHODS
All patients undergoing submental flap reconstruction between 2014 and 2016 were reviewed. Those patients undergoing reconstructions specifically after a total parotidectomy defect were included in the study. Patients requiring a second simultaneous reconstructive flap were excluded. For each patient, their demographics, cancer characteristics, and follow‐up were recorded. For each flap, harvest technique and flap outcomes including viability and maintenance of flap volume were recorded. We noted the presence of postoperative complications including but not limited to wound complications (hematoma, seroma, sialocele, dehiscence) and flap viability. This review was approved by the Massachusetts Eye and Ear Infirmary Institutional Review Board.
Surgical Technique
The oncologic resection was performed in the standard fashion for a total parotidectomy with or without facial nerve preservation as indicated by the tumor extent. Typically, we used a modified Blair incision.
After a satisfactory oncologic resection, the team then proceeded with the reconstructive effort. The goal of using the submental flap was to restore the lost volume from removing the parotid. The neck incision was extended anteriorly and a skin paddle sufficient for the volume loss was marked. A Doppler was used to ensure arterial flow along the submental vessels and to identity the perforator to the skin. The skin ellipse was planned to adequately incorporate the perforator. Typically, no specific measurements were taken and instead a “pinch” test was used to help determine the maximal skin that could be harvested and still allow for a tension‐free primary closure (Fig. 1A).
Figure 1.
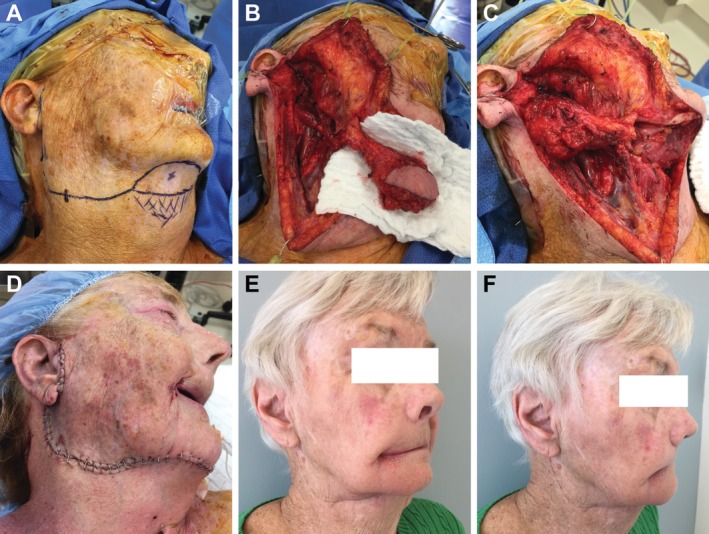
Intraoperative photography of submental flap marking (A), elevation (B), inset (C), and closure (D). Postoperative photography demonstrate an oblique (E) and profile (F) view with good contouring and minimal scar deformity.
The technique for submental flap elevation has been described in prior articles by Patel, Bayles and Hayden, and others.8, 9 Briefly, the marked skin paddle was sharply incised through the epidermis and dermis. The underlying platysma was also harvested with the flap. The submandibular fascia was elevated to preserve the marginal mandibular nerve and facial vein was preserved. The perifacial nodes and nodes of Level 1B were dissected during this segment and removed with the submandibular gland. The ipsilateral anterior belly of the digastric and mylohyoid were harvested with the flap to help preserve the submental artery and vein which reside between these two muscles. While Level 1A nodal metastasis is uncommon with parotid malignancies, the flap was examined for the presence of palpable or suspicious nodes. In the event one or two nodes were identified they would be meticulously dissected free (none in our series). In the event of bulky nodal disease in Level 1A, the submental flap would not be a suitable reconstructive option.10
With the myocutaneous flap freed and attached only by its pedicle, the pedicle was dissected in a retrograde fashion to allow for a greater arc of rotation (Fig. 1B). After demonstrating that the flap was able to reach the parotidectomy defect, we proceeded to sharply de‐epithelialize the flap using a #15 blade while keeping the dermis intact (Fig. 1C).
The flap was secured to the parotidectomy wound with absorbable suture. One or two suction drains were placed into the wound as typically done for a parotidectomy defect. The wound was then closed with advancement of the neck skin (Fig. 1D). Patients stayed in the hospital 2 days before being discharged home.
RESULTS
Over this period, eight patients underwent this reconstruction. Table 1 summarizes their demographics and relevant data regarding the surgery, employed flap, and postoperative follow‐up. All of the included patients underwent a total parotidectomy, neck dissection and facial nerve sacrifice. In one patient (patient F) overlying skin was resected but did not require skin reconstruction. In seven out of eight patients the pedicle did not require ligation of the distal facial artery after the take‐off of the submental artery. In each case, the operative time for the submental flap elevation was not specifically recorded, though the average time ranged between 30 to 45 minutes based on the experience of the senior author (DGD). There were no complications during their hospitalization or in the follow‐up period.
Table 1.
Details regarding surgical resection, follow up, and soft tissue volume outcomes.
| Patient | Oncologic Defect | Complications | Post‐Op Radiation | Follow up (o) | Tissue Volume |
|---|---|---|---|---|---|
| A | Total Parotid, ND, FN Sacrifice | None | Yes | 31.5 | Stable |
| B | Total Parotid, ND, FN Sacrifice | None | No | 22.9 | Stable |
| C | Total Parotid, ND, FN Sacrifice | None | Yes | 19.2 | Stable |
| D | Total Parotid, ND, FN Sacrifice | None | Yes | 22.2 | Stable |
| E | Total Parotid, ND, FN Sacrifice | None | Yes | 9.9 | Stable |
| F | Total Parotid, ND, FN Sacrifice | None | Yes | 10.8 | Stable |
| G | Total Parotid, ND, FN Sacrifice | None | Yes | 21.9 | Stable |
| H | Total Parotid, ND, FN Sacrifice | None | Yes | 37.5 | Stable |
ND = Neck Dissection; FN = Facial Nerve.
Patient follow‐up ranged from 9.9 to 37.5 months (mean 22.0 months). Eight patients received adjuvant treatment following surgical resection. All patients were discharged within 2 days of hospitalization with no complications or concerns regarding the viability of the flap. Up to the last‐known follow‐up with each patient, there was stable flap volume and suitable reconstruction of the parotidectomy defect with subjective symmetry when compared to the contralateral, unoperated parotid (Fig. 1E, F; Fig. 2A‐C). The flap did not inhibit the ability to use magnetic resonance imaging (MRI) for surveillance imaging (Fig. 2D).
Figure 2.
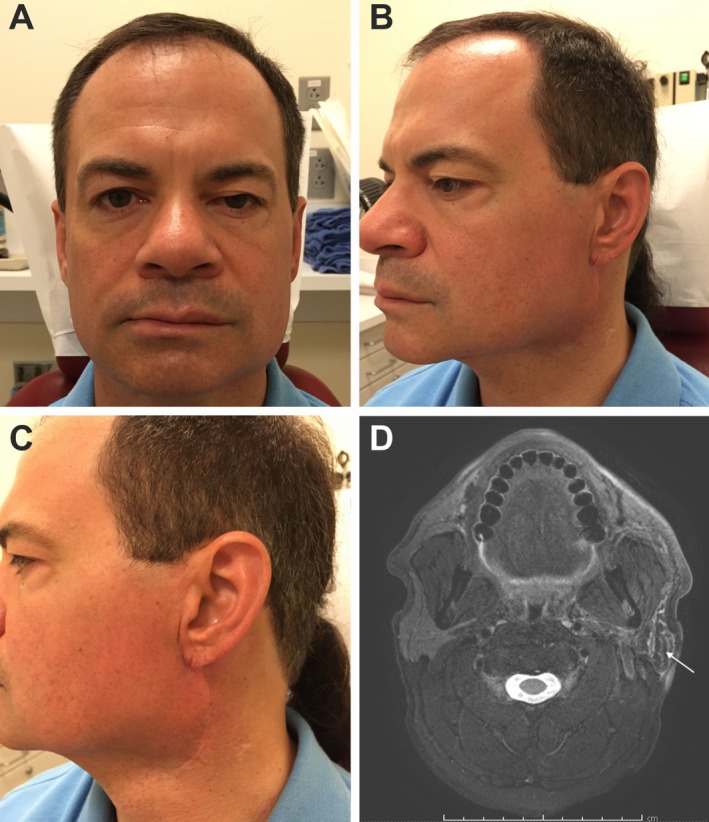
Postoperative photography demonstrating a portrait (A), oblique (B), and profile (C) view of the scar and reconstructed area noting good contour and minimal scar deformity. Surveillance MRI can also easily distinguish the flap from the underlying wound bed as noted by the white arrow (D).
DISCUSSION
Patients requiring a total parotidectomy for oncologic management of their parotid malignancy are often faced with a large resultant surgical defect. Our reported case series demonstrates an effective way to reconstruct these wounds with suitable reconstructed volume that persists over time. The ideal reconstruction for this defect should provide adequate soft tissue to prevent contour deformity and ear contraction and rotation. Additionally, the ideal reconstruction should not add substantial operative time, increase intra‐ and perioperative complexity, and have limited associated morbidity. The submental flap meets all of these goals.
Additionally, the harvest technique does not require the use of a separate incision or surgical wound related to donor material utilized. Instead, the standard parotidectomy incision is extended anteriorly to incorporate the planned harvest. The postoperative closure also allows for the incision to heal in a way that is not deforming or drastically different that the typical parotidectomy incision.
Prior studies have evaluated the use of a variety of reconstructive options including autologous fat, local tissue advancement, and cadaveric material (eg, Alloderm). These options all provide a relatively easy and simple approach to reconstruction. They add little time to the procedure and require no additional perioperative care. However, of these options only a large fat graft can offer enough volume to adequately reconstruct this space.1, 11 Nonvascularized tissue suffers the limitation of volume loss over time or unpredictable end results, especially in the setting of adjuvant radiation. Additionally, reports of using cadaveric material have been associated with increased infection and seroma risk.11
Free tissue transfer is another viable option for reconstruction of this defect. The use of free tissue transfer such as a radial forearm, lateral arm,5 and anterolateral thigh flap6, 7 necessitate a separate surgical site and the associated risks with the respective donor harvest. These techniques also require a longer total operative time.9
Several reports have discussed the use of local or regional pedicled flaps which include sternocleidomastoid muscle flaps or the supraclavicular flap. The sternocleidomastoid muscle flap relies on a variable arterial anatomy and also can be associated with postoperative morbidity related to harvesting this muscle. Additionally, the overall defect volume that can be replaced is limited. It however, does not require a separate incision or extension of the modified Blair incision. The supraclavicular flap, while viable, and not associated with long‐term shoulder weakness or morbidity,12 does still employ a separate incision. Additionally, depending on body habitus, the amount of tissue volume that can be replaced can vary significantly.
The submental flap is a well‐described flap for reconstruction of head and neck defects. It has a reliable blood supply off of the submental artery (terminal branch of the facial artery) and submental vein (drains into the facial vein and internal jugular system). Additionally, well‐reported techniques demonstrate a reliable way to harvest the flap and preserve the blood supply, especially with the inclusion of the mylohyoid muscle.8 Flap harvest routinely takes less than hour and does not require special instruments or microscopes. Additionally, no other postoperative monitoring is needed. This can help avoid ICU admission and patients can be discharged when their drains meet criteria, typically 48 hours following surgery.
In this series, we found that inclusion of the dermis can help with contouring underneath the native skin. While in this series, all patients underwent facial nerve sacrifice, other reconstructive efforts demonstrate that harvesting this flap can be done safely without injury to the marginal mandibular nerve. Further research demonstrating long term viability in a larger series would help solidify its utility as a reconstructive option. A last observation from this series relates to facial nerve reconstruction. Initial facial nerve reconstruction was performed primarily in all patients. The flap did not limit, impede, or otherwise impact choice of facial nerve reconstruction. For patients undergoing static sling procedures and cable grafting this soft tissue can help support this reconstruction. Additionally, by avoiding microvascular surgery, the distal facial vessels remain intact and available for future reconstruction such as a gracilis free flap for facial reanimation.
In addition to providing adequate aesthetic reconstruction and contouring, it is important that the submental flap does not compromise the oncologic resection and nodal dissection. For the primary parotid cancer, level 1A nodal disease is an atypical presentation, as compared to oral cavity. In the case of oral cavity tumors, several studies have demonstrated safety with using the submental flap.10, 13 In 50 patients with oral cavity malignancies undergoing submental flap reconstruction, Howard et al. demonstrated occult nodal disease in level 1A in 10% of patients, without compromise to the patient's cancer care in using this flap.10 Authors have also published video dissections of level 1A while preserving the submental flap vasculature.14 In our population, level 1 was able to be dissected safely without compromising the viability of the flap, without any noted flap loss in our series.
This series is intended to provide an initial report of this technique and observations from this experience. Further research demonstrating long‐term viability in a larger series would help solidify its utility as an excellent reconstructive option for this surgical defect. More complex questions directly comparing this reconstruction to others by measures of appearance, volume, and utility measurements such as operative time, inpatient length of stay, and cost will be considered for the future when a larger volume of experience and follow‐up time is available.
CONCLUSION
The submental flap is an excellent option for reconstruction of the total parotidectomy defect even when skin reconstruction is not required. This flap allows the surgeon to use the same surgical region as the oncologic resection without introducing additional complexity, significant operative time, hospital length of stay, or morbidity. It provides an aesthetically pleasing and durable reconstruction of a total parotidectomy defect.
This manuscript was presented as a poster presentation at the American Head and Neck Society Annual Meeting, July 16–20, 2016; Seattle, Washington.
Funding Disclosure: There was no grant support for this manuscript.
Conflict of Interests: None of the authors have any conflicts of interest.
BIBLIOGRAPHY
- 1. Loyo M, Gourin CG. Free abdominal fat transfer (FAT) for partial and total parotidectomy defect reconstruction. Laryngoscope May 2016;126(12):2694–2698. [DOI] [PubMed] [Google Scholar]
- 2. Bonanno PC. Re: Dermis‐fat graft after parotidectomy to prevent “Frey's syndrome and the concave deformity.” Ann Plast Surg 1994;33(2):235. [DOI] [PubMed] [Google Scholar]
- 3. Ioannides C, Fossion E. Reconstruction of extensive defects of the parotid region: experience with the pectoralis major and free latissimus dorsi flaps. J Craniomaxillofac Surg 1997;25(2):57–62. [DOI] [PubMed] [Google Scholar]
- 4. Emerick KS, Herr MW, Lin DT, Santos F, Deschler DG. Supraclavicular artery Island flap for reconstruction of complex parotidectomy, lateral skull base, and total auriculectomy defects. JAMA Otolaryngol Head Neck Surg 2014;140(9):861–866. [DOI] [PubMed] [Google Scholar]
- 5. Teknos TN, Nussenbaum B, Bradford CR, Prince ME, El‐Kashlan H, Chepeha DB. Reconstruction of complex parotidectomy defects using the lateral arm free tissue transfer. Otolaryngol Head Neck Surg 2003;129(3):183–191. [DOI] [PubMed] [Google Scholar]
- 6. Cannady SB, Seth R, Fritz MA, Alam DS, Wax MK. Total parotidectomy defect reconstruction using the buried free flap. Otolaryngol Head Neck 2010;143(5):637–643. [DOI] [PubMed] [Google Scholar]
- 7. Hanasono MM, Skoracki RJ, Silva AK, Yu P. Adipofascial perforator flaps for “aesthetic” head and neck reconstruction. Head Neck 2011;33(10):1513–1519. [DOI] [PubMed] [Google Scholar]
- 8. Patel UA, Bayles SW, Hayden RE. The submental flap: a modified technique for resident training. Laryngoscope 2007;117(1):186–189. [DOI] [PubMed] [Google Scholar]
- 9. Howard BE, Nagel TH, Barrs DM, Donald CB, Hayden RE. Reconstruction of lateral skull base defects: a comparison of the submental flap to free and regional flaps. Otolaryngol Head Neck Surg 2016;154(6):1014–1018. [DOI] [PubMed] [Google Scholar]
- 10. Howard BE, Nagel TH, Donald CB, Hinni ML, Hayden RE. Oncologic safety of the submental flap for reconstruction in oral cavity malignancies. Otolaryngol Head Neck Surg 2014;150(4):558–562. [DOI] [PubMed] [Google Scholar]
- 11. Athavale SM, Phillips S, Mangus B, et al. Complications of alloderm and dermamatrix for parotidectomy reconstruction. Head Neck 2012;34(1):88–93. [DOI] [PubMed] [Google Scholar]
- 12. Herr MW, Bonanno A, Montalbano LA, Deschler DG, Emerick KS. Shoulder function following reconstruction with the supraclavicular artery Island flap. Laryngoscope 2014;124(11):2478–2483. [DOI] [PubMed] [Google Scholar]
- 13. Elzahaby IA, Roshdy S, Shahatto F, Hussein O. The adequacy of lymph node harvest in concomitant neck block dissection and submental Island flap reconstruction for oral squamous cell carcinoma; a case series from a single Egyptian institution. BMC Oral Health 2015;15(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eskander A, Strigenz D, Seim N, Ozer E. Submental artery Island flap with simultaneous level I neck dissection. Head Neck 2018;40(4):842–845. [DOI] [PubMed] [Google Scholar]


