Abstract
Objective:
To compare the relative quantity of talk between providers, caregivers, and adolescents and young adults (AYAs) with chronic kidney disease (CKD) and how communication differs by age.
Methods:
During nephrology clinic visits, conversations between AYAs with CKD (N = 99, ages 11 –20, median = 15), their caregivers, and providers (N=19) were audiotaped and coded using the Roter Interaction Analysis System. Linear mixed models tested AYA age differences in talk frequency by AYAs, caregivers, and providers. Post-hoc analyses tested differences in talk using AYA age groups.
Results:
During clinic visits, providers spoke the most (63.7%), and caregivers spoke more (22.6%) than AYAs (13.7%). Overall talk differed by AYA age in AYAs (p < 0.001) and caregivers (p <0.05), but not providers. Higher AYA age was associated with more AYA talk (biomedical information-giving, partnering, rapport-oriented) and less caregiver biomedical information-giving (ps < 0.001 –0.05). In post-hoc analyses, young adults talked more than adolescents; caregiver talk decreased in the middle- adolescent group.
Conclusions:
Increases in AYA talk occur primarily in young adulthood, whereas caregiver talk decreases in middle adolescence. This may indicate an appropriate developmental shift but raises concerns about conversational gaps during middle-adolescence.
Practice implications:
During transition-oriented treatment planning, providers should engage both AYAs and caregivers to avoid potential gaps in communication.
Keywords: Patient-provider communication, Adolescents, Young adults, Caregivers, Chronic kidney disease, Nephrology, Transition, Subspecialty, Pediatric
1. Introduction
Provider-patient communication is essential for providing high-quality health care and effective communication between medical providers and patients is linked to higher family satisfaction in pediatric settings [1], better adherence across pediatric and adult care [2], and better health outcomes in adolescents and adults [3]. Still, more rigorous approaches are needed to define the active components of “effective communication” [4]. Patient-provider communication is complicated in pediatric care, with developmental complexities affecting parents’ and children’s participation during visits [5,6]. In primary care settings, findings are mixed on the influence of child age on providers’, children’s and parents’ communication [6,7], while talk in pediatric subspecialty settings is not well studied.
Previous pediatric studies have included wide age ranges without accounting for developmentally distinct phases. Similarly, young adults are often grouped into studies of adults, neglecting the unique aspects of young adulthood. There is a remarkable dearth of research on healthcare communication during the transition from pediatric to adult care. For adolescent and young adult patients (AYAs), understanding the relative participation of conversation by all participants has the potential to promote successful transitions for AYAs with chronic health conditions [8].
1.1. Developmental shifts in communication during pediatric medical visits
Unsurprisingly, providers speak the most and primarily discuss biomedical topics in pediatric primary care [5,9–11]. With older child age, there is more direct provider-child communication [11]. Providers may allocate more verbal turns to older children, but actual duration of child talk does not necessarily increase. In a study with children ages 3 months to 18 years, family physicians and pediatricians used more information-gathering with older children and spent less time relationship-building [5]. Similarly, older children contribute more information during medical encounters, often in exchange for less caregiver talk [6,11]. Qualitatively, adolescents have reported that they want more health-related communication with their health professionals [12]. Unfortunately, they describe providers and caregivers as dominating the conversation and report feeling passive and inhibited. Parents may also act as gatekeepers to manage their condition and/or withhold information from their children [12,13]. However, no studies of communication in pediatric settings have examined AYA as a distinct developmental period by directly observing talk in subspecialty care visits.
1.2. Communication in AYA subspecialty care
Childhood chronic illness imposes high demands on children, families and providers, which is further strained during adolescence. With chronic illness, there is more information to exchange about health status, medication regimen, and treatment plans [14,15]. Further, there is an expectation for AYAs to assume more responsibility for their care [6,11,16], which is particularly important as individuation and self-management become increasingly crucial [12,16]. The American Academy of Pediatrics (AAP) and Society of Adolescent Medicine (SAM) stress the importance of involving adolescents in a gradual transition towards selfmanagement [17,18]. However, in specialty pediatric care settings, providers dominate medical interactions and discuss primarily biomedical topics, similar to observations in primary care [11,19]. Ineffective communication may reduce AYAs’ self-efficacy, adherence to medical regimens, and providers’ accurate assessment of AYAs’ readiness for transition to adult care [20]. Adolescence marks the beginning of a sensitive period in which self-efficacy, biomedical knowledge, and self-management skills could either promote or interfere with this transition [20]. Despite its theoretical and preliminary data highlighting its importance, there is relatively little research on communication in AYA subspecialty care.
The shortcomings of available literature are three-fold: first, although distinct from primary care, communication in pediatric subspecialty care is not well-studied; second, medical communication has not been examined during the crucial developmental stage of adolescence and young adulthood; third, AYA participation during medical encounters has been less well-studied than that of providers and caregivers. Quantitative studies are needed to identify the nature of medical communication among all participants during AYA subspecialty visits to inform the future development of interventions.
1.3. Study aims
This study was designed to examine communication during outpatient nephrology clinic visits among providers, caregivers and AYAs with chronic kidney disease (CKD). CKD is a progressive disease which requires continuous, often complex medical care [15]. Despite a heterogeneous etiology of CKD, antihypertensive medications can slow the progression of the disease and thus are commonly prescribed [21]. Thus, the subspecialty care setting associated with CKD care may be generalizable to other chronic illnesses. Given the need for more thorough examination of medical communication among AYAs in subspecialty care, we had two primary aims: (1) Compare the relative quantity of talk between providers, caregivers, and AYAs and (2) examine how communication changes at different AYA ages. We hypothesized that: (1) providers would speak the most during the medical encounter, followed by caregivers, with the least talk by AYAs and (2) talk by AYAs would increase with age, and caregiver talk would decrease. A key goal of this study was to explore which types of talk change with higher AYA age.
2. Methods
2.1. Participants
As part of a larger longitudinal, observational study about communication, antihypertensive medication adherence and health outcomes, AYAs with CKD and their caregivers were recruited from pediatric nephrology patient rosters of three academic medical centers in the Mid-Atlantic region of the United States. AYA inclusion criteria included a confirmed diagnosis of CKD stages 1–5, age 11–19 years at consent, and being prescribed an antihypertensive medication for at least 6 months. AYAs were excluded if they were unable to comprehend spoken English, pregnant, unwilling to use electronic medication monitors, had developmental delays that would interfere with study procedures, had a sibling enrolled in the study, or had undergone a kidney transplant less than 6 months ago. All participants were established patients at the participating clinic; the recorded visit was never the first clinical encounter at the site. Of 128 enrolled participants, 102 completed baseline surveys and a clinic visit (13 never attended a clinic visit, 5 withdrew, 3 were on dialysis/ transferred care, 2 discontinued antihypertensive medications, 1 moved, 1 provider refused to be recorded, 1 clinic visit included a non-enrolled sibling). For this study, an additional inclusion criterion was that a caregiver was present at the clinic visit; 3 visits had no caregiver present, resulting in a final sample size of 99.
2.2. Procedures
After providing informed consent/assent, AYAs and caregivers completed a baseline assessment and an audio-recorded clinic visit. The clinic visit was completed separately from the baseline assessment (median = 16 days after baseline, IQR = −14–78). Families were compensated $100 for the baseline assessment and $50 for audiotaping a clinic visit; providers received no payment. Research staff accompanied the family to a scheduled clinic visit, started the audio recorder, and left the room. Nineteen pediatric nephrology care physicians consented to audiotaping medical encounters. All other clinic staff who entered the room to provide care (medical technicians, nurses, residents, and social workers) consented to be audiotaped. For simplicity, we refer to nephrologists and other members of the care team as “providers”. Providers and families were informed they could stop recording at any time. Three recordings were paused or turned off briefly during the visit (2 by providers, 1 when caregiver received a phone call), but these visits were included after determining that the majority of the visit was captured.
2.3. Measures
At the baseline assessment, caregivers and AYAs completed a survey that included patient (age, gender, race) and caregiver (education, relationship to patient, and household income) characteristics as well as the duration of the relationship with their nephrology provider. Health comorbidities (dialysis, transplant, stage) were abstracted from the medical record. Height and weight were collected at the baseline assessment to compute body mass index (BMI) percentiles using the CDC’s pediatric BMI calculator (https://nccd.cdc.gov/dnpabmi/calculator.aspx).
Audiotaped encounters were coded using the Roter Interactive Assessment System (RIAS) [22], the most widely used method of coding patient-provider interactions. The unit of analysis (“talk”) is defined as a complete thought, usually a simple sentence, clause, or word that conveys sufficient information to be categorized under a single code [22]. All codes are mutually exclusive and exhaustive. RIAS has high reliability (Pearson r= 0.70–0.90) and predictive validity for a variety of patient outcomes [23]. We used well- established RIAS composite counts [24,25] by each speaker (number of times each speaker used the following: biomedical information-giving, biomedical information-gathering, psychosocial information-giving, psychosocial information-gathering, partnering and activation, positive talk, emotional talk, social talk, negative talk, and procedural talk). In addition, overall talk (total count of all units of talk) was examined for each speaker (providers, caregivers, or AYAs) and across all speakers during the visit. Talk by all providers, including nephrologists and other participating providers, was combined to form “provider talk” count variables. Similarly, when two caregivers were present (i.e., mother and father), talk by both caregivers comprised “caregiver talk” variables. Each type of caregiver (mother, father, other caregiver, or both parents) was tracked using a grouping variable. One coder with 4 years of experience and established levels of high reliability in previous studies coded the study recordings. Coding was monitored throughout the coding period with a random selection of 24 recordings double-coded by the same individual. In the RIAS categories occurring greater than once per session, average Pearson’s correlation coefficients were 0.956 (0.80–0.99) for providers (23 codes) and 0.941 (0.86–0.99) for patients and caregivers (13 codes).
2.4. Analytic plan
All analyses were executed with SPSS. Proportions were computed for relative contribution to overall talk in the visit as well as within-speaker talk. For raw scores (frequency counts) and proportions, descriptive analyses were conducted to examine the distribution of data and outliers. Linear mixed models (LMMs) with post-hoc contrasts were used for all other analyses to account for patient clustering within providers. The following variables were tested for potential confounding effects: visit duration, total count of talk, caregiver type, AYA race, AYA gender, and household income.
LMMs were used to examine the differences between speakers on the frequency count for each talk variable, controlling for non-independence of cases occurring from nesting of data within provider. Next, to examine effects of age on talk, LMMs were computed for each speaker group (providers, caregivers, and AYAs) predicting frequency count of overall talk as the dependent variable. To further examine the underlying nature of the effect of age on talk, additional post-hoc LMMs were computed with frequency count of specific subtypes of talk as dependent variables. For each model, age was entered as a fixed effect, and provider was entered as a random effect to control for nesting within provider. Covariates were frequency count of overall talk and duration of visit (due to their high correlations with individual composites of talk), as well as the type of caregiver.
As a follow-up exploration of the results in the prior LMMs, a grouping variable for AYA age was calculated which included three age groups (11–14,15–17, and 18–20 years). LMMs were computed again using age group as the fixed effect with post-hoc contrasts to explore the differences between age groups. Bar graphs were created from this analysis to illustrate within-group differences among the talk variables that yielded significant results.
3. Results
Table 1 shows diverse demographic and caregiver characteristics of AYAs (median age = 15 years, IQR= 13–17) at their baseline assessment. Families reported a median relationship of 6 years (IQR = 3–11) with the provider. Mothers primarily attended the clinic visit (71%). Nineteen nephrology providers (79% female and 58% Caucasian) participated in clinic visits, with a median of 3 (range = 1–18) patients per provider (within-provider intra-class coefficients for overall talk by adolescents = 0.15; caregivers = 0.24; providers = 0.61). In 49 clinic observations, between one and three additional providers participated in the visit, often a registered nurse (N = 32) or medical resident (N = 14). Median duration was 23.82 min (IQR= 18.03–31.48). For all analyses, patient race, gender, household income, duration of relationship with provider, and illness severity (transplant and dialysis status, CKD stage) were tested as covariates; these variables did not significantly affect results and were removed from final analyses.
Table 1.
Demographic and caregiver characteristics of the adolescents and young adults (AYA) evaluated at the baseline visit.
| N | % | |
|---|---|---|
| AYA Age | ||
| 11–14 | 45 | 45.5 |
| 15–17 | 31 | 31.3 |
| 18–20 | 23 | 23.2 |
| AYA Race | ||
| African American | 51 | 51.5 |
| Caucasian | 38 | 38.4 |
| Asian | 4 | 4.0 |
| Hispanic/Latino | 2 | 2.0 |
| Other/Not reported | 4 | 4.0 |
| AYA Gender | ||
| Male | 54 | 54.5 |
| Female | 45 | 45.5 |
| AYA Health Status | ||
| Post-transplant | 24 | 24.2 |
| Hypertension diagnosis | 41 | 41.4 |
| Obesity diagnosis | 31 | 31.3 |
| Household Income | ||
| <$50,000 | 29 | 29.3 |
| $50,000–100,000 | 27 | 27.3 |
| ≥$100,000 | 28 | 28.3 |
| Not reported | 15 | 15.2 |
| Health Insurance | ||
| Public | 42 | 42.4 |
| Private | 40 | 40.4 |
| Both (Public and Private) | 5 | 5.1 |
| Military | 6 | 6.1 |
| Unknown/Other | 6 | 6.1 |
| Caregiver Relationship to AYA | ||
| Mother | 70 | 70.7 |
| Father | 13 | 13.1 |
| Both (Mother & Father) | 10 | 10.1 |
| Other | 6 | 6.0 |
Note: Total N = 99. IQR = Inter-Quartile Range. Other race consisted of Spanish (N = 1); not reported (N = 3). Other caregivers attending visit consisted of grandmother (N = 4), godmother (N = 1), and older sister (N = 1). Obesity diagnosis was calculated as a BMI ≥95th percentile.
3.1. Overall talk
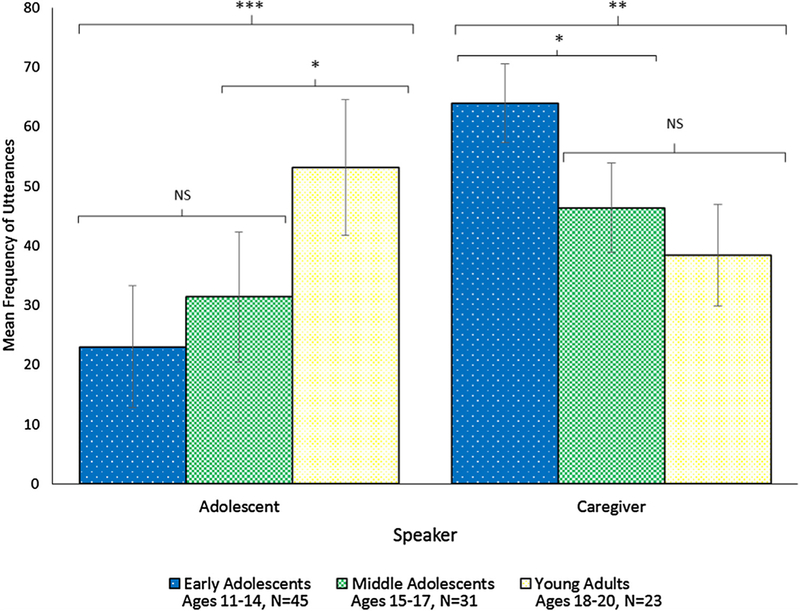
Table 2 shows descriptive statistics of talk for each speaker group (providers, caregivers, and AYAs). Overall talk differed by speaker (F(2,196) = 226.59, p <0.001); providers talked more than caregivers and AYAs, and caregivers talked more than AYAs (all p <0.001). Proportionally, providers contributed a mean of 63.7% (SD = 7.6) of all talk in the visits, caregivers contributed 22.6% (SD = 9.0), and AYAs contributed 13.7% (SD = 9.4). Providers had higher RIAS scores (p < 0.05) than caregivers and AYAs for all composites except for psychosocial information-giving and negative talk. Caregivers also had higher RIAS scores (p < 0.05) than AYAs for all composites except for lifestyle/psychosocial information-giving and –gathering as well as procedural talk. Biomedical information-giving was the highest frequency form of talk among each group, consisting of 28.9% of talk by providers, 32.3% of talk by caregivers, and 36.7% of talk by AYAs.
Table 2.
Descriptive statistics for frequency counts of talk composites by adolescents and young adults (AYA), caregivers, and providers during their baseline clinic appointment.
| AYA |
Caregivers |
Providers |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Min | Max | Median | IQR | Min | Max | Median | IQR | Min | Max | |
| Overall Talk | 70 | 36–124 | 3 | 592 | 156 | 84–220 | 18 | 488 | 432 | 300–566 | 127 | 1205 |
| Biomedical | ||||||||||||
| Information-Giving | 26 | 14–41 | 0 | 265 | 47 | 21–76 | 5 | 193 | 116 | 74–166 | 14 | 557 |
| Information-Gathering | 1 | 0–3 | 0 | 22 | 3 | 2–7 | 0 | 34 | 37 | 23–47 | 5 | 91 |
| Lifestyle/Psychosocial | ||||||||||||
| Information-Giving | 14 | 7–27 | 0 | 161 | 17 | 6–39 | 1 | 167 | 18 | 7–37 | 0 | 116 |
| Information-Gathering | 0 | 0–0 | 0 | 3 | 0 | 0–1 | 0 | 7 | 13 | 8–19 | 0 | 53 |
| Partnering | ||||||||||||
| Partnering and Activation | 6 | 2–12 | 0 | 91 | 24 | 12–51 | 2 | 107 | 66 | 46–99 | 18 | 239 |
| Rapport-Oriented | ||||||||||||
| Positive Talk | 9 | 4–20 | 0 | 64 | 23 | 14–36 | 1 | 88 | 64 | 38–93 | 10 | 204 |
| Emotional Talk | 4 | 2–8 | 0 | 64 | 8 | 4–17 | 0 | 59 | 43 | 28–66 | 0 | 159 |
| Social Talk | 1 | 0–2 | 0 | 20 | 2 | 0–6 | 0 | 59 | 6 | 2–13 | 0 | 90 |
| Negative Talk | 0 | 0–1 | 0 | 14 | 1 | 0–2 | 0 | 15 | 1 | 0–2 | 0 | 8 |
| Procedural | ||||||||||||
| Procedural Talk | 1 | 0–2 | 0 | 11 | 1 | 0–3 | 0 | 19 | 39 | 25–50 | 5 | 100 |
Note: IQR = Inter-Quartile Range. Min = Minimum. Max = Maximum All talk variables are frequency counts of units of talk, each characterized by a complete thought (the smallest distinct statement that can be classified according to the RIAS system).
3.2. Differences in talk by AYA age
There were significant linear associations of AYA age on frequency count of talk for caregivers and AYAs, but not providers (Table 3). Higher AYA age was associated with more overall AYA talk (p < 0.001) and less caregiver talk (p < 0.01). In addition, with increased age, AYA used more biomedical information-giving (p < 0.001), partnering and activation (p < 0.001), positive (p < 0.001), emotional (p < 0.01), and social (p < 0.05) talk. With increased AYA age, caregivers used less biomedical information-giving (p < 0.01), lifestyle/psychosocial information-giving (p < 0.05), lifestyle/psychosocial information-gathering (p < 0.05), positive (p < 0.05), emotional (p < 0.05), and negative talk (p < 0.05). Neither biomedical information-gathering nor procedural talk were associated with AYA age for any speaker. Provider talk was not associated with AYA age for any talk variable.
Table 3.
Fixed effects of age on talk in adolescents and young adults (AYA), caregivers, and providers from linear mixed models.
| AYA |
Caregivers |
Providers |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Estimate | SE | 95% CI | F | Estimate | SE | 95% CI | F | Estimate | SE | 95% CI | |
| Overall Talk | 16.81*** | 13.78 | 3.36 | 7.10–20.46 | 9.04** | –11.39 | 3.79 | –18.92-−.86 | 0.01 | –0.91 | 8.14 | –17.11–15.28 |
| Biomedicai | ||||||||||||
| Information-Giving | 13.63*** | 4.77 | 1.29 | 2.20–7.34 | 9.45** | –4.30 | 1.40 | –7.09-−1.52 | 0.31 | 2.21 | 3.96 | –5.65–10.08 |
| Information-Gathering | 1.95 | 0.17 | 0.12 | –0.07–0.42 | 0.56 | 0.17 | 0.23 | –0.29–0.63 | 0.82 | 0.67 | 0.74 | –0.80–2.13 |
| Lifestyle/Psychosocial | ||||||||||||
| Information-Giving | 1.99 | 1.43 | 1.02 | –0.58–3.45 | 6.47* | –2.51 | 0.99 | –4.47-−0.55 | 1.10 | –1.00 | 0.96 | –2.90–0.90 |
| Information-Gathering | 0.02 | 0.00 | 0.04 | –0.07–0.07 | 4.85* | –0.11 | 0.05 | –0.20-−0.01 | 1.32 | –0.43 | 0.38 | –1.18–0.32 |
| Partnering | ||||||||||||
| Partnering and Activation | 30.50*** | 3.26 | 0.59 | 2.09–4.43 | 3.72 | –1.84 | 0.95 | –3.74–0.06 | 0.18 | –0.68 | 1.58 | –3.83–2.47 |
| Rapport-Oriented | ||||||||||||
| Positive Talk | 24.31*** | 2.43 | 0.49 | 1.45–3.42 | 4.76* | –1.54 | 0.71 | –2.94-−0.14 | 0.19 | –0.53 | 1.22 | –2.95–1.89 |
| Emotional Talk | 6.67** | 0.99 | 0.38 | 0.23–1.74 | 5.39* | –1.07 | 0.46 | –1.99-−0.15 | 1.34 | –1.08 | 0.94 | –2.95–0.78 |
| Social Talk | 6.57* | 0.35 | 0.14 | 0.08–0.62 | 0.14 | –0.15 | 0.41 | –0.67–0.98 | 0.09 | 0.20 | 0.66 | –1.10–1.50 |
| Negative Talk | 2.17 | 0.11 | 0.07 | –0.04–0.26 | 5.80* | –0.22 | 0.09 | –0.40-−0.04 | 0.07 | –0.02 | 0.07 | –0.16–0.12 |
| Procedural | ||||||||||||
| Procedural Talk | 0.90 | 0.08 | 0.09 | –0.09–0.25 | 0.35 | –0.06 | 0.10 | –0.26–0.14 | 0.41 | –0.39 | 0.61 | –1.61–0.82 |
Note: Each linear mixed model included age as a continuous variable and provider as a random effect, controlling for total length of visit (minutes), total talk in visit (count), and caregiver type.
p < 0.001.
p ≤0.01.
p < 0.05.
Figs. 1–3 visually illustrate the differences in talk across developmental stages using three age groups: “early adolescent” (ages 11–14) “middle adolescent” (ages 15–17) and “young adult” (ages 18–20). Regardless of age group, providers talked the most, whereas AYA and caregiver talk differed between age groups (Fig. 1). Young adults talked proportionately more than adolescents, and caregivers of young adults talked less than caregivers of adolescents. Post-hoc contrasts indicated that young adults used significantly more biomedical information-giving (p < 0.05; Fig. 2), partnering and activation (p < 0.001; Fig. 3A), emotional talk (p < 0.05; Fig. 3B), and positive talk (p < 0.001; Fig. 3C) compared to either adolescent group. There were no differences in RIAS composite scores between early and middle adolescents. In contrast, caregivers engaged in significantly less biomedical information-giving for the middle adolescent (p < 0.05) and young adult (p <0.001) age groups compared to the early adolescent group with no difference between caregivers in the middle adolescent and young adult groups (Fig. 2).
Fig. 1.
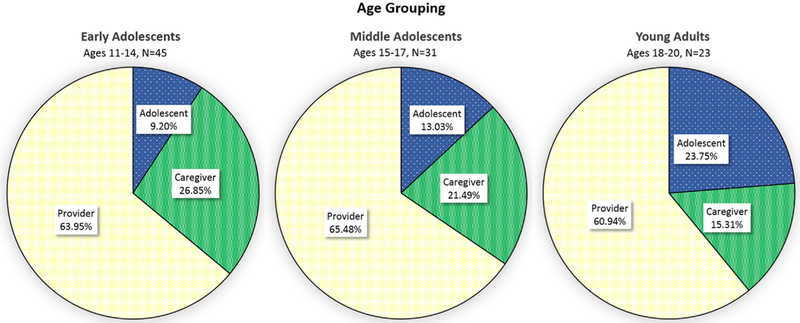
Mean proportion of overall talk by age group in adolescents and young adults (AYA), caregivers, and providers.
Fig. 3.
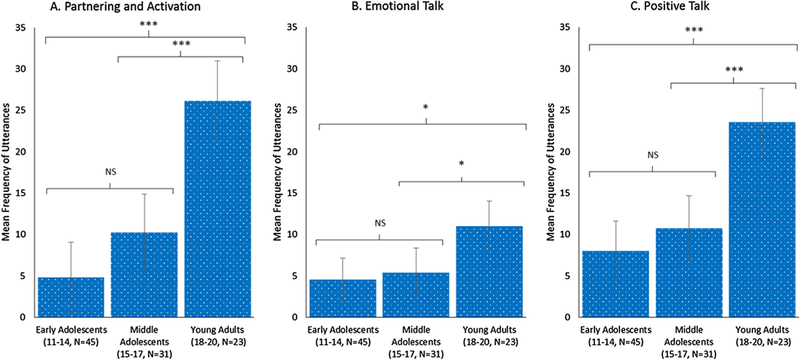
Marginal means and differences by age group from linear mixed models with post-hoc contrasts by age group for adolescents and young adults (AYA) rapport-oriented talk. Note: ***p < 0.001, *p < 0.05, NS = not significant.
Fig. 2.
Marginal means and differences by age group from linear mixed models with post-hoc contrasts for biomedical information-giving in adolescents and young adults (AYA) and caregivers. Note: ***p < 0.001, **p ≤ 0.01, *p < 0.05, NS = not significant.
To explore the aspects of biomedical talk underlying age differences, additional post-hoc testing using LMMs (Table 4) was completed on the two codes that comprised the composite: “therapeutic regimen information-giving” (e.g., past, current and future medications and treatment planning) and “medical information-giving” (e.g., medical history and symptoms). AYAs’ therapeutic regimen information-giving was lower in the early adolescent group compared to either of the older groups (ps <0.05). Young adults used more medical information-giving compared to either of the younger groups (ps < 0.05). Turning to caregivers, medical information-giving was higher among care-givers in the early adolescent group compared to middle adolescent and young adult groups (ps < 0.05). Therapeutic regimen information-giving by caregivers did not differ across age groups. For providers, therapeutic regimen information-giving was higher in the middle adolescent and young adult groups compared to the early adolescent group (ps < 0.05) whereas there were no differences on medical information-giving.
Table 4.
Marginal means and post-hoc contrasts from linear mixed models testing differences in talk by age group for therapeutic regimen and medical information-giving.
| Information-Giving Talk | Age Group |
|||||
|---|---|---|---|---|---|---|
|
Early Adolescents (ages 11–14) |
Middle Adolescents (ages 15–17) |
Young Adults (ages 18–20) |
||||
| Marginal Mean | SE | Marginal Mean | SE | Marginal Mean | SE | |
| AYA Talk | ||||||
| Therapeutic Regimen | 8.79a,c | 4.38 | 18.28a | 4.85 | 24.40c | 5.03 |
| Medical | 14.60c | 4.47 | 14.43b | 4.89 | 28.85b,c | 5.6 |
| Caregiver Talk | ||||||
| Therapeutic Regimen | 28.79 | 6.93 | 20.93 | 7.51 | 17.44 | 9.59 |
| Medical | 30.93a,c | 3.29 | 20.40a | 3.53 | 18.82c | 5.25 |
| Provider Talk | ||||||
| Therapeutic Regimen | 46.10a | 18.20 | 79.31a | 19.99 | 54.57 | 26.31 |
| Medical | 50.70 | 8.01 | 48.68 | 8.48 | 45.84 | 12.14 |
Note: SE = standard error. Variables with matching superscript (a, b, c) indicate significant differences of at least p < 0.05 according to post-hoc contrasts between the following age groups.
Early vs. middle adolescent groups.
Middle adolescent vs. young adult groups.
Early adolescent vs. young adult groups.
4. Discussion and conclusion
4.1. Discussion
As the first quantitative study evaluating communication during AYA subspecialty care visits, our findings offer innovative insights into the patient-provider relationship during this sensitive developmental period. We found differences in the proportion of conversation and developmentally-relevant age differences in AYA and caregiver talk. These differences highlight the importance of in-depth investigation of patient-provider communication among all speakers and identify several clinical considerations for successful transition to adult care.
4.1.1. Overall characteristics of communication during visits
Proportionately, providers talked the most, similar to other subspecialty care settings, such as gastroenterology and rheumatology clinics [19], specialized outpatient pediatric consultations in the Netherlands [11], family practice acute visits for children [5,9,10], and adult primary care in which physicians contribute 60% of the conversation [26]. The nature of CKD makes it reasonable to expect provider-driven sharing of biomedical information in nephrology appointments. Interestingly, quantity of provider talk did not vary by AYA age, which has been noted elsewhere in pediatric visits [5]. Biomedical information-giving was the most common form of talk, consistent with research in other settings with adults [23]. Overall, adolescents contributed relatively little talk during the clinic visit, similar to prior studies [5,11,27], but notably low given our focus on AYAs rather than younger children. Low child participation is often attributed to physicians’ roles as both an adult and an institutional authority [6]. Qualitative studies have shown that chronically ill adolescents anticipate criticism or disapproval from providers if they disclose worsening symptoms of their condition or reveal poor adherence [12].
4.1.2. Adolescents and young adults
As hypothesized, young adults demonstrated more overall talk than adolescents. While there were linear trends that AYA age was associated with more biomedical information-giving, partnering and activation, emotional talk, and positive talk, our group analyses demonstrate that the increase occurred primarily in the young adult group. Therefore, the transition from adolescence to adulthood likely brings higher expectations, motivation and/or skills to actively participate through sharing health information, showing sustained interest, and seeking reassurance. Related, qualitative studies show that young adults have the interest in a trusting, mutually respectful relationship with their providers [28]. We found no effects of age on psychosocial and lifestyle-related conversation, in contrast to other studies, which have shown increases in child psychosocial talk with higher age [5]. Differences in findings may be due to our specific focus on adolescence, our inclusion of multiple categories of talk, or the nature of conversation during a subspecialty care visit.
The biomedical aspects of a chronic illness may be complex enough that adolescents are not developmentally ready to independently navigate a medical encounter [29], which would explain the relatively consistent quantity of talk between the early and middle adolescent groups. Our data suggest that middle adolescence may be a key age for becoming more independent in knowing one’s therapeutic regimen–that is, the names, doses, and functions of medications. Subsequently, young adults may build upon earlier acquired knowledge about therapeutic regimen to gain additional responsibility for their medical history and more actively participate in visits. This progression follows many developmental models about the gradual progression needed for successful transitioning to adult care [18,30].
Correspondingly, for middle adolescents, providers focus more of the visit on the therapeutic regimen– specifically, medical reconciliation which likely contributed to the increase in the middle adolescents’ talk on this topic. This is consistent with the International Society of Nephrology (ISN) and International Pediatric Nephrology Association (IPNA) consensus statement on transition, which recommends encouraging adolescents to gradually assume self-management responsibilities (e.g., knowing their medications, adhering to their regimen, and acquiring skills) [30]. While knowledge of the therapeutic regimen is a necessary first step, it is likely insufficient for effective transitioning to independent self-management. For example, transition models such as the TRxANSITION Scale [30] not only identify proficiencies in managing therapeutic regimen, but also include other aspects of medical knowledge and history, as well as psychosocial functioning and planning. Given our findings, it is possible that in practice, therapeutic management is prioritized above other topics, perhaps due to the perceived importance of medication adherence and time constraints of a clinic visit.
4.1.3. Caregiver participation
Because our study required a caregiver to be present, increased AYA talk is not merely an artifact of older adolescents or young adults attending appointments alone. Instead, this likely reflects a true developmental shift. Whereas prior data has suggested that caregivers may interfere with doctor-patient communication [31], our findings do not support this, at least in regards to the quantity of talk during visits. However, the timing of changes in conversation by caregivers compared to adolescents appear somewhat uncoordinated. Specifically, caregivers of 15- to 17-year-olds show less biomedical talk, yet the quantity of their adolescents’ biomedical information-giving is not correspondingly higher. Therefore, discussion of the teenagers’ interim medical history and symptoms may be under-discussed during middle adolescence, leaving the teenager vulnerable to improper treatment or poorer quality of life.
4.1.4. Limitations
Several limitations should be considered in interpreting the current findings. First, this is cross-sectional data; although we can speculate about developmental shifts, we cannot comment on longitudinal trajectories of communication. Related, although duration of patient-provider relationship did not affect our results in a single encounter, we are not able to comment on the trajectory of change in communication over many years. Second, as this study was exploratory, our analyses were not designed to correct for Type I error and should be interpreted with care. Nonetheless, we controlled for potential confounders and accounted for nesting of data. Related, we may have been underpowered to detect differences in low-frequency psychosocial talk, especially with a relatively small sample size; its potential to facilitate a positive patient-provider relationship should not be discounted [32]. Third, because approximately half of visits included more than one provider, this study population may differ from primary care. Finally, the RIAS coding system uses a single rather than multiple coders; further, it differs from discourse and conversational analysis and is not a method of content analysis. Nonetheless, RIAS is well-validated and the most widely used tool for quantifying and summarizing communication patterns in medical encounters.
4.1.5. Future directions
Patient-provider communication has not been as well-studied in AYA subspecialty care, and our findings support a need for several areas to investigate further. First, longitudinal research of communication will more effectively assess changes in trajectories of talk within individuals over time. Second, communication research in AYA subspecialty care should examine the actual content of talk, which likely shifts as life transitions occur (e.g., puberty, entering high school/college). Third, research should test the generalizability of subspecialty care communication across populations and compared to primary care. Finally, future work should test the assumption that certain features of communication, other characteristics such as gender and race and provider concordance, and their timing in development could affect outcomes as proposed by professional societies [7,33], are beneficial to AYA transition of care, medication adherence, and health outcomes. If these assumptions are upheld, they would support the development of empirically-based communication interventions. Integrating complementary coding methods with longitudinal models and outcomes will identify qualities of patient-provider communication that may have an effect on health across development.
4.2. Conclusion
This study provided the unique opportunity to compare conversations by AYAs with CKD, their caregivers, and their providers to understand developmental differences across ages. We found that talk during clinic visits involves substantial contribution by providers, with an increasingly active role among AYAs. However, changes across AYA age are not necessarily linear, although theoretical models suggest that consistent support may facilitate the achievement of independent self-care. Our findings reinforce the importance of investigating transition in subspecialty care as a developmental process that involves providers, caregivers and adolescents, and spans a wide age range from early adolescence into adulthood.
4.3. Practice implications
The AAP, SAM, International Society of Nephrology, and the International Pediatric Nephrology Association stress the importance of involving adolescents and their families in a gradual transition process well before the point of transition [17,18,30]. Our finding that there may be gaps in medical information-giving during mid-adolescence suggests that providers need to guide AYAs, with support from caregivers, through multiple competencies. Specifically, in addition to knowing medication names and dosages, AYAs should also be able to name and describe their medical conditions, make appointments and refill medications, understand their dietary needs, and be informed about sexual health and substance use issues [18,30]. Developmentally appropriate encouragement of adolescent self-management can be facilitated through comprehensive individualized treatment plans that consider the needs and abilities of AYAs. Importantly, providers should communicate expectations for patients to assume an increasingly active role in their medical appointments. Future interventions may encourage longer visits during middle adolescence to allow for gathering information and teaching of skills, with support from caregivers. This approach could provide the scaffolding and monitoring needed to acquire self-management skills needed for adult care [30]. Communication training focused on collaborative and developmentally-sensitive treatment planning may encourage AYAs’ involvement in their healthcare and foster an open dialogue throughout the transition process.
Acknowledgements
This study was supported by a grant, NIH R01 DK092919, PI: K. Riekert. We would like to thank our research staff as well as the adolescents and young adults, their caregivers, and providers who participated in this study.
Role of funding
The funding source had no involvement in the conduct of this research or preparation of the article.
Footnotes
Conflict of interest
Debra Roter is the author of the Roter Interaction Analysis System (RIAS) and holds the copyright for the system. Johns Hopkins University also has rights to some enhancements of the system. Neither Debra Roter nor Johns Hopkins collects royalties for use of the system in research as is the case for the current study. Debra Roter is an owner of RIASWorks LLC, a company that provides RIAS coding services for non-university projects and it is possible that RIASWorks would benefit indirectly from dissemination of the current research.
All other authors of this manuscript have no conflicts of interest, including financial interests or gains.
References
- [1].Swedlund MP,Schumacher JB, Young HN, Cox ED, Effect of communication style and physician-family relationships on satisfactionwith pediatric chronic disease care, Health Commun. 27 (2012) 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zolnierek KB, Dimatteo MR, Physician communication and patient adherence to treatment: a meta-analysis, Med. Care 47 (2009) 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stewart MA, Effective physician-patient communication and health outcomes: a review, CMAJ 152 (1995) 1423–1433. [PMC free article] [PubMed] [Google Scholar]
- [4].Street RL, Makoul G, Arora NK, Epstein RM, How does communication heal- pathways linking clinician-patient communication to health outcomes, Patient Educ. Couns. 74 (2009) 295–301. [DOI] [PubMed] [Google Scholar]
- [5].Cox ED, Smith MA, Brown RL, Fitzpatrick MA, Learning to participate: effect of child age and parental education on participation in pediatric visits, Health Commun. 24 (2009) 249–258. [DOI] [PubMed] [Google Scholar]
- [6].Tates K, Meeuwesen L, ‘Let Mum have her say’: turntaking in doctor-parent- child communication, Patient Educ. Couns. 40 (2000) 151–162. [DOI] [PubMed] [Google Scholar]
- [7].Wissow LS, Roter D, Bauman LJ, Crain E,Kercsmar C, Weiss K, Mitchell H, Mohr B, Patient-provider communication during the emergency department care of children with asthma, Med. Care 36 (1998) 1439–1450. [DOI] [PubMed] [Google Scholar]
- [8].Modi AC, Pai AL, Hommel KA, Hood KK, Cortina S, Hilliard ME, Guilfoyle SM, Gray WN, Drotar D, Pediatric self-management: a framework for research, practice, and policy, Pediatrics 129 (2012) 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carpenter DM, Ayala GX, Williams DM, Yeatts KB, Davis S, Sleath B, The relationship between patient-provider communication and quality of life for children with asthma and their caregivers, J. Asthma 50 (2013) 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sleath B, Carpenter DM, Slota C, Williams D, Tudor G, Yeatts K, Davis S, Ayala GX, Communication during pediatric asthma visits and self-reported asthma medication adherence, Pediatrics 130 (2012) 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van Dulmen AM, Children’s contributions to pediatric outpatient encounters, Pediatrics 102 (1998) 563–568. [DOI] [PubMed] [Google Scholar]
- [12].Beresford BA, Sloper P, Chronically ill adolescents’ experiences of communicating with doctors: a qualitative study, J. Adolesc. Health 33 (2003) 172–179. [DOI] [PubMed] [Google Scholar]
- [13].Ellis R, Leventhal B, Information needs and decision-making preferences of children with cancer, Psychooncology 2 (1993) 277–284. [Google Scholar]
- [14].Whyte DA, Fine RN, Chronic kidney disease in children, Pediatr. Rev. 29 (2008) 335. [DOI] [PubMed] [Google Scholar]
- [15].Tong A, Lowe A, Sainsbury P, Craig J, Parental perspectives on caring for a child with chronic kidney disease: an in-depth interview study, Child Care Health Dev. 36 (2010) 549–557. [DOI] [PubMed] [Google Scholar]
- [16].Michaud PA,Suris JC, Viner R, The adolescent with a chronic condition: part II: healthcare provision, Arch. Dis. Child. 89 (2004) 943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Blum RW, Garell D, Hodgman CH, Jorissen TW, Okinow NA, Orr DP, Slap GB, Transition from child-centered to adult health-care systems for adolescents with chronic conditions: a position paper of the Society for Adolescent Medicine, J. Adolesc. Health 14 (1993) 570–576. [DOI] [PubMed] [Google Scholar]
- [18].American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians-American Society of Internal Medicine, A consensus statement on health care transitions for young adults with special health care needs, Pediatrics 110 (2002) 1304–1306. [PubMed] [Google Scholar]
- [19].Lipstein EA, Dodds CM, Britto MT, Real life clinic visits do not match the ideals of shared decision making, J. Pediatr. 165 (2014) 178–183e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stinson J, Kohut SA, Spiegel L, White M, Gill N, Colbourne G, Sigurdson S, Duffy KW, Tucker L, Stringer E, A systematic review of transition readiness and transfer satisfaction measures for adolescents with chronic illness, Int. J. Adolesc. Med. Health 26 (2014) 159–174. [DOI] [PubMed] [Google Scholar]
- [21].ESCAPE Trial Group, Strict blood-pressure control and progression of renal failure in children, N. Engl. J. Med. 361 (2009) 1639–1650. [DOI] [PubMed] [Google Scholar]
- [22].Roter D, Larson S, The Roter interaction analysis system (RIAS): utility and flexibility for analysis of medical interactions, Patient Educ. Couns. 46 (2002) 243–251. [DOI] [PubMed] [Google Scholar]
- [23].Roter DL, Stewart M, Putnam SM, Lipkin M, Stiles W, Inui TS, Communication patterns of primary care physicians, J Amer Med Assoc 277 (1997) 350–356. [PubMed] [Google Scholar]
- [24].Roter D, The medical visit context of treatment decision-making and the therapeutic relationship, Health Expect. 3 (2000) 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wissow L, Gadomski A, Roter D, Larson S, Lewis B, Brown J, Aspects of mental health communication skills training that predict parent and child outcomes in pediatric primary care, Patient Educ. Couns. 82 (2011) 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Roter D,Hall JA, DoctorsTalkingwith Patients/patientsTalkingwith Doctors: Improving Communication in Medical Visits, Greenwood Publishing Group, 2006. [Google Scholar]
- [27].Vigilante VA,Hossain J, Wysocki T, Sharif I, Correlates of type and quantityof child communication during pediatric subspecialty encounters, Patient Educ. Couns. 98 (2015) 1352–1359. [DOI] [PubMed] [Google Scholar]
- [28].Freake H, Barley V, Kent G, Adolescents’ views of helping professionals: a review of the literature, J. Adolesc. 30 (2007) 639–653. [DOI] [PubMed] [Google Scholar]
- [29].Ferris ME, Ferris MT, Stewart HD, Fenton N, Haberman C, Iglesia EA, Harward DH, O’Neill J, Imperial R, Ko Z, The self-management and transition to adulthood program UNC STARx: instruments and lessons from the field, Int. J. Child Adolesc. Health 6 (2013) 137. [Google Scholar]
- [30].Watson AR, Harden PN, Ferris ME, Kerr PG, Mahan JD, Ramzy MF, Transition from pediatric to adult renal services: a consensus statement by the International Society of Nephrology (ISN) and the International Pediatric Nephrology Association (IPNA), Kidney Int. 80 (2011) 704–707. [DOI] [PubMed] [Google Scholar]
- [31].Tates K, Meeuwesen L, Doctor-parent-child communication. A (re) view of the literature, Soc. Sci. Med. 52 (2001) 839–851. [DOI] [PubMed] [Google Scholar]
- [32].Butalid L, Bensing JM, Verhaak PF, Talking about psychosocial problems: an observational study on changes in doctor-patient communication in general practice between 1977 and 2008, Patient Educ. Couns. 94 (2014) 314–321. [DOI] [PubMed] [Google Scholar]
- [33].Drotar D, Physician behavior in the care of pediatric chronic illness: association with health outcomes and treatment adherence, J. Dev. Behav. Pediatr. 30 (2009) 246–254. [DOI] [PubMed] [Google Scholar]


