Summary
The isolation and biochemical characterization of lipid droplet-associated mitochondria revealed the capacity of the cell to produce and maintain distinct mitochondrial populations carrying disparate proteome and dissimilar capacities to oxidize fatty acids and pyruvate. With mitochondrial motility being a central parameter determining mitochondrial fusion, adherence to lipid droplets provides a mechanism by which peridroplet mitochondria (PDM) remain segregated from cytoplasmic mitochondria (CM). The existence of metabolically distinct subpopulations provide an explanation for the capacity of mitochondria within the individual cell to be involved simultaneously in fatty acid oxidation and lipid droplet (LD) formation. The mechanisms that deploy mitochondria to the LD and the dysfunction that results from unbalanced proportions of PDM and CM remains to be explored. Understanding the roles and regulation of mitochondrial tethering to lipid droplets offers new points of intervention in metabolic diseases.
Keywords: Mitochondria, lipid droplet, peridroplet mitochondria, obesity, adipose tissue, fat, lipid, triacylglyceride, triacylglycerol, fatty acid oxidation
eTOC blurb
Benador et al. describe arrest of mitochondrial motility and fusion as a mechanism by which mitochondria segregate and develop specialized proteome and metabolic capacity within the same cell. Specialized mitochondrial subpopulations explain how a single cell can be involved simultaneously in fatty acid oxidation and lipid droplet formation.
Introduction
Biochemistry taught us that mitochondrial involvement in lipogenesis and triacylglyceride synthesis are antagonistic to mitochondrial fat oxidation by beta oxidation. Specifically, increased malonyl-CoA production in the synthetic pathway has been shown to block mitochondrial fatty acid entry through CPT1/CAT/CPT2 system (McGarry et al., 1973, 1978). This regulatory mechanism established the notion that mitochondria within a cell can either support the synthesis or the oxidation of lipids, never both at the same time. However, this cornerstone biochemical finding was directly challenged by recent reports that brown adipocytes and immune cells can simultaneously accommodate both synthesis and oxidation of fatty acids (Mottillo et al., 2014; O’Sullivan et al., 2014; Sanchez-Gurmaches et al., 2018). The concurrency of these two antagonistic mitochondrial processes within the same cell could not be explained biochemically.
It is the field of mitochondrial dynamics (fusion, fission, and motility) that provides the tools to conceptualize the mechanisms to explain the concurrency of antagonistic mitochondrial metabolic processes inside the same cell. Early studies suggested that the entire population of mitochondria with a cell goes though frequent fusion event, leading to all mitochondria sharing similar content. In the past decade, an evolving understanding that mitochondria are involved in fusion in a selective manner has revealed the possibility that the cellular population of mitochondria is divided into distinct subpopulations (Twig et al., 2008). Segregation of mitochondria into disparate subpopulations was initially found to be a key mechanism to separate and eliminate dysfunctional mitochondria. However, the recent report that mitochondria bound to lipid droplets (peridroplet mitochondria) are segregated from cytoplasmic mitochondria and selectively promote triacylglyceride synthesis represents a new utility for segregation as it enables the cell to perform antagonistic metabolic processes (Benador et al., 2018).
Segregation of mitochondria into subpopulations is achieved by the suppression of fusion activity of a subset of mitochondria, allowing them to develop disparate cristae structures, proteomes and super-complexes. Interestingly, elimination of dysfunctional mitochondria relies on preventing their fusion with the rest of the mitochondrial network, a task achieved by the inactivation of their fusion proteins. In contrast, segregation of peri-droplet mitochondria (PDM) is achieved while keeping their fusion proteins intact. In turn, another key component required for fusion event is eliminated: mitochondrial motility (Twig et al., 2010). PDM are found to be anchored to the lipid droplets, which reduces their motility and suggests that inter-organelle binding is a mechanism for reduced motility and segregation (Benador et al., 2018). The finding that PDM have distinct proteome and metabolic capabilities put forward the concept that inter-organelle linkers represent a mechanism for subcellular specialization of groups of mitochondria. Here we review the concept of PDM as the foundation of a novel node of control for lipid metabolism.
What is the definition of peridroplet mitochondria?
Mitochondria associated with lipid droplets (LDs) have been observed as early as 1959 (Palade) and have since been reported in a variety of cell culture models and tissue types (Figure 1). Despite this, the metabolic role of PDM remained unclear until recently. To better understand the role and regulation of PDM, we need a formal definition of what constitutes a peridroplet mitochondrion based on quantifiable parameters. We suggest to define a peridroplet mitochondrion as a mitochondrion that meets one or more of the following essential criteria:
A mitochondrion that has significant membrane contact with LD (>20% of the mitochondria perimeter) visualized by electron microscopy. At least in the brown adipose tissue, the contact area is characterized by electron dense structure between the mitochondria and the LD surface. (Benador et al., 2018; Bleck et al., 2018; Herms et al., 2015; Palade, George, 1959; Tarnopolsky et al., 2007)
A mitochondrion that remains adherent to LD after mechanical cell disruption and/or restrictive purification conditions such as trypsinization and high salt wash (Benador et al., 2018; Yu et al., 2015).
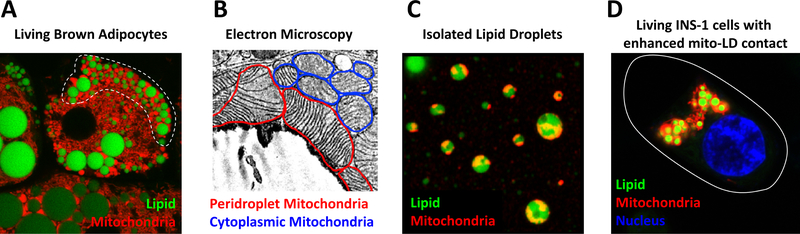
Figure 1.
Images of peridroplet mitochondria. A. Living cultured brown adipocytes with lipid droplets stained with BODIPY (green) and mitochondria stained with MitoTracker Red (red). White line denotes peridroplet mitochondria. B. Electron micrograph of brown adipose tissue demonstrating mitochondria (red line) associated with a lipid droplet (LD). C. Isolated brown adipose tissue lipid droplets stained with BODIPY and MitoTracker Red, note the adherence of mitochondrial particles to lipid droplets. D. Living cultured INS-1 insulinoma cells over-expressing the lipid coating protein Plin5, which recruits mitochondria to lipid surface, stained with DAPI, BODIPY, and MitoTracker. Cell boarder marked by white line.
Additional features that can serve as supporting criteria may include the following:
A mitochondrion with >50% of its cross sectional area within 0.5 micrometers of LD border visualized by light microscopy (Benador et al., 2018; Herms et al., 2015; Nguyen et al., 2017; Rambold et al., 2015; Stone et al., 2009)
A mitochondrion that has significantly lower motility and fusion rates compared to the cell’s average cytoplasmic mitochondrion (Benador et al., 2018).
A mitochondrion associated with LD surface via the mitochondrion’s long-axis rather than the short-axis (Benador et al., 2018). In such orientation, the cristae of the mitochondrion are perpendicular to the LD surface.
As described in the criteria above, a mitochondrion must have imaging or physical evidence of adherence to a lipid droplet in order to be defined as a peridroplet mitochondrion. Importantly, the physical association between mitochondria and LDs requires a modified approach to mitochondrial isolation in order to separate PDM from cytoplasmic mitochondria as traditional preparations discard and/or dilute PDM (Benador et al., 2018). In summary, we suggest two essential criteria for the definition of PDM and the considerations of additional features involving PDM dynamics. We look forward to the evolution of these criteria as more discoveries are made about the composition, function, and regulation of PDM in different tissues.
What are the roles of peridroplet mitochondria in lipid metabolism?
Clues toward the physiological roles of PDM in lipid metabolism can be found in conditions during which PDM assume larger or lower portions of the total cellular mitochondrial content (Table 1). If PDM primarily play a role in LD accumulation, one would expect that a higher proportion of mitochondria will be bound to LDs under physiological conditions of increased LD synthesis. On the other hand, if PDM are part of a cellular adaptation for fatty acid oxidation (FAO) in mitochondria an increase in abundance of PDM would be expected under conditions in which fatty acid oxidation rate is maximized. Comparing the relative abundance of PDM under different physiological conditions can therefore provide evidence toward the physiological roles of PDM.
Table 1.
Conditions that increase mitochondrial recruitment to LDs
| Cell type | Condition | Mito-LD interaction | LD Mass | FAO | |
|---|---|---|---|---|---|
| Brown adipose tissue | |||||
| (Benador et al., 2018) | Mouse BAT | Thermoneutrality compared to cold exposure | Higher | Higher | Lower |
| (Yu et al., 2015) | Mouse BAT | Thermoneutrality compared to cold exposure | Higher (Table S6) | Higher | Lower |
| Cell culture | |||||
| (Wang et al., 2011a) | CHO, AML12, HL-1 cells | Oleate loading in Plin5 overexpression vs GFP control | Higher | Higher | Lower |
| (Benador et al., 2018) | Primary brown adipocytes | Plin5 vs C-truncated Plin5 | Higher | Higher | N/A |
| (Stone et al., 2009) | COS-7 | Oleate loading DGAT2 overexpression vs DGAT1 and MGAT2 | Higher | Higher | N/A |
| (Rambold et al., 2015) | MEF | Nutrient deprivation compared to complete medium | Higher | Higher | Higher |
| (Nguyen et al., 2017) | MEF | Nutrient deprivation compared to complete medium | Higher | Higher | Higher |
| Striated muscle | |||||
| (Tarnopolsky et al., 2007) | Human Vastus Lateralis muscle | Post-endurance training vs pre-training | Higher | Higher | Higher |
| (Wang et al., 2013) | Mouse Heart | Plin5 overexpression vs Wild Type | Higher | Higher | Lower |
| (Wang et al., 2013) | Mouse Heart | Fasting vs feeding | Higher | Higher | Lower |
| Liver | |||||
| (Arruda et al., 2014) | Mouse liver | Ob/Ob vs wt HFD vs chow | Higher | Higher | N/A |
What can brown adipose tissue teach us about peridroplet mitochondria?
Brown adipocytes tissue (BAT) is a tissue specialized in heat generation (thermogenesis) to protect the body against hypothermia. Under baseline thermoneutral conditions, fatty acids are primarily stored rather than oxidized. Fatty acids destined for storage first undergo ATP-dependent ligation to coenzyme A by acyl-CoA synthetases (ACSLs) followed by esterification to glycerol backbone resulting in synthesis of triacylglycerides (TAGs) and their storage in lipid droplets (Wang et al., 2017). Upon cold exposure, lipase enzymes are rapidly recruited to the LD surface where they liberate free fatty acids that are again converted to acyl-CoA in an ATP-dependent manner before being processed into acyl-carnitines for mitochondrial import, uncoupled oxidation and heat generation. The robust shift from lipid storage to oxidation makes BAT an ideal system to explore the role of PDM.
Proteomic analysis of lipid droplets isolated from mouse BAT identified 130 mitochondrial proteins under thermoneutral conditions, when fatty acids are primary directed toward LD expansion (Yu et al., 2015). In contrast, 85 of these 130 mitochondrial proteins (65%) were undetectable or decreased in LD fraction when mice were under cold exposure, a condition where BAT FAO is maximized for thermogenesis. This remarkable reduction in PDM under cold exposure was confirmed by electron microscopy analysis (Benador et al., 2018). Furthermore, PDM isolated from BAT were found to have lower fatty acid oxidation capacity compared to cytoplasmic mitochondria (CM). Taken together, these reports suggest a primary role for PDM in LD expansion rather than oxidation in brown adipose tissue.
Intriguingly, the presence of PDM may also provide an explanation for a bioenergetic paradox in BAT research: mitochondria isolated from BAT using traditional techniques have remarkably low levels of ATP synthase elementary particles and activity compared to other tissues (Cannon and Vogel, 1977; Lindberg et al., 1967). Initially, this was suspected to be a feature of BAT mitochondria that facilitated uncoupled respiration. However, this low ATP synthesis capacity could not be reconciled with high levels of ATP required for rapid acyl-CoA synthesis and lipid cycling required for cold-induced thermogenesis nor the ATP requirements for triglyceride synthesis occurring during thermoneutrality. The paradox could be reconciled if we take into account that traditionally, isolation of mitochondria from adipose tissue involves the disposal of the floating fat cake thereby depleting PDM from the sample. Remarkably, PDM, purified exclusively from the floating fat cake are found to be enriched in ATP synthase with ATP synthesis capacity over 2-fold higher compared to cytoplasmic mitochondria.
Do peridroplet mitochondria play an active role in lipid droplet expansion?
Mammalian cells are exposed to fatty acids introduced by a variety of different sources, including circulating lipoproteins and albumin-bound lipids, intracellular lipid droplet stores, lipids released by autophagic membrane degradation, and de novo lipid synthesis (Olzmann and Carvalho, 2018). Excess fatty acids are quickly processed into triacylglycerides (TAG) to maintain energy homeostasis and to prevent lipotoxicity. TAG accumulation between the inner and outer membrane leaflets of the endoplasmic reticulum give rise to nascent lipid droplets (Walther et al., 2017). As lipid droplets grow larger and mature, they depart from the ER and acquire a unique set of peridroplet proteins that help regulate their contents and interact with other organelles and enzymes (Olzmann and Carvalho, 2018).
Remarkably, Carole Sztalryd’s group demonstrated that overexpressing the lipid droplet coat protein Perilipin5 (PLIN5) forces mitochondrial recruitment to LDs and enhances LD expansion in CHO, hepatic AML12, and cardiac HL-1 cells, as well as in cardiac tissue in transgenic mice (Wang et al., 2011a, 2013). However, since these studies compared PLIN5 overexpression to GFP overexpression, it was not possible to discern whether the observed changes in LD mass resulted from mitochondrial recruitment to the LD surfaces, lipolysis inhibition by Plin5 (Wang et al., 2011b), or both. Subsequent studies specifically assessed the role of mitochondrial recruitment to LDs by comparing the effect of overexpressing the full transcript of Plin5 to the effect of truncated Plin5 in which the C-terminal mitochondrial targeting sequence was deleted (Benador et al. 2018). These experiments specifically demonstrated that mitochondrial recruitment to LDs promoted a doubling in LD mass and increased the rate of TAG synthesis in brown adipocytes and INS1 cells independently of lipolysis. The association between mitochondrial recruitment to lipid droplet surface and the expansion of the lipid droplet has also been demonstrated by Stone and colleagues, who examined the TAG synthesizing enzyme diacylglycerol acyltransferase (DGAT2). Similar to PLIN5, DGAT2 can recruit mitochondria to LDs and double LD size (Stone et al., 2009). Overexpression of the enzymes DGAT1 or MGAT2, which lack a mitochondrial targeting sequence, do not recruit mitochondria nor increase LD size. These results thus support a role for mitochondrial recruitment in LD expansion in excess nutrient environment.
Evidence for PDM involvement in LD biogenesis has also been observed in cells exposed to excess fatty acids from autophagic membrane degradation under conditions of nutrient deprivation. When cellular nutrient sources are depleted, autophagosomes hydrolyze phospholipids and rapidly release free fatty acids. The liberated fatty acids are ligated to CoA groups before being converted to acyl-carnitine on the outer mitochondrial membrane to enter the mitochondrial matrix via the carnitine palmitoyltransferase system. Surprisingly, Nguyen and colleagues have demonstrated that autophagy activation under nutrient deprivation increases LD mass despite increased reliance on fatty acids for ATP production (Nguyen et al., 2017). This was strongly associated with increased peridroplet mitochondria abundance and demonstrated in multiple cell types. Inhibition of LD formation under these conditions resulted in fatty acid-mediated mitochondrial depolarization and cell death, suggesting that LDs biogenesis protects against lipotoxic insult of rapid autophagosome-mediated fatty acid release.
Rambold and colleagues have similarly observed that nutrient deprivation leads to expansion of LD number with increased mitochondrial elongation and association with LDs (Rambold et al. 2015). Interestingly, inhibition of mitochondrial fusion by Mfn1KO and Opa1KO led to robust mitochondrial dissociation from LDs concomitant with impaired FAO, leading this group to propose that PDM facilitate fatty acids trafficking toward mitochondrial beta oxidation.
Rambold and Nguyen are thus consistent in their observation that PDM are associated with LD expansion in starved cells. However, while Nguyen proposes that the primary function of PDM formation in starved cells is to protect against lipotoxicity, Rambold proposed that PDM formation is essential for cellular adaptation to fatty acid oxidation. Our model of two segregated mitochondrial populations, one specialized in FFA oxidation and the other in LD expansion, reconciles these apparently contradictory findings: PDM can promote sequestration of FFA while protecting against lipotoxic injury and preserving function of cytoplasmic mitochondria under metabolic stress, where fatty acid oxidation can occur. Mechanistically, enhanced pyruvate oxidation by PDM may increase malonyl-CoA levels thus preventing acyl-CoA entry into these specific mitochondria (Figure 2).
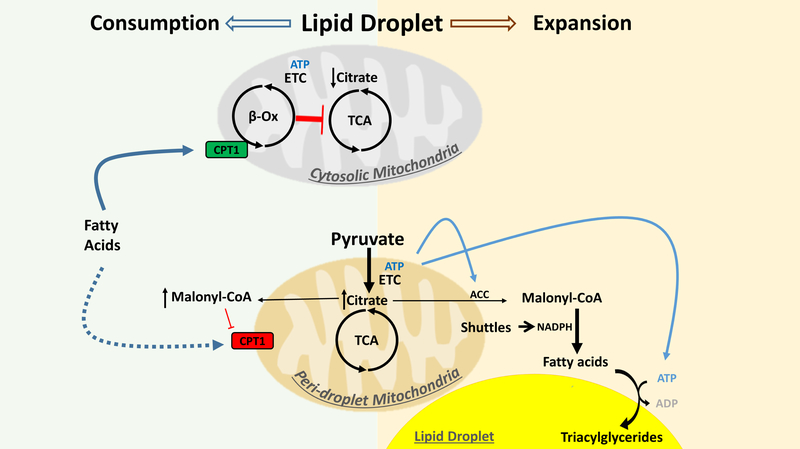
Figure 2.
Scheme of metabolic specialization model of CM and PDM. Metabolic specialization in PDM and CM: Mitochondria support lipogenesis with malonyl-CoA, a metabolite that inhibits FA oxidation by the mitochondria. Separation of mitochondria into PDM and CM may allow for both fatty acid oxidation and lipid synthesis to occur in the same cell at the same time. High pyruvate oxidation capacity in PDM may lead to increased malonyl-CoA pools. Malonyl-CoA can act as a negative regulator of Carnitine palmitoyltransferase I (CPT1) and thereby block fatty acid entry into mitochondria. In addition, malonyl-CoA is a building block for de novo lipid synthesis. Higher ATP synthesis capacity in PDM supports fatty acid esterification into triacylglycerides for lipid droplet expansion. Lower pyruvate oxidation in CM leads to reduced citrate and malonyl-CoA pools, which allow CPT1 to import fatty acids for β-oxidation (β-Ox). Increased β-oxidation products inhibit PDH and several steps of the tricarboxylic acid (TCA) cycle (for details see Figure S1). ETC: Electron transport system, ACC: acetyl-CoA carboxylase.
The expansion of LD mass observed by the Nguyen and Rambold groups in nutrient deprived cultured cells may have important implications when observed in a specialized tissue that is both adipogenic and dependent on fat oxidation for ATP production under starvation conditions: the liver. Despite decreased caloric and fat intake during organismal starvation, the liver receives more fatty acids from white adipose tissue than needed for ATP demand, causing the liver to simultaneously increase fatty acid oxidation and accumulate LDs under starvation (Gan and Watts, 2008). PDM could thus play a role in livers of starved animals where excess fatty acids are stored as LDs while FAO continues to generate ATP. This remains to be established.
What are the mechanisms by which PDM expand lipid droplets?
Recent studies demonstrated that LD are capable of synthesizing TAGs in a cell-free environment when provided with ATP and CoA (Zhang et al., 2016). This suggests that PDM may support LD expansion by providing ATP for TAG synthesis. ATP levels may be especially important for TAG synthesis given the exceptionally high Km of ATP of the enzyme Acyl CoA Synthetase (4.6mM) (Bar-Tana and Shapiro, 1975). Consistent with this interpretation, we demonstrated that increasing PDM recruitment enhances LD expansion by providing ATP to power TAG synthesis in brown adipose cells (Benador et al., 2018). Overall the evidence indicates that PDM promote expansion of LDs by providing ATP for acyl-CoA synthesis in the TAG synthetic pathway. Whether PDM additionally enhance TAG synthesis by providing citrate derived malonyl-CoA for de novo lipogenesis remains an open question.
What are the tethering mechanisms that maintain mitochondria-lipid droplet association and how are they regulated?
Inter-organellar interaction is a rapidly growing field in cell biology that appears to play a key role in cellular adaptation to nutrient environment (Valm et al., 2017). The robust analysis of mitochondria-ER interactions and the linkers involved can teach us some principles that may apply to mitochondria-LD interaction. First is the utilization of artificial linkers. Using artificial drug-inducible linkers, mitochondria-ER tethering has been shown to strongly affect calcium homeostasis and autophagosome formation (Arruda et al., 2014; Csordás et al., 2010). Second is the plethora of proteins that can promote ER-Mitochondria linkage demonstrating the concept that different proteins link ER to mitochondria under different conditions and for different functions (de Brito and Scorrano, 2008; Elsenberg-Bord et al., 2016; Gomez-Suaga et al., 2017; Stoica et al., 2014).
Lipid droplets are unique among cellular organelles in having a phospholipid monolayer with distinct biophysical requirements for interaction. Integral membrane protein complexes that have been implicated in LD interactions with mitochondria include DGAT2-FATP1 and RAB18-NRZ/SNARE. However, LDs have also been shown to interact with other organelles via fusion of the outer membrane leaflet. This type of interaction allows the direct exchange of metabolites and enzymes with the docking organelle (Thazar-Poulot et al., 2015). Despite the wealth of data available on mitochondria-LD interactions, the molecular composition and the regulation of mitochondria-LD tethering remains largely unknown.
Jagerstrom and colleagues first demonstrated that isolated mitochondria can interact with isolated LDs in a cell-free environment, suggesting that components anchored to the outer organellar membranes are sufficient to mediate mitochondria-LD interaction (Jägerström et al., 2009) (Table 2). One such protein is PLIN5, a lipid droplet coating protein that has been shown to strongly recruit mitochondria to the LDs in multiple cell and tissue types, including CHO cells, hepatic AML12, cardiac HL-1 cells, primary brown adipocytes, INS1 cells, and mouse heart (Benador et al., 2018; Kimmel and Sztalryd, 2014; Wang et al., 2011a, 2013). Super-resolution microscopy has confirmed that PLIN5 specifically localizes to the mitochondria-LD contact sites (Gemmink et al., 2018). Site-directed mutagenesis of Plin5 revealed a highly conserved sequence of the C-terminus necessary to recruit mitochondria to LDs. Fusion of the Plin5 mitochondrial recruiting sequence to Plin2, which does not recruit mitochondria on its own, was sufficient to induce mitochondrial recruitment. However, the mechanisms by which Plin5 promotes mitochondria-LD interaction remains to be elucidated. For example, fusing the Plin5 mitochondrial recruiting sequence to a fluorescent probe was not sufficient to target the probe to mitochondria (unpublished data and personal communication), suggesting the sequence is necessary but not sufficient to target mitochondria. Furthermore, despite considerable research efforts, a specific mitochondrial outer membrane protein that partners with PLIN5 has yet to be identified (Dr. Carole Sztalyrd, personal communication). The recent discovery that PLIN5 can translocate to the nucleus and regulate gene transcription (Gallardo-Montejano et al., 2016) suggests the possibility that recruitment of mitochondria to LD by PLIN5 involves the induction of expression of the components linking PLIN5 to the mitochondrial outer membrane.
Table 2.
Hypothesized tethers and regulators of Mitochondria-LD interaction
| Cell type | Technique | Potential mediators of mitochondria-LD association | |
|---|---|---|---|
| (Jägerström et al., 2009) | NIH 3T3 | Isolated mitochondria and lipid droplets in a cell-free system | Factors on outer surfaces of organelles. |
| (Wang et al., 2011a) Benador et al. 2018 | CHO, AML12, HL-1, Primary brown adipocytes, INS1 | Site-directed mutagenesis and confocal fluorescence microscopy. | C-terminus of Plin5 as potential regulator (Mitochondrial target unknown) |
| (Stone et al., 2009) | COS-7 | Site-directed mutagenesis, confocal fluorescence microscopy, and cell fractionation. | N-terminus of DGAT2 as potential recruiter (DGAT2 dimers) |
| (Boutant et al., 2017) | Primary brown adipocytes | Co-IP | Plin1-Mfn2 |
| (Yu et al., 2015) | BAT | Western blot analysis of mitochondrial proteins in isolated lipid droplets subjected to tryptic digestion, high salt wash, and detergent wash. | Membrane hemifusion |
Additional proteins involved in mitochondrial recruitment to LD are currently being investigated. Stone and colleagues demonstrated that DGAT2, which localizes to the ER and LDs membranes, strongly recruited mitochondria to lipid droplets in COS-7 cells (Stone et al., 2009). Site-directed mutagenesis revealed that DGAT2 possesses an N-terminal mitochondrial targeting sequence that is necessary and sufficient to recruit mitochondria to LD. Unlike the mitochondrial targeting sequence of Plin5, the sequence identified in DGAT2 was sufficient to target fluorescent probes to mitochondria. Since DGAT2 has been shown to form dimers (Jin et al., 2014), its dual localization to mitochondria and LDs and ability to form dimers could promote mitochondria-LD tethering. Interestingly, Mitoguardin-2 (MIGA2) is a third protein that has been recently reported to recruit mitochondria to LD in WAT (Dr. Robin Klemm, personal communication). As with mitochondria-ER tethers, different tissues may utilize distinct tethers. For example, WAT expresses higher levels of DGAT2 and MIGA2 compared to Plin5.
Lastly, mitochondria-LD tethering has been proposed to involve mechanisms beyond protein-protein interaction. Studies by Yu and colleagues found that mitochondria-LD association is resistant to tryptic digestion and high salt wash (Yu et al., 2015). This suggests that protein-protein interaction may not entirely explain the mechanism of mitochondria-LD tethering. The sensitivity of mitochondria-LD association to detergents suggests that mitochondria-LD association may be further stabilized by membrane-membrane interactions, such as fusion with the outer leaflet of the mitochondrial outer membrane. Much experimental work remains to be done to elucidate the mechanisms and regulation of mitochondria-LD interaction.
What is the role of peridroplet mitochondria in lipid handling in different tissues?
White adipose tissue
White adipose tissue is specialized in storing excess lipids for later use. Pre-adipocytes progressively accumulate small lipid droplets as they differentiate toward mature adipocytes, where the majority of the cytoplasm is occupied by a large unilocular lipid droplet. Adipocytes in early stages of differentiation, where de novo lipogenesis and LD synthesis rate is highest, have high levels of mitochondrial mass compared to adipocytes in late stages of differentiation, where lipid synthesis is relatively lower (Goldman et al., 2011). The observed increase in pyruvate oxidation capacity of PDM relative to cytoplasmic mitochondria (Benador et al., 2018) may promote high rates of malonyl-CoA formation, leading to enhanced lipid synthesis, reduced lipolysis, and reduced lipid oxidation (Benador et al., 2018). The higher levels of Complex I+III super assembly observed in PDM compared to cytoplasmic mitochondria (Benador et al., 2018) may provide a mechanism for increased utilization of pyruvate-derived NADH and elevated ATP formation (Acín-Pérez et al., 2008). Thus, while not directly studied, the correlation between mitochondrial mass and LD expansion rate suggests that PDM may play a role in de novo lipogenesis and LD building in the early stages of adipocyte differentiation.
Skeletal muscle
LDs have been shown to expand in skeletal muscle tissue in both obese and trained athletes with opposite effects on insulin sensitivity (athlete’s paradox). Electron microscopy studies have shown that LDs primarily localize to the interfibrillar compartment in athletes, while obese individuals accumulated LDs in the subsarcolemmal region (Nielsen et al., 2010). Additional studies have demonstrated that increased PDM are associated with endurance exercise and increased interfibrillar LD accumulation in human skeletal muscle (Tarnopolsky et al., 2007). This suggests that muscle PDM play a role in healthy, rather than pathogenic, LD expansion. In support of this concept, reports have shown that muscle-specific Plin5 overexpression increased PDM and protected muscle tissue from high fat diet-induced lipotoxicity (Bosma et al., 2012; Laurens et al., 2016). However, as mentioned before, it remains unclear whether Plin5 effects were mediated directly by PLIN5 interaction with the LD, by mitochondrial recruitment to the LD, or both. Further studies are needed to determine the precise role of PDM in physiological and pathological LD accumulation in vivo.
Liver
The liver is specialized in synthesizing lipids post-prandially for distribution to other tissues by secreting lipoproteins, a specialized form of lipid packaging for fat delivery to peripheral tissues. The liver significantly expands intrahepatocyte lipid droplet stores both in long-term fasting and under pathological conditions of excess dietary fatty acids, the latter eventually progressing to a chronic inflammatory state and cirrhosis. Although PDM have been observed in liver as early as 1959 (Palade), the physiological role of PDM in hepatocytes remains unknown. There is evidence to suggest that PDM recruitment is increased with liver steatosis. For example, electron microscopy images of livers from HFD and genetic models of obesity appear to have increased levels of PDM (Arruda et al., 2014). Furthermore, high-fat feeding increases the expression of the mitochondrial recruiting protein Plin5 in both human and mouse liver tissue. Finally, overexpression of Plin5 and DGAT2 increased LD mass and protected against lipotoxic liver injury in mice (Monetti et al., 2007; Trevino et al., 2015; Wang et al., 2015). These reports thus suggest that PDM expand LD to protect against lipotoxic liver injury induced by nutrient excess.
Do peridroplet mitochondria play a role in cancer?
Alterations in lipid handling are not only observed in metabolic diseases but are also an important hallmark in cancer (Petan et al., 2018; Tirinato et al., 2017). Upregulation of TAG-FFA cycling has been proposed to be involved in the maintenance of human breast cancer cell survival (Przybytkowski et al., 2007) and LD accumulation has been shown to be associated with tumor aggressiveness and chemotherapy resistance in colorectal, glioblastoma, and prostate cancer (Cotte et al., 2018; Geng et al., 2016; Yue et al., 2014). If PDM support LD expansion in cancer cells, blocking PDM recruitment could provide an attractive therapeutic target to stop tumor progression and promote chemotherapy sensitivity. However, PDM have not been directly studied in cancer to date.
Further implications of PDM in mitochondrial biology
Conditions characterized by increased mitochondrial ATP synthesis capacity have been associated with elongated mitochondrial networks (Liesa and Shirihai, 2013). On the other hand, induction of mitochondrial uncoupling lead to mitochondrial fragmentation (Wikstrom et al., 2014). While these associations describe the behavior of the entire mitochondrial population within a cell, a closer examination of the brown adipocyte reveals the co-existence of elongated and fragmented mitochondria within each individual cell (Benador et al., 2018). Mitochondria associated with LD appear to be distinctly elongated compared to cytoplasmic mitochondria. The bioenergetic characteristics of elongated vs fragmented mitochondria match the architecture of the two groups, where PDM have increased coupled respiration, while CM have increased uncoupling. These observations also imply an association between mitochondrial fragmentation and detachment of mitochondria from LDs.
Various studies have examined the link between mitochondrial dynamics and mitochondrial metabolic functions. The existence of PDM as a metabolically distinct group requires fusion selectivity that prevents PDM from mixing with CM. As such, impaired control of mitochondrial fusion or motility may affect the capacity of the cell to maintain the unique functions of PDM, CM or both. In this context, PDM offers a link between dysfunctional mitochondrial dynamics and metabolic dysregulation.
While previous studies assumed the life cycle of the mitochondria to be a singular cycle encompassing all mitochondria equally, additional studies have reported on the observations that some mitochondria tend to be multi-fusers, while other mitochondria in the same cell fuse at lower frequency (Twig et al., 2010). With mitochondrial motility being a central parameter determining fusion likelihood, mitochondrial adherence to other organelles provides a mechanism by which such populations can remain segregated and develop disparate structure and composition allowing for subsets of mitochondria to be simultaneously involved in antagonistic and competing processes. Furthermore, the distinct life cycles of mitochondrial subsets suggests the possibility that the different groups of mitochondria within the same cell have different levels of turnover and quality control. If that is the case, we expect that PDM may have different capacity to handle damage, a prediction yet to be tested.
There is much to be learned about the stringency of segregation between PDM and CM. One would expect a relationship between the extent of content exchanged between PDM and CM, and the differences that will be evolving between them over time. While shorter periods of segregation may limit the differences between the PDM and CM proteomes to short living proteins, prolonged segregation could extend the differences to long living proteins such as ATP synthase and/or affect mtDNA content and integrity. This may have important implications for pathological conditions involving accumulated mtDNA damage, such as mtDNA mutations, oxidative injury, and aging.
Conclusions
The existence of PDM demonstrates that essential fat metabolism processes can be selectively confined to exclusive and segregated subsets of mitochondria, offering a novel mode of metabolic regulation to be explored. Understanding the impact of PDM/CM ratio and the mechanism that control this ratio may provide a new form of mitochondrial function and dysfunction that is beyond the mere bioenergetics capacities.
Supplementary Material
Acknowledgements
We would like to thank Dr. Carole Sztalryd for guiding our lab into the field of Plin5 biology. We would like to thank Harold Sacks for insightful discussions on the role of PDM and human adipose tissue in clinical contexts. We would like to thank Saverio Cinti for insightful discussions on white adipose tissue biology. We would like to thank Dr. Marc Prentki and Dani Dagan for editorial help and Scott Wilde for cartoon illustrations. We would like to thank Drs. Rebeca Acin-Perez, Linsey Stiles, Anton Petcherski, Guy Las, Essam Assali, Marcus Oliveira, Jakob Wikstrom, Barbara Corkey, Linsey Stiles, Karen Reue, Jonathan Wanagat, David Shackelford, Anthony Jones, Heather Christofk, Sarah Cohen, Gyorgy Hajnoczky and Scot Stone for insightful discussions. Our work is supported by NIHNIDDK 5-R01DK099618-02, RO1DK56690, by the UCLA Department of Medicine Chair commitment, UCSD/UCLA Diabetes Research Center pilot grant NIH P30 DK063491 and T32DK007201 Training Program in Metabolism, Endocrinology, and Obesity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acín-Pérez R, Fernández-Silva P, Peleato ML, Pérez-Martos A, and Enriquez JA (2008). Respiratory active mitochondrial supercomplexes. Mol. Cell 32, 529–539. [DOI] [PubMed] [Google Scholar]
- Arruda AP, Pers BM, Parlakgül G, Güney E, Inouye K, and Hotamisligil GS (2014). Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat. Med 20, 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Tana J, and Shapiro B (1975). Long-chain fatty acyl-CoA synthetase from rat liver microsomes. EC 6.2.1.3 fatty acyl-CoA ligase (ATP). Methods Enzymol. 35, 117–122. [DOI] [PubMed] [Google Scholar]
- Benador IY, Veliova M, Mahdaviani K, Petcherski A, Wikstrom JD, Assali EA, Acín-Pérez R, Shum M, Oliveira MF, Cinti S, et al. (2018). Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab. 27, 869–885.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleck CKE, Kim Y, Willingham TB, and Glancy B (2018). Subcellular connectomic analyses of energy networks in striated muscle. Nat. Commun 9, 5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma M, Minnaard R, Sparks LM, Schaart G, Losen M, de Baets MH, Duimel H, Kersten S, Bickel PE, Schrauwen P, et al. (2012). The lipid droplet coat protein perilipin 5 also localizes to muscle mitochondria. Histochem. Cell Biol 137, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, and Scorrano L (2008). Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610. [DOI] [PubMed] [Google Scholar]
- Cannon B, and Vogel G (1977). The mitochondrial ATPase of brown adipose tissue. Purification and comparison with the mitochondrial ATPase from beef heart. FEBS Lett. 76, 284–289. [DOI] [PubMed] [Google Scholar]
- Cotte AK, Aires V, Fredon M, Limagne E, Derangère V, Thibaudin M, Humblin E, Scagliarini A, Barros J.-P.P. de, Hillon P, et al. (2018). Lysophosphatidylcholine acyltransferase 2mediated lipid droplet production supports colorectal cancer chemoresistance. Nat. Commun 9, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G, Várnai P, Golenár T, Roy S, Purkins G, Schneider TG, Balla T, and Hajnóczky G (2010). Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol. Cell 39, 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenberg-Bord M, Shai N, Schuldiner M, and Bohnert M (2016). A tether is a tether is a tether: tethering at membrane contact sites. Dev. Cell 39, 395–409. [DOI] [PubMed] [Google Scholar]
- Gallardo-Montejano VI, Saxena G, Kusminski CM, Yang C, McAfee JL, Hahner L, Hoch K, Dubinsky W, Narkar VA, and Bickel PE (2016). Nuclear Perilipin 5 integrates lipid droplet lipolysis with PGC-1α/SIRT1-dependent transcriptional regulation of mitochondrial function. Nat. Commun 7, 12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S, and Watts G (2008). Is adipose tissue lipolysis always an adaptive response to starvation? Implications for non-alcoholic liver disease. Clin. Sci 114, 543–545. [DOI] [PubMed] [Google Scholar]
- Gemmink A, Daemen S, Kuijpers HJH, Schaart G, Duimel H, López-Iglesias C, van Zandvoort MAMJ, Knoops K, and Hesselink MKC (2018). Super-resolution microscopy localizes perilipin 5 at lipid droplet-mitochondria interaction sites and at lipid droplets juxtaposing to perilipin 2. Biochim. Biophys. Acta BBA - Mol. Cell Biol. Lipids [DOI] [PubMed] [Google Scholar]
- Geng F, Cheng X, Wu X, Yoo JY, Cheng C, Guo JY, Mo X, Ru P, Hurwitz B, Kim S-H, et al. (2016). Inhibition of SOAT1 Suppresses Glioblastoma Growth via Blocking SREBP-1-Mediated Lipogenesis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res 22, 5337–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SJ, Zhang Y, and Jin S (2011). Autophagic Degradation of Mitochondria in White Adipose Tissue Differentiation. Antioxid. Redox Signal. 14, 1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Suaga P, Paillusson S, Stoica R, Noble W, Hanger DP, and Miller CCJ (2017). The ER-Mitochondria Tethering Complex VAPB-PTPIP51 Regulates Autophagy. Curr. Biol 27, 371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms A, Bosch M, Reddy BJN, Schieber NL, Fajardo A, Rupérez C, Fernández-Vidal A, Ferguson C, Rentero C, Tebar F, et al. (2015). AMPK activation promotes lipid droplet dispersion on detyrosinated microtubules to increase mitochondrial fatty acid oxidation. Nat. Commun 6, 7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jägerström S, Polesie S, Wickström Y, Johansson BR, Schröder HD, Højlund K, and Boström P (2009). Lipid droplets interact with mitochondria using SNAP23. Cell Biol. Int 33, 934–940. [DOI] [PubMed] [Google Scholar]
- Jin Y, McFie PJ, Banman SL, Brandt C, and Stone SJ (2014). Diacylglycerol acyltransferase2 (DGAT2) and monoacylglycerol acyltransferase-2 (MGAT2) interact to promote triacylglycerol synthesis. J. Biol. Chem 289, 28237–28248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel AR, and Sztalryd C (2014). Perilipin 5, a lipid droplet protein adapted to mitochondrial energy utilization. Curr. Opin. Lipidol 25, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens C, Bourlier V, Mairal A, Louche K, Badin P-M, Mouisel E, Montagner A, Marette A, Tremblay A, Weisnagel JS, et al. (2016). Perilipin 5 fine-tunes lipid oxidation to metabolic demand and protects against lipotoxicity in skeletal muscle. Sci. Rep 6, 38310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesa M, and Shirihai OS (2013). Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 17, 491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg O, de Pierre J, Rylander E, and Afzelius BA (1967). Studies of the mitochondrial energy-transfer system of brown adipose tissue. J. Cell Biol 34, 293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD, Meier JM, and Foster DW (1973). The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J. Biol. Chem 248, 270–278. [PubMed] [Google Scholar]
- McGarry JD, Leatherman GF, and Foster DW (1978). Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J. Biol. Chem 253, 4128–4136. [PubMed] [Google Scholar]
- Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, et al. (2007). Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6, 69–78. [DOI] [PubMed] [Google Scholar]
- Mottillo EP, Balasubramanian P, Lee Y-H, Weng C, Kershaw EE, and Granneman JG (2014). Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J. Lipid Res jlr.M050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TB, Louie SM, Daniele JR, Tran Q, Dillin A, Zoncu R, Nomura DK, and Olzmann JA (2017). DGAT1-Dependent Lipid Droplet Biogenesis Protects Mitochondrial Function during Starvation-Induced Autophagy. Dev. Cell 42, 9–21.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Mogensen M, Vind BF, Sahlin K, Højlund K, Schrøder HD, and Ortenblad N (2010). Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. Am. J. Physiol. Endocrinol. Metab 298, E706–713. [DOI] [PubMed] [Google Scholar]
- Olzmann JA, and Carvalho P (2018). Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan D, van der Windt GJW, Huang SC-C, Curtis JD, Chang C-H, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, et al. (2014). Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade George (1959). Subcellular Particles (The Ronald Press Company, New York: ). [Google Scholar]
- Petan T, Jarc E, Jusović M, Petan T, Jarc E, and Jusović M (2018). Lipid Droplets in Cancer: Guardians of Fat in a Stressful World. Molecules 23, 1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybytkowski E, Joly E, Nolan CJ, Hardy S, Francoeur A-M, Langelier Y, and Prentki M (2007). Upregulation of cellular triacylglycerol - free fatty acid cycling by oleate is associated with long-term serum-free survival of human breast cancer cells. Biochem. Cell Biol. Biochim. Biol. Cell 85, 301–310. [DOI] [PubMed] [Google Scholar]
- Rambold AS, Cohen S, and Lippincott-Schwartz J (2015). Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell 32, 678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Tang Y, Jespersen NZ, Wallace M, Martinez Calejman C, Gujja S, Li H, Edwards YJK, Wolfrum C, Metallo CM, et al. (2018). Brown Fat AKT2 Is a Cold-Induced Kinase that Stimulates ChREBP-Mediated De Novo Lipogenesis to Optimize Fuel Storage and Thermogenesis. Cell Metab. 27, 195–209.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica R, De Vos KJ, Paillusson S, Mueller S, Sancho RM, Lau K-F, Vizcay-Barrena G, Lin W-L, Xu Y-F, Lewis J, et al. (2014). ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat. Commun 5, 3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SJ, Levin MC, Zhou P, Han J, Walther TC, and Farese RV (2009). The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J. Biol. Chem 284, 5352–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, and Hamadeh MJ (2007). Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am. J. Physiol. Regul. Integr. Comp. Physiol 292, R1271–1278. [DOI] [PubMed] [Google Scholar]
- Thazar-Poulot N, Miquel M, Fobis-Loisy I, and Gaude T (2015). Peroxisome extensions deliver the Arabidopsis SDP1 lipase to oil bodies. Proc. Natl. Acad. Sci 112, 4158–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirinato L, Pagliari F, Limongi T, Marini M, Falqui A, Seco J, Candeloro P, Liberale C, and Di Fabrizio E (2017). An Overview of Lipid Droplets in Cancer and Cancer Stem Cells. [DOI] [PMC free article] [PubMed]
- Trevino MB, Mazur-Hart D, Machida Y, King T, Nadler J, Galkina EV, Poddar A, Dutta S, and Imai Y (2015). Liver Perilipin 5 Expression Worsens Hepatosteatosis But Not Insulin Resistance in High Fat-Fed Mice. Mol. Endocrinol. Baltim. Md 29, 1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al. (2008). Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27, 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Liu X, Liesa M, Wikstrom JD, Molina AJA, Las G, Yaniv G, Hajnóczky G, and Shirihai OS (2010). Biophysical properties of mitochondrial fusion events in pancreatic β-cells and cardiac cells unravel potential control mechanisms of its selectivity. Am. J. Physiol. - Cell Physiol 299, C477–C487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, Cohen AR, Davidson MW, Betzig E, and Lippincott-Schwartz J (2017). Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 546, 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC, Chung J, and Farese RV (2017). Lipid Droplet Biogenesis. Annu. Rev. Cell Dev. Biol 33, 491–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhao Y, Gao X, Li L, Yuan Y, Liu F, Zhang L, Wu J, Hu P, Zhang X, et al. (2015). Perilipin 5 improves hepatic lipotoxicity by inhibiting lipolysis. Hepatol. Baltim. Md 61, 870–882. [DOI] [PubMed] [Google Scholar]
- Wang H, Sreenivasan U, Sreenevasan U, Hu H, Saladino A, Polster BM, Lund LM, Gong D, Stanley WC, and Sztalryd C (2011a). Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J. Lipid Res 52, 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bell M, Sreenivasan U, Sreenevasan U, Hu H, Liu J, Dalen K, Londos C, Yamaguchi T, Rizzo MA, et al. (2011b). Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J. Biol. Chem 286, 15707–15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sreenivasan U, Gong D-W, O’Connell KA, Dabkowski ER, Hecker PA, Ionica N, Konig M, Mahurkar A, Sun Y, et al. (2013). Cardiomyocyte-specific perilipin 5 overexpression leads to myocardial steatosis and modest cardiac dysfunction1. J. Lipid Res 54, 953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Airola MV, and Reue K (2017). How lipid droplets “TAG” along: Glycerolipid synthetic enzymes and lipid storage. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862, 1131–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom JD, Mahdaviani K, Liesa M, Sereda SB, Si Y, Las G, Twig G, Petrovic N, Zingaretti C, Graham A, et al. (2014). Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J. 33, 418–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang S, Cui L, Wang W, Na H, Zhu X, Li L, Xu G, Yang F, Christian M, et al. (2015). Lipid droplet remodeling and interaction with mitochondria in mouse brown adipose tissue during cold treatment. Biochim. Biophys. Acta 1853, 918–928. [DOI] [PubMed] [Google Scholar]
- Yue S, Li J, Lee S-Y, Lee HJ, Shao T, Song B, Cheng L, Masterson TA, Liu X, Ratliff TL, et al. (2014). Cholesteryl Ester Accumulation Induced by PTEN Loss and PI3K/AKT Activation Underlies Human Prostate Cancer Aggressiveness. Cell Metab. 19, 393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang Y, Cui L, Deng Y, Xu S, Yu J, Cichello S, Serrero G, Ying Y, and Liu P (2016). Morphologically and Functionally Distinct Lipid Droplet Subpopulations. Sci. Rep 6, 29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.