Abstract
A female rhesus macaque developed two episodes of generalized convulsions during transcutaneous spinal cord stimulation (TSCS) and urodynamic studies under ketamine anesthesia. The seizures took place in the absence of active TSCS and bladder pressure elevation. Ketamine anesthesia remains the primary risk factor for the convulsions during these experimental procedures.
Keywords: cystometrogram, electromyography, ketamine, non-human primate
1 |. INTRODUCTION
Transcutaneous spinal cord stimulation (TSCS) has recently emerged as a potentially useful non-invasive intervention to augment neurological function, including standing and locomotion, after spinal cord injury.1,2 However, it is not known whether transcutaneous electrical stimulation may also modulate lower urinary tract function. We have therefore initiated a feasibility study to determine the potential utility of thoracolumbar TSCS for neurourological applications.
Here, we report the onset of generalized convulsions in one of a total of nine female rhesus macaques undergoing TSCS and urodynamic studies. The seizures took place while the animal was under ketamine anesthesia but not receiving any active electrical stimulation or bladder filling. Possible contributing causes of the seizures are discussed, as TSCS represents a new experimental procedure for lower urinary tract studies in rhesus macaques.
2 |. CASE REPORT
The case subject was a 14-year-old female rhesus macaque in good health, weighed 6.3 kg, and was housed at the California National Primate Research Center (CNPRC). All procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals (2011) and approved by the UC Davis Institutional Animal Care and Use Committee (IACUC).
The subject was immobilized by an intramuscular (IM) dose of ketamine (10 mg/kg) followed by a ketamine constant rate infusion at 12 mg/kg/h IV. The dose was adjusted to achieve immobilization and light sedation. A 7-French triple-Iumen transurethral bladder catheter (Life-Tech, Stafford, TX, USA) was placed and the bladder emptied. Bilateral pairs of wire-electrodes (1215A-F; Life-Tech) were inserted into the external urethral sphincter (EUS), which includes an outer skeletal muscle layer. Wire electrode placement allows for EUS electromyographic (EMG) recordings with minimal disruption of adjacent tissues. Paired 22 gauge needle electrodes (Hamilton Company, Reno, NV, USA) were placed into the external anal sphincter (EAS). The tip of each needle electrode was placed in the EAS at a depth of approximately 1 cm from the skin surface. The use of stiff needle electrodes allow for precise electrode placement within the EAS muscle without bending of the electrode tip. Surface patch electrodes were attached to the pelvic floor, adjacent and lateral to the EAS to record from the levator ani muscle group. An MP150 recording system (Biopac Systems, Goleta, CA, USA) was used for combined urodynamic and EMG recordings. TSCS was applied at thoracolumbar interspinous processes as a monophasic rectangular pulse at 100 V and 1 Hz with a pulse width of 1–5 ms in steps of 1 ms using a 2.0 cm circular adhesive electrode as cathode and a 5.0 × 10 cm rectangular electrode as anode over the abdomen (Axelgard, ValuTrode® Cloth). A range of stimulation parameters was tested to determine thresholds and optimal stimulation settings for pressure changes and EMG responses. A total of five stimulation cycles were performed for each pulse width setting. Evoked pressure recordings from the bladder and urethra as well as EMG activity from the EUS, EAS, and levator ani muscle group were obtained to determine the effects of TSCS on peripheral targets that receive different peripheral nerve innervation and have different spinal cord representations.
Following TSCS at the T11/T12 level at 1, 2, and 3 ms pulse widths, the case subject developed generalized convulsions involving the trunk and extremities. The seizures began 17 seconds after the stimulation series, and no active stimulation was present at the onset of the convulsions. The convulsions lasted 59 seconds, stopped spontaneously, and were captured by the cystometrogram and EMG recordings (Figure 1). The subject maintained stable vital signs, including heart rate (HR), respiratory rate (RR), SpO2, and EtCO2 before and after the seizures, and a decision to continue the functional mapping studies was made after consultation with the veterinary staff. The remainder of the TSCS studies, including stimulation at the T12/L1, L1/ L2, L2/L3, and L3/L4 vertebral levels and using a 1–5 ms pulse width range at each segmental level, was tolerated well without any additional seizures.
FIGURE 1.

Urodynamic recordings of bladder pressure (Pves), urethra pressure (Pure), external urethral sphincter EMG (EUS), and external anal sphincter EMG (EAS). TSCS was administered over the interspace between the T11 and T12 spinous processes. Note two sets of responses to TSCS in the EUS trace and one set of stimulation responses from the Pves and Pure recordings. Generalized seizures with convulsions started 17 seconds after the ending of the last stimulation train (100 V, 3 ms, 1 Hz). The mid-portion of the recordings demonstrates a period of irregular and large amplitude changes in bladder and urethral pressures as well as in EUS and EAS EMG activity. The pressure and EMG changes corresponded to the onset and spontaneous cessation of generalized convulsive seizures. No active electrical stimulation took place at the seizure onset or during its course. A stable and quiescent baseline was re-established after the seizures had ended
Next, saline was manually infused into an emptied bladder to raise the intravesical pressure to approximately 20 cm H2O in attempts to trigger reflex micturition. Saline infusions of 90 mL and 69 mL, separated by bladder emptying, resulted in non-voiding contractions (Figure 2A). Generalized convulsions, including upper body and facial muscles, started after another 11 mL of saline had been infused as part of a third cycle but not caused any reflex bladder contraction (Figure 2B). Diazepam (3.5 mg IV) was administered and the seizures stopped after 15 seconds. Vital signs, including HR, RR, SpO2, and EtCO2 remained stable. Post-procedure, flumazenil (0.1 mg IV and 0.1 mg IM) was administered to reverse the effects of diazepam and expedite the recovery. Some blood was noted in the urine, possibly secondary to localized trauma from the bladder catheter during the convulsions. Cefazolin (25 mg/kg IM BID × 5 days) and ketoprofen (5 mg/kg IM SID × 3 days) were administered to reduce the risk for infection and discomfort. The subject recovered well and resumed normal eating, drinking, and cage activities. No hematuria or seizures recurred.
FIGURE 2.
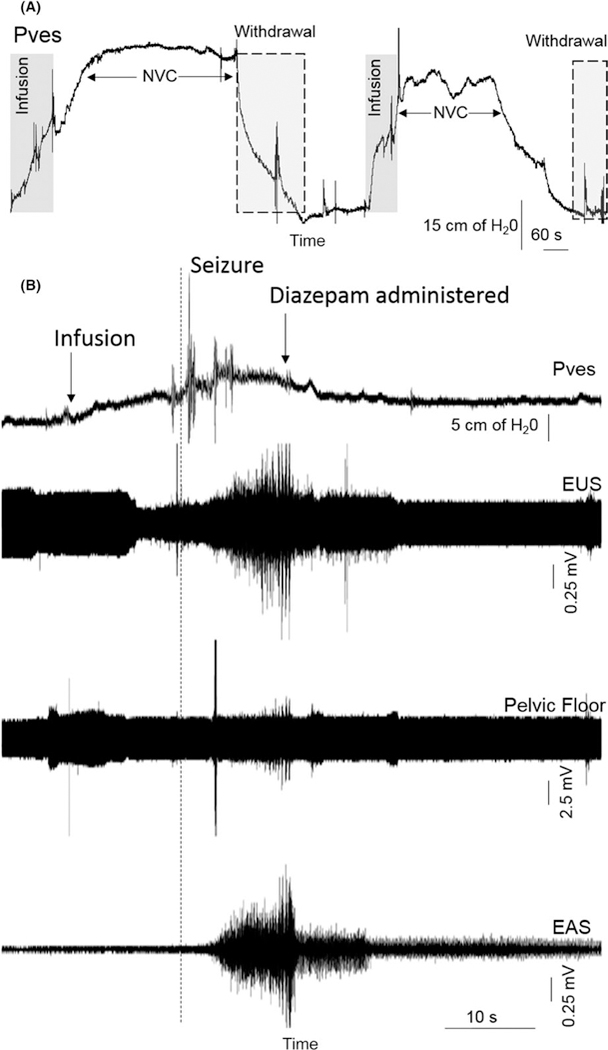
(A) Urodynamic recordings of two consecutive reflex bladder contractions in response to partial bladder filling with saline. Volumes of 90 and 69 mL of saline were infused to raise the bladder from baseline (0–5 cm H2O) to about 20 cm H2O for the two evoked reflex responses, which consisted of non-voiding contractions (NVC). Following each NVC, the infused saline was withdrawn using the fill-port of the triple lumen bladder catheter. No seizures took place during these reflex micturition studies. (B) Following the two evoked micturition reflex responses detailed in A, a third attempt was made to evoke a voiding bladder contraction. Urodynamic recordings show the onset of large amplitude changes in bladder pressure (Pves) as well as in EUS, EAS, and pelvic floor EMG activity corresponding to the onset of clinical seizures with generalized convulsions. The seizures started during the early phase of saline infusions, after a total of 11 mL of saline had been administered. Diazepam was administered by IV infusion and the convulsions aborted. The total seizure duration was 15 seconds
3 |. DISCUSSION
TSCS has been used extensively in humans to augment neurological function, including standing and locomotion, after spinal cord injury.1,2 No episodes of seizures were reported in these prior human TSCS research studies. Similarly, epidural stimulation over the thoracolumbar spinal cord in rats to activate micturition reflexes have been well tolerated and without any reports of seizures.3–5 In our case subject, seizures took place in the absence of any active electrical stimulation, and subsequent additional trains of electrical stimulation using an even longer pulse width was tolerated well. Our observations suggest that TSCS was not a significant trigger of seizures.
Seizures associated with clinical urodynamic evaluations are very rare. However, two patients have had seizure onset reported as a complication to diagnostic uroflowmetry.6,7 In both of these cases, the subject drank a large volume of fluids over a relatively short period of time in an attempt to quickly fill the bladder but also developed water intoxication, hyponatremia, and seizures. Therefore, the seizures in these clinical cases may be attributed to the electrolyte abnormality rather than the diagnostic procedure. Of note, urodynamic studies in humans are normally performed in awake subjects and without sedation.
Ketamine represents an established cause of seizures in experimental models and humans.8–12 However, reports on convulsive seizures associated with ketamine anesthesia in non-human primates are sparse, and firm assessments on the relative frequency of seizures associated with ketamine in macaque monkeys are not presently available. We have previously reported generalized convulsions taking place in a rhesus macaque sedated by ketamine and undergoing urodynamic studies, but we were at the time unable to determine a definitive cause for the seizure.13 In the present case, both seizures took place in the absence of any active electrical stimulation, bladder filling, or voiding. Therefore, the single common risk factor for seizures shared by the rhesus macaques in our present and prior studies is the presence of ketamine anesthesia.
We conclude that rhesus macaques are at an increased risk for generalized convulsions while undergoing experimental procedures under ketamine anesthesia. The seizures may be self-Iimited or aborted by early parenteral administration of diazepam.
ACKNOWLEDGMENTS
The studies were supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the National Institutes of Health (NIH) Office of the Director (P51 OD011107), and the NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant (UL1TR001881).
Funding information
Dr. Miriam and Sheldon G. Adelson Medical Research Foundation; National Institutes of Health (NIH), Grant/Award Number: P51 OD011107; NIH National Center for Advancing Translational Science (NCATS); UCLA CTSI, Grant/Award Number: UL1TR001881
REFERENCES
- 1.Gerasimenko Y, Gorodnichev R, Moshonkina T, Sayenko D, Gad P. Edgerton VR. Transcutaneous electrical spinal-cord stimulation in humans. Ann Phys Rehab Med. 2015;58:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sayenko DG, Atkinson DA, Dy CJ, et al. Spinal segment-specific transcutaneous stimulation differentially shapes activation pattern among motor pools in humans. J Appl Physiol. 2015;118: 1364–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abud EM, Ichiyama RM, Havton LA, Chang HH. Spinal stimulation of the upper lumbar spinal cord modulates urethral sphincter activity in rats after spinal cord injury. Am J Physiol Renal Physiol. 2015;308:F1032–F1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gad PN, Roy RR, Zhong H, Gerasimenko YP, Taccola G, Edgerton VR. Neuromodulation of the neural circuits controlling the lower urinary tract. Exp Neurol. 2016;285(PtB):182–189. pii: S0014–4886(16)301996. 10.1016/j.expneurol.2016.06.034. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gad PN, Roy RR, Zhong H, Lu DC, Gerasimenko YP, Edgerton VR. Initiation of bladder voiding with epidural stimulation in paralyzed, step trained rats. PLoS One. 2014;9:e108184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Issa MM, Pruthi RS, Vial C, McNamara DE, Terris MK. An unusual complication following uroflowmetry: water intoxication resulting in hyponatremia and seizure. Urol Int. 1997;59:129–130. [DOI] [PubMed] [Google Scholar]
- 7.Vishwajeet S, Aneesh S. Water intoxication leading to hyponatremia and seizures: a rare complication of uroflowmetry. Int Urol Nephrol. 2005;37:275–276. [DOI] [PubMed] [Google Scholar]
- 8.Adami C, Spadavecchia C, Casoni D. Seizure activity occurring in two dogs after S-ketamine-induction. Schweiz Arch Tierheilkd. 2013;155:569–572. [DOI] [PubMed] [Google Scholar]
- 9.Gourie-Devi M, Cherian L, Shankar SK. Seizures in cats induced by ketamine hydrochloride anaesthesia-a preliminary report. Indian J Med Res. 1983;77:525–528. [PubMed] [Google Scholar]
- 10.Kayama Y Ketamine and e.e.g. seizure waves: interaction with antiepileptic drugs. Br J Anaesth. 1982;54:879–883. [DOI] [PubMed] [Google Scholar]
- 11.Voss LJ, Sleigh JW, Barnard JP, Kirsch HE. The howling cortex: seizures and general anesthetic drugs. Anesth Analg. 2008;107:1689–1703. [DOI] [PubMed] [Google Scholar]
- 12.Yoshizawa K, Oishi Y, Matsumoto M, Nyska A. Ischemic brain damage after ketamine and xylazine treatment in a young laboratory monkey (Macaca fascicularis). Contemp Top Lab Anim Sci. 2005;44:19–24. [PubMed] [Google Scholar]
- 13.Christe KL, Lee UJ, Lemoy MJ, Havton LA. Generalized seizure activity in an adult rhesus macaque (Macaca mulatta) during ketamine anesthesia and urodynamic studies. Comp Med. 2013;63:445–447. [PMC free article] [PubMed] [Google Scholar]


