Abstract
Background:
The tau protein plays a central role in Alzheimer’s disease (AD) and there is huge interest in measuring tau in blood and CSF.
Methods:
We developed a set of immunoassays to measure tau in specimens from humans diagnosed based on current best clinical and CSF biomarker criteria.
Results:
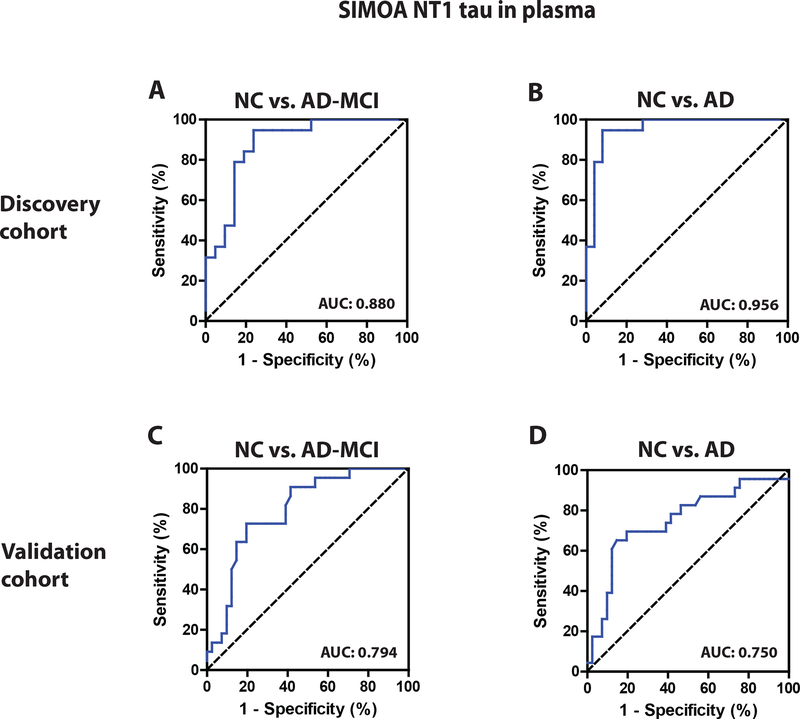
In CSF, mid-region-detected and N-terminal-detected tau predominated and rose in disease. In plasma, an N-terminal assay (NT1) detected elevated levels of tau in AD and AD-mild cognitive impairment (MCI). Plasma NT1 measurements separated controls from AD-MCI (area under the curve, AUC=0.88) and AD (AUC=0.96) in a Discovery Cohort; and in a Validation Cohort (with AUCs=0.79 and 0.75, respectively).
Conclusions:
The forms of tau in CSF and plasma are distinct, but in each specimen type the levels of certain fragments are increased in AD. Measurement of plasma NT1 tau should be aggressively pursued as a potential blood-based screening test for AD/AD-MCI.
Keywords: Biomarker, dementia, enzyme-linked immunoassay, mild cognitive impairment, plasma, Single molecule array
Introduction
Alzheimer’s disease (AD) research is entering the exciting phase in which the hard-won knowledge about the molecular basis of this disorder is being translated into therapeutics. However, recent drug trials have highlighted a need for better diagnosis of trial participants, and development of biomarkers that can be used to monitor response to therapy [1, 2]. Measurement of tau and the amyloid β-protein (Aβ) in CSF, and quantitation of amyloid pathology by positron-emission tomography (PET) imaging are now incorporated into clinical criteria for the diagnosis of AD-dementia, and mild cognitive impairment (MCI) due to AD pathogenesis. But PET is expensive and limited in availability, and CSF sampling is unpopular with patients and is rarely done serially. Thus, there is an urgent need for less costly and intrusive, blood-based biomarkers that can replace or supplement current CSF and PET markers.
Numerous studies have shown that in AD CSF tau is increased [3] and in several cases this has been validated by autopsy confirmation of disease status [3, 4]. Typically, tau assays employ mid-region-directed monoclonal antibodies (mAbs) and often are erroneously referred to as total tau assays. Such assays (including the widely used INNOTEST ELISAs) cannot detect fragments of tau that lack all or part of the mid-region domain. This is important given the growing recognition that the primary structure of extracellular tau is heterogeneous [5–10].
Recent reports suggest that minute amounts of tau can be detected in plasma [11–20] and some studies indicate that certain forms of plasma tau may be increased in AD [14, 15, 18, 21] and are associated with neurodegeneration and cognitive dysfunction [17]. However, there is a poor correlation between tau measured in CSF and in blood, and differences in the antibodies used and hence the forms of tau detected, limit comparisons between studies. Moreover, in the few studies that have measured plasma tau in more than one cohort the results have been conflicting [18].
We report the development and application of a set of validated tau immunoassays capable of detecting different populations of tau fragments in CSF and blood. We applied these assays to the analysis of specimens from three pre-specified diagnostic groups: (1) patients with mild AD and CSF INNOTEST Aβ42 and tau values consistent with a diagnosis of AD, (2) individuals with MCI and CSF INNOTEST values as in (1) (referred to as AD-MCI), and (3) cognitively intact subjects with CSF INNOTEST Aβ42 and tau values in the normal range. By analysing CSF samples using five distinct immunoassays we demonstrate that full-length (FL) tau constitutes only a small fraction of the tau present in both normal and AD CSF, whereas mid-region- and N-terminal-containing fragments are much more abundant and increase with disease. Applying some of the same ultra-sensitive assays to plasma we show that certain N-terminal fragments of tau are elevated in AD and in individuals with AD-MCI. Elevation was seen with an N-terminal assay (NT1) which requires a minimal sequence of 6–198, but not an N-terminal assay (NT2) that requires a larger sequence (6–224). These results suggest that our NT1 assay may be useful as a screening blood-based test for AD.
Materials and Methods
Reagents
Chemicals and reagents were from Sigma-Aldrich unless otherwise noted.
Antibodies
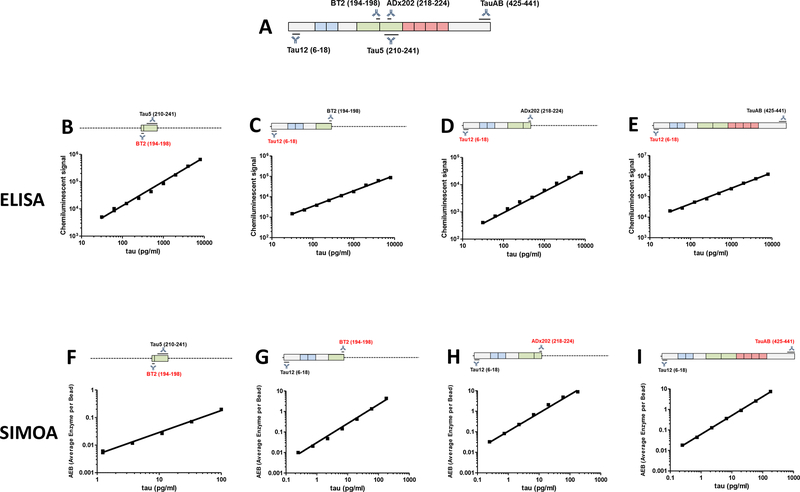
Antibodies used and their source are described in Supplementary Table 1. The anti-tau antibody, TauAB, is a novel mouse monoclonal antibody (mAb) which was produced by traditional hybridoma technology [22] from mice immunized with recombinant human tau441. Subsequent epitope mapping revealed that TauAB recognized an epitope within residues 425–441 (Figure 1A).
Figure 1. Standard curves of plate-based tau ELISAs and ultra-sensitive tau Simoa assays.
(A) Schematic representation of human tau441 and epitopes of the relevant antibodies. (B) Mid-region (BT2-Tau5), (C) NT1 (Tau12-BT2), (D) NT2 (Tau12-ADx202) and (E) full-length (Tau12-TauAB) ELISAs readily detect human tau with LLoQs ~30 pg/ml. Simoa-based (F) mid-region (BT2-Tau5) (LLoQ ~3.70 pg/ml), and Simoa-based (G) NT1 (BT2-Tau12), (H) NT2 (ADx202-Tau12), and full-length (TauAB-Tau12) assays are shown (LLoQ ~0.27 pg/ml). Each data point is the average ±SEM of three technical replicates. Where the error bars are not visible, the SEM is smaller than the size of the symbol.
Immunoassays and assay validation
Detailed protocols describing immunoassays, the recombinant proteins used as standards, and the methods used to test sensitivity, specificity and reproducibility are provided in the Supplementary Methods.
Study Participants
Studies were conducted in accordance with the declaration of Helsinki [23] and patients were only included after giving their written informed consent. Subjects were from: (1) the Harvard Aging Brain Study (HABS); (2) research participants seen at UCL; and (3) research participants in the UCSD Shiley-Marcos Alzheimer’s Disease Research Center (ADRC). Each participant donated both plasma and CSF. Collectively the samples from the HABS and UCL studies are referred to as our Discovery Cohort, and specimens from UCSD are referred to as our Validation Cohort. We first analyzed the Discovery cohort and upon finding that NT-1 was elevated in both AD and AD-MCI, we obtained a second cohort to examine the robustness of our initial result. Subject enrollment, sample collection and sharing of samples across sites was approved by the BWH and UCSD Ethics Committees, and by the London region ethics committee. Table 1 shows clinical and demographic characteristics, and further details of about the cohorts, and the procedures used for blood and CSF collection are described in the Supplementary Methods.
Table 1 -.
Antibodies, sources, and working concentration
| Antibody | Epitope | Source | Concentration for ELISA | Concentration for SIMOA | Concentration for Immunodepletion |
|---|---|---|---|---|---|
| Tau12 | Tau 6–18 | EMD Millipore | 2.5 μg/ml (capture) | 0.6 μg/ml (detection) | - |
| BT2 | Tau 194–198 | Thermo Scientific | 2.5 μg/ml (capture) 1.7 μg/ml (detection) |
2 mg/ml (conjugation to beads) | - |
| Tau5 | Tau 210–241 | Biolegend | 1.7 μg/ml (detection) | - | 5 μg/ml |
| ADx202 | Tau 218–224 | ADx | 1.7 μg/ml (detection) | 2 mg/ml (conjugation to beads) | - |
| TauAB | Tau 425–441 | MedImmune | 1.7 μg/ml (detection) | 1 mg/ml (conjugation to beads) | - |
| 4–64 | HIV glycoprotein 120 | ATCC | - | - | 5 μg/ml |
Clinical categorization of participants
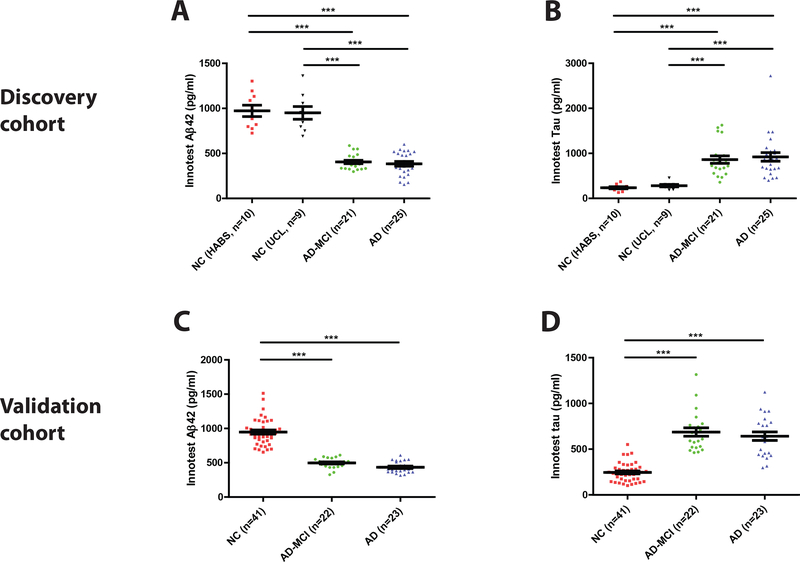
Subjects were designated as AD, AD-MCI, or controls based on cognitive testing (MMSE) and CSF biomarker criteria. The applied CSF Aβ1–42 and tau/Aβ1–42 values were taken from a prior study where the cut-offs were validated against amyloid PET [24]. Control subjects had an MMSE score ≥28, tau/Aβ1–42 ratio <0.5, and Aβ1–42 concentration >630 pg/ml. AD-MCI and AD subjects had a tau/Aβ1–42 ratio >0.88 and Aβ1–42 ≤630 pg/ml; those with an MMSE score of 15–24 points were defined as AD and those with a CSF AD biomarker profile and an MMSE score of 25–29 points as AD-MCI (Figure 2).
Figure 2. CSF biomarkers of AD concur with the clinical diagnosis of subjects in the Discovery and Validation cohorts.
(A and C) Aβ42 and (B and D) tau were measured in CSF using validated INNOTEST ELISAs. In the Discovery Cohort, approximately equal numbers of healthy control subjects were from two distinct sources the HABS study (NC HABS) and the UCL study (NC UCL). Subjects with AD biomarker positive-mild cognitive impairment (AD-MCI) and AD biomarker positive-clinical AD were from the UCL study. The NC, AD-MCI and AD groups in the Validation Cohort were all from the UCSD study. Each point is the average of duplicate measurement for a single individual, and group means ± SEM are indicated. Differences between groups were assessed with Kruskal-Wallis H test followed by Dunn’s post-hoc test. Aβ42 levels were lower in the AD-MCI and AD groups than in the NC groups, whereas tau levels were elevated in the AD-MCI and AD groups. n.s., non-significant; p>0.05; *** p<0.001.
Statistical Analysis
Statistical analyses were carried out using SPSS statistics, version 24.0 (IBM, Armonk, NY, USA). For normally distributed data, ANOVA followed by Tukey’s post-hoc test was performed, and for non-normal distributions, a Kruskal-Wallis H test followed by Dunn`s post-hoc test was performed. True positive rate (sensitivity) was plotted against the false positive rate (1-specificity) to obtain receiver operating characteristics (ROC) curves and area under the curve was calculated using non-parametric methods. The significance threshold was set to a two-sided p ≤ 0.05.
Results
Development and characterization of ELISAs for N-terminal fragments of tau.
A goal of this study was to produce new immunoassays to enable the detection and quantification of distinct sub-groups of extracellular tau in both human CSF and plasma. As a template we used a validated in-house mid-region ELISA [8] to develop two novel N-terminal ELISAs. NT1 utilizes the anti-tau mAbs Tau12 and BT2, and NT2 employs Tau12 and ADx202 (Figure 1). These new ELISAs, which have lower limits of quantitation (LLoQs) of less than 30 pg/ml, readily detect tau in human CSF (Figure 1C and D and Supplementary Figure 1B and C) and show excellent response to dilution of sample, and recovery of exogenously added tau (Supplementary Figure 1B and C).
Tau populations detected by mid-region and N-terminal ELISAs are elevated in AD CSF.
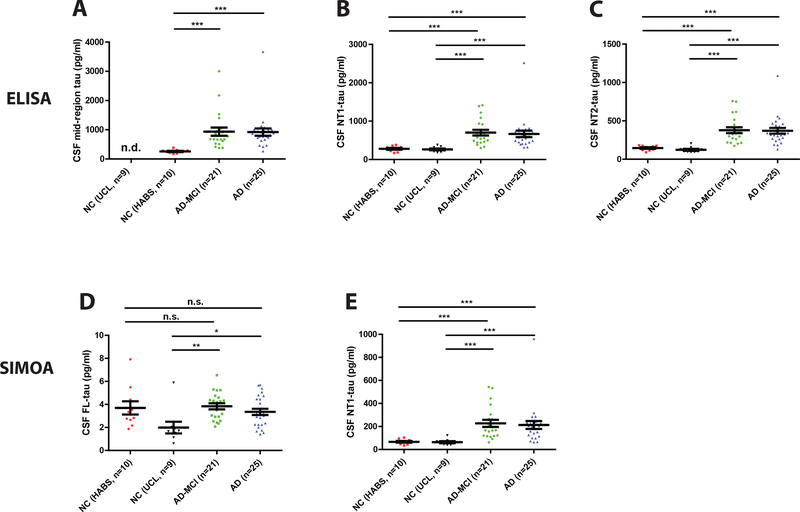
When used to measure tau in CSF from the Discovery Cohort both the NT1 and NT2 ELISAs allowed good discrimination between the AD-MCI and AD groups versus controls (Figure 3B and C). As might be anticipated for assays that utilize highly similar antibodies, the absolute amounts of tau detected by the INNOTEST assay (Figure 2B) and our in-house mid-region (Figure 3A) were closely comparable (Supplementary Table 2). For instance, the average concentration of tau detected in controls by the INNOTEST and in-house mid-region assay were 236 ± 23 pg/ml and 258 ± 19.5 pg/ml, respectively, and for AD were 921 ± 96 pg/ml and 920 ±124 pg/ml (Supplementary Table 2). Moreover, the fold increase seen in the AD-MCI and AD groups relative to normal controls was approximately 3.6 for both assays.
Figure 3. CSF Mid-region and N-terminal-containing fragments of tau, but not full-length tau are elevated in AD and AD-MCI.
Tau was measured in the CSF of subjects from the Discovery Cohort using three distinct ELISAs for (A) mid-region tau (BT2-Tau5), (B) NT1 tau (Tau12-BT2), and (C) NT2 tau (Tau12-ADx202), and two Simoa-based assays for (D) FL tau (TauAB-Tau12) and (E) NT1 tau (BT2-Tau12). Healthy control subjects were from the HABS study (NC HABS) and UCL study (NC UCL). Each point represents a single individual and means ± SEM are indicated. Differences between groups were assessed with Kruskal-Wallis H test followed by Dunn’s post-hoc test. All assays, except the FL assay detected higher levels of tau in AD-MCI and AD, than NC. n.s., non-significant; p>0.05; *p<0.05; ***p<0.001.
To enable comparison of absolute amounts of tau detected by different ELISAs the same recombinant tau standard was used for each assay. The concentration of tau measured in controls by our NT1 assay (278 ± 22 pg/ml) was comparable to that measured by the mid-region assays, but in the AD-MCI and AD groups the levels of tau detected by NT1 were on average ~25% lower than those detected by the mid-region ELISAs (Figure 2B, Figure 3A and B, Supplementary Table 2). While tau measured by the NT2 ELISA was elevated in AD and AD-MCI versus controls (Figure 3C), the amount of tau detected by the NT2 assay was considerably lower than that measured by the other assays (Supplementary Table 2). The relative elevation of tau measured by NT1 and NT2 in AD-MCI and AD versus controls was approximately 2.5 for both assays.
Collectively, these results indicate that tau detected by the different assays comprise some overlapping and some distinct populations of fragments and that the distribution of tau species in CSF changes with disease. These findings are congruent with the very recent report that used mass spectrometry and stable isotope labeling to study the kinetics of tau production and turnover in human CSF [9].
Development and characterization of ultrasensitive immunoassays for tau.
Next, we sought to develop ultrasensitive immunoassays to search for low abundant species in CSF and blood. For this purpose, we employed Simoa (named for Single molecule array) technology and replicated our mid-region, NT1, NT2, and FL ELISAs as Simoa assays. Preliminary experiments focused on identifying antibody combinations which produced the best standard curve and lowest LLoQs, and involved flipping the mAbs used for capture and detection (not shown). In three cases we were able to generate highly sensitive assays with LLoQs of less than 0.7 pg/ml (Figure 1G–I). The NT1, NT2 and FL assays all readily detected tau in human CSF and most importantly in blood plasma (Supplementary Figures 2–4). For as yet unknown reasons, various iterations of Simoa-based assays that employed the Tau5 mAb did not allow reliable detection of tau in either CSF or plasma (data not shown). However, for the NT1, NT2 and FL Simoa-based assays the signal detected in human fluids was specific and could be greatly diminished by immunoprecipitation (Supplementary Figures 2–4). Each assay exhibited good response to dilution of sample, and excellent recovery of exogenously added tau (Supplementary Figures 2–4).
Simoa-measured N-terminal, but not full-length tau, is elevated in AD CSF.
Next, we applied NT1 and FL Simoa assays to analyze the Discovery CSF samples tested using the corresponding ELISAs (Figure 3). Results from Simoa NT1 assay and NT1 ELISA were highly correlated (R2 = 0.885) and both allowed similarly good discrimination between the AD-MCI and AD groups, versus controls (Figure 3B and E). Unexpectedly, the absolute levels of tau detected by the Simoa assay were always significantly lower than those detected by ELISA; this despite the fact that the same tau standard was used for both assays. In general, we have found that Simoa assays tend to detect less biological analyte than ELISAs employing the same mAbs and protein standards.
Until relatively recently, tau was considered to be an exclusively intra-neuronal protein, and the presence of tau in human CSF was a product of axonal damage or neuronal death [25]. The results presented here (Figure 3D and Supplementary Table 3) and elsewhere [5–7, 9, 10] demonstrate that the vast majority of tau in CSF is not full-length, and therefore indicate that most CSF tau is not released as a consequence of neuronal death. To determine if FL tau might serve as an indicator of neurodegeneration, we measured FL tau in CSF from the Discovery Cohort (Figure 3D). All CSF specimens contained small, but measurable values (Figure 3D). On average FL tau accounted for less than 5% of the tau detected by the NT1 Simoa assay and never exceeded 16% of tau detected by the NT1 Simoa assay (Figure 3D and E, Supplementary Table 2). The contribution of FL tau relative to the mid-region ELISA-measured tau was even smaller, on average ≤0.5%. Moreover, the levels of FL tau in CSF overlapped between controls, AD-MCI and AD (Figure 3D and Supplementary Table 2).
Plasma NT1 tau is elevated in AD-MCI and AD subjects from the Discovery Cohort.
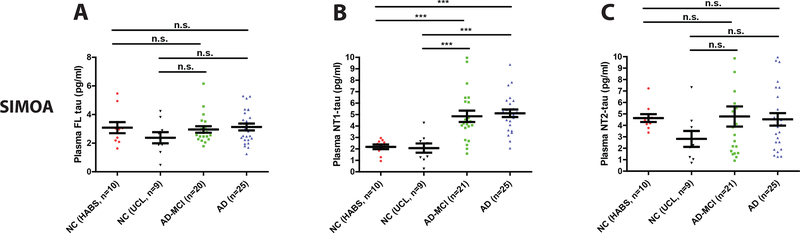
Next, we investigated whether the levels of tau were elevated in plasma of subjects with AD or AD-MCI. In the Discovery Cohort, tau concentrations measured by the FL and NT2 Simoa assays were similar in normal controls and subjects with AD-MCI and AD, and there were no significant differences between any of the groups (Figure 4A and C). In contrast, the levels of tau measured using the NT1 assay were significantly elevated in AD-MCI and AD compared to the two normal control sub-groups (Figure 4B). ROC analysis (Figure 4D and E) showed that plasma NT1 measurements separated controls from AD-MCI, with an AUC of 0.88 (95% CI: 0.77 – 0.99). Similarly, NT1 tau levels discriminated controls from patients with AD-dementia with an AUC of 0.96 (95% CI: 0.90 – 1.00). A cut-off value (determined by Youden’s index) of 3.07 pg/ml yielded excellent sensitivity (for AD: 0.95; 95% CI: 0.74 – 1.00, AD-MCI: 0.95, 95% CI: 0.74 – 1.00) and good specificity (for AD: 0.92; 95% CI: 0.74 – 0.99; AD-MCI: 0.76; 95% CI: 0.53 – 0.92). Interestingly, plasma NT1 tau levels were similar in AD and AD-MCI (Figure 4B).
Figure 4. Plasma NT1 tau is elevated in AD-MCI and AD subjects from the Discovery Cohort.
Plasma samples from the Discovery Cohort were analyzed with ultrasensitive Simoa assays for (A) FL tau (TauAB-Tau12), (B) NT1 tau (Tau12-BT2) and (C) NT2 tau (Tau12-ADx202). As before, healthy controls were from the HABS study (NC HABS) and UCL study (NC UCL). Subjects with mild cognitive impairment (AD-MCI) and AD were from the UCL cohort. Each point represents a single individual and means ± SEM are indicated. Differences between groups were assessed with Kruskal-Wallis H test followed by Dunn`s post-hoc test. NT1 tau was elevated in AD-MCI and AD versus controls, but both NT2 and FL tau were similar in all groups. ROC curves of plasma NT1 tau values distinguishes (D) NC from AD-MCI, and (E) NC from AD. Area under the curves (AUC) for ROC analyses were calculated using a non-parametric approach. n.s., non-significant; p>0.05; ***p<0.001.
Plasma NT1 tau is also elevated in AD-MCI and AD subjects from the Validation Cohort.
To further examine the discriminatory power of measuring NT1, we analyzed a second separate cohort. Our Validation Cohort was from the UCSD ARDC, and as with the Discovery Cohort, subjects were assigned to gold standard diagnostic groups based on clinical assessment and INNOTEST Aβ42 and tau CSF levels. In addition to testing the usefulness of measuring NT1 in plasma from the Validation Cohort, we also investigated the robustness of the assay by having 2 different operators measure NT1 in duplicate samples.
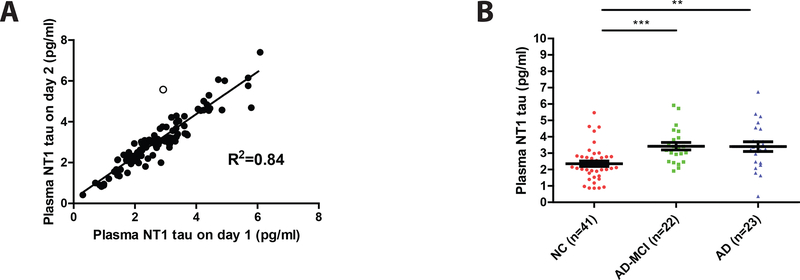
Plasma was available from a total of 87 subjects and repeated testing of samples on separate days was highly reproducible, yielding an R2 value of 0.84 (Figure 5A). Thereafter, we proceeded to analyze NT1 results based on diagnostic groups. This was done by comparing: (i) day 1 results for all 87 samples (not shown), (ii) day 2 results for all 87 samples (not shown), and (iii) by using the average of day 1 and day 2 results for the 86 samples which yielded day-to-day variance <20% (Figure 5B). As in the Discovery Cohort, the levels of NT1 in the AD-MCI and AD groups were significantly elevated compared to the controls (Figure 5B). Moreover, the levels of NT1 were similar in AD-MCI and AD (Figure 5B), thus suggesting that NT1 elevation is unlikely to be useful for monitoring progression from AD-MCI to AD. ROC analysis (Figure 5C and D) revealed that plasma NT1 measurements separated controls from AD-MCI with an AUC of 0.79 (95% CI: 0.62 – 0.88), and controls from AD-dementia with an AUC of 0.75 (95% CI: 0.68 – 0.91). To test how the cut-off derived from the Discovery Cohort might apply to the Validation Cohort, we generated a Youden’s index for the Validation Cohort. Using the cut-off of 3.07 pg/ml which produced excellent separation of AD, and AD-MCI from controls in our Discovery Cohort to our Validation Cohort, produced high specificity (for AD: 0.83, 95% CI: 0.68 – 0.93, and AD-MCI: 0.85, 0.71 – 0.94), but modest sensitivity (for AD: 0.65, 95% CI: 0.42 – 0.84, and AD-MCI: 0.59, 95% CI: 0.36 – 0.79).
Figure 5. Plasma NT1 tau is elevated in AD-MCI and AD subjects from the Validation Cohort.
Plasma from a total of 87 Validation Cohort subjects was analyzed using the Simoa-based NT1 (BT2-Tau12) assay. (A) Samples were measured by two different operators on different days and the values obtained on day 1 and day 2 were plotted one versus the other. Results obtained on day 1 and day 2 were highly similar and had an R2 value of 0.84. Each symbol represents an individual subject, and the straight line indicates the line of best fit from a linear regression model. All duplicate values, except one set, deviated from the line of best fit by less than 20% (filled circles). (B) Day 1 and day 2 results that deviated from the line of best fit by less than 20% were averaged and these values segregated based on diagnosis (normal controls, NC; mild cognitive impairment, MCI and AD). Each point represents a single individual and means ± SEM are indicated. Differences between groups were assessed with Kruskal-Wallis H test followed by Dunn`s post-hoc test. As in the Discovery Cohort, NT1 tau was elevated in AD-MCI and AD versus controls and (C) there was a good separation of NC versus AD-MCI, and (D) NC versus AD. Area under the curves (AUC) for ROC analyses were calculated using a non-parametric approach. **p<0.01; ***p<0.001.
A likely explanation for the poor transfer of cut-off values from the Discovery Cohort to the Validation Cohort is the fact that the NT1 values were higher for AD-MCI and AD groups in the Discovery Cohort compared to the Validation Cohort (4.85 vs. 3.42 pg/ml, and 5.12 vs. 3.40 pg/ml), thus complicating the use of a common cut-off. However, establishing a new cut-off (2.88 pg/ml) based on Youden’s index analysis within the Validation Cohort produced good sensitivity (for AD: 0.70; 95% CI: 0.47 – 0.87, and AD-MCI: 0.73; 95% CI: 0.50 – 0.89) and specificity (for AD: 0.78; 95% CI: 0.62 – 0.89, and 0.80; 95% CI: 0.65 – 0.91). Moreover, when data from the Discovery and Validation cohorts were pooled and used for ROC analysis (Supplementary Figure 5), Youden’s index analysis produced good sensitivity (for AD: 0.79; 95% CI: 0.65 – 0.90, and AD-MCI: 0.70; 95% CI: 0.54 – 0.83) and specificity (for AD: 0.88; 95% CI: 0.77 – 0.95, and 0.88; 95% CI: 0.77 – 0.95).
Discussion
While it has been known for over two decades that CSF tau is elevated in AD [26, 27] little attention has been paid to the forms of tau that are elevated and why the elevation measured in AD CSF is not seen in other neurodegenerative disorders [7, 28]. To date most efforts at measuring tau in CSF have employed mid-region-directed mAbs incapable of detecting fragments of tau that lack all or part of the mid-region domain. Similarly, while rapid progress has been made on measuring tau in human plasma only a small number of tau immunoassays have been used and these have been applied without regard to the limitations imposed by the antibodies employed [14–18, 21, 29]. Here, we report the development and application of an assay to measure full-length tau in CSF and plasma, which when used in tandem with other tau immunoassays is capable of differentiating between N-terminal fragments and full-length tau.
In AD, diagnostic assignment is challenging, and it is now widely appreciated that a sizeable number of individuals defined by clinical criteria have cognitive dysfunction as a result of non-AD processes [1, 30]. To minimize this problem and to exclude cognitively intact controls that might have incipient AD we defined our diagnostic groups based on both clinical criteria and the best available AD CSF biomarkers. In parallel with the attention given to diagnosis, sample collection and handling, we were careful to validate the novel assays we developed. This involved confirming the specificity of the analyte detected by immunodepleting specimens with an anti-tau mAb, diluting to ensure: (i) linearity of response, and (ii) identify conditions that minimize matrix interference.
Using well validated assays and CSF specimens we measured tau using four in-house ELISAs and three in-house Simoa assays. All but one of the seven assays allowed detection of tau in CSF. Our FL tau ELISA which on the day of testing had an LLoQ ~31 pg/ml did not detect signal in any CSF samples. However, the more sensitive FL Simoa assay (LLOQ ~0.7 pg/ml) detected tau in all 76 CSF samples examined, although the levels were very low and never exceeded more than 4% of the tau detected by mid-region ELISA (Supplementary Tables 2 and 3, and Supplementary Figure 4). To our knowledge this is the first reliable demonstration of full-length tau in CSF. Importantly, unlike the other tau forms measured, full-length tau which could be passively released as a consequence of neuronal injury, does not appear to change with AD. This is in contrast to tau fragments which appear to be actively secreted from neurons in response to Aβ-exposed [9] and indicates that N-terminal fragments which contain a plasma membrane-interacting domain escape neurons more readily than C-terminal fragments. Whilst our results provide important insights into tau processing and release in the context of AD, it will be of considerable interest to extend this work to non-AD tauopathies.
Tau measured in CSF by our NT1 and NT2 ELISAs was elevated in AD and AD-MCI versus controls, but the elevation was lower than that measured by either the INNOTEST ELISA or our in-house mid-region ELISA. These results suggest that the tau detected by the different assays comprise both overlapping and distinct populations of fragments and the population measured by mid-region ELISAs change more with disease than do tau species that contain an intact N-terminus. These data together with our finding of only low levels of full-length tau support the notion that in CSF, smaller fragments predominate [7].
When applied to the analysis of plasma from the same subjects as used for CSF studies, all three of our Simoa-based assays readily detected tau. In controls the levels of tau measured by our FL, NT1 and NT2 assays spanned comparable ranges, but in general NT2 detected the highest levels of tau, NT1 the lowest, and the FL assay intermediate values. This outcome indicates that most plasma tau is full-length. In line with the observation that CSF and plasma contain different populations of tau, the levels of tau measured in CSF and plasma show little correlation. This finding raises the intriguing proposition that a substantial portion of plasma tau comes from a peripheral source and not the brain. Indeed, it is known that peripheral neurons express a form of tau referred to as “Big tau” which contains an additional large exon (4a) [31, 32]. In future studies it will be important to generate Big tau-specific mAbs and determine if this form of peripheral tau changes in disease and whether AD-related genetic factors alter expression or turnover of peripherally expressed tau.
Notwithstanding the possible contribution of peripheral sources of tau, it is striking that the NT1 assay showed a significant disease-related increase. Consistent with prior studies that used a commercial Simoa assay analogous to our NT2 assay, levels of tau measured using the NT2 assay tended to be elevated in AD/AD-MCI, but exhibited broad overlap with controls [17, 18, 33]. On an individual subject basis, the NT1 assay often measured higher levels of tau in AD and AD-MCI than either the NT2 or FL assay, suggesting specific elevation of a tau species detected by the NT1 assay. Importantly, ROC analysis of the Discovery Cohort indicated that measurement of tau in plasma using our NT1 assay showed sufficient diagnostic sensitivity (the ability to predict AD cases) and specificity (the ability to exclude controls) to pursue its use as a potential screening assay for AD.
When cut-offs determined in the Discovery Cohort were applied to the Validation Cohort they exhibited good specificity (0.83; 95% CI: 0.68 – 0.93), but poor sensitivity (0.59; 95% CI: 0.36 – 0.79). Conversely, Youden’s index analysis within: (i) the Validation Cohort, and (ii) a pool of the Validation Cohort and the Discovery Cohort, produced much more encouraging results. A possible explanation for the difficulty in applying a common cut-off to the Validation Cohort may lie in the fact that the levels of NT1-measured tau tended to be lower in this cohort, than in the Discovery Cohort. Why this should be, is not yet clear. However, it is worth noting that the AD and AD-MCI groups in the Validation Cohort had lower CSF tau than the corresponding groups in the Discovery Cohort (Supplementary Table 2) and on average the subjects in the Validation Cohort were ~10 years older. This might suggest a less aggressive disease process and hence slightly lower levels of NT1 tau. Additional studies using larger and preferably longitudinal cohorts will be required to determine cut-offs. Longitudinal studies will also resolve whether NT1 can serve as a biomarker of disease progression. However, given that NT1 values are similar for AD and AD-MCI it would appear that plasma NT1 will not be a useful measure to monitor conversion of AD-MCI to AD. Future studies should also help clarify whether NT1 levels, as has been suggested for other tau analytes [7], are affected by age and/or gender.
Despite these caveats, it is important to emphasize that a blood-based screening assay for AD does not need to display perfect diagnostic sensitivity and specificity. A relative crude marker that could be optimized to minimizing false negatives would significantly reduce the numbers of individuals that might then need CSF analysis or PET imaging, and thus streamline participant selection for clinical trials. As such, the application of our novel NT1 assay merits further investigation, both as a single predictor of disease and in combination with other emerging blood-based biomarkers.
Supplementary Material
Figure 6.
Table 2 -.
Patient characteristics
| Discovery cohort (HABS + UCL) | Validation cohort (UCSD) | ||||||
|---|---|---|---|---|---|---|---|
| NC (HABS, n=10) | NC (UCL, n=9) | AD-MCI (n=21) | AD (n=25) | NC (n=41) | AD-MCI (n=22) | AD (n=23) | |
| N (%) or mean ± SD | N (%) or mean ± SD | N (%) or mean ± SD | N (%) or mean ± SD | N (%) or mean ± SD | N (%) or mean ± SD | N (%) or mean ± SD | |
| Gender (female) | 7 (70.0) | 7 (77.8) | 16 (76.2) | 13 (52.0) | 27 (56.3) | 10 (45.5) | 12 (52.2) |
| Age (years) | 69.8 ± 9.8 | 59.8 ± 6.5 | 65.3 ± 6.7 | 61.0 ± 6.3 | 71.8 ± 6.0 | 73.2 ± 8.1 | 72.4 ± 8.3 |
| MMSE | n. a. | 29.4 ± 0.5 | 26.1 ± 1.2 | 20.0 ± 2.9 | 29.3 ± 0.8 | 26.9 ± 1.4 | 19.8 ± 2.4 |
| CSF Aβ (pg/ml) | 972.7 ± 199.7 | 950.4 ± 212.0 | 405.2 ± 83.7 | 384.0 ± 130.3 | 947.0 ± 193.3 | 497.3 ± 72.0 | 433.4 ± 84.7 |
| CSF Tau (pg/ml) | 236.1 ± 72.2 | 282.1 ± 78.5 | 861.3 ± 373.3 | 921.2 ± 480.4 | 245.6 ± 104.4 | 687.3 ± 214.7 | 641.8 ± 222.3 |
| CSF Tau/Aβ ratio | 0.2 ± 0.1 | 0.3 ± 0.1 | 2.4 ± 1.4 | 2.5 ± 1.0 | 0.3 ± 0.1 | 1.4 ± 0.4 | 1.5 ± 0.4 |
Acknowledgements
This work was made possible by support to DMW from an Alzheimer’s Association Zenith Award, the Harvard NeuroDiscovery Center through a major gift from Rick and Nancy Moskovitz and a grant from Medimmune Plc. ADx202 was obtained from ADx under an MTA with Dr Eugeen Vanmechelen. Assays performed at UCL were made possible through funding from the Leonard Wolfson Experimental Neurology Centre. JMS and AK acknowledge funding from the Weston Brain Institute and Wolfson Foundation. JMS acknowledges the support of the National Institute for Health Research University College London Hospitals Biomedical Research Centre, MRC Dementias Platform UK (MR/L023784/1), and Alzheimer’s Research UK. DM is a research fellow funded by the German Research Foundation (DFG). We are grateful to clinician colleagues for referring patients and performing lumbar punctures.
Footnotes
Disclosures
None of the authors have biomedical financial interests or potential conflicts of interest.
References
- [1].Monsell SE, Kukull WA, Roher AE, Maarouf CL, Serrano G, Beach TG, et al. Characterizing Apolipoprotein E epsilon4 Carriers and Noncarriers With the Clinical Diagnosis of Mild to Moderate Alzheimer Dementia and Minimal beta-Amyloid Peptide Plaques. JAMA Neurol. 2015;72:1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Salloway S, Sperling R, Brashear HR. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer’s disease. N Engl J Med. 2014;370:1460. [DOI] [PubMed] [Google Scholar]
- [3].Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–84. [DOI] [PubMed] [Google Scholar]
- [4].Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Johnson GV, Seubert P, Cox TM, Motter R, Brown JP, Galasko D. The tau protein in human cerebrospinal fluid in Alzheimer’s disease consists of proteolytically derived fragments. J Neurochem. 1997;68:430–3. [DOI] [PubMed] [Google Scholar]
- [6].Meredith JE Jr., Sankaranarayanan S, Guss V, Lanzetti AJ, Berisha F, Neely RJ, et al. Characterization of novel CSF Tau and ptau biomarkers for Alzheimer’s disease. PLoS One. 2013;8:e76523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wagshal D, Sankaranarayanan S, Guss V, Hall T, Berisha F, Lobach I, et al. Divergent CSF tau alterations in two common tauopathies: Alzheimer’s disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2015;86:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kanmert D, Cantlon A, Muratore CR, Jin M, O’Malley TT, Lee G, et al. C-Terminally Truncated Forms of Tau, But Not Full-Length Tau or Its C-Terminal Fragments, Are Released from Neurons Independently of Cell Death. J Neurosci. 2015;35:10851–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sato C, Barthelemy NR, Mawuenyega KG, Patterson BW, Gordon BA, Jockel-Balsarotti J, et al. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron. 2018;98:861–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guix FX, Corbett GT, Cha DJ, Mustapic M, Liu W, Mengel D, et al. Detection of Aggregation-Competent Tau in Neuron-Derived Extracellular Vesicles. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Randall J, Mortberg E, Provuncher GK, Fournier DR, Duffy DC, Rubertsson S, et al. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation. 2013;84:351–6. [DOI] [PubMed] [Google Scholar]
- [12].Neselius S, Zetterberg H, Blennow K, Randall J, Wilson D, Marcusson J, et al. Olympic boxing is associated with elevated levels of the neuronal protein tau in plasma. Brain Inj. 2013;27:425–33. [DOI] [PubMed] [Google Scholar]
- [13].Shahim P, Blennow K, Zetterberg H. Tau, s-100 calcium-binding protein B, and neuron-specific enolase as biomarkers of concussion-reply. JAMA Neurol. 2014;71:926–7. [DOI] [PubMed] [Google Scholar]
- [14].Chiu MJ, Chen YF, Chen TF, Yang SY, Yang FP, Tseng TW, et al. Plasma tau as a window to the brain-negative associations with brain volume and memory function in mild cognitive impairment and early Alzheimer’s disease. Hum Brain Mapp. 2014;35:3132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chiu MJ, Yang SY, Horng HE, Yang CC, Chen TF, Chieh JJ, et al. Combined plasma biomarkers for diagnosing mild cognition impairment and Alzheimer’s disease. ACS Chem Neurosci. 2013;4:1530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J, et al. Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther. 2013;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dage JL, Wennberg AMV, Airey DC, Hagen CE, Knopman DS, Machulda MM, et al. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimers Dement. 2016;12:1226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, et al. Plasma tau in Alzheimer disease. Neurology. 2016;87:1827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yanamandra K, Patel TK, Jiang H, Schindler S, Ulrich JD, Boxer AL, et al. Anti-tau antibody administration increases plasma tau in transgenic mice and patients with tauopathy. Sci Transl Med. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kasai T, Tatebe H, Kondo M, Ishii R, Ohmichi T, Yeung WTE, et al. Increased levels of plasma total tau in adult Down syndrome. PLoS One. 2017;12:e0188802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mielke MM, Hagen CE, Wennberg AMV, Airey DC, Savica R, Knopman DS, et al. Association of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in the Mayo Clinic Study on Aging. JAMA Neurol. 2017;74:1073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].NRC. Committee on Methods of Producing Monoclonal Antibodies Monoclonal Antibody Production. Washington (DC): National Academies Press (US)1999. [Google Scholar]
- [23].Goodyear MD, Krleza-Jeric K, Lemmens T. The Declaration of Helsinki. BMJ. 2007;335:624–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Weston PS, Paterson RW, Modat M, Burgos N, Cardoso MJ, Magdalinou N, et al. Using florbetapir positron emission tomography to explore cerebrospinal fluid cut points and gray zones in small sample sizes. Alzheimers Dement (Amst). 2015;1:440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jack CR Jr., Holtzman DM. Biomarker modeling of Alzheimer’s disease. Neuron. 2013;80:1347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tato RE, Frank A, Hernanz A. Tau protein concentrations in cerebrospinal fluid of patients with dementia of the Alzheimer type. J Neurol Neurosurg Psychiatry. 1995;59:280–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Andreasen N, Vanmechelen E, Van de Voorde A, Davidsson P, Hesse C, Tarvonen S, et al. Cerebrospinal fluid tau protein as a biochemical marker for Alzheimer’s disease: a community based follow up study. J Neurol Neurosurg Psychiatry. 1998;64:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].O’Dowd ST, Ardah MT, Johansson P, Lomakin A, Benedek GB, Roberts KA, et al. The ELISA-Measured Increase in Cerebrospinal Fluid Tau that Discriminates Alzheimer’s Disease from other Neurodegenerative Disorders is not Attributable to Differential Recognition of Tau Assembly Forms. J Alz Dis. 2013;33:923–8. [DOI] [PubMed] [Google Scholar]
- [29].Kovacs GG, Andreasson U, Liman V, Regelsberger G, Lutz MI, Danics K, et al. Plasma and cerebrospinal fluid tau and neurofilament concentrations in rapidly progressive neurological syndromes: a neuropathology-based cohort. Eur J Neurol. 2017;24:1326–e77. [DOI] [PubMed] [Google Scholar]
- [30].FDA. Early Alzheimer’s Disease: Developing Drugs for Treatment Guidance for Industry. 2018. [DOI] [PMC free article] [PubMed]
- [31].Goedert M, Spillantini MG, Crowther RA. Cloning of a big tau microtubule-associated protein characteristic of the peripheral nervous system. Proc Natl Acad Sci U S A. 1992;89:1983–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Boyne LJ, Tessler A, Murray M, Fischer I. Distribution of Big tau in the central nervous system of the adult and developing rat. J Comp Neurol. 1995;358:279–93. [DOI] [PubMed] [Google Scholar]
- [33].Deters KD, Risacher SL, Kim S, Nho K, West JD, Blennow K, et al. Plasma Tau Association with Brain Atrophy in Mild Cognitive Impairment and Alzheimer’s Disease. J Alzheimers Dis. 2017;58:1245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.