Abstract
We sought to test the efficacy of extended-release naltrexone (XR-NTX) on HIV-related and drinking outcomes. From April 2011-February 2015, we conducted a 4-site randomized doubleblind placebo controlled clinical trial involving 51 HIV-positive patients with heavy drinking and <95% antiretroviral (ART) adherence. All participants received counseling. The primary outcome was proportion with ≥95% ART adherence. Secondary outcomes included HIV biomarkers, VACS Index score, and past 30-day heavy drinking days. Based on receipt of ≥5 injections, 23 participants were retained at 24 weeks. We did not detect an effect of XR-NTX on ART adherence (p=.38); undetectable HIV viral load (p=0.26); CD4 cell count (p=.75) or VACS Index score (p=.70). XR-NTX was associated with fewer heavy drinking days (p=.03). While XR-NTX decreases heavy drinking days, we did not detect improvements in ART adherence or HIV outcomes. Strategies to improve retention in alcohol treatment and HIV-related outcomes among heavy drinking HIV-positive patients are needed.
Keywords: HIV, alcohol, extended-release naltrexone, randomized clinical trial
INTRODUCTION
Heavy alcohol use(1) is of major concern among HIV-positive patients(2, 3). Largely explained by negative effects on antiretroviral therapy adherence, heavy alcohol use is associated with uncontrolled HIV disease(4–7) and is independently associated with greater mortality risk(3). Despite demonstrated benefits with integrating substance use treatment and HIV care(8–14), alcohol use is not widely addressed in HIV treatment settings and there are few studies addressing ways to mitigate the role of heavy alcohol use on ART adherence(15–19).
Naltrexone, is an opioid receptor antagonist, which in conjunction with counseling, decreases heavy drinking among the general population. Naltrexone is safe in HIV-positive patients thus offering a potential solution(20–22). In contrast to an oral formulation, an injectable, extended-release formulation of naltrexone (XR-NTX) improves adherence and does not add to pill burden while decreasing heavy alcohol use(23). Prior work demonstrates it can be integrated into primary care(24). Since XR-NTX’s Food and Drug Administration’s approval in 2006 for treating alcohol dependence, few studies have evaluated the impact of XR-NTX on HIV-related outcomes. Prior studies are limited to one that enrolled individuals released from prison and a pilot study conducted in HIV treatment settings(25, 26).
We conducted the current study, entitled Project DAWN, in HIV clinics to determine the efficacy of XR-NTX with counseling, compared to placebo with counseling, on HIV-related and drinking outcomes among HIV-positive patients with heavy alcohol use and suboptimal ART adherence. Given data demonstrating the impact of heavy alcohol use on health behaviors and outcomes, we hypothesized that by intervening upon heavy alcohol use, HIV related outcomes would also improve.
METHODS
Settings and Participants
Project DAWN was conducted in four HIV clinics from three healthcare systems. We employed a multi-pronged approach to recruitment including: 1) community-based flyers and radio advertisement; 2) provider-based referral from organizations serving people living with HIV; 3) peer referral; 4) chart review; and 5) clinic screening using the Alcohol Use Disorders Identification Test-Consumption Test (AUDIT-C)(27, 28). Patients were eligible if they met the following criteria: 1) HIV-positive; 2) currently prescribed antiretroviral therapy (ART); 3) had evidence of <95% ART adherence by pharmacy fill/refill data(29) or self-report using the Visual Analog Scale(30, 31); 4) reported heavy drinking ≥four times in the past 4 weeks based on the Timeline Followback (TLFB)(32) or met criteria for alcohol use disorder (based on the Diagnostic and Statistical Manual (DSM)-IVcriteria for alcohol abuse and dependence)(33); 5) ≥18 years old; and 6) able to understand English and provide informed consent. Patients were excluded if they met any of the following criteria: 1) were psychotic or severely psychiatrically disabled; 2) enrolled in formal treatment for alcohol (excluding self-help, e.g. Alcoholics Anonymous); 3) had a serious medical condition that would preclude study participation; 4) had significant liver dysfunction (alanine aminotransferase [ALT] or aspartate aminotransferase [AST] greater than 5 times the upper limit of the normal range) or cirrhosis with a Child-Pugh classification greater than A or B; 5) had a contraindication to naltrexone (e.g. required opioid medication for pain), polylactide-co-glycolide (PLG), carboxymethylcellulose, or any other components of the diluent; 6) were a woman who was pregnant, nursing or unable to use an effective method of birth control; or 7) tested opioid positive on a urine test. To assess for eligibility, potential patients underwent evaluation for alcohol, drug or psychiatric disorders, a physical examination, blood work, urine sample and pregnancy test for women. Assessments included the Structured Clinical Interview (SCID) for alcohol(34) and the Addiction Severity Index Lite from which a composite alcohol subscale score was calculated(35). Participants were enrolled from April 2011 through February 2015. The study was approved by the Human Investigation Committee of Yale School of Medicine, VA Connecticut Healthcare System, and the James J. Peters VA Medical Center. A certificate of confidentiality was obtained from the National Institute on Alcohol Abuse and Alcoholism. Patients provided written informed consent.
Treatment Conditions
Enrolled participants were randomized to either XR-NTX or placebo. All patients received counseling.
Medication: extended-release naltrexone and placebo
Participants received their randomly assigned treatment (XR-NTX or placebo) throughout the 24-week treatment period, administered by a study nurse or physician. Those assigned to XR-NTX received 380mg (4mL) administered as an intramuscular gluteal injection at four-week intervals.
To maintain blinding, injections were prepared in colored syringes that made XR-NTX and placebo indistinguishable. Participants who missed a monthly dose were scheduled as soon as possible for their next injection to maximize the number of injections during the study period.
Counseling intervention: medical management with medication coaching
The manualized intervention was delivered to participants randomized to either XR-NTX or placebo, and integrated two efficacious behavioral treatments: Medical Management (MM) and Medication Coaching (MC). MM incorporates skills and advice used by primary care practitioners, coupled with referrals to Alcoholics Anonymous(36). MC’s focus was on addressing ART adherence among people with substance use(8, 37).
The initial counseling visit lasted approximately 45 minutes, while eight follow-up visits were approximately 20–30 minutes each and occurred biweekly for the first two months and then monthly. Content from these original manuals (i.e., MM and MC) was integrated to avoid overlap and to emphasize areas of relevance to HIV (Appendix I). This was led by one of the investigative team’s psychologists and then refined with input from other members of the investigative team. Trained study nurses followed the manual and sessions were taped and reviewed to monitor fidelity. Physician visits followed a structured visit form that prompted review of alcohol and other drug use, the associated impact on health and social function, changes in medications, adherence to injections and any associated side effects, and indicated adjustments in treatment plans.
Assignment of Treatment
Participants were randomly assigned 1:1 to XR-NTX 380mg (4mL) or placebo (4mL injections of microspheres without XR-NTX) within a web-based clinical trials management system(38). The randomization scheme was written in blocks of 6 and 8 and stratified by site and presence of alcohol use disorder and executed by the study pharmacist. All other research staff and the participant were blinded to treatment allocation.
Outcome Measures
The primary outcome, the proportion of participants with ≥95% ART adherence, was defined using pharmacy fill/refill data to derive the medication possession ratio (MPR: the total days supply/refill interval over the prior 90 day interval)(39). In cases where the MPR might not be reliable, including automatic pharmacy fills, use of visiting nurse agency or mobile health van, and/or recent initiation of ART in the past 30 days, a Visual Analog Scale (VAS) was used to measure medication adherence(30, 31, 40). For participants where the MPR data was deemed unreliable at any time point (n=24), VAS data were used throughout the study; otherwise, MPR data (n=27) were used to enhance reliability within a participant. Type of adherence measure used did not differ by assigned condition (p=.32). However, because VAS adherence values were higher than MPR (p<001), we controlled for measure type for all analyses. In sensitivity analyses, given estimates that the necessary levels of adherence to achieve an undetectable viral load occur between 90–95%, we also examined the proportion of participants with ≥90% ART adherence(41).
Secondary outcomes included the proportion of participants with an undetectable HIV viral load (defined as <50 copies/mL), CD4 cell count, and VACS Index score based on laboratory data. The VACS Index is a validated biomarker(42–44) that predicts morbidity and mortality and is sensitive to changes in health-related behaviors, including ART adherence and alcohol use(3, 45). Drinking outcomes included number of heavy drinking days and, in sensitivity analyses, the number of any drinking days in the past 30 days using the TLFB(20, 32). In post-hoc analysis, alcohol use was assessed with phosphatidylethanol (PEth), an alcohol biomarker that reflects alcohol exposure over the prior 21 days with higher levels consistent with greater alcohol use(46).
Adverse events (AEs) were assessed during physician visits with the Systematic Assessment for Treatment Emergent Effects (SAFTEE)(47).
Sample size calculations and statistical analysis
The power calculation was based on detecting a difference, over the 24-week intervention period, in ART adherence and heavy drinking days in the XR-NTX group compared to the placebo group. Regarding adherence, we estimated that 40% of the XR-NTX group and 20% of the placebo group would demonstrate ≥95% ART adherence. Regarding the heavy drinking outcomes, using a 25% difference in days of heavy drinking between XR-NTX and placebo(48) and prior work demonstrating that non-heavy drinking days is highly correlated with ART adherence(4), we anticipated a moderate effect size of 0.50 of XR-NTX, compared to placebo, on heavy drinking. For a power (β) of 72% and a two-tailed α=0.05, a random effect of slope for individual, and a test of the between groups linear trend, our target sample size was 154 participants allowing for a 5% attrition rate.
For baseline characteristics, we evaluated differences between assigned conditions using t-test for continuous measures (with non-parametric alternatives for highly non-normal distributions) and chi-square for categorical measures. For proportion achieving ≥95% ART adherence and an undetectable HIV viral load we used General Estimating Equations (GEE) logistic regression model with robust variance estimation and autoregressive (AR1) working correlation structure with intercept to evaluate the effects of condition over time(49, 50). For CD4 cell count, VACS Index score, number of heavy drinking days and number of any drinking days, we used Linear Mixed Models with random effect of patient using autoregressive (AR1) working correlation structure and using baseline values as covariates for cases with baseline differences(49, 50). In post-hoc analysis, alcohol use was assessed based on PEth using Poisson regression GEE. Drinking estimates were rounded up to the full day for heavy drinking or any drinking days. Condition was determined based on intention-to-treat principles.
To address missing HIV biomarker data, we used two procedures. First, we conducted multiple imputation (NORM 2.03)(51, 52) of estimates for missing values for viral load categorization <50 copies/mL and CD4 cell count using other demographics (age, sex and race) and other biomarkers CD4 (for HIV viral load only), WBC count, hemoglobin, hematocrit, platelets, glucose, creatinine, albumin, alanine aminotransferase and aspartate aminotransferase, gamma-glutamyl transferase, hepatitis B status, hepatitis C status). Twenty-four cases included sufficient biomarker data to impute viral load categorization. Seventeen cases were imputed for CD4. Second, we consolidated data into 12 week blocks. Because the 12 week block could have up to three points of data collection, there were instances in which viral load was available for one or more of the timepoints, but the other timepoints were imputed. For cases in which imputed values and observed values were available for the same time frame, the viral load categorization (above or below 50 copies/mL) was the same for all available data for 75% (39/52) of the cases. We considered p<.05 as statistically significant. All analyses were conducted using SPSS 22 (Armonk, NY: IBM Corp.).
RESULTS
Baseline measures
Demographic and clinical characteristics
Based on 1000 reviewed charts, including 837 individuals screened, 81 of whom were assessed for eligibility, 51 were enrolled into the study and randomized (Figure I), reflecting 33% of targeted enrollment. Among these 51 enrolled participants, 48% (n=24) were seeking treatment with referral based on self, a friend or peer, an advertisement, or a community organization. Among participants randomized to XR-NTX group, five were lost to follow-up and one discontinued the intervention; among those randomized to placebo group, seven were lost to follow-up and two discontinued the intervention. Demographic and clinical characteristics of enrolled patients are described in Table I.
Figure I.
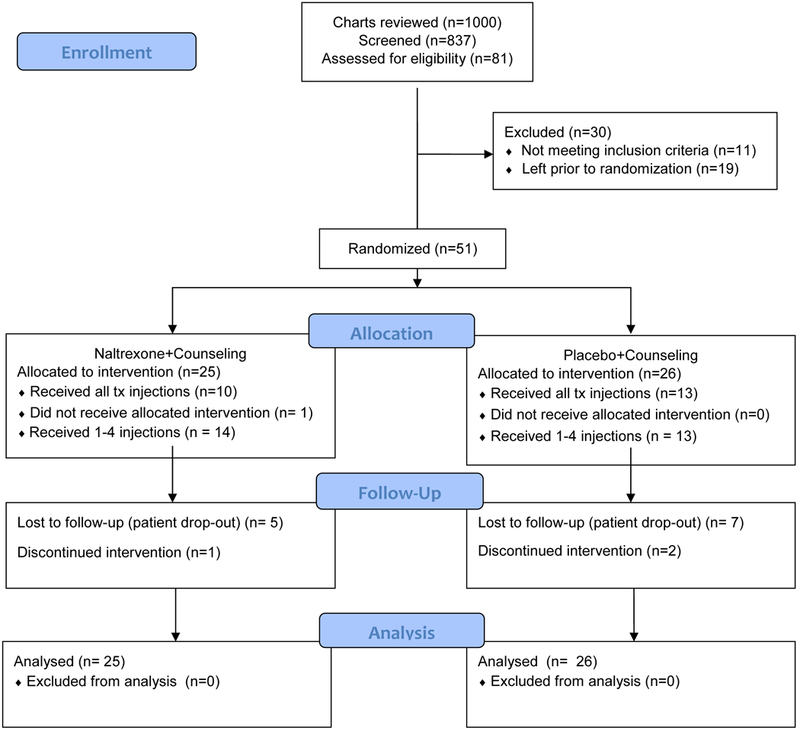
CONSORT Flow Diagram
Table I.
Participant Demographic and Clinical Characteristics
| Characteristic, % (n) | Overall, N=51 | Placebo + Counseling, N=26 | Extended-release Naltrexone + Counseling, N=25 | p value |
|---|---|---|---|---|
| Gender, male | 71% (36) | 73% (19) | 68% (17) | .69 |
| Race/ethnicity | .33 | |||
| White-non Hispanic | 16% (7) | 8% (2) | 24% (6) | |
| Black-non-Hispanic | 71% (36) | 73% (19) | 68% (17) | |
| Hispanic | 8% (4) | 12% (3) | 4% (1) | |
| Other | 6% (3) | 8% (2) | 4% (1) | |
| Age, mean (SD), years | 51.2 (8.2) | 51.2 (9.2) | 51.2 (7.6) | .99 |
| Education (n = 48) | .93 | |||
| Less than high school graduate | 23% (11) | 25% (6) | 21% (5) | |
| High school graduate or equivalent | 35% (17) | 33% (8) | 38% (9) | |
| At least some college | 42% (20) | 42% (10) | 42% (10) | |
| Employed full-time or part-time | 12% (6) | 16% (4) | 8% (2) | |
| Marital Status (n = 50) | .59 | |||
| Never married | 68% (34) | 68% (17) | 68% (17) | |
| Married/cohabitating | 12% (6) | 8% (2) | 16% (4) | |
| Separated/divorced/widowed | 20% (10) | 24% (6) | 16% (4) | |
| ART Adherence, HIV Biomarkers. VACS Index | ||||
| ART Adherence, mean (SD) | 55% (32) | 59% (31) | 51% (33) | .38 |
| Undetectable HIV viral loadᶧ | 51% (26) | 62% (16) | 40% (10) | .12 |
| CD4 count, cells/mm3, mean (SD)ᶧ | 522 (380) | 513 (423) | 457 (313) | ..62 |
| VACS Index score, mean (SD)ᶧ | 43 (26) | 44 (26) | 42 (26) | .28 |
| Substance Use Disorder and Treatment History | ||||
| Number of heavv drinkina davs in the past 30 days, mean (SD) | 14.7 (9.8) | 16.4 (8.4) | 11.3 (8.4) | .03 |
| Number of days with any drinking in the past 30 days, mean (SD) | 17.9 (8.8) | 19.2 (7.5) | 14.8 (8.7) | .06 |
| Alcohol abuse/dependence | 69% (35) | 65% (17) | 72% (18) | .26 |
| Drug abuse/dependence | 76% (39) | 73% (19) | 80% (20) | .56 |
| Addiction Severity Index alcohol composite | 0.26 (0.20) |
0.29 (0.22) | 0.23 (0.16) | .38 |
| Prior alcohol or drug treatment? | 65% (32) | 63% (15) | 68% (17) | .69 |
| Prior receipt for medications to help with drinking? | 12% (6) | 12% (3) | 12% (3) | .96 |
| Prior use of naltrexone | 4% (2) | 8% (2) | 0% (0) | |
| Prior use of acamprosate | 2% (1) | 0% (0) | 2% (1) | |
| Prior use of disulfiram | 6% (3) | 4% (1) | 8% (2) | |
Asterisk (*): Mann-Whitney U test.
indicates based on raw data.
Outcome measures
ART adherence
Based on adjusted means, the proportion of participants who achieved ≥95% ART adherence did not differ by treatment group (p=.38) or by treatment group over time (p=.97) (Figure II). In sensitivity analyses evaluating the proportion of participants who achieved ≥90% ART adherence, there was also no difference by treatment group or treatment group over time (p values >.55).
Figure II.
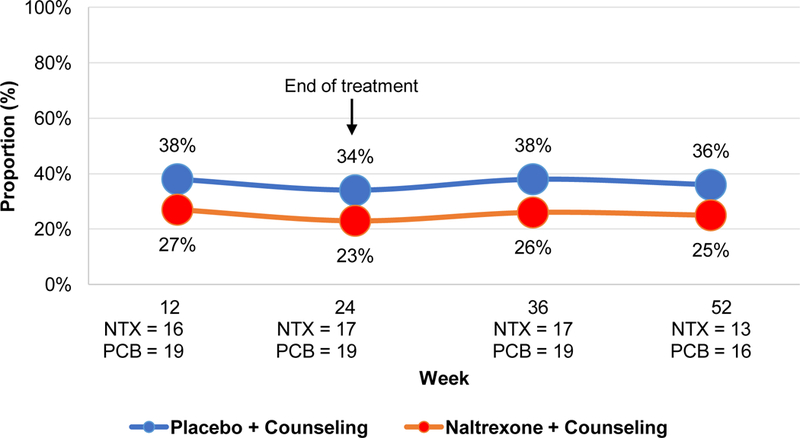
Estimated proportion achieving ≥95% ART Adherence
Note: p value for group=0.38, group*time=0.97. NTX=extended release naltrexone; PCB=placebo. Presented values are adjusted for baseline differences.
HIV biomarkers
Based on adjusted means, there were no differences across conditions or across conditions over time for the proportion achieving an undetectable HIV viral load (Figure III). The proportion of patients with an undetectable HIV viral load increased after baseline but this failed to reach stochastic significance (p=.06). There was a significant interaction of condition by time for CD4 value (p=.01). Based on adjusted means, CD4 was lower for the XR-NTX group compared to the placebo group at baseline, although not significantly so (p=.06), but were similar for all assessments during and after treatment (p value for group=.75) (Figure III). Based on adjusted means, VACS Index scores did not differ by condition (p=.70), or over time (p=.63), nor did the pattern over time differ by condition (p=.83) (data not shown).
Figure III.
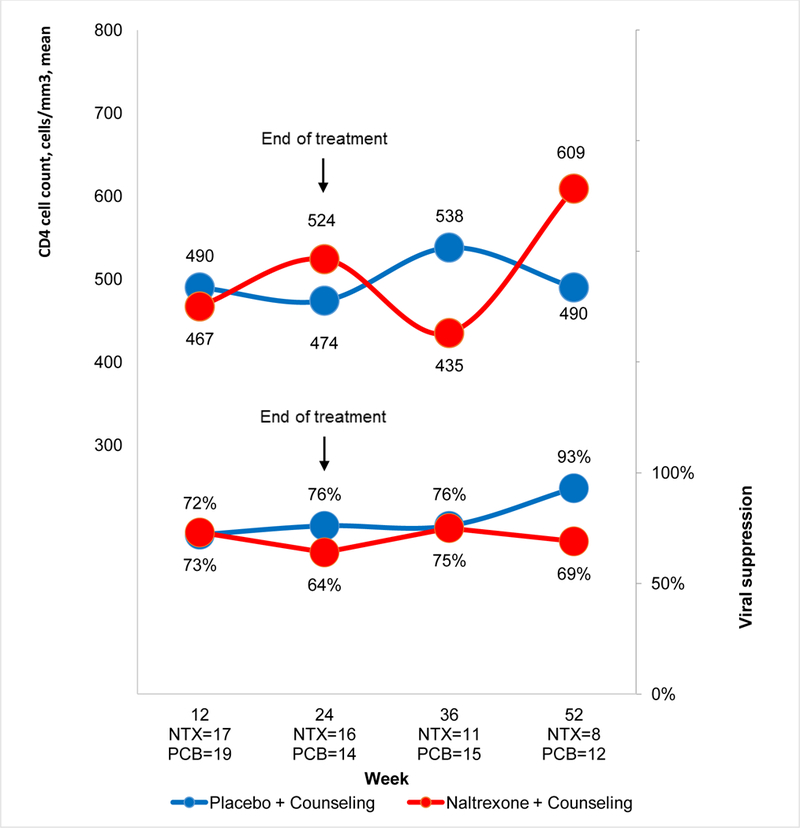
HIV biomarkers: Proportion achieving an undetectable HIV viral load and mean CD4 cell count
Notes: Proportion achieving an undetectable viral load: p value for group=0.26, group*time=0.20; Estimated mean CD4 cell count: p value for group=0.75, group*time=0.01. NTX=extended release naltrexone; PCB=placebo. Presented values are adjusted for baseline differences and using imputed values.
Alcohol use
Based on adjusted means, compared to those assigned to the placebo group, participants assigned to the XR-NTX group had fewer past 30-day heavy drinking days (p value for group=.03); this treatment effect did not differ over time (p=.63) (Figure IV). Similar findings were observed for past 30-day any drinking days (p value for group=.02; p value for group*time interaction=.31). In post-hoc analysis, PEth values appeared to decrease over time for the XR-NTX group (p=.03), but not the placebo group (p=0.94, p value for group=.64) (Appendix Figure I). The interaction term was not significant (group*time interaction =.12).
Figure IV.
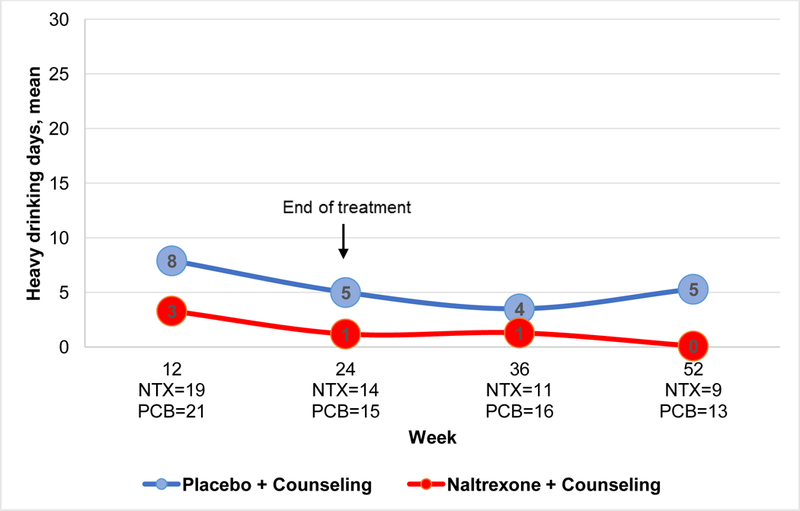
Estimated number of heavy drinking days, past 30 days
Notes: p value for group=0.03, group*time=0.63; NTX=extended release naltrexone; PCB=placebo. Presented values are adjusted for baseline differences.
Process measures
Receipt of the intervention
Over the 24-week intervention period, patients randomized to XR-NTX and placebo received a similar number of injections [mean of 3.62 (SD=2.08, range 0–6) versus 3.65 (SD=2.17, range 1–6), respectively; p=0.96], physician visits [XR-NTX, 3.6 (SD=2.1), placebo, 3.8 (SD=2.0), p=.82], and nurse provided counseling sessions [XR-NTX, 4.9 (SD=2.3), placebo, 4.3 (SD=2.7), p=.45]. Treatment completion was also similar across conditions [XR-NTX 76% (19), placebo 65% (17), p=.42].
Adverse events
While 51% of patients (n = 26) experienced one or more AEs most were judged to be of mild to moderate severity and 18% (n=9) of patients had a serious adverse event (SAE). The proportion of patients in each condition who had an AE (p=.48) or a SAE (p=.24) did not differ by condition, nor did the severity of the event (p=.16) or the number of days of the event (p=.75). Only one AE (and no SAEs) was judged to be possibly related to the study. Type of AEs varied substantially from gastrointestinal, neurological, muscular, psychiatric, slips and falls, legal, family and social problems, and problems related to alcohol and drug use.
DISCUSSION
This is one of few randomized controlled trials to investigate the efficacy of XR-NTX among HIV-positive patients with heavy alcohol use to address HIV-related outcomes. It yields several important findings. First, engagement and retention of HIV-positive individuals with heavy alcohol use in HIV treatment settings for an alcohol treatment intervention is challenging; despite a multi-pronged approach, we only recruited one third of planned participants. Second, compared to placebo, we were unable to detect an impact of XR-NTX on ART adherence or HIV biomarkers. Third, XR-NTX was associated with a clinically and statistically significant decrease in heavy drinking days and any drinking days with consistent though non-significant findings observed based on PEth. Interestingly, findings persisted beyond the treatment period.
Prior work demonstrates benefits of integrating treatment of substance use disorder into HIV treatment settings to reach patients and improve substance use and HIV-related outcomes(10, 12, 13). Notably, our study is one of the first to examine the impact of XR-NTX targeting HIV-positive patients and specifically in HIV treatment settings(25). A 16-week, HIV clinic-based single site study evaluated the feasibility and preliminary efficacy of XR-NTX versus treatment as usual among HIV-positive patients seeking treatment for alcohol use disorder (n=27), opioid use disorder (n=16) or both (n=8)(25). They found that XR-NTX was feasible in an HIV clinic. Our study extends these findings as we focused on alcohol, targeted non-treatment and treatment seeking patients; and had longer-term follow-up. Despite a multipronged recruitment strategy, we did not meet recruitment targets. This likely reflects low motivation among HIV-positive patients attending routine health visits given use of alcohol as a maladaptive coping mechanism and unawareness of associated medical risks(15, 53–55). One qualitative study focused on barriers to XR-NTX initiation among a sample of 15 HIV-positive individuals found that a third of participants did not believe their alcohol use was a big enough of a problem to warrant medication treatment and that distance to treatment was problematic(56). To address these factors, in our study the counseling intervention was designed to help participants understand the impact of alcohol on their health, with a focus on its potential impact on ART adherence. In addition, we provided bus passes to help with the transportation challenges. Another barrier to recruitment was that alcohol use and its consequences may have been under-recognized by providers(16, 56, 57) and potentially eligible patients may have not been referred for the study. Nonetheless, we successfully engaged patients who had largely been untreated for their alcohol use previously highlighting a need for such interventions in HIV treatment settings. Our findings that decreases in drinking persisted among those randomized to XR-NTX after the active treatment phase ended are consistent with promising recent real-world data demonstrating decreased craving and alcohol use while in treatment as well as 30 day and 60 days post-injection (58).
Contrary to our hypotheses, compared to placebo, we did not find that XR-NTX was associated with increases in the proportion of participants achieving ≥95% ART adherence or any associated improvement in HIV-related biomarkers. This was despite evidence that XR-NTX was associated with a decrease in both past 30-day heavy drinking and any drinking days. Prior work demonstrates an association between heavy alcohol use and ART adherence(4, 59). That we did not observe an effect of XR-NTX on the proportion achieving high levels of ART adherence may relate to our sample size as we were underpowered to detect the anticipated effect of XR-NTX on ART adherence. Additionally, findings may relate to our choice of control condition as regardless of treatment condition, participants received an evidence-based counseling intervention to promote medication adherence. Furthermore, our adherence measures may have lacked sensitivity to detect smaller changes as captured by other metrics (e.g. TLFB-based methods, drug levels). Similarly, XR-NTX was not associated with improvements in HIV biomarkers. This is consistent with the existing literature, which does not reveal a consistent effect of alcohol or XR-NTX(22, 25) on HIV disease progression(2). A prior study reported that compared to baseline, among those with alcohol use disorder only, the proportion with an undetectable HIV viral load decreased among those assigned to XR-NTX (92% vs. 82%) and remained unchanged among those assigned to treatment as usual (100% vs. 100%) at 16 weeks(25). While a recent study by our group found that patterns of alcohol use over time are associated with VACS Index scores, patients were observed over eight years; how quickly changes in alcohol use translate into changes in VACS Index is unknown(60).
Consistent with the literature in non-HIV positive patients(20), XR-NTX was associated with a decrease in heavy drinking days and any drinking days in this sample of HIV-positive participants. PEth results supported these findings. While these findings contrast with findings from a recent study examining the impact of XR-NTX vs. placebo among HIV-positive people released from prison, in which an effect on alcohol was not observed in the main analysis, they are consistent with findings in the afore referenced pilot(25, 26). Interestingly, our findings were observed as an overall group effect with significant differences between groups at each time point during the treatment phase and at 12 months. These findings are encouraging and suggest durability of treatment effects after the study treatment phase and may relate to participants receiving treatment through routine clinical care and/or sustained behavior change in the absence of treatment. Furthermore, that participants in both groups experienced a decrease in estimated number of heavy drinking days post-treatment may relate to sustained effects of the counseling intervention.
Our study should be interpreted in the context of its limitations. First, given our sample size, we were underpowered to detect anticipated differences by treatment condition. Second, given counseling provided in our control condition, we are unable to comment on how these findings would compare if participants had received usual care. Third, missing data is a challenge to the field(61, 62). However, we applied multiple imputation to address these concerns, consistent with standards in the field(63). Fourth, these findings may not be generalizable to patients not engaged in HIV care. Lastly, as is standard in the field, for our primary outcome, we relied on participant self-reported measures of alcohol use that may be subject to social desirability and recall bias. Due to blinding, this should not differ by treatment condition. Further, PEth findings are consistent with the self-reported data; however, the sample size limits definitive conclusions.
In summary, by offering XR-NTX in HIV treatment settings, we engaged HIV-positive heavy drinkers with suboptimal ART adherence who generally had limited prior alcohol treatment. While we were unable to detect any benefits to ART adherence and HIV biomarkers, XR-NTX led to clinically and statistically significant decreases in alcohol use that were sustained over time. Alternative strategies to promote ART adherence and improve HIV biomarkers among HIV-positive heavy drinkers are needed and future evaluations of the impact of XR-NTX in larger sample sizes are warranted.
Supplementary Material
ACKNOWLEDGEMENTS
Contributors: We would like to acknowledge Ms. Orli Florsheim, Dr. Lydia Barakat, Dr. Michael Kozal and Mr. Steven Farber for their contributions and support in conducting this work.
Source of Funding: This study was funded by the U.S. National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (5R01AA018923). EJ Edelman was supported as a Yale Drug Abuse, Addiction, and HIV Research Scholar during the conduct of this work (NIDA K12 DA033312). Alkermes donated medication for this study.
Prior Presentations: Findings from this study were presented at the Annual Research Society on Alcoholism Conference, June 24, 2017 in Denver, Colorado.
Footnotes
Disclosures: An earlier version of this work was presented at the Research Society on Alcoholism Annual Conference, June 26, 2017, in Denver, Colorado.
Conflicts of Interest: The authors have no conflicts of interest.
Conflict of Interest: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much: A Clinician’s Guide In: Services DoHaH, editor. 2005. [Google Scholar]
- 2.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcohol Clin Exp Res. 2016;40(10):2056–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 2016;161:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29(7):1190–7. [DOI] [PubMed] [Google Scholar]
- 5.Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J Gen Intern Med. 2001;16(2):83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn JA, Samet JH. Alcohol and HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep. 2010;7(4):226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahler CW, Liu T, Cioe PA, Bryant V, Pinkston MM, Kojic EM, et al. Direct and Indirect Effects of Heavy Alcohol Use on Clinical Outcomes in a Longitudinal Study of HIV Patients on ART. AIDS Behav. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan LE, Barry D, Moore BA, Chawarski MC, Tetrault JM, Pantalon MV, et al. A trial of integrated buprenorphine/naloxone and HIV clinical care. Clin Infect Dis. 2006;43 Suppl 4:S184–90. [DOI] [PubMed] [Google Scholar]
- 9.Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56 Suppl 1:S22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiellin DA, Weiss L, Botsko M, Egan JE, Altice FL, Bazerman LB, et al. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr. 2011;56 Suppl 1 :S33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng TY, Krebs P, Schoenthaler A, Wong S, Sherman S, Gonzalez M, et al. Combining Text Messaging and Telephone Counseling to Increase Varenicline Adherence and Smoking Abstinence Among Cigarette Smokers Living with HIV: A Randomized Controlled Study. AIDS Behav. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walley AY, Palmisano J, Sorensen-Alawad A, Chaisson C, Raj A, Samet JH, et al. Engagement and Substance Dependence in a Primary Care-Based Addiction Treatment Program for People Infected with HIV and People at High-Risk for HIV Infection. J Subst Abuse Treat. 2015;59:59–66. [DOI] [PubMed] [Google Scholar]
- 13.Korthuis PT, Fiellin DA, Fu R, Lum PJ, Altice FL, Sohler N, et al. Improving adherence to HIV quality of care indicators in persons with opioid dependence: the role of buprenorphine. J Acquir Immune Defic Syndr. 2011;56 Suppl 1 :S83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drainoni ML, Farrell C, Sorensen-Alawad A, Palmisano JN, Chaisson C, Walley AY. Patient perspectives of an integrated program of medical care and substance use treatment. AIDS Patient Care STDS. 2014;28(2):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredericksen RJ, Edwards TC, Merlin JS, Gibbons LE, Rao D, Batey DS, et al. Patient and provider priorities for self-reported domains of HIV clinical care. AIDS Care. 2015;27(10):1255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chander G, Monroe AK, Crane HM, Hutton HE, Saag MS, Cropsey K, et al. HIV primary care providers--Screening, knowledge, attitudes and behaviors related to alcohol interventions. Drug Alcohol Depend. 2016;161:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasin DS, Aharonovich E, O’Leary A, Greenstein E, Pavlicova M, Arunajadai S, et al. Reducing heavy drinking in HIV primary care: a randomized trial of brief intervention, with and without technological enhancement. Addiction. 2013;108(7):1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samet JH, Horton NJ, Meli S, Dukes K, Tripps T, Sullivan L, et al. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antivir Ther. 2005;10(1):83–93. [DOI] [PubMed] [Google Scholar]
- 19.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46(4):443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889–900. [DOI] [PubMed] [Google Scholar]
- 21.Vagenas P, Di Paola A, Herme M, Lincoln T, Skiest DJ, Altice FL, et al. An evaluation of hepatic enzyme elevations among HIV-infected released prisoners enrolled in two randomized placebo-controlled trials of extended release naltrexone. J Subst Abuse Treat. 2014;47(1):35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tetrault JM, Tate JP, McGinnis KA, Goulet JL, Sullivan LE, Bryant K, et al. Hepatic Safety and Antiretroviral Effectiveness in HIV-Infected Patients Receiving Naltrexone. Alcohol Clin Exp Res. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garbutt JC, Greenblatt AM, West SL, Morgan LC, Kampov-Polevoy A, Jordan HS, et al. Clinical and biological moderators of response to naltrexone in alcohol dependence: a systematic review of the evidence. Addiction. 2014;109(8):1274–84. [DOI] [PubMed] [Google Scholar]
- 24.Lee JD, Grossman E, Huben L, Manseau M, McNeely J, Rotrosen J, et al. Extended-release naltrexone plus medical management alcohol treatment in primary care: findings at 15 months. J Subst Abuse Treat. 2012;43(4):458–62. [DOI] [PubMed] [Google Scholar]
- 25.Korthuis PT, Lum PJ, Vergara-Rodriguez P, Ahamad K, Wood E, Kunkel LE, et al. Feasibility and safety of extended-release naltrexone treatment of opioid and alcohol use disorder in HIV clinics: a pilot/feasibility randomized trial. Addiction. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Springer SA, Di Paola A, Azar MM, Barbour R, Krishnan A, Altice FL. Extended-release naltrexone reduces alcohol consumption among released prisoners with HIV disease as they transition to the community. Drug Alcohol Depend. 2017;174:158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson DA, Smith SM, Saha TD, Rubinsky AD, Grant BF. Comparative performance of the AUDIT-C in screening for DSM-IV and DSM-5 alcohol use disorders. Drug Alcohol Depend. 2012;126(3):384–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–95. [DOI] [PubMed] [Google Scholar]
- 29.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–74; discussion 75–7. [DOI] [PubMed] [Google Scholar]
- 30.Kalichman SC, Amaral CM, Swetzes C, Jones M, Macy R, Kalichman MO, et al. A simple single-item rating scale to measure medication adherence: further evidence for convergent validity. J Int Assoc Physicians AIDS Care (Chic). 2009;8(6):367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5(2):74–9. [DOI] [PubMed] [Google Scholar]
- 32.Sobell LC, Sobell SM. Alcohol Timeline Followback (TLFB); Handbook of Psychiatric Measures. Washington, D.C. : American Psychiatric Association; 1996. [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (fifth edition) Washington, D.C.: American Psychiatric Press; 2013. [Google Scholar]
- 34.Kranzler HR. Pharmacotherapy of alcoholism: gaps in knowledge and opportunities for research. Alcohol Alcohol. 2000;35(6):537–47. [DOI] [PubMed] [Google Scholar]
- 35.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. [DOI] [PubMed] [Google Scholar]
- 36.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–17. [DOI] [PubMed] [Google Scholar]
- 37.Haug NA, Sorensen JL, Gruber VA, Lollo N, Roth G. HAART adherence strategies for methadone clients who are HIV-positive: a treatment manual for implementing contingency management and medication coaching. Behav Modif. 2006;30(6):752–81. [DOI] [PubMed] [Google Scholar]
- 38.Nadkarni PM, Brandt C, Frawley S, Sayward FG, Einbinder R, Zelterman D, et al. Managing attribute--value clinical trials data using the ACT/DB client-server database system. J Am Med Inform Assoc. 1998;5(2):139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988;26(8):814–23. [DOI] [PubMed] [Google Scholar]
- 40.Oyugi JH, Byakika-Tusiime J, Charlebois ED, Kityo C, Mugerwa R, Mugyenyi P, et al. Multiple validated measures of adherence indicate high levels of adherence to generic HIV antiretroviral therapy in a resource-limited setting. J Acquir Immune Defic Syndr. 2004;36(5):1100–2. [DOI] [PubMed] [Google Scholar]
- 41.Ortego C, Huedo-Medina TB, Llorca J, Sevilla L, Santos P, Rodriguez E, et al. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS Behav. 2011;15(7):1381–96. [DOI] [PubMed] [Google Scholar]
- 42.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54(7):984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Justice AC, Modur SP, Tate JP, Althoff KN, Jacobson LP, Gebo KA, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62(2):149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. Aids. 2013;27(4):563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bebu I, Tate J, Rimland D, Mesner O, Macalino GE, Ganesan A, et al. The VACS index predicts mortality in a young, healthy HIV population starting highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;65(2):226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wurst FM, Thon N, Weinmann W, Tippetts S, Marques P, Hahn JA, et al. Characterization of sialic acid index of plasma apolipoprotein J and phosphatidylethanol during alcohol detoxification--a pilot study. Alcohol Clin Exp Res. 2012;36(2):251–7. [DOI] [PubMed] [Google Scholar]
- 47.Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22(2):343–81. [PubMed] [Google Scholar]
- 48.O’Malley SS, Rounsaville BJ, Farren C, Namkoong K, Wu R, Robinson J, et al. Initial and maintenance naltrexone treatment for alcohol dependence using primary care vs specialty care: a nested sequence of 3 randomized trials. Arch Intern Med. 2003;163(14):1695–704. [DOI] [PubMed] [Google Scholar]
- 49.Brown CH, Wang W, Kellam SG, Muthen BO, Petras H, Toyinbo P, et al. Methods for testing theory and evaluating impact in randomized field trials: intent-to-treat analyses for integrating the perspectives of person, place, and time. Drug Alcohol Depend. 2008;95 Suppl 1 :S74–S104. PMC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.West BT. Analyzing longitudinal data with the linear mixed models procedure in SPSS. Eval Health Prof. 2009;32(3):207–28. [DOI] [PubMed] [Google Scholar]
- 51.Schafer JL. Analysis of incomplete multivariate data in Monographs on statistics and applied probability 72, 1st Ed Boca Raton, Florida: Chapman & Hall/CRC; 1997. [Google Scholar]
- 52.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. [DOI] [PubMed] [Google Scholar]
- 53.Elliott JC, Aharonovich E, O’Leary A, Wainberg M, Hasin DS. Drinking motives among HIV primary care patients. AIDS Behav. 2014;18(7):1315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elliott JC, Aharonovich E, O’Leary A, Johnston B, Hasin DS. Perceived medical risks of drinking, alcohol consumption, and hepatitis C status among heavily drinking HIV primary care patients. Alcohol Clin Exp Res. 2014;38(12):3052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elliott JC, Stohl M, Aharonovich E, O’Leary A, Hasin DS. Reasons for drinking as predictors of alcohol involvement one year later among HIV-infected individuals with and without hepatitis C. Annals of medicine. 2016;48(8):634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chokron Garneau H, Venegas A, Rawson R, Ray LA, Glasner S. Barriers to initiation of extended release naltrexone among HIV-infected adults with alcohol use disorders. J Subst Abuse Treat. 2018;85:34–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montague BT, Kahler CW, Colby SM, McHugh RK, Squires D, Fitzgerald B, et al. Attitudes and Training Needs of New England HIV Care and Addiction Treatment Providers: Opportunities for Better Integration of HIV and Alcohol Treatment Services. Addict Disord Their Treat. 2015;14(1):16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crevecoeur-MacPhail D, Cousins SJ, Denering L, Kim T, Rawson RA. Effectiveness of extended release naltrexone to reduce alcohol cravings and use behaviors during treatment and at follow-up. J Subst Abuse Treat. 2018;85:105–8. [DOI] [PubMed] [Google Scholar]
- 59.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2): 180–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marshall BDL, Tate JP, McGinnis KA, Bryant KJ, Cook RL, Edelman EJ, et al. Long-term alcohol use patterns and HIV disease severity. AIDS. 2017;31(9):1313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Witkiewitz K, Finney JW, Harris AH, Kivlahan DR, Kranzler HR. Recommendations for the Design and Analysis of Treatment Trials for Alcohol Use Disorders. Alcohol Clin Exp Res. 2015;39(9):1557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Witkiewitz K, Falk DE, Kranzler HR, Litten RZ, Hallgren KA, O’Malley SS, et al. Methods to analyze treatment effects in the presence of missing data for a continuous heavy drinking outcome measure when participants drop out from treatment in alcohol clinical trials. Alcohol Clin Exp Res. 2014;38(11):2826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hallgren KA, Witkiewitz K. Missing data in alcohol clinical trials: a comparison of methods. Alcohol Clin Exp Res. 2013;37(12):2152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


