Abstract
One emerging strategy to address the opioid crisis is the development of mu opioid receptor (MOR) ligands that preferentially signal the G-protein vs. β-arrestin pathway. The present study compared the relative potency and effectiveness of two G-protein biased (GPB)-MOR ligands TRV130 and SR-14968 to five unbiased MOR ligands (NAQ, nalbuphine, buprenorphine, morphine, and methadone) on therapeutic-related (e.g. antinociception) and abuse-related (e.g. discriminative stimulus effects) endpoints. Male and female rats were tested in a warm water tail-withdrawal procedure (50°C) or trained to discriminate fentanyl (0.04 mg/kg, SC) from saline in a two-lever food-reinforced discrimination procedure. TRV130 and SR-14968 were approximately two-fold more potent to produce fentanyl stimulus effects vs. antinociception. Morphine, fentanyl, and methadone were significantly more potent in the fentanyl discrimination vs. tail withdrawal procedure. In addition, maximum antinociceptive and discriminative stimulus effects of fixed-proportion fentanyl/naltrexone mixtures (1:0.018, 1:0.054, 1:0.18, 1:0.3, and 1:0.54) were used to quantify 1) the relative in vivo efficacy of the two GPB-MOR agonists and five unbiased MOR ligands, and 2) potential species differences in MOR ligand effects between rats and monkeys. The efficacy-effect function generated from the fentanyl/naltrexone mixtures stratified the five unbiased ligands consistent with agonist-stimulated GTPγS binding (NAQ=nalbuphine<buprenorphine<morphine<methadone). However, TRV130 and SR-14968 produced greater antinociception and less fentanyl-like stimulus effects than was predicted. Furthermore, there was a significant positive correlation between rat and monkey antinociceptive effects. Overall, these results demonstrate GPB-MOR agonists produce undesirable abuse-related effects, albeit with slightly better potency and efficacy ratios compared to unbiased agonists.
Keywords: mu-opioid receptor, efficacy, fentanyl, drug discrimination, G-protein biased ligands, warm water tail-withdrawal
1.0. Introduction
The current opioid crisis in the United States is a significant public health issue. For example, recent estimates suggest that opioid-related deaths accounted for 66.4% of all drug overdose deaths in the United States in 2016 (Seth et al., 2018). Mu opioid receptor (MOR) agonists, such as fentanyl or morphine, are clinically effective for the treatment of various pain states. However, their clinical utility is also limited by undesirable effects including abuse liability and respiratory depression (Corbett et al., 2006). Thus, there is a need for basic research to develop effective analgesics with reduced undesirable effects and the National Institutes of Health and National Institute on Drug Abuse have outlined several research strategies to address this need (Volkow and Collins, 2017).
One research strategy to address the opioid crisis is the development of MOR agonists that differentially activate the MOR G-protein vs. β-arrestin pathways. Emerging evidence suggests G-protein signaling at the MOR mediates some of the therapeutic effects (e.g. antinociception) and β-arrestin signaling mediates some of the undesirable effects (e.g. respiratory depression) (Rankovic et al., 2016). For example, TRV130 (oliceridine) is a G-protein Biased (GPB)-MOR agonist currently being evaluated in humans for the treatment of acute pain (Singla et al., 2017). One proposed method to scale MOR ligand selectivity to differentially signal through the G-protein vs. β-arrestin pathways has been to calculate a “bias factor” (Kenakin, 2015; Stahl et al., 2015). TRV130 has a reported bias factor of 3 (DeWire et al., 2013); however, recently published GPB-MOR ligands such as SR-14968 have similar in vitro G-protein MOR efficacy as TRV130, but larger bias factors suggesting greater selectivity for G-protein vs. β-arrestin signaling (Schmid et al., 2017).
Thermal nociception procedures, such as warm-water tail-withdrawal, are routinely utilized to assess basic pharmacological effects of novel MOR ligands in the preclinical pain research field (Whiteside et al., 2008; Whiteside et al., 2013). In addition, novel MOR ligands can be evaluated in a drug discrimination procedure to assess basic pharmacological effects related to abuse potential (Holtzman, 1985). The deployment of these two behavioral procedures in tandem to assess novel MOR ligand potency and effectiveness has yielded two main findings. First, drug discrimination is a lower MOR efficacy requiring behavioral endpoint compared to thermal nociception suggesting that low efficacy MOR agonists may produce discriminative stimulus effects without producing antinociception (Bergman et al., 2000; Morgan and Picker, 1996). In addition, MOR ligands are generally more potent to produce discriminative stimulus vs. antinociceptive effects and this dose-ratio analysis has been used as an experimental index of behavioral selectivity (Morgan and Picker, 1996). Second, sex differences have been reported in MOR ligand effects in both the warm water tail-withdrawal and drug discrimination procedures (Craft, 2008; Terner et al., 2003). However, GPB-MOR agonist effects in drug discrimination studies have not been published nor have potential sex differences in GPB-MOR agonist effects been examined.
The aim of the present study was to compare two GPB-MOR agonists TRV130 (DeWire et al., 2013) and SR-14968 (Schmid et al., 2017) with five different MOR ligands 17-cyclopropylmethyl-3,14βdihyroxy-4,5α-epoxy-6α-[(3´-isoquinolyl)acetamindo]morphinan (NAQ), nalbuphine, buprenorphine, morphine, and methadone that vary in agonist-stimulated GTPγS binding (Alt et al., 2001; Emmerson et al., 1996; Selley et al., 1998; Thompson et al., 2004) in both warm water tail-withdrawal and drug discrimination procedures in female and male rats. GPB-MOR and unbiased ligands were compared using two different methods. First, the relative potency of each drug in the two behavioral procedures was quantified using dose-ratio analysis as described previously (Banks et al., 2010; Negus et al., 2009; Negus et al., 2008). Second, the relative effectiveness of each drug in the two behavioral procedures was quantified using receptor theory applications to fixed-proportion fentanyl/naltrexone mixtures (Cornelissen et al., 2018). For example, fixed-proportion fentanyl/naltrexone mixtures in male rhesus monkeys was utilized to quantify the relative in vivo effectiveness of six MOR ligands in a warm-water trail-withdrawal procedure at two noxious stimulus intensities (Cornelissen et al., 2018). The present study extended these previous findings in rhesus monkeys to in vivo effectiveness comparisons between two different experimental endpoints and sex as a biological variable.
2. Materials and Methods
2.1. Subjects:
A total of 64 adult male (31) and female (33) Sprague Dawley rats (Envigo, Frederick, MD, USA) served as subjects. Estrous cycle was not monitored because recent guidelines have suggested that when sex differences are present, they are often sufficiently robust for detection without reference to estrous cycle phase (Becker and Koob, 2016). Rats in the drug discrimination studies were fed sufficient amounts of standard rodent chow (Tekland LM-485 Mouse/Rat 7012, Envigo) after behavioral sessions to maintain stable 85%-of-free-feeding body weights. Rats in the tail withdrawal studies were fed ad lib rodent chow. All rats had unlimited access to water in the home cage and were individually housed in a temperature- and humidity-controlled vivarium maintained on a 12-h light-dark cycle (lights on from 0600 to 1800). The vivarium was accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Both experimental and enrichment protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (Council, 2011).
2.2. Drugs:
Fentanyl HCl, (−)-naltrexone HCl, and (−)-morphine sulfate were supplied by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). (−)-Nalbuphine HCl was provided by Dr. Kenner Rice (Drug Design and Synthesis Section, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD). (±)-Methadone HCl and (±)-buprenorphine HCl were purchased from Spectrum Chemicals (Gardena, CA). NAQ HCl was synthesized and provided by Drs. Sam Obeng and Yan Zhang. (±)-SR-14968 and (+)-TRV130 HCl were synthesized and provided by Dr. Bruce Blough. Fentanyl, naltrexone, buprenorphine, morphine, methadone, and all fentanyl/naltrexone mixtures were dissolved in sterile water. Nalbuphine was dissolved in 10% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) and 90% sterile water. NAQ was dissolved in 40% DMSO (Sigma-Aldrich) and 60% sterile water. SR-14968 was dissolved in a 1:1:8 vehicle of DMSO:Tween 80:sterile water and made fresh each test day immediately before administration. TRV130 was dissolved in a vehicle of 1:1:8 ethanol:cremophor:sterile water. All drugs were administered subcutaneously (SC) at an injection volume of 1–2 ml/kg and all drug doses are expressed as the salt forms listed above except SR-14968 which is expressed as the free base.
Individual drug effects were compared to effects of fentanyl/naltrexone mixtures as a method for stratifying the relative in vivo efficacy of drugs (Cornelissen et al., 2018). The initial fentanyl and naltrexone proportion was based on the published affinities (Kd) of fentanyl (0.16 nM) and naltrexone (0.087 nM) at the MOR in C6 glioma cells transfected with cloned rat MOR (Emmerson et al., 1996). Specifically, the fixed-proportion of fentanyl to naltrexone for the initial mixture was set to the proportion of their Kd values (0.16:0.087 = 1:0.54). Additional mixtures were tested in which the proportion of naltrexone in the mixture was reduced in 0.25 or 0.5 log units, yielding fentanyl/naltrexone mixtures of 1:0.30, 1:0.18, 1:0.054, and 1:0.018. When testing effects of a given fentanyl/naltrexone mixture, the proportion of fentanyl/naltrexone doses was held constant, and doses of both drugs were manipulated in unison.
2.3. Thermal nociception procedure
A total of 19 male and 20 female rats served as subjects and were wrapped in an absorbent bench underpad (VWR, Radnor, PA) such that their tails hung freely. The bottom 5 cm of the tail was immersed in water maintained at either 40 or 50°C in separate water baths (Thermo Precision 2833, Thermo Fisher Scientific, Waltham, MA, USA). Mercury thermometers were used to monitor water temperature. The latency for the rat to withdrawal its tail was measured by the same experimenter throughout the entire study using a hand operated digital stopwatch with a time resolution of 1/100 s. If the rat did not remove its tail by 20 s, the experimenter removed the tail and a latency of 20 s was assigned. Baseline latencies at both 40 and 50°C were obtained in each daily test session before drug administration and test sessions only occurred if tail-withdrawal latencies from 40°C did not occur before the 20 s cut off. This criterion was met in every rat during every test session. After baseline latencies were collected, rats were administered a single MOR-ligand or drug-mixture dose, and tail-withdrawal latencies were determined 30 min later unless otherwise noted. Drug or drug-mixture doses were tested no more than twice per week in an individual rat, and test sessions were separated by at least three days. Final sample sizes for each drug or drug mixture were six males and six females.
Three types of experiments were conducted. First, acute dose-effect functions were determined for a series of five MOR ligands that vary from low to high efficacy at MOR as determined by agonist-stimulated GTPγS binding (Alt et al., 2001; Emmerson et al., 1996; Selley et al., 1998; Thompson et al., 2004): NAQ (10–32 mg/kg), nalbuphine (32–100 mg/kg), buprenorphine (0.32–3.2 mg/kg), morphine (3.2–32 mg/kg), and methadone (3.2–10 mg/kg). Drugs were tested up to doses that produced maximal antinociception, undesirable physiological effects such as respiratory depression, reached solubility limits, or antagonized fentanyl effects in other studies. In addition, two GPB-MOR ligands TRV130 and SR-14968 were evaluated. TRV130 (0.1–10 mg/kg) was administered as a 30-min pretreatment based on time course studies. Time course studies with SR14968 (0.1–1 mg/kg) indicated peak effects occurred at 300 min, so dose-effect results represent the 300-min time point. Second, antagonism studies were conducted with nalbuphine (100 mg/kg) and NAQ (32 mg/kg) because neither drug produced >10%MPE in the acute dose-effect studies. For these studies, tail-withdrawal latencies were initially assessed 30 min after nalbuphine or NAQ administration. Fentanyl (0.1 mg/kg) was then administered, and tail-withdrawal latencies were redetermined 10 and 30 min after fentanyl administration. Naltrexone antagonism studies were also conducted for SR-14968. Tail-withdrawal latencies were initially assessed 300 min after SR-14968 (1 mg/kg). Subsequently, naltrexone (0.1 mg/kg) was administered, and tail-withdrawal latencies were redetermined 10 and 30 min after naltrexone administration.
Lastly, studies determined effects of a range of fentanyl doses (0.01–0.1 mg/kg) and a single naltrexone dose (0.32 mg/kg) alone. Subsequently, three fixed-proportion fentanyl/naltrexone mixtures were examined (fentanyl/naltrexone proportions of 1:0.18, 1:0.054, 1:0.018) across a range of mixture doses, and each mixture dose-effect function was determined once. In addition, drug interactions can be influenced not only by the relative proportions of drugs in a mixture, but also by the individual drug time courses. Accordingly, the time courses were determined for fentanyl alone (0.1 mg/kg), 0.1 mg/kg fentanyl in combination with 0.0018 mg/kg naltrexone (1:0.018), 1 mg/kg fentanyl in combination with 0.054 mg/kg naltrexone (1:0.054), and 1 mg/kg fentanyl in combination with 0.18 mg/kg naltrexone (1:0.18) at 10, 30, 100, and 300 min after administration.
2.4. Drug Discrimination studies
2.4.1. Apparatus:
Drug discrimination studies were conducted in sound-attenuating boxes containing modular acrylic and metal test chambers (29.2 × 30.5 × 24.1 cm; Med Associates, St. Albans, VT, USA). Each chamber contained two retractable response levers (4.5 cm wide, 2.0 cm deep, 3.0 cm above the floor), three stimulus lights (red, yellow, and green) centered 7.6 cm above each lever, and a retractable “dipper” cup (0.1 ml) located between the levers. Sonalert modules (2900 Hz and 4500 Hz; Med Associates) were placed in the upper left and right corners of the chambers, respectively. Chambers interfaced with a Windows-based computer running custom programs for controlling behavioral sessions and processing data collection (Med PC-IV, Med Associates).
2.4.2. Discrimination Training:
Rats (n=25) were trained to discriminate fentanyl from saline in a two-lever food-reinforced discrimination procedure. The position of the fentanyl-appropriate lever was the right lever for 17 rats (8 males and 9 females) and the left lever for 8 rats (4 males and 4 females). Fentanyl and saline training sessions were conducted 5–7 days per week and presented in a double alternating sequence. On training days, 0.04 mg/kg fentanyl or saline was administered 30 min before the operant session. At the start of the 15-min response period, both levers extended and stimulus lights above both levers were illuminated. Completion of the ratio requirement on the injection-appropriate lever resulted in delivery of 0.1 ml liquid food (Ensure Original Vanilla Nutrition Shake, Abbott Laboratories, Illinois, USA) and presentation of a 3 ms tone from both sonalert modules. Reinforcement was initially delivered under a fixed-ratio (FR) 1 schedule on the injection-appropriate lever, however the response requirement increased to the terminal FR10 schedule, based on individual discrimination performance. Responding on the injection-inappropriate lever reset the ratio requirement of the injection-appropriate lever. The response period terminated after 15 min or after 10 reinforcers were earned. The criterion for accurate discrimination at terminal schedule conditions was 1) greater than 80% appropriate responding for the first reinforcer 2) greater than 80% overall appropriate responding and 3) presentation of at least one reinforcer for 5 out of 6 consecutive training sessions.
2.4.2. Discrimination Testing:
Once rats met drug discrimination training criteria described above, test sessions were introduced in addition to training sessions. Test sessions were identical to training sessions, except that response requirement completion on either lever resulted in the presentation of liquid food. Test sessions were typically conducted on Tuesdays and Fridays and training sessions were conducted on all other weekdays. Test sessions were only conducted if a rat met training criteria for the two preceding training sessions. Two types of experiments were conducted. First, the same five MOR ligands (NAQ, nalbuphine, buprenorphine, morphine, and methadone) and two GPB-MOR agonists (TRV130 and SR-14968) examined in the warm water tail-withdrawal studies were also evaluated for their potency and effectiveness to produce fentanyl-appropriate responding. NAQ (1–32 mg/kg), nalbuphine (0.32–100 mg/kg), buprenorphine (0.0032–0.32 mg/kg), morphine (1–10 mg/kg), methadone (0.32–3.2 mg/kg), TRV130 (0.1–1 mg/kg), and SR-14968 (0.1–1 mg/kg) were tested up to doses that produced maximal (≥90% FAR), suppressed rates of responding, reached solubility limits, or antagonized fentanyl effects in other studies. All MOR ligands, except SR-14968, were administered as a 30-min pretreatment. Similar to the thermal nociception studies, time course studies revealed peak SR-14968 effects occurred at 300 min. The order of MOR ligands and MOR ligand doses were counterbalanced across rats. Second, acute dose-effect functions for fentanyl (0.0032–0.056 mg/kg) and naltrexone alone (0.032–0.32 mg/kg) were determined. Subsequently, four fentanyl/naltrexone fixed-proportion mixtures were evaluated: 1:0.054, 1:0.18, 1:0.30, and 1:0.54. Mixtures were tested up to doses that produced maximal fentanyl-appropriate responding, suppression of operant responding, undesirable physiological effects such as respiratory depression, or were at least 10-fold larger than the fentanyl dose (0.032 mg/kg) that alone produced maximal fentanyl-appropriate responding. All fentanyl/naltrexone dose combinations were determined once and administered as a 30-min pretreatment.
2.5. Data Analysis:
For the thermal nociception studies, raw tail-withdrawal latencies were converted to percent maximal possible effect (%MPE) using the following equation: %MPE = [(Test latency – Baseline latency) / (20 s – Baseline latency)] × 100 where “test latency” was the latency from 50°C after drug or drug mixture administration, and “control latency” was the latency from 50°C taken during the baseline period prior to drug or drug mixture administration. For the drug discrimination studies, the primary dependent measures were (1) percent fentanyl-appropriate responding (%FAR) calculated as: %FAR = [(Number of responses on the fentanyl-associated lever) / (Number of responses on both the fentanyl-and-saline-associated levers)] × 100 and (2) percent control rate (%control rate) calculated as: [(Raw rates of responding during the test session) / (Average rate of responding of all saline training sessions for each individual rat for that set of experiments)] × 100. These dependent measures were then plotted as a function of MOR ligand dose or time. In addition, ED50 values were defined as the MOR ligand dose that produced 50%MPE or 50%FAR and calculated using nonlinear regression using the [Agonist] vs. normalized response equation (GraphPad Prism, La Jolla, CA). A drug was considered to produce full generalization to the fentanyl stimulus or full antinociception when the %FAR or %MPE was ≥90%.
Maximum fentanyl/naltrexone mixture or MOR ligand effects for each dependent measure described above were determined and defined as the greatest effect regardless of drug or drug mixture dose. Individual values for females and males were calculated for each measure regardless of dose and were averaged to yield a group mean. Group mean maximum effects were compared using a two-way analysis of variance (ANOVA) with sex and fentanyl/naltrexone proportion as the main factors. In the absence of a significant sex × fentanyl/naltrexone proportion interaction, the analysis defaulted to one-way ANOVAs. A Tukey post-hoc test was conducted following a significant main effect. Maximum effect values were also used in the analysis described in the next paragraph.
Fentanyl/naltrexone mixtures have been shown to generate precise increments in efficacy that can be used 1) to calibrate efficacy requirements for drug effects in different procedures, and 2) to infer efficacies of other MOR ligands tested in those procedures (Cornelissen et al., 2018). Mixtures of 1:0.018, 1:0.054, 1:0.18, 1:0.3, and 1:0.54 fentanyl/naltrexone will have relative efficacies along this continuum of 55.56, 18.52, 5.56, 3.33, and 1.85 respectively (i.e. relative efficacy = fractional contribution of fentanyl in the mixture, 1/0.018 = 55.56). The efficacy requirement for thermal antinociception at 50°C and drug discrimination were then quantified by (a) testing effects of fentanyl and naltrexone alone and of fentanyl/naltrexone mixtures, (b) generating efficacy-effect graphs to relate maximum effects of each drug and mixture to the fentanyl proportion and associated relative efficacy, and (c) using nonlinear regression to determine the EP50 value, defined as the “effective proportion” of fentanyl to produce a maximum effect equal to 50% MPE or 50% FAR. EP50 values were then compared across procedures. Additionally, once efficacy-effect relationships are established, efficacies of test MOR ligands were then estimated as the fentanyl proportion that produced maximum effects equivalent to that of the test ligand. Efficacy-effect functions were generated using nonlinear regression (GraphPad Prism) to fit maximum effects data for each fentanyl/naltrexone mixture for %MPE and %FAR using the equation: Effect = 100 × [(fentanyl proportionHill Slope) / (EP50Hill Slope + (fentanyl proportionHill Slope))] where fentanyl proportion was the fractional contribution of fentanyl to the total drug in the mixture, and EP50 was the fentanyl proportion that produced a maximum effect equivalent to 50% MPE or 50% FAR. Relative efficacies of MOR ligands were then estimated by comparing maximum effects of each MOR ligand at each dependent measure (i.e., %MPE and %FAR) with the efficacy-effect function. Specifically, relative efficacy was defined as the fentanyl proportion at which maximum effects of the MOR ligand deviated least from the efficacy-effect function. Deviation was quantified as the sum of the differences between MOR ligand maximum effect and efficacy-effect function, and the fentanyl proportion was identified at which deviation was smallest.
3. Results
3.1. Unbiased and G-protein biased mu-opioid receptor ligands in warm water tail-withdrawal and drug discrimination procedures
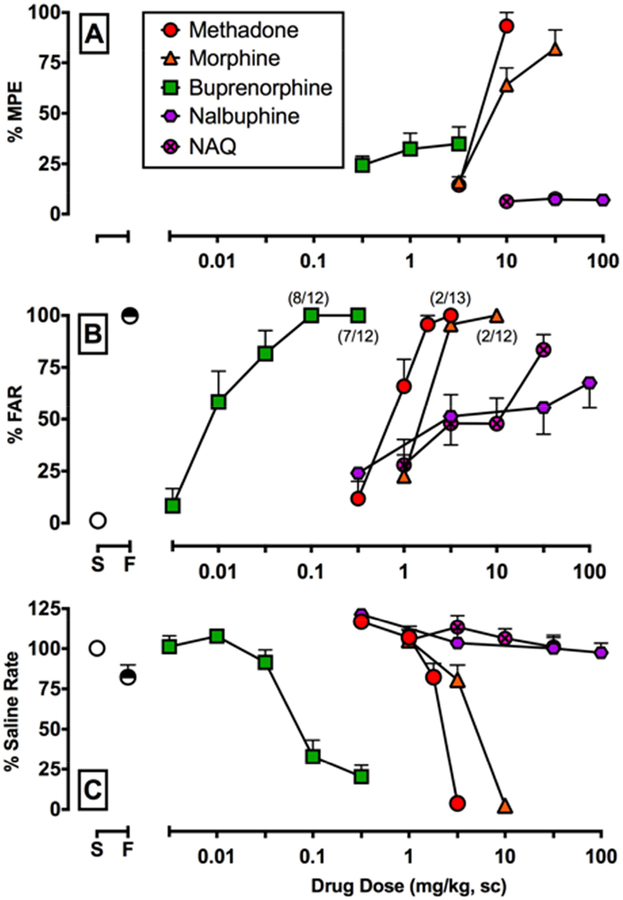
Across all baseline sessions prior to drug administration, tail-withdrawal latencies were 20 ± 0.0 and 3.9 ± 0.1 s at 40°C and 50°C, respectively. Figure 1 shows the antinociceptive (Panel A), discriminative stimulus (Panel B), and rate-altering (Panel C) effects of NAQ, nalbuphine, buprenorphine, morphine, methadone. Fentanyl effects are shown in Figure 4. MOR ligand ED50 values at each endpoint are shown in Table 1 and maximum effect values determined from individual dose-effect functions are shown in Table 2. MOR ligand ED50 values separated by sex are shown in Supplementary Table 1 and only sex differences in the potency of fentanyl and buprenorphine to produce discriminative stimulus effects were noted. Figure 3A shows potency ratios for MOR ligands to produce antinociception vs. discriminative stimulus effects for males and females combined. Fentanyl, methadone, and morphine were significantly more potent in the drug discrimination vs. warm water tail-withdrawal procedure as denoted by nonoverlapping confidence limits. There were no significant potency differences between rate-decreasing and antinociceptive effects (Table 1). Methadone produced maximal (≥90% MPE) antinociceptive effects at 50°C, and the %MPEmax rank order (from highest to lowest) was methadone = fentanyl > morphine > buprenorphine > NAQ = nalbuphine (Table 2). Because both nalbuphine and NAQ produced < 10% MPE, nalbuphine (100 mg/kg, SC) and NAQ (32 mg/kg, SC) were administered as a 30-min pretreatment to fentanyl. Both nalbuphine and NAQ antagonized fentanyl-induced antinociception (Supplementary Figure 1A). In drug discrimination, fentanyl, methadone, morphine, and buprenorphine produced maximal (≥90% FAR) fentanyl-like stimulus effects. The %FARmax rank order (from highest to lowest) was fentanyl = buprenorphine = methadone = morphine > nalbuphine > NAQ (Table 2). Figure 3B shows relative efficacies of MOR ligands to produce %MPEmax vs. %FARmax. Fentanyl, methadone, and morphine were equi-effective between the two procedures. However, buprenorphine, nalbuphine, and NAQ produced significantly greater discriminative stimulus vs. antinociceptive effects as denoted by nonoverlapping confidence limits between the two endpoints.
Figure 1:
Effects of five different MOR ligands in a warm water tail-withdrawal and fentanyl discrimination procedure in male and female rats. Panel A shows effects at 50°C. Panels B and C show effects in the fentanyl discrimination procedure. All five MOR ligands were administered as a 30-min pretreatment. Abscissae: acute drug dose alone in milligrams per kilogram administered subcutaneously (SC). Symbols above “S” and “F” represent mean ± s.e.m. for all training sessions that preceded test sessions when the saline- and fentanyl-associated keys were correct, respectively. Top ordinate: percent maximum possible effect (%MPE). Middle ordinate: percent fentanyl-appropriate responding (%FAR). Bottom ordinate: percent saline rate. All points represent mean ± s.e.m. of 12–13 rats. Numbers in parentheses denote the number of subjects (numerator) contributing to that data point if less than the total number of subjects tested (denominator) and indicated dose where at least one rat failed to complete at least one ratio requirement.
Figure 4:
Effects of fixed-proportion fentanyl/naltrexone mixtures in a warm water tail-withdrawal (50°C; Panel A) and fentanyl discrimination procedure (Panels B and C) in male and female rats. Fentanyl, naltrexone, and fentanyl/naltrexone mixtures were administered as a 30-min pretreatment. Abscissae: acute drug dose alone in milligrams per kilogram administered subcutaneously (SC). For the fentanyl/naltrexone mixtures, the abscissae show the fentanyl dose and the naltrexone dose = fentanyl dos × naltrexone proportion. Symbols above “S” and “F” represent mean ± s.e.m. for all training sessions that preceded test sessions when the saline- and fentanyl-associated keys were correct, respectively. Top ordinate: percent maximum possible effect (%MPE). Middle ordinate: percent fentanyl-appropriate responding (%FAR). Bottom ordinate: percent saline rate. All points represent mean ± s.e.m. of 12–14 rats. Numbers in parentheses denote the number of subjects (numerator) contributing to that data point if less than the total number of subjects tested (denominator) and indicated dose where at least one rat failed to complete at least one ratio requirement.
Table 1.
MOR ligand ED50 values and (95% confidence limits; CL) in the warm water tail-withdrawal (50°C) and drug discrimination procedure in male and female rats (n=11–14).
| Ligand | Warm-water Tail Withdrawal (WWTW) | Drug Discrimination (DD) | Rate Suppression (RS) |
|---|---|---|---|
| ED50 in mg/kg (95% CL) | ED50 in mg/kg (95% CL) | ED50 in mg/kg (95% CL) | |
| Fentanyl | 0.049 (0.034, 0.071) | 0.014 (0.0099, 0.020) | 0.069 (0.047, 0.11) |
| Methadone | 4.97 (2.92, 8.36) | 0.68 (0.40, 1.15) | 2.95 (1.86, 4.95) |
| Morphine | 8.07 (5.34, 12.18) | 1.29 (0.74, 2.17) | 5.42 (3.43, 8.76) |
| Buprenorphine | N/C | 0.0096 (0.0057, 0.016) | 0.097 (0.066, 0.15) |
| Nalbuphine | N/C | 10.52 (1.43, 51.41) | N/C |
| NAQ | N/C | 5.40 (2.59, 11.13) | N/C |
| TRV130 | 0.89 (0.60, 1.32) | 0.49 (0.31, 0.78) | 1.04 (0.61, 1.93) |
| SR-14968 | 0.65 (0.46, 0.95) | 0.33 (0.17, 0.70) | 0.35 (0.21, 0.58) |
N/C: not calculated because no drug dose produced >50% group effect for that endpoint
Table 2.
Group mean %MPEmax and %FARmax values and (95% confidence limits; CL) for each test MOR ligand administered in male and female rats (n=11–13). Estimated efficacy of each compound relative to the naltrexone-to-fentanyl continuum in proportion of fentanyl units.
| Drug | %MPEmax (95% CL) | %FARmax (95% CL) | Proportion Fentanyl |
|---|---|---|---|
| (±)-Methadone | 93.77 (81.55, 100) | 97.62 (93.1, 100) | 47.3 |
| (−)-Morphine | 85.94 (71.72, 100) | 96.03 (90.87, 100) | 31.4 |
| (+)-TRV130 | 100 | 74.47 (51.58, 97.36) | 25.4 |
| (±)-Buprenorphine | 45.2 (31.5, 58.9) | 100 | 14.6 |
| SR-14968 | 82.32 (67.64, 96.99) | 51.02 (25.64, 76.4) | 12.1 |
| (−)-Nalbuphine | 10.63 (5.99, 15.27) | 93.42 (86.8, 100) | 7.8 |
| NAQ | 12.29 (9.06, 15.51) | 86.18 (72.05, 100) | 7.2 |
Figure 3:
Relative potency (Panel A) and efficacy (Panel B) ratios of GPB-MOR (SR-14968, TRV130) and unbiased MOR (methadone, fentanyl, morphine, buprenorphine, nalbuphine, and NAQ) ligands in male and female rats. Relative potency ratios were calculated as ED50 values in drug discrimination ÷ ED50 values in warm-water tail withdrawal for each MOR ligand (Table 1). Relative efficacy ratios were calculated as %MPEmax ÷ %FARmax for each MOR ligand (Table 2). Asterisks denote nonoverlapping 95% confidence intervals between the two procedures. An “X” denotes a ratio could not be calculated because no MOR ligand dose produced >50% group effect for one of the two endpoints (Table 1).
Figure 2 shows the antinociceptive (Panel A), discriminative stimulus (Panel B), and rate-altering (Panel C) effects of TRV130 and SR-14968. Table 1 shows aggregate ED50 values in both males and females and Supplementary Table 1 shows ED50 values separated by sex. In contrast to fentanyl, methadone, and morphine which were significantly more potent to produce discriminative stimulus vs. antinociceptive effects, potency ratios for either GPB-MOR agonist were not significantly different suggestive of a trend towards a better potency ratio profile (Figure 3A). There were also no significant potency differences between rate-decreasing and antinociceptive effects (Table 1). Maximum effect values at each endpoint for TRV130 and SR-14968 determined from individual dose-effect functions are shown in Table 2 and relative efficacies are shown in Figure 3B. Both SR-14968 and TRV130 had relative efficacies greater than 1.0 indicative of both GPB-MOR agonists trending towards producing %MPEmax vs. %FARmax. Larger SR-14968 doses (3.2 mg/kg) were examined in a subset of animals and discovered to be toxic. Figure 2B also shows the antinociceptive time course of TRV130 (10 mg/kg) and SR-14968 (1 mg/kg). TRV130 produced peak antinociceptive effects at 10 and 30 min, whereas SR-14968 produced peak antinociceptive effects at 300 min. Supplementary Figure 1B shows SR-14968-induced antinociception was reversed by 0.1 mg/kg naltrexone. Supplementary Figure 2 shows the time course of SR-14968 discriminative stimulus and rate-altering effects. Supplementary Figure 3 shows naltrexone (0.1 mg/kg, sc) antagonism of TRV130 discriminative stimulus and TRV130 and SR-14968 rate-altering effects.
Figure 2:
Effects of two different GPB-MOR ligands TRV130 and SR-14968 in a warm water tail-withdrawal and fentanyl discrimination procedure in male and female rats. Panels A (dose effect) and B (time course) show antinociceptive effects at 50°C. Panels C and D show discriminative stimulus and rate-altering effects, respectively in the fentanyl discrimination procedure. TRV130 was administered as a 30-min pretreatment whereas SR-14968 was a 300-min pretreatment. Abscissae: acute drug dose alone in milligrams per kilogram administered subcutaneously (SC) or time in min or h post administration. Symbols above “S” and “F” represent mean ± s.e.m. for all training sessions that preceded test sessions when the saline- and fentanyl-associated keys were correct, respectively. Top ordinate: percent maximum possible effect (%MPE). Middle ordinate: percent fentanyl-appropriate responding (%FAR). Bottom ordinate: percent saline rate. All points represent mean ± s.e.m. of 11–12 rats. Numbers in parentheses denote the number of subjects (numerator) contributing to that data point if less than the total number of subjects tested (denominator) and indicated dose where at least one rat failed to complete at least one ratio requirement.
3.2. Fentanyl and fentanyl-naltrexone fixed-proportion mixtures effects in warm water tail-withdrawal and drug discrimination procedures
Figure 4A shows the antinociceptive effects of fentanyl alone, naltrexone alone, and fentanyl/naltrexone mixtures. Fentanyl alone produced dose-dependent antinociception; whereas, naltrexone alone produced <5% MPE. Maximum effect values (%MPEmax) are shown in Table 3. Because there was no significant main effect of sex or a significant sex × fentanyl/naltrexone proportion interaction, results from males and females were combined for statistical analysis. Fentanyl/naltrexone mixtures produced a naltrexone proportion-dependent decrease in maximum antinociception (F4,55=115.6, p<0.0001). Fentanyl produced maximum antinociception that was significantly different from all other fentanyl/naltrexone mixtures except the 1:0.018 fentanyl/naltrexone mixture. All other fentanyl/naltrexone mixtures were significantly different from each other. Supplementary Figure 4 shows there were no significant differences between the antinociception time courses of 0.1 mg/kg fentanyl alone and 0.1 mg/kg fentanyl in the 1:0.018 fentanyl/naltrexone mixture. The antinociceptive time courses of 1 mg/kg fentanyl in the 1:0.054 and 1:0.18 fentanyl/naltrexone mixture are also shown for comparison.
Table 3.
Group mean %MPEmax and %FARmax values and (±SEM) for fentanyl, naltrexone, or each fentanyl/naltrexone mixture administered in the warm water tail withdrawal or drug discrimination procedure in male and female rats (n=12–14).
| Drug or Drug Mixture | %MPEmax (± SEM) | %FARmax (± SEM) |
|---|---|---|
| Fentanyl Alone | 100 ± 0 | 98.88 ± 0.79 |
| Fentanyl/Naltrexone (1:0.018) | 95.27 ± 4.73 | NT |
| Fentanyl/Naltrexone (1:0.054) | 54.18 ± 8.72 | 100 ± 0 |
| Fentanyl/Naltrexone (1:0.18) | 9.42 ± 2.44 | 68.71 ± 10.65 |
| Fentanyl/Naltrexone (1:0.30) | NT | 30.32 ± 8.95 |
| Fentanyl/Naltrexone (1:0.54) | NT | 25.16 ± 10.46 |
| (−)-Naltrexone | −0.56 ± 1.88 | 2.75 ± 1.54 |
NT: not tested
On fentanyl and saline training days preceding all test days, mean ± SEM percent injection-appropriate responding was 97.96 ± 0.61 and 99.11 ± 0.23, respectively. Rates of responding (mean ± SEM) during fentanyl and saline training days were 0.52 ± 0.06 and 0.84 ± 0.04 responses/s, respectively. Figure 4 also shows the discriminative stimulus (Panel B) and rate-altering (Panel C) effects of fentanyl alone, naltrexone alone, and four fentanyl/naltrexone mixtures. Fentanyl alone produced dose-dependent and maximal (≥90%) %FAR and decreases in rates of responding; whereas, naltrexone alone produced ≤5%FAR and did not alter rates of responding up to the largest dose (0.32 mg/kg) examined. Maximum effect values (%FARmax) are shown in Table 3. Because there was no significant main effect of sex or a significant sex × fentanyl/naltrexone proportion interaction, results from males and females were combined for statistical analysis. Fentanyl/naltrexone mixtures produced a naltrexone proportion-dependent decrease in maximum effects (F5,77=30.67, p<0.0001). Fentanyl/naltrexone mixtures of 1:0.3 and 1:0.54 produced significantly less %FAR compared to fentanyl alone. Fentanyl/naltrexone mixtures of 1:0.18 and 1:0.054 produced significantly greater %FAR compared to naltrexone alone. Maximum effects on rates of responding were not analyzed.
3.3. Efficacy Estimates of MOR Ligands Relative to Fentanyl and Naltrexone.
Figure 5A shows efficacy-effect curves that relate %MPEmax and %FARmax effects of each fentanyl/naltrexone mixture to the proportion of fentanyl in the mixture. An extra sum-of-squares F-test demonstrated that each dependent measure was best fit by different nonlinear functions (F2,5=21.94, p=0.0033). For 50°C (rats), the Hill slope was 2.05, the EP50 value (95% confidence limits) was 13.9 (12.27, 15.66) and the R2 value was 0.99. For drug discrimination, the Hill slope was 1.85, the EP50 value was 3.69 (2.07, 5.93) and the R2 value was 0.94. The EP50 value for discriminative stimulus effects was significantly smaller than the EP50 value for thermal antinociception as demonstrated by nonoverlapping 95% confidence limits. Figure 5B shows previous results from fentanyl/naltrexone mixtures in rhesus monkeys (Cornelissen et al., 2018) replotted using the same methods as for Figure 5A. For 50°C (monkeys), the Hill slope was 2.39, the EP50 value (95% confidence limits) was 8.98 (5.42, 14.82) and the R2 value was 0.66. For 54°C (monkeys), the Hill slope was 1.93, the EP50 value (95% confidence limits) was 14.14 (8.72, 21.36) and the R2 value was 0.74. The EP50 values for thermal antinociception between rats and monkeys were not significantly different (Figure 5E). Figures 5C and 5D shows the best fit for maximum effects of NAQ, nalbuphine, buprenorphine, morphine, and methadone to the efficacy-effect function defined by the naltrexone-to-fentanyl continuum in rats (5C) and rhesus monkeys (5D), respectively. Figure 5F shows a significant (p=0.034) positive correlation (R=0.91) between proportion fentanyl values in rats and rhesus monkeys at 50°C for the five MOR ligands examined. The corresponding proportion fentanyl values for the five MOR ligands are reported in Table 2.
Figure 5:
Maximum antinociceptive effect at 50°C (square; solid line) and discriminative stimulus effect (circle; dashed line) as a function of the fentanyl proportion in the fentanyl/naltrexone mixture in male and female rats (Panel A). Panel B shows previously published results of fentanyl/naltrexone mixtures in rhesus monkeys (Cornelissen et al., 2018) replotted using fentanyl proportion calculations as described in Methods 2.5. Panels C and D shows empirically determined maximum antinociceptive and discriminative stimulus effects of NAQ, nalbuphine, buprenorphine, morphine, and methadone in rats (C) and rhesus monkeys (D). Abscissae: Relative efficacy expressed as proportion fentanyl as described in Methods 2.5. Ordinates: maximum possible effect. Panel E shows EP50 values and 95% confidence intervals for rat drug discrimination, rat antinociception (50°C), monkey antinociception (50°C), and monkey antinociception (54°C). Panel F shows the correlation of proportion fentanyl values between rats and monkeys for NAQ, nalbuphine, buprenorphine, morphine, and methadone. All points represent mean ± s.e.m. of 11–14 rats or 4 monkeys.
Figure 6 shows the best fit for maximum effects of TRV130 and SR-14968 to the efficacy-effect function defined by the naltrexone-to-fentanyl continuum. The corresponding proportion fentanyl values are reported in Table 2. Both TRV130 and SR-14968 produced greater %MPEmax and less %FARmax than would have been predicted based on the efficacy-effect function.
Figure 6:
Empirically determined maximum antinociceptive and discriminative stimulus effects of TRV130 and SR-14968. Both GPB-MOR agonists produced greater antinociceptive (square) and less discriminative stimulus (circle) effects than predicted from fentanyl/naltrexone mixtures (antinociception: solid line; discrimination: dashed line). All points represent mean ± s.e.m. of 11–12 rats. Abscissa: Relative efficacy expressed as proportion fentanyl as described in Methods 2.5. Ordinate: maximum possible effect.
4. Discussion
The present study compared the effects of two GPB-MOR agonists (SR-14968, TRV130) and five other MOR ligands (methadone, fentanyl, morphine, nalbuphine, NAQ) in warm water tail-withdrawal and drug discrimination procedures in male and female rats. There were three main findings. First, all but the low-efficacy MOR agonists nalbuphine and NAQ produced dose-dependent antinociception, and all seven drugs produced dose-dependent fentanyl-like discriminative stimulus effects. Second, the potency ratios and efficacy ratios for each drug to produce antinociception vs. fentanyl-like stimulus effects were compared, and the GPB-MOR agonists had more favorable potency ratios and efficacy ratios than the other five opioids to produce antinociception. Lastly, a range of fixed-proportion fentanyl/naltrexone mixtures was also evaluated to establish an efficacy-effect function in each procedure, and data from each drug were fit to this efficacy-effect function. Data for methadone, fentanyl, morphine, nalbuphine, and NAQ fit well to the function, and efficacy estimates for these drugs in rats correlated with efficacy estimates reported previously for these same drugs in rhesus monkeys (Cornelissen et al., 2018). By contrast, data for the two GPB-MOR agonists did not fit well to the fentanyl/naltrexone efficacy-effect function. Taken together, these results provide evidence to suggest that GPB-MOR agonists may have a distinct pharmacology that includes better potency and efficacy ratios than existing opioid analgesics to produce therapeutic antinociception vs. abuse-related discriminative stimulus effects.
Fentanyl, methadone, and morphine produced dose-dependent antinociception and discriminative stimulus effects in both male and female rats and all three MOR agonists were significantly more potent to produce fentanyl-like stimulus vs. antinociceptive effects. The present results are consistent with the extant literature demonstrating the antinociceptive and discriminative stimulus effects of these MOR agonists in humans (Finch and DeKornfeld, 1967), nonhuman primates (Cornelissen et al., 2018; Maguire and France, 2014), and rats (Colpaert et al., 1976; Morgan and Picker, 1996). NAQ failed to produce antinociception in male and female rats up to doses that antagonized the antinociceptive effects of fentanyl. These NAQ results are consistent with previous findings in male mice (Zhang et al., 2014), male and female rats in a chronic pain model (Siemian et al., 2016), and male rhesus monkeys (Cornelissen et al., 2018). In contrast to the warm water tail-withdrawal studies, NAQ produced dose-dependent fentanyl-like stimulus effects. These NAQ results were consistent with and extend previous results demonstrating NAQ produced facilitation of intracranial brain stimulation in opioid-experienced male rats (Altarifi et al., 2017). Overall, the present results with these unbiased MOR ligands were consistent with the extant literature in male and female Sprague Dawley rats (Morgan et al., 1999; Terner et al., 2003) and provide an empirical foundation for comparison with novel GPB-MOR agonists.
TRV130 and SR-14968 also produced dose-dependent antinociception and discriminative stimulus effects and there were three main findings. First, no sex differences in potency or effectiveness were detected for either GPB-MOR agonist. The antinociceptive effects of TRV130 and SR-14968 in the present study were consistent with previous studies in male mice (DeWire et al., 2013; Liang et al., 2018; Schmid et al., 2017) and male rats (DeWire et al., 2013; Zamarripa et al., 2018) and extend these previous findings to females. Second, the fentanyl-like stimulus effects of TRV130 were consistent with and extended previous preclinical studies examining the abuse-related effects of TRV130 using intracranial self-stimulation (Altarifi et al., 2017) and drug self-administration procedures (Zamarripa et al., 2018) in male rats and human subjective reports with TRV130 (Soergel et al., 2014). Overall, the present TRV130 results are consistent with the growing body of literature (Negus and Freeman, 2018) demonstrating that GPB-MOR agonists produce prototypic MOR agonist abuse-related effects.
Third, SR-14968 served as a useful pharmacological tool to assess the role of G-protein bias in MOR agonist abuse-related effects because SR-14968 and TRV130 are relatively similar in agonist-stimulated GTPγS binding, but SR-14968 is approximately 10-fold more G-protein biased than TRV130 (DeWire et al., 2013; Schmid et al., 2017). Out of eleven rats tested, SR-14968 produced greater than 80%FAR in only four rats. In contrast, TRV130 produced greater than 80%FAR in eight out of eleven rats tested. Although SR-14968 produced less %FARMax effects than TRV130, maximum fentanyl-like discriminative stimulus effects between SR-14968 and TRV130 were not significantly different (t20=1.35, p=0.2). One potential implication of these SR-14968 and TRV130 results could be that even more G-protein vs. β-arrestin biased MOR ligands than SR-14968 may have even better selectivity for therapeutic vs. undesirable abuse-related effects.
Increasing amounts of naltrexone to fentanyl in the fixed-proportion fentanyl/naltrexone mixture produced a naltrexone-proportion dependent decrease in maximal antinociception and fentanyl-like stimulus effects. There were no significant sex differences in maximal effects of fentanyl/naltrexone mixtures on either behavioral endpoint. The present study extends previous fentanyl/naltrexone mixture findings in rhesus monkeys (Cornelissen et al., 2018) to discriminative stimulus effects and confirms previous findings using irreversible MOR antagonists that discriminative stimulus effects require lower MOR efficacy than thermal antinociception (Adams et al., 1990; Holtzman, 1997; Morgan and Picker, 1996; Zernig et al., 1994). Furthermore, the EP50 value (95% confidence limits) for thermal antinociception at 50°C were not significantly different between rats (present results) and monkeys (Cornelissen et al., 2018). In fact, the EP50 value for thermal antinociception in rats was more similar to the EP50 value for thermal antinociception at 54°C in monkeys. Overall, the present results further demonstrate the utility of fixed-proportion agonist:antagonist mixtures to manipulate in vivo effectiveness that translates across at least two species and potentially to humans for basic research or therapeutic applications.
Fentanyl/naltrexone mixtures were also used to generate efficacy-effect functions for stratifying various MOR ligands based on in vivo antinociceptive and discriminative stimulus efficacy. For example, NAQ effects were quantified as a 7.2 proportion fentanyl and were roughly equivalent to effects with the 1:0.18 fentanyl/naltrexone mixture (5.56 proportion fentanyl). The rank order of lowest-to-highest in vivo efficacy NAQ < nalbuphine < buprenorphine < morphine < methadone using the efficacy-effect function from the fentanyl/naltrexone mixtures agreed with the rank efficacy order as determined by in vitro agonist-stimulated GTPγS binding (Alt et al., 2001; Selley et al., 1998; Yuan et al., 2015). With the exception of nalbuphine and buprenorphine being switched, this rank order of in vivo efficacy in rats also agreed with previous rankings in monkeys (Cornelissen et al., 2018). Overall, fentanyl/naltrexone mixtures offer one flexible strategy to quantify MOR agonists across multiple different experimental endpoints and species.
In contrast to the five unbiased MOR ligands, TRV130 and SR-14968 efficacy to produce antinociception was greater than and efficacy to produce fentanyl-like stimulus effects was less than that predicted based on the efficacy-effect functions from the fentanyl/naltrexone mixtures. Although TRV130 and SR-14968 rate-decreasing effects may have contributed to the antinociceptive effects, potency ratios for rate-decreasing vs. antinociceptive effects were not significantly different for any MOR ligand examined. Thus, this explanation does not adequately explain the poor efficacy-effect function fits for TRV130 and SR-14968. One future direction might be to generate an efficacy-effect function with a GPB-MOR agonist such as TRV130 and naltrexone to determine how both GPB-MOR and unbiased MOR agonists would be stratified on therapeutic and undesirable abuse-related endpoints. In conclusion, although the present results support the potential for GPB-MOR agonists to produce a more desirable profile of analgesia with reduced abuse potential, the separation in potency for signaling via the G-protein vs. β-arrestin pathways that is currently achievable limits the interpretation of whether these preclinical results will translate into improved clinical utility compared to currently utilized MOR agonists.
Supplementary Material
Highlights.
G-protein biased mu agonists produced fentanyl-like discriminative stimulus effects
GPB-MOR agonists produced better potency and efficacy ratios than existing opioids
Fixed-proportion fentanyl/naltrexone mixtures manipulate in vivo drug efficacy
Mu agonist antinociceptive effects in rats and monkeys were positively correlated
Acknowledgements
We thank Dr. Katherine Nicholson for lending the hot-water baths used in this study and Kevin Costa for programming assistance.
Funding Sources:
This work was supported by the National Institutes of Health grants [F31DA043921; R01DA037287; R01DA024022]. A portion of this work was also supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA) Intramural Research Programs. NIAAA and NIDA had no role in study design, collection, analysis, and interpretation of the data, in the writing or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAAA or NIDA.
Abbreviation:
- MOR
mu-opioid receptor
- DMSO
dimethyl sulfoxide
- GPB
G-Protein Biased
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- Adams JU, Paronis CA, Holtzman SG, 1990. Assessment of relative intrinsic activity of muopioid analgesics in vivo by using beta-funaltrexamine. J Pharmacol Exp Ther 255, 1027–1032. [PubMed] [Google Scholar]
- Alt A, McFadyen IJ, Fan CD, Woods JH, Traynor JR, 2001. Stimulation of Guanosine-5′-o-(3-[35S]thio)triphosphate Binding in Digitonin-Permeabilized C6 Rat Glioma Cells: Evidence for an Organized Association of μ-Opioid Receptors and G Protein. J Pharmacol Exp Ther 298, 116–121. [PubMed] [Google Scholar]
- Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS, 2017. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol 31, 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Rice KC, Negus SS, 2010. Antinociceptive Interactions between Mu-Opioid Receptor Agonists and the Serotonin Uptake Inhibitor Clomipramine in Rhesus Monkeys: Role of Mu Agonist Efficacy. J Pharmacol Exp Ther 335, 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68, 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN, 2000. Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAA-ergic ligands. Psychopharmacology 153, 67–84. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJ, Janssen PA, 1976. Theoretical and methodological considerations on drug discrimination learning. Psychopharmacologia 46, 169–177. [DOI] [PubMed] [Google Scholar]
- Corbett AD, Henderson G, McKnight AT, Paterson SJ, 2006. 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br J Pharmacol 147, S153–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen JC, Obeng S, Rice KC, Zhang Y, Negus SS, Banks ML, 2018. Application of Receptor Theory to the Design and Use of Fixed-Proportion Mu-Opioid Agonist and Antagonist Mixtures in Rhesus Monkeys. J Pharmacol Exp Ther 365, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council, National Research, 2011. Guide for the care and use of laboratory animals. National Academies Press, Washington DC. [Google Scholar]
- Craft RM, 2008. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Exp Clin Psychopharmacol 16, 376–385. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen X-T, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD, 2013. A G Protein-Biased Ligand at the μ-Opioid Receptor Is Potently Analgesic with Reduced Gastrointestinal and Respiratory Dysfunction Compared with Morphine. J Pharmacol Exp Ther 344, 708–717. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F, 1996. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J Pharmacol Exp Ther 278, 1121–1127. [PubMed] [Google Scholar]
- Finch JS, DeKornfeld TJ, 1967. Clinical Investigation of the Analgesic Potency and Respiratory Depressant Activity of Fentanyl, a New Narcotic Analgesic. J Clin Pharmacol J New Drugs 7, 46–51. [DOI] [PubMed] [Google Scholar]
- Holtzman SG, 1985. Drug discrimination studies. Drug Alcohol Depend 14, 263–282. [DOI] [PubMed] [Google Scholar]
- Holtzman SG, 1997. Antagonism of Morphine-Like Discriminative Effects by β-Funaltrexamine. Pharmacol Biochem Behav 57, 771–777. [DOI] [PubMed] [Google Scholar]
- Kenakin T, 2015. The Effective Application of Biased Signaling to New Drug Discovery. Mol Pharmacol 88, 1055–1061. [DOI] [PubMed] [Google Scholar]
- Liang DY, Li WW, Nwaneshiudu C, Irvine KA, Clark JD, 2018. Pharmacological Characters of Oliceridine, a mu-Opioid Receptor G-Protein-Biased Ligand in Mice. Anesth Analg. [DOI] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2014. Impact of Efficacy at the Mu-Opioid Receptor on Antinociceptive Effects of Combinations of Mu-Opioid Receptor Agonists and Cannabinoid Receptor Agonists. J Pharmacol Exp Ther 351, 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Cook CD, Picker MJ, 1999. Sensitivity to the Discriminative Stimulus and Antinociceptive Effects of μ Opioids: Role of Strain of Rat, Stimulus Intensity, and Intrinsic Efficacy at the μ Opioid Receptor. J Pharmacol Exp Ther 289, 965–975. [PubMed] [Google Scholar]
- Morgan D, Picker MJ, 1996. Contribution of individual differences to discriminative stimulus, antinociceptive and rate-decreasing effects of opioids: importance of the drug’s relative intrinsic efficacy at the mu receptor. Behav Pharmacol 7, 261–284. [PubMed] [Google Scholar]
- Negus SS, Bear AE, Folk JE, Rice KC, 2009. Role of delta opioid efficacy as a determinant of mu/delta opioid interactions in rhesus monkeys. Eur J Pharmacol 602, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Freeman KB, 2018. Abuse potential of biased mu opioid receptor agonists. Trends Pharmacol Sci 39, 916–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Schrode K, Stevenson GW, 2008. Mu/kappa opioid interactions in rhesus monkeys: implications for analgesia and abuse liability. Exp Clin Psychopharmacol 16, 386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankovic Z, Brust TF, Bohn LM, 2016. Biased agonism: An emerging paradigm in GPCR drug discovery. Bioorg Med Chem Lett 26, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C, NM K, Ross N, Lovell K, Yue Z, Morgenweck J, Cameron M, Bannister M, Bohn LM, 2017. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171, 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley DE, Liu Q, Childers SR, 1998. Signal Transduction Correlates of Mu Opioid Agonist Intrinsic Efficacy: Receptor-Stimulated [35S]GTPγS Binding in mMOR-CHO Cells and Rat Thalamus. J Pharmacol Exp Ther 285, 496–505. [PubMed] [Google Scholar]
- Seth P, Rudd RA, Noonan RK, Haegerich TM, 2018. Quantifying the Epidemic of Prescription Opioid Overdose Deaths. Am J Pub Health 108, 500–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemian JN, Obeng S, Zhang Y, Zhang Y, Li J-X, 2016. Antinociceptive Interactions between the Imidazoline I2 Receptor Agonist 2-BFI and Opioids in Rats: Role of Efficacy at the μ-Opioid Receptor. J Pharmacol Exp Ther 357, 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla N, Minkowitz HS, Soergel DG, Burt DA, Subach RA, Salamea MY, Fossler MJ, Skobieranda F, 2017. A randomized, Phase IIb study investigating oliceridine (TRV130), a novel mu-receptor G-protein pathway selective (mu-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J Pain Res 10, 2413–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soergel DG, Subach RA, Burnham N, Lark MW, James IE, Sadler BM, Skobieranda F, Violin JD, Webster LR, 2014. Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 155, 1829–1835. [DOI] [PubMed] [Google Scholar]
- Stahl EL, Zhou L, Ehlert FJ, Bohn LM, 2015. A Novel Method for Analyzing Extremely Biased Agonism at G Protein–Coupled Receptors. Mol Pharmacol 87, 866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terner JM, Lomas LM, Smith ES, Barrett AC, Picker MJ, 2003. Pharmacogenetic analysis of sex differences in opioid antinociception in rats. Pain 106, 381–391. [DOI] [PubMed] [Google Scholar]
- Thompson CM, Wojno H, Greiner E, May EL, Rice KC, Selley DE, 2004. Activation of G-Proteins by Morphine and Codeine Congeners: Insights to the Relevance of O- and N-Demethylated Metabolites at μ- and δ-Opioid Receptors. J Pharmacol Exp Ther 308, 547–554. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Collins FS, 2017. The Role of Science in Addressing the Opioid Crisis. N Engl J Med 377, 391–394. [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Adedoyin A, Leventhal L, 2008. Predictive validity of animal pain models? A comparison of the pharmacokinetic–pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology 54, 767–775. [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Pomonis JD, Kennedy JD, 2013. An industry perspective on the role and utility of animal models of pain in drug discovery. Neurosci Lett 557, 65–72. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zaidi SA, Stevens DL, Scoggins KL, Mosier PD, Kellogg GE, Dewey WL, Selley DE, Zhang Y, 2015. Design, syntheses, and pharmacological characterization of 17-cyclopropylmethyl-3,14beta-dihydroxy-4,5alpha-epoxy-6alpha-(isoquinoline-3’-ca rboxamido)morphinan analogues as opioid receptor ligands. Bioorg Med Chem 23, 1701–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarripa A, Edwards SR, Qureshi HN, Yi JN, Blough BE, Freeman KB, 2018. The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug Alcohol Depend 192, 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Butelman ER, Lewis JW, Walker EA, Woods JH, 1994. In vivo determination of mu opioid receptor turnover in rhesus monkeys after irreversible blockade with clocinnamox. J Pharmacol Exp Ther 269, 57–65. [PubMed] [Google Scholar]
- Zhang Y, Braithwaite A, Yuan Y, Streicher JM, Bilsky EJ, 2014. Behavioral and cellular pharmacology characterization of 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6α-(isoquinoline-3′-carboxamido)morphinan (NAQ) as a mu opioid receptor selective ligand. Eur J Pharmacol 736, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.