Abstract
Objectives
To assess the safety and effectiveness of in‐office bipolar radiofrequency treatment of nasal valve obstruction
Study Design
Prospective, nonrandomized, multicenter case series
Methods
Adult patients with a Nasal Obstruction Symptom Evaluation scale (NOSE) score ≥60 were selected. Patients were clinically diagnosed with dynamic or static internal nasal valve obstruction as primary or significant contributor to obstruction and were required to have a positive response to nasal mechanical dilators or lateralization maneuvers. Bilateral radio‐frequency treatment was applied intranasally using a novel device, under local anesthesia in a single session. Safety and tolerance were assessed by event reporting, inspection, and Visual Analogue Scale (VAS) for pain. Efficacy was determined using the NOSE score and patient‐reported satisfaction survey at 26 weeks.
Results
Fifty patients were treated. No device or procedure‐related serious adverse events occurred. Soreness, edema, and crusting resolved by 1 month. The mean baseline NOSE score was 79.9 (SD 10.8, range 60–100), and all had severe or extreme obstruction. At 26 weeks, mean NOSE score was 69% lower at 24.7 (P < .0001) with 95% two‐sided confidence intervals 48.5 to 61.1 for decrease. The decrease in NOSE score did not differ significantly between patients who did or did not have prior nasal surgery. Patient satisfaction mean by survey was 8.2 of 10.
Conclusion
In office treatment of internal nasal valve obstruction using a bipolar radiofrequency device is safe and well‐tolerated. Nasal obstruction, as assessed using the NOSE questionnaire at 26 weeks, was markedly improved with high patient satisfaction.
Level of Evidence
2b, prospective cohort
Keywords: Nasal valve, nasal obstruction, radio‐frequency, nasal surgery, minimally invasive surgery
INTRODUCTION
Nasal obstruction is a highly prevalent, multifactorial disorder. Nasal obstruction may be mediated by mucosal inflammation from infection, allergenic, or nonallergenic irritants. Chronic nasal obstruction may be mediated by persistent inflammation or structural factors such as nasal masses or polyps, nasal septal deviation, and nasal valve narrowing or collapse.1, 2
Chronic nasal obstruction may result in sleep disruption and daytime sleepiness, snoring, congestion, and headaches. Overall quality of life is also decreased.3, 4 Given its high prevalence, treatment of nasal obstruction is costly, with large expenditures for prescriptions, over‐the‐counter medications, and doctor's visits.
Surgical treatment of nasal obstruction most commonly consists of repair of a deviated nasal septum and reduction of the inferior turbinates. Recently there has been a greater focus on treatment of the nasal valve region. The nasal valve is defined by the caudal cartilaginous nasal septum, the anterior head of the inferior turbinate, and the caudal end of the upper lateral cartilage. This region is critical in the development of nasal obstruction and represents the narrowest part of the nasal airway.5, 6, 7, 8 As described by Poiseuille's law, minute changes in the diameter of a tube will result in exponential changes in airflow.
Noninvasive management of nasal valve obstruction includes use of over‐the‐counter devices such as external nasal dilator strips or internal nasal dilators. Surgical treatments involve various suturing and or grafting techniques using autologous cartilage or synthetic biomaterials.5, 9, 10, 11, 12 These treatments usually require the significant recovery period of surgery and the risks of infection, bleeding, scarring and graft complications.9, 12, 13, 14
Radiofrequency, electrical current or laser‐mediated heating of cartilage can change its shape, as shown in several ex vivo studies on rib and septal cartilage.15, 16, 17 Curvature and transient softening of septal cartilage can be achieved at temperatures of 60–75°C, depending on rate of thermal transfer, and posttreatment, the cartilage retains its elastic properties for anatomical loading. Radiofrequency treatment of the lateral nasal wall has been used for patients with nasal valve collapse in one investigation, but an incisional approach was required.18 In this previous investigation, three treatments were applied per side, based on a predicted coagulation zone, with intent to produce tissue retraction and volume reduction. The resultant Visual Analogue Scale (VAS) obstruction score decreased from mean of 8.2 to 3.7.
In the current study, temperature‐controlled radiofrequency treatment was applied intranasally using a bipolar stylus to the internal nasal valve region. Under local anesthesia, radiofrequency energy along with outward pressure was applied to the mucosa at the region of the caudal end of the upper lateral cartilage to induce mechanical deformation and potentially change the shape of the lateral nasal wall. Safety of this approach, as well as its efficacy for symptomatic relief of nasal obstruction was subsequently assessed after 6 months.
MATERIALS AND METHODS
Study Population
Study design was a prospective, nonrandomized multicenter trial to evaluate the safety and efficacy of bipolar, temperature‐controlled radiofrequency treatment of the nasal valve. The study received IRB approval (Western Institutional Review Board, Puyallup, WA) and was registered with ClinicalTrials.gov (NCT02914236).
The study was performed on adults age 22 to 75 years (inclusively), seeking treatment for nasal obstruction and willing to undergo an office‐based procedure. Nasal obstruction, as assessed by the NOSE scale,19 needed to be ≥60, as it indicates that on average the nasal symptoms were “a fairly bad problem” for the patient, scoring 3 out of 4 in severity for each question. The nasal valve was required to be a primary or significant contributor to the subject's nasal obstruction as determined by the investigator, based on clinical presentation, physical examination, or nasal endoscopy. Subjects were required to have positive symptomatic improvement with use of external or internal nasal dilators, Q‐Tip or curette test (manual intranasal lateralization), or the Cottle Maneuver (manual lateral retraction of the cheek).
Subjects were excluded if they had:
Prior surgery to the nasal valve, rhinoplasty, septoplasty, inferior turbinate reduction or other surgical nasal procedures within the past 12 months;
Severe and/or chronic sinusitis, recurrent sinusitis, or allergies leading to nasal obstruction and currently requiring oral corticosteroid therapy—the presence of treated allergic rhinitis was not a contraindication and patients were allowed to continue use of oral and/or topical medications during the trial;
Severe case of any of the following; septal deviation, turbinate hypertrophy, polyps, or ptotic nasal tip believed to be the primary contributor to the subject's nasal obstruction symptoms and warranting surgical intervention;
Known or suspected allergies or contraindications to the anesthetic agents and/or antibiotic medications to be used during the study procedure session;
Known or suspected pregnancy, or lactation; or
Other medical conditions that the investigator believed would predispose subject to poor wound healing or increased surgical risk.
Nasal Valve Treatment Protocol
The region of the upper lateral cartilage was anesthetized intranasally using either topical lidocaine 4% or tetracaine 4%. Lidocaine 1% with epinephrine was subsequently injected to achieve complete local anesthesia.
Bilateral radiofrequency treatment was applied in a single visit using Aerin Medical's Vivaer Stylus with a Model ORA‐50S generator (Fig. 1) at a setting of 60°C and 4 watts. The stylus was placed intranasally onto the mucosa overlying the lower edge of the upper lateral cartilage and three nonoverlapping loci along the nasal valve angle were treated on each side (Fig. 2). No incisions were made. Tissue temperature feedback was constantly provided by the stylus before, during and after treatment to allow the generator to modulate power for maintaining treatment temperature at 60°C, and procedure safety.
Figure 1.

Aerin Medical's generator and vivaer stylus with close‐up of stylus tip
Figure 2.

Treatment area scheme
Treatment was applied to the nasal valve region at the caudal end of the upper lateral cartilage bilaterally in three nonoverlapping zones, marked by circles in the figure.
Each treatment cycle consisted of an 18‐second treatment pulse and 12‐second cooling time while applying continuous upward pressure with the stylus in an outward direction perpendicular to the upper lateral cartilage at each position. A standardized approach was used based on animal and human pilot studies where temperature and duration parameters were determined for optimal safety and efficacy.20
Outcome Assessment
Safety and tolerance were assessed by event reporting, inspection, and VAS for pain at follow‐up visits performed at 4, 12, and 26 weeks postprocedure. Adverse events were categorized as serious or nonserious and relatedness to the device or treatment. Nasal status assessment was performed including intranasal examination, the presence of saddling, bruising, pain, numbness. Medication logs were obtained.
Efficacy endpoint was determined at 26 weeks using the NOSE scale survey. NOSE scores were assessed at baseline, 4, 12, and 26 weeks postprocedure and the responder rate, defined as a ≥15‐point decrease, was determined. In two studies the minimally clinically important difference (MCID) in the NOSE score was calculated as approximately 4 to 6.3 points, so a ≥15‐point was selected as the minimal clinically relevant change.21, 22 A satisfaction survey of five questions was administered to all subjects at the 26‐week visit inquiring about procedure tolerance, recovery, nasal breathing, overall satisfaction, and whether the procedure would be recommended to others. Subjects were allowed to continue their preprocedure concurrent treatments during the follow up period, including topical, oral medications and nasal dilators, and usage was queried at all follow‐up visits.
Statistical Methodology
The primary study objective was to demonstrate that the mean improvement in NOSE score from baseline to 26 weeks exceeded 15 points. The null and alternative hypotheses were defined as:
where μd represents the mean change in NOSE score from baseline to 26 weeks. The hypothesis was to be tested at the one‐sided alpha 0.05 level. The change from baseline to the 26‐week time point was analyzed using a paired t‐test and presented as the mean change with associated 95% one‐sided lower confidence interval.
The sample size estimated for this study was predicated on information from a pilot study20 that estimated the expected mean change (28.5) and standard deviation (30.3) for the change in NOSE score from baseline to 6 months (26 weeks). A sample of 45 subjects was estimated to provide 90% power to test the study hypothesis with one‐sided alpha = 0.05. To allow for up to 10% attrition, 50 subjects were enrolled.
For comparison of patient with past nasal surgery history to those without, Student's t‐test was performed to compare NOSE means. For the responder rate comparison, Fisher's Exact test was used. Wilson confidence intervals for a binomial proportion are reported. Significance level was set to P < .05.
Statistical analysis was performed using SAS/STAT Version 14.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Fifty‐five patients were screened and fifty who met the inclusion criteria were included in the study. Five patients were not treated due to withdrawal of consent or due to screening error. Treatment was performed at nine sites by 16 investigators, between September 2016 and January 2017. Patients were followed up to 26 weeks postprocedure with only one patient unavailable for follow up at the end‐point.
Patient mean age was 51 years, with a range of 24 to 78 years, with similar numbers of male and female patients treated (Table 1). Patients were predominantly of Caucasian descent, with mean BMI of 28, and 74% were overweight or obese. Nasal obstruction was present for at least 1 year in almost all patients. Bilateral, dynamic nasal valve collapse was diagnosed clinically in 76% with the remainder having nasal valve stenosis or unilateral collapse. Seventy percent of the patients had tried mechanical nasal dilators prior. Some 56% of patients had previously undergone nasal procedures, most commonly septoplasty, followed by inferior turbinate reduction.
Table 1.
Patient Characteristics.
| Measure | N (%) | Mean ± SD |
|---|---|---|
| BMI | 50 (100) | 28.1 ± 5.2 |
| Sex | ||
| Male | 28 (56) | |
| Female | 22 (44) | |
| Age | 50.9 ± 12.6 | |
| <50 | 24 (48) | |
| ≥50 | 26 (52) | |
| Nasal Obstruction Duration | ||
| <1 year | 2 (4) | |
| ≥1 year | 48 (96) | |
| Dynamic Valve Collapse | ||
| Bilateral | 38 (76) | |
| Unilateral | 2 (4) | |
| None | 10 (20) | |
| Nasal Surgery History * | ||
| Yes | 28 (56) | |
| No | 22 (44) |
BMI = Body Mass Index
Septoplasty (18), inferior turbinate reduction (6), sinus surgery (6), rhinoplasty (4), polyp removal (2), sinuplasty (2). Some subjects had multiple procedures.
Nasal valve treatment using bipolar radiofrequency was performed after application of topical anesthetic and injection of approximately 1 cc of local anesthetic to the nasal valve. All patients except one were treated bilaterally. Three sites along the internal nasal valve were treated on each side in 98% of patients. From the time of local anesthetic administration to completion, procedure time ranged from 7 to 37 minutes with a mean of 18 minutes.
There were no device or procedure‐related serious adverse events. Minor adverse events of nasal congestion, swelling and pain were limited to the first month with only two patients reporting pharyngitis in the subsequent follow‐up period visits. There were no cases of nasal bleeding requiring intervention. At 1 month, only 6% of patients reported soreness of the nose, which gradually subsided. Crusting was noted by about 33% of patients at 1 month and subsided by the next follow‐up visit. Mean VAS pain reported immediately after the procedure was 29 out of 100, and at 4 weeks was 9 out of 100. No adverse changes to nasal shape or changes in the aesthetic appearance of the nose were reported or observed. No other nasal procedures were performed during the follow‐up period.
Effectiveness of treatment was assessed using the NOSE questionnaire, administered at baseline, 4, 12, and 26 weeks from treatment. Mean and individual patient NOSE outcomes are shown (Fig. 3, Table 2). The mean baseline score was severely elevated at 80, with 46% noting severe and 54% noting extreme obstruction. At 26 weeks severe and extreme obstruction were only present in 8% and 2% respectively, with one patient's score affected by acute sinusitis. Conversely, at the 26 week end‐point, 65% of patients reported having no problem or very mild problem by the NOSE score, as compared with baseline of 0%. The mean NOSE score declined from 80 to 37, 27, and 25 at 4, 12, and 26 weeks, respectively. The change in the mean score at 26 weeks was 55 points, with P < .0001 for testing the hypothesis that the change was greater than 15 points (95% one‐sided lower bound on the difference = 49.6) and 95% two‐sided confidence intervals of 48.5 to 61.1 change from baseline.
Figure 3.

Mean NOSE score at BL, 4, 12, and 26 weeks evaluations
Mean NOSE score was 79.9 at baseline and 37.2, 27.3, and 24.7 at 4, 12, and 26 weeks, respectively. 95% confidence intervals are displayed.
BL = baseline; NOSE = Nasal Obstruction Symptom Evaluation.
Table 2.
NOSE Scores Outcome.
| Subjects N = 50 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 4 Weeks | 12 Weeks | 26 Weeks | |||||
| NOSE Score | n | % | n | % | n | % | n | % |
| No Problems | – | – | – | – | 4 | 8 | 5 | 10.2 |
| Mild | – | – | 21 | 42 | 22 | 44 | 27 | 55.1 |
| Moderate | – | – | 18 | 36 | 19 | 38 | 12 | 24.5 |
| Severe | 23 | 46 | 10 | 20 | 5 | 10 | 4 | 8.2 |
| Extreme | 27 | 54 | 1 | 2 | – | – | 1 | 2 |
| n evaluated | 50 | 100 | 50 | 100 | 50 | 100 | 49 | 100 |
| Mean | 79.9 | 37.2 | 27.3 | 24.7 | ||||
| SD | 10.8 | 18.3 | 18 | 20.4 | ||||
| Median | 80 | 32.5 | 25 | 20 | ||||
| Min – Max | 60–100 | 5–80 | 0–70 | 0–90 | ||||
| Change from Baseline | ||||||||
| Mean | – | – | 42.7 | 52.6 | 54.8 | |||
| SD | – | – | 20.9 | 19.6 | 21.9 | |||
| Median | – | – | 45 | 55 | 55 | |||
| Min – Max | – | – | 0–85 | 15–90 | 0–95 | |||
| % Change from Baseline | ||||||||
| Mean | – | – | −52.8 | −65.6 | −68.7 | |||
| SD | – | – | 23.5 | 22.8 | 25.3 | |||
| Median | – | – | −58.2 | −67.6 | −71.4 | |||
| Min ‐ Max | – | – | 0 ‐ −94 | −21 ‐ −100 | 0 ‐ −100 | |||
NOSE score outcomes are shown as a function of severity category, mean score and change in NOSE score from baseline. NOSE Severity scoring was as follows: Mild (5–25), Moderate (30–50), Severe (55–75), Extreme (80–100).
NOSE = Nasal Obstruction Symptom Evaluation.
Outcome was compared between patients who had undergone nasal surgery prior to treatment and those who did not. The reductions in NOSE scores were not statistically different between the groups (P = .459) nor was the rate of response of at least 15‐point drop in the NOSE score. (Table 3, Fig. 4)
Table 3.
NOSE Score Outcome and Response Rate in Patients with and Without Prior Nasal Surgery.
| Group | Number Evaluated | 26‐Week NOSE mean† | SD | 95% CI | Min‐Max | Responder (yes/n) | Responder (%)‡ | 95% CI§ | |
|---|---|---|---|---|---|---|---|---|---|
| No Prior Surgery | 22 | 22.3 | 19.2 | 13.8–30.8 | 0–90 | 21/22 | 95.5 | 78.2–99.2 | |
| Prior Surgery | 27* | 26.7 | 21.5 | 18.2–35.2 | 0–75 | 25/27 | 92.6 | 76.6–97.9 |
There were 28 subjects with prior surgery but one missed the 26‐week evaluation.
NOSE mean scores t‐test result's P‐value = .459.
Responder rates Fisher's exact test's P‐value = 1.
Wilson's confidence intervals for a binomial proportion.
CI = confidence interval; NOSE = Nasal Obstruction Symptom Evaluation; SD = standard deviation.
Figure 4.
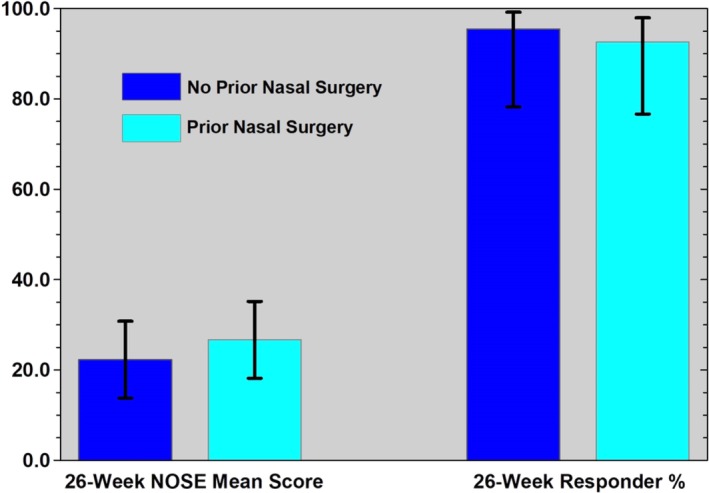
Mean NOSE score outcome and response rate in patients with and without prior nasal surgery
Mean NOSE scores were 22.3 and 26.7 (P = .459) and responder percentages were 95.5 and 92.6 (P = 1), for no prior nasal surgery and prior nasal surgery, respectively. 95% confidence intervals displayed.
NOSE = Nasal Obstruction Symptom Evaluation.
The mean score for individual items on the NOSE questionnaire ranged from 3.08/4–3.29/4 at baseline (Fig. 5). At 26 weeks there was approximately a 2‐ to 4‐point drop in each category on the NOSE questionnaire (range 1.82–2.49). Self‐reported sleep quality was improved following treatment. At baseline, patients’ score on the “Trouble Sleeping” item of the NOSE questionnaire was 3.12/4 which progressively declined to a mean of 0.90/4 at 26 weeks. Ability to breathe nasally during exercise improved similarly with baseline report of exercise‐associated dysfunction of 3.24/4 and 26‐week score of 0.76/4 on the NOSE questionnaire. These scores reflect patient perception of sleeping and breathing during exercise difficulties as being very mild or not problematic following nasal treatment.
Figure 5.

NOSE questionnaire individual item mean responses at baseline and 26 weeks posttreatment
95% confidence intervals displayed.
NOSE = Nasal Obstruction Symptom Evaluation.
Patient satisfaction was assessed by a five‐question satisfaction survey, where each question was answered on a 10‐point scale, with a higher score indicating greater satisfaction. Results were available for 49 out of 50 patients with a mean score of 7.3 to 8.7 (Table 4). The highest mean score of 8.7 was for whether the patient would recommend the treatment for a friend with nasal congestion.
Table 4.
Satisfaction Survey Outcome.
| Survey Question | Subjects N = 50 | |||||
|---|---|---|---|---|---|---|
| n | Mean | SD | Median | Min | Max | |
| How tolerable was the treatment procedure to receive? | 49 | 7.3 | 2.4 | 8 | 2 | 10 |
| How easy was your recovery from the treatment? | 49 | 8.3 | 1.7 | 9 | 2 | 10 |
| How has breathing through your nose changed since the treatment? | 49 | 8.3 | 1.8 | 9 | 1 | 10 |
| How satisfied are you with the treatment? | 49 | 8.4 | 2.1 | 9 | 2 | 10 |
| Would you recommend the treatment to a friend who suffered from congestion? | 49 | 8.7 | 2.1 | 10 | 1 | 10 |
Satisfaction Survey administered at the 26‐week evaluation visit. Responses were on 1–10 scale where higher scores indicate more favorable response.
DISCUSSION
The internal nasal valve is the narrowest part of the nasal airway and provides approximately two‐thirds of its resistance to airflow. Dynamic collapse or stenosis of the valve is a recognized but often overlooked cause of symptomatic nasal obstruction. Treatment using mechanical dilating devices such as external strips or intranasal inserts require repeated application and are not always tolerated. Surgical treatments often require general anesthesia with extensive dissection and graft harvest as well as risk of relapse, scarring, and postoperative external deformity. Less invasive surgical options, such as suspension sutures may be prone to infection, granulation, extrusion, breakage, and relapse.23 Recently, an absorbable nasal valve implant, deployed via a hollow cannula has been used to treat the nasal valve with a reduction of NOSE scale mean from 77 to 35 with standard deviation of 29 at 12 months.24 While this is a simple, rapid procedure, potential problems include improper placement, cosmetic changes, migration, foreign body sensation or reaction and need for removal. It also requires alar rim anesthesia for placement in the office.
In this prospective, multicenter study, the preliminary safety and effectiveness of radiofrequency energy application under local anesthesia to the internal nasal valve for treatment of nasal obstruction was studied. No incisions were made. There were no serious device or procedure‐related adverse events and minor adverse effects were transient and localized to the treatment site. There were no cases of nasal cosmetic changes noted by the investigators or reported by the patients. A novel radiofrequency delivery device was used to increase and maintain treated tissue temperatures at 60°C for the duration of the treatment. The treatment was performed under local anesthesia in a single visit and was well‐tolerated. Mean procedure time including local anesthesia administration was 18 minutes.
The response rate was high with 94% of subjects having improvement in NOSE score by at least 15 points over 26 weeks. Some investigators have suggested that a 25‐ to 30‐point NOSE score change is required to deem whether a nasal intervention was successful,25, 26 but even by this definition, improvement in the mean NOSE score for our study was 55 points from baseline, thus far greater. The NOSE score improvement was similar to that reported in metanalyses and systematic reviews of invasive surgical treatments for nasal obstruction including septo‐rhinoplasty procedures where the mean NOSE score change was 42 to 50 points.25, 27, 28
The percentage of subjects with severe or extreme obstruction scores declined from 100% to 10%. Each of the five individual domains on the NOSE questionnaire improved significantly, including self‐reported sleep quality and ability to breath nasally during exercise. This suggests broad and significant improvement in quality of life after the procedure. Patient‐reported satisfaction rate was high. Close to half the subjects had prior nasal surgery, and their outcome was not different than that of those with no prior surgery. Thus, the procedure could be applied to the population of patients post‐septoplasty, rhinoplasty, and/or turbinate reduction with residual obstruction.
Over 50% of patients in this study were 50 years old or older and 78% were 40 or older. Presumably, this age group has a lower elastic modulus or greater collapsibility of the nasal side walls. The possible actions of radiofrequency energy treatment of the nasal valve region can include stabilizing it via tissue retraction as reported in a previous study18 and perhaps widen it via a change in nasal valve angle or cross‐sectional area. This is inferred, as some patients did not have dynamic lateral nasal collapse and yet benefitted from treatment. Objective change in the nasal valve was not demonstrated in this investigation, as quantitative techniques such acoustic rhinometry, rhinomanometry, imaging, and peak nasal inspiratory flow are not widely believed to correlate with patient‐reported outcome.29, 30
The strengths of this study include the large treatment response, also supported by a 95% one‐sided lower bound of 31.7 on the decrease from baseline for the NOSE score. The treatment was performed by multiple investigators in clinical, nonacademic setting, and did not appear to have a significant learning curve. Thus, it appears promising as a technique that could be used by a wide pool of otolaryngologists. Weaknesses of this study include its uncontrolled, nonrandomized, unblinded design which can be prone to selection bias. The NOSE score is a validated outcome tool, but consists of subjective reporting, and thus the outcome is susceptible to the Hawthorne effect where subjects may respond differently due to being in a study. While a placebo effect cannot be excluded, the high magnitude of response is supportive for a true clinical response. With respect to the patient population, the cohort was almost entirely Caucasian, and limited to 50 subjects. A placebo‐controlled study with a larger and more diverse population would be desirable. Also, the endpoint analysis was performed at 26 weeks postprocedure, thus relatively short term. Follow‐up for outcome over several years is needed to assess longevity of the patients’ outcome and this study will be performed.
CONCLUSION
In office treatment of internal nasal valve obstruction using a bipolar radiofrequency device is safe, simple and well‐tolerated. Nasal obstruction, as assessed using the NOSE questionnaire at 26 weeks, was markedly improved with high patient satisfaction.
Editor's Note: This Manuscript was accepted for publication 03 January 2019.
Podium presentation at the Annual Meeting of the Triological Society, April 21, 2018, National Harbor, Maryland, U.S.A.
We thank Jeff Doerzbacher for assistance with statistical analysis.
Funding was provided for the FDA trial by Aerin Medical, Inc. (ClinicalTrials.gov Identifier: NCT02914236).
Conflict of Interest: Drs. Jacobowitz and Ephrat became consultants for Aerin Medical, Inc., subsequent to completion of the research study and clinical trial FDA submission.
BIBLIOGRAPHY
- 1. Recker C, Hamilton G. Evaluation of the patient with nasal obstruction. Facial Plast Surg 2016;32(1):3–8. [DOI] [PubMed] [Google Scholar]
- 2. Chandra R, Patadia M, Raviv J. Diagnosis of nasal airway obstruction. Otolaryngol Clin North Am 2009;42(2):207–225, vii. [DOI] [PubMed] [Google Scholar]
- 3. Udaka T, Suzuki H, Kitamura T, et al. Relationships among nasal obstruction, daytime sleepiness, and quality of life. Laryngoscope 2006;116(12):2129–2132. [DOI] [PubMed] [Google Scholar]
- 4. Rhee J, Book D, Burzynski M, et al. Quality of life assessment in nasal airway obstruction. Laryngoscope 2003;113(7):1118–1122. [DOI] [PubMed] [Google Scholar]
- 5. Barrett D, Casanueva F, Cook T. Management of the nasal valve. Facial Plast Surg Clin North Am 2016;24(3):219–234. [DOI] [PubMed] [Google Scholar]
- 6. Cole P. The four components of the nasal valve. Am J Rhinol 2003;17(2):107–110. [PubMed] [Google Scholar]
- 7. Wexler D, Davidson T. The nasal valve: a review of the anatomy, imaging, and physiology. Am J Rhinol 2004;18(3):143–150. [PubMed] [Google Scholar]
- 8. Rhee J, Weaver E, Park S, et al. Clinical consensus statement: Diagnosis and management of nasal valve compromise. Otolaryngol Head Neck Surg 2010;143(1):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khosh M, Jen A, Honrado C, Pearlman S. Nasal valve reconstruction: experience in 53 consecutive patients. Arch Facial Plast Surg 2004;6(3):167–171. [DOI] [PubMed] [Google Scholar]
- 10. Fischer H, Gubisch W. Nasal valves‐‐importance and surgical procedures. Facial Plast Surg 2006;22(4):266–280. [DOI] [PubMed] [Google Scholar]
- 11. Spielmann P, White P, Hussain S. Surgical techniques for the treatment of nasal valve collapse: a systematic review. Laryngoscope 2009;119(7):1281–1290. [DOI] [PubMed] [Google Scholar]
- 12. Rhee J, Arganbright J, McMullin B, Hannley M. Evidence supporting functional rhinoplasty or nasal valve repair: a 25‐year systematic review. Otolaryngol Head Neck Surg 2008;139(1):1020. [DOI] [PubMed] [Google Scholar]
- 13. Sufyan A, Ziebarth M, Crousore N, Berguson T, Kokoska MS. Nasal batten grafts: are patients satisfied? Arch Facial Plast Surg 2012;14(1):14–19. [DOI] [PubMed] [Google Scholar]
- 14. de Pochat V, Alonso N, Mendes R, et al. Nasal patency after open rhinoplasty with spreader grafts. J Plast Reconstr Aesthet Surg 2012;65(6):732–738. [DOI] [PubMed] [Google Scholar]
- 15. Keefe M, Rasouli A, Telenkov S, et al Radiofrequency cartilage reshaping: efficacy, biophysical measurements, and tissue viability. Arch Facial Plast Surg 2003;5(1):46–52. [DOI] [PubMed] [Google Scholar]
- 16. Manuel C, Foulad A, Protsenko D, et al. Needle electrode‐based electromechanical reshaping of cartilage. Ann Biomed Engin 2010;38(11):3389–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zemek A, Protsenko D, Wong B. Mechanical properties of porcine cartilage after uniform RF heating. Lasers Surg Med 2012;44(7):72–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seren E. A new surgical method of dynamic nasal valve collapse. Arch Otolaryngol Head Neck Surg 2009;135(10):1010–1014. [DOI] [PubMed] [Google Scholar]
- 19. Stewart M, Witsell D, Smith T, et al. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg 2004;130(2):157–163. [DOI] [PubMed] [Google Scholar]
- 20.InFlux System for Nasal Breathing Improvement clinical trial NCT01960816. Available at: https://clinicaltrials.gov/ct2/show/NCT01960816. Accessed April 8, 2018
- 21. Stewart M, Smith T, Weaver E, et al. Outcomes after nasal septoplasty: results from the Nasal Obstruction Septoplasty Effectiveness (NOSE) study. Otolaryngol Head Neck Surg 2004;130(3):283–290. [DOI] [PubMed] [Google Scholar]
- 22. Lipan M, Most S. Development of a severity classification system for subjective nasal obstruction. JAMA Facial Plast Surg 2013;15(5):358–361. [DOI] [PubMed] [Google Scholar]
- 23. Nuara M, Mobley S. Nasal valve suspension revisited. Laryngoscope 2007;117(12):2100–2106 [DOI] [PubMed] [Google Scholar]
- 24. San Nicoló M, Stelter K, Sadick H, et al . Absorbable implant to treat nasal valve collapse. Facial Plast Surg 2017;33(2):233–240. [DOI] [PubMed] [Google Scholar]
- 25. Rhee J, Sullivan C, Frank D, et al. A systematic review of patient‐reported nasal obstruction scores: defining normative and symptomatic ranges in surgical patients. JAMA Facial Plast Surg 2014;16(3):219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ziai H, Bonaparte P. determining a successful nasal airway surgery: calculation of the patient‐centered minimum important difference. Otolaryngol Head Neck Surg 2017;157(2):325–330. [DOI] [PubMed] [Google Scholar]
- 27. Kandathil C, Spataro E, Laimi K, et al. Repair of the lateral nasal wall in nasal airway obstruction: A systematic review and meta‐analysis. JAMA Facial Plast Surg 2018;20(4):307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Floyd EM, Ho S, Patel P, et al. Systematic review and meta‐analysis of studies evaluating functional rhinoplasty outcomes with the NOSE score. Otolaryngol Head Neck Surg 2017;156(5):809–815. [DOI] [PubMed] [Google Scholar]
- 29. André RF, Vuyk HD, Ahmed A, et al. Correlation between subjective and objective evaluation of the nasal airway. A systematic review of the highest level of evidence. Clin Otolaryngol 2009;34(6):518–525. [DOI] [PubMed] [Google Scholar]
- 30. Andrews PJ, Choudhury N, Takhar A, et al. The need for an objective measure in septorhinoplasty surgery: are we any closer to finding an answer? Clin Otolaryngol 2015;40(6):698–703. [DOI] [PubMed] [Google Scholar]


