Abstract
Objectives:
To evaluate the effect of laterality, gender, age and body mass index (BMI) on fat fraction (FF) measurements of both parotid glands (PGs) and submandibular glands (SMGs) by using: Iterative decomposition of water and fat with echo asymmetry and least-squares estimation method (IDEAL-IQ).
Methods:
A total of 87 healthy participants were enrolled in our study. IDEAL-IQ image was scanned using a 3.0 T scanner. Paired t test was performed to compare the difference on FF of both PGs and SMGs between left and right side. The FF of two glands between male and female healthy participants were compared using an unpaired t-test. The correlation between the FF of two glands and participant age or BMI were analyzed by Pearson’s correlation.
Results:
Excellent inter- and intrareader agreements were obtained during the measurements of FF by IDEAL-IQ method (ICC, 0.952–0.981). FF values correlated positively with the age and BMI in both left and right PGs and SMGs (p < 0.05). No significant difference was found on FF between left and right PGs and SMGs (p > 0.05). There was also no difference on FF between male and female healthy participants (p > 0.05).
Conclusions:
FFs of PGs and SMGs were age- and BMI- dependent, but not laterality- and gender-dependent. The effect of age and BMI need to be considered in further studies using Ideal-IQ technology to evaluate FFs of salivary gland diseases.
Keywords: fat fraction, IDEAL-IQ, MR imaging, parotid gland, submandibular gland
Introduction
Fatty tissue is an important component within salivary glands. Dynamic change of fatty tissue within salivary glands may indicate several diseases of salivary glands, such as Sjögren’s syndrome, malnutrition, alcoholism, parotid lipomatosis, and radiation-related injury.1–4 In previous studies, ultrasonography, CT and structural MR imaging have been used to analyze the fat changes within salivary glands.3–6 However, each imaging technique has its own disadvantages. Ultrasonography is an operator-dependent technique, while radiation exposure limits the wide application of CT. Signal intensity on structural T 1and T 2 weighted image can reflect fat content changes, however they are usually assessed via subjective scoring, which is mainly limited by unsatisfactory reproducibility.
Some quantitative MR technology have been reported in detecting tissue fat fraction. 1H-MR spectroscopy (MRS) has been proven to be a useful technique for assessing the fat fraction (FF) of liver7 and bone marrow,8 however it is limited by long scan time, small imaging range without morphological information and an intricate post-processing. These disadvantages limit the wide clinical application of 1H-MRS, especially in small organs.9 Recently, Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) imaging attracts more and more attention. It can steadily separate fat and water by using three asymmetric echo times and three-point Dixon method.10 Chang HC et al reported that IDEAL method provided the highest success rate for measuring parotid fat content compared with T1 and T2 method, even in subjects with metallic dental implants.2 The latest IDEAL technique, named IDEAL-IQ, can automatically obtain FF images without off-line processing, and calculate FF with less scanning and measuring time.11,12 A recent study demonstrated that IDEAL-IQ could be clinically useful for evaluating the salivary gland FF compared to MRS.11 Previously, several studies demonstrated the fat content in PG and bone marrow could be influenced by some individual differences, such as the age, gender and body mass index (BMI).4,13,14 However, researches testified the influence of these individual differences on FF measurements of both PGs and SMGs using IDEAL-IQ method were still in lack until now.
Therefore, the purpose of our study was to evaluate the effect of laterality, gender, age and BMI on FF measurements of both PGs and SMGs by using IDEAL-IQ method.
Methods and materials
Study population
Our study was approved by the institutional review board of our hospital, informed consents were obtained from all healthy volunteers. From December 2016 to September 2017, a total of 87 healthy participants were enrolled in our study based on the following inclusion criteria: (1) no long history of smoking, excessive drinking or drug abuse; (2) no medical history of radiation therapy for head and neck tumors; (3) no previous history of salivary gland disease, such as Sjögren syndrome or salivary gland tumor. These 87 volunteers included 42 male (mean age, 54.8 ± 15.5 years; age range, 13–80 years) and 45 female (mean age, 50.4 ± 13.7 years; age range, 18–83 years) participants. All healthy volunteers were required to rest at least one hour before MR scan.
MR examinations
All examinations were acquired using a 3.0T MR system (Discovery MR750W, GE Healthcare, Waukesha, WI) with a 29-channel phased array head and neck coil. Both PGs and SMGs were covered in each MR scan. Structural MR imaging protocol included unenhanced axial T 1 weighted imaging (repetition time/echo time, 900/10 msec; section thickness, 4 mm; slices, 25; intersection gap, 1 mm; and field of view, 20 cm), axial T 2 weighted imaging with fat saturation (repetition time/echo time, 5000/99 msec; section thickness, 4 mm; slices, 25; intersection gap, 1 mm; and field of view, 20 cm), and coronal T 2 weighted imaging with fat saturation (repetition time/echo time, 5000/100 msec; slices, 15; section thickness, 4 mm; intersection gap, 1 mm; field of view, 22 cm). For IDEAL-IQ sequence, detailed imaging parameters were 6 echoes per repetition time;repetition time, 12 msec; echo time, 4 msec; echo time spacing, 0.66 msec; slice thickness, 4 mm; field of view, 24 cm; matrix, 160; auto flip angle, 5; and scan time, 45 s.
Image analyses
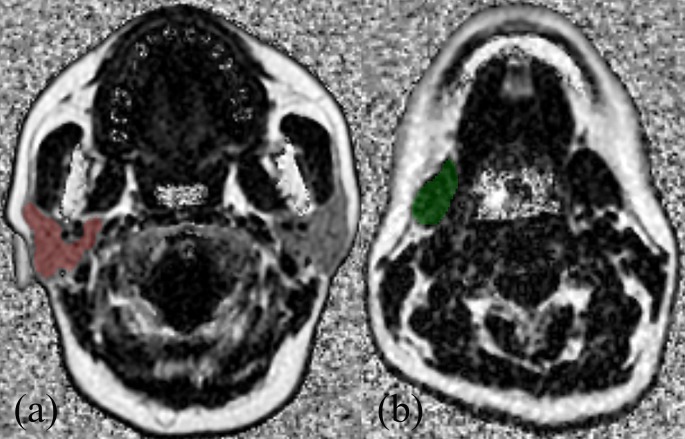
All IDEAL-IQ data were digitally transferred to offline with in-house software (FireVoxel; CAI2R; New York University School of Medicine, New York, NY) for post-processing. FF measurements of PGs and SMGs were assessed by two radiologists (with 4 and 16 years’ experience in head and neck radiology, respectively) independently. Regions of interest (ROIs) were delineated in the largest section of both PGs and SMGs in FF maps (Figure 1). The regions containing large vessels and parotid duct were excluded with reference to the T 2 weighted images with fat saturation. The results of the measurements results of the two radiologists were used to calculate inter-reader reproducibility. To assess the intrareader reproducibility, the first reader performed all the measurements again, with an interval of more than 1 month. The average result of two measurement of the first radiologist were adopted into the statistical analysis.
Figure 1.
Example of region of interest selection in right parotid gland (a) and submandibular gland (b) based on FF mapping in a 50-year-old female participant.
Statistical analysis
Numeric data were averaged over all volunteers, and reported as mean ± standard deviation. Normality of FF of PGs and SMGs was assessed using Kolmogorov–Smirnov test. Paired t test was performed to compare the difference on FF of both PGs and SMGs between left and right side. The correlations between FF of two glands and age or BMI were analyzed by Pearson’s correlation analyses. FF of PGs and SMGs between male and female healthy participants were compared with an unpaired t-test.
The intraclass correlation coefficient (ICC) with 95% confidence intervals (CI) was used to evaluate the inter- and intrareader reproducibility. ICC ranged between 0 and 1.00, and the values closer to 1.00 meant better reproducibility. ICC was interpreted as follows: r < 0.20, poor; r = 0.20 – 0.40, fair; r = 0.41 – 0.60, moderate; r = 0.61 –0.80, good; r > 0.81, excellent. Statistical analysis was performed by using the SPSS software package (v. 22.0; SPSS, Chicago, IL). A two-sided p-value less than 0.05 indicated statistical significance.
Results
IDEAL-IQ MR images showed adequate imaging quality for imaging analysis in all 87 healthy participants. There was no significant difference on FF between left and right PGs (37.279 ± 10.631 vs 37.721 ± 10.334, p = 0.253), and SMGs (12.870 ± 4.723 vs 12.954 ± 4.603, p = 0.679). Within the subgroup analyses of either female or male participants, there were also no significant differences on FF between left and right PGs and SMGs (all p > 0.05).
Between female and male participants, there were no significant difference on FF in left PGs (36.970 ± 10.828 vs 37.609 ± 10.536, p = 0.781), right PGs (37.093 ± 10.225 vs 38.394 ± 10.298, p = 0.093), left SMGs (12.047 ± 4.711 vs 13.751 ± 4.630, p = 0.260) and right SMGs (12.246 ± 4.286 vs 13.713 ± 4.857, p = 0.138). Effect of laterality and gender on FF of PGs and SMGs is shown in Table 1.
Table 1.
Effect of laterality and gender on FFs of parotid and submandibular glands
| Parameter | PG | p value | SMG | p value | ||
| Female | Male | Female | Male | |||
| Left | 36.970 ± 10.828 | 37.609 ± 10.536 | 0.781 | 12.047 ± 4.711 | 13.751 ± 4.630 | 0.260 |
| Right | 37.093 ± 10.225 | 38.394 ± 10.298 | 0.093 | 12.246 ± 4.286 | 13.713 ± 4.857 | 0.138 |
| p-value | 0.837 | 0.112 | 0.505 | 0.891 | ||
FF, indicates fat fraction; PG, parotid gland; SMG, submandibular gland;
Data are expressed as mean ± standard deviation.
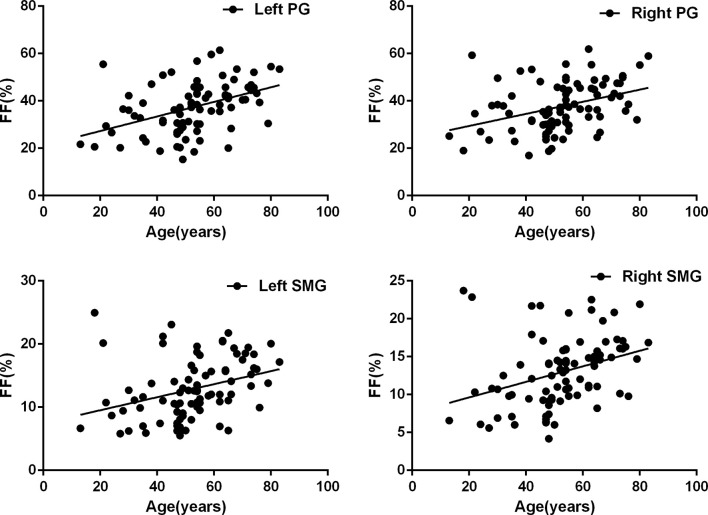
FF of both PGs and SMGs in both side showed an age-related increase (left PGs: r = 0.432 p < 0.001; right PGs: r = 0.369 p < 0.001; left SMGs: r = 0.326 p = 0.002; right SMGs: r = 0.332 p = 0.002). Detailed results about the correlation between FF and age is shown in Figure 2.
Figure 2.
Scatter plots of age effects on the measurement of FFs in bilateral parotid and submandibular glands. Correlation analyses indicated that there were significant positive correlations between age and FF of PGs and SMGs in both sides (left PGs: r = 0.432 p < 0.001; right PGs: r = 0.369 p < 0.001; left SMGs: r = 0.326 p = 0.002; right SMGs: r = 0.332 p = 0.002).
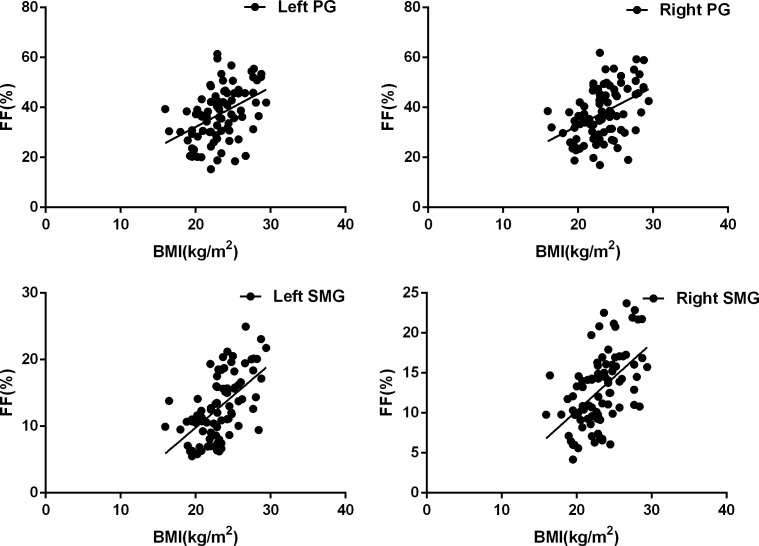
Meanwhile, significant positive correlations were found between BMI and FF in both sides of PGs and SMGs (left PGs: r = 0.431 p < 0.001; right PGs: r = 0.433 p < 0.001; left SMGs: r = 0.586 p = 0.002; right SMGs: r = 0.531 p = 0.002). Detailed results about the correlation between FF and BMI is shown in Figure 3.
Figure 3.
Scatter plots of BMI effects on the measurement of FFs in bilateral parotid and submandibular glands. Correlation analyses indicated that there were significant positive correlations between BMI and FF of PGs and SMGs in both sides (left PGs: r = 0.431 p < 0.001; right PGs: r = 0.433 p < 0.001; left SMGs: r = 0.586 p = 0.002; right SMGs: r = 0.531 p = 0.002).
Excellent inter- and intrareader ICCs were acquired for the measurements of FF in both sides of PGs and SMGs (ICC, 0.952 – 0.981). Detailed inter- and intrareader ICCs for the measurements of FF are shown in Table 2.
Table 2.
Inter-reader and intrareader agreements for the measurements of FFs
| Parameters | Inter-reader ICC | Intrareader ICC |
| Left PG | 0.970 (0.954 – 0.988) | 0.981 (0.970 – 0.992) |
| Right PG | 0.968 (0.952 – 0.987) | 0.977 (0.965 – 0.989) |
| Left SMG | 0.955 (0.939 – 0.972) | 0.962 (0.943 – 0.978) |
| Right SMG | 0.952 (0.936 – 0.971) | 0.959 (0.939 – 0.979) |
ICC, indicates intraclass correlation coefficient; PG, parotid gland; SMG, submandibular gland;
Data in parentheses are 95% confidence intervals.
Discussion
MR imaging has been used more and more for assessing the neoplastic and diffuse diseases of salivary gland.15 Conventional structural MR imaging can provide detailed information about localization and perineural extension of salivary gland tumors,15 and can also assess the morphological changes of the diffuse diseases of salivary gland, including Sjögren’s Syndrome,16 sialadenitis17 and radiation-related injury.18 Besides that, multiple functional MR techniques, including various advanced diffusion-weighted imaging model and dynamic contrast-enhanced MR imaging, can supply additional information about cell density or vascular permeability in salivary gland disease.19,20 They have been proven to be useful for differentiating malignant from benign salivary gland tumors, detecting radiation- and radioiodine-induced sialadenitis, and early diagnosing and staging of Sjögren’s syndrome.19,20
Fat infiltration is a representative pathological change in a lot of salivary gland disease.1–4 In previous study, 1H-MRS, T 1 mapping and T 2 mapping technique have been used to measure the FF of PGs and SMGs. However, the long acquisition limited their wide clinical application. Meanwhile, MRS is also limited by small imaging range without morphological information and an intricate post-processing.2,7–9 Recently IDEAL-IQ technique attracted more and more attention for its potential in the quantification of FF in salivary gland dasease.10–12 Our study demonstrated significant age-related and BMI-related changes on FF of PGs and SMGs in healthy volunteers. Along with the age or BMI increasing, FF of both PGs and SMGs also increases. However, there was no significant difference on FF between male and female participants, left and right PGs or SMGs. Our study results indicated that the influence of age and BMI should be considered, and these two variables should be matched when using IDEAL-IQ technique to assess the FF in salivary gland disease.
In our study, we found that the FF of both PGs and SMGs showed a tendency to increase along with age in healthy volunteers, which was similar to previous studies.4,5,13,14 Both Izumi et al4 and Anton et al5 found that the CT attenuation of PGs and SMGs decreased along with increasing age. Sumi reported that the T 1 weighted signal intensity of PGs increased with age.13 Chang et al found a positively association between FF and age in PGs.14 The tendency of increasing FF in PGs and SMGs along with increasing age could be explained by the characteristic age-related histopathological changes in salivary glands, such as oncocyte proliferation, fatty infiltration, squamous and mucous metaplasia, hyperplasia, atrophy, and regeneration.21–23 Thus, our finding of age-related FF changes could be explained by an increase in fibrofatty tissue and a decrease in mean acinar volume in the PGs and SMGs of elder participants.
In prior study, Takashima indicated that BMI showed correlation with extra myocellular lipids concentration using MRS.24 In our study, a similar positive correlation between FF of the salivary glands and BMI was also found. Based on our results, we thought that BMI should also be strictly matched when we assessed the FF within salivary glands in various disease.
Conflicting results existed in previous studies on the effect of gender on the FF in salivary glands.5,14 Mahne et al found that there were no significant difference on fat content of both PGs and SMGs between two genders via measuring CT attenuation.5 However, Chang et al reported that parotid gland fat content was larger in males than that in females by about ten percentage.14 Our study results were in accordance with that of the former study, and no significant difference was found between male and female participants on FF of salivary glands. In our opinion, these paradoxical results might be associated with different MR scanner, imaging parameters and sample sizes. Further study unifying these variables would be more valuable for clarifying the influence of gender on FF in salivary glands. In addition, we found no significant difference on the FF existed between left and right salivary glands. Therefore, the laterality was not a crucial confounding factor when assessing the FF in salivary glands.
Excellent inter- and intrareader ICC indicated that Ideal-IQ was a highly reproducible imaging technology, and could serve as a promising diagnostic imaging technique for assessing the fat contents in salivary gland disease that characterized by dynamic changes of fat content, such as Sjögren’s syndrome, malnutrition, alcoholism, parotid lipomatosis, and radiation-related injury.1–4
Our study had several limitations. Firstly, the sample size was relatively small. Further studies with larger numbers of volunteers will be needed to validate our results. Secondly, our results were not compared with tissue fat content measurements that directly assessed by pathologic biopsy of salivary gland. Thirdly, the stability of Ideal-IQ technology was influenced by MRI parameters such as matrix and slice thickness, which should be considered in the study.
In conclusion, our preliminary study demonstrated that FFs of PGs and SMGs were age- and BMI- dependent, but not laterality- and gender- dependent. Our study results indicated that the influence of age and BMI need to be considered in future studies that use IDEAL-IQ technology to evaluate the change of FF in salivary gland diseases.
Footnotes
The authors Guo-Yi Su and Chuan-Bing Wang contributed equally to the work.
Contributor Information
Xiao-Quan Xu, Email: xiaoquanxu_1987@163.com.
Fei-Yun Wu, Email: wfy_njmu@163.com.
REFERENCES
- 1. Fox RI . Sjögren's syndrome . Lancet 2005. ; 366 : 321 – 31 . doi: 10.1016/S0140-6736(05)66990-5 [DOI] [PubMed] [Google Scholar]
- 2. Chang HC , Juan CJ , Chiu HC , Liu YJ , Cheng CC , Chiu SC , et al. . Parotid fat contents in healthy subjects evaluated with iterative decomposition with echo asymmetry and least squares fat-water separation . Radiology 2013. ; 267 : 918 – 23 . doi: 10.1148/radiol.12112599 [DOI] [PubMed] [Google Scholar]
- 3. Izumi M , Eguchi K , Ohki M , Uetani M , Hayashi K , Kita M , et al. . MR imaging of the parotid gland in Sjögren's syndrome:A proposal for new diagnostic criteria . AJR Am J Roentgenol 1996. ; 166 : 1483 – 7 . doi: 10.2214/ajr.166.6.8633469 [DOI] [PubMed] [Google Scholar]
- 4. Izumi M , Eguchi K , Nakamura H , Nagataki S , Nakamura T . Premature fat deposition in the salivary glands associated with Sjögren syndrome: MR and CT evidence . AJNR Am J Neuroradiol 1997. ; 18 : 951 – 8 . [PMC free article] [PubMed] [Google Scholar]
- 5. Mahne A , El-Haddad G , Alavi A , Houseni M , Moonis G , Mong A , et al. . Assessment of age-related morphological and functional changes of selected structures of the head and neck by computed tomography, magnetic resonance imaging, and positron emission tomography . Semin Nucl Med 2007. ; 37 : 88 – 102 . doi: 10.1053/j.semnuclmed.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 6. Howlett DC . High resolution ultrasound assessment of the parotid gland . Br J Radiol 2003. ; 76 : 271 – 7 . doi: 10.1259/bjr/33081866 [DOI] [PubMed] [Google Scholar]
- 7. Hamilton G , Yokoo T , Bydder M , Cruite I , Schroeder ME , Sirlin CB , et al. . In vivo characterization of the liver fat 1HMR spectrum . NMR Biomed 2011. ; 24 : 784 – 90 . doi: 10.1002/nbm.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karampinos DC , Ruschke S , Dieckmeyer M , Diefenbach M , Franz D , Gersing AS , et al. . Quantitative MRI and spectroscopy of bone marrow . J Magn Reson Imaging 2018. ; 47 : 332 – 53 . doi: 10.1002/jmri.25769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernard CP , Liney GP , Manton DJ , Turnbull LW , Langton CM . Comparison of fat quantification methods: a phantom study at 3.0T . J Magn Reson Imaging 2008. ; 27 : 192 – 7 . doi: 10.1002/jmri.21201 [DOI] [PubMed] [Google Scholar]
- 10. Reeder SB , Wen Z , Yu H , Pineda AR , Gold GE , Markl M , et al. . Multicoil Dixon chemical species separation with an iterative least-squares estimation method . Magn Reson Med 2004. ; 51 : 35 – 45 . doi: 10.1002/mrm.10675 [DOI] [PubMed] [Google Scholar]
- 11. Kise Y , Chikui T , Yamashita Y , Kobayashi K , Yoshiura K . Clinical usefulness of the mDIXON Quant the method for estimation of the salivary gland fat fraction: comparison with MR spectroscopy . Br J Radiol 2017. ; 90 : 20160704 . doi: 10.1259/bjr.20160704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aoki T , Yamaguchi S , Kinoshita S , Hayashida Y , Korogi Y . Quantification of bone marrow fat content using iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): reproducibility, site variation and correlation with age and menopause . Br J Radiol 2016. ; 89 : 20150538 . doi: 10.1259/bjr.20150538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sumi M , Izumi M , Yonetsu K , Nakamura T . Sublingual gland: MR features of normal and diseased states . AJR Am J Roentgenol 1999. ; 172 : 717 – 22 . doi: 10.2214/ajr.172.3.10063867 [DOI] [PubMed] [Google Scholar]
- 14. Chang HC , Juan CJ , Chiu HC , Cheng CC , Chiu SC , Liu YJ , et al. . Effects of gender, age, and body mass index on fat contents and apparent diffusion coefficients in healthy parotid glands: an MRI evaluation . Eur Radiol 2014. ; 24 : 2069 – 76 . doi: 10.1007/s00330-014-3265-z [DOI] [PubMed] [Google Scholar]
- 15. Abdel Razek AAK , Mukherji SK . State-of-the-Art Imaging of Salivary Gland Tumors . Neuroimaging Clin N Am 2018. ; 28 : 303 – 17 . doi: 10.1016/j.nic.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 16. Fujita A . Imaging of Sjögren Syndrome and Immunoglobulin G4-Related Disease of the Salivary Glands . Neuroimaging Clin N Am 2018. ; 28 : 183 – 97 . doi: 10.1016/j.nic.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 17. Abdel Razek AAK , Mukherji S , Suresh M . Imaging of sialadenitis . Neuroradiol J 2017. ; 30 : 205 – 15 . doi: 10.1177/1971400916682752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdel Razek AAK , Mukherji SK . Imaging of posttreatment salivary gland tumors . Neuroimaging Clin N Am 2018. ; 28 : 199 – 208 . doi: 10.1016/j.nic.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 19. Abdel Razek AAK . Routine and advanced diffusion imaging modules of the salivary glands . Neuroimaging Clin N Am 2018. ; 28 : 245 – 54 . doi: 10.1016/j.nic.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 20. Zhou N , Chu C , Dou X , Li M , Liu S , Zhu L , et al. . Early evaluation of irradiated parotid glands with intravoxel incoherent motion MR imaging: correlation with dynamic contrast-enhanced MR imaging . BMC Cancer 2016. ; 16 : 865 . doi: 10.1186/s12885-016-2900-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martinez-Madrigal F , Micheau C . Histology of the major salivary glands . Am J Surg Pathol 1989. ; 13 : 879 – 99 . doi: 10.1097/00000478-198910000-00008 [DOI] [PubMed] [Google Scholar]
- 22. Dayan D , Vered M , Paz T , Buchner A . Aging of human palatal salivary glands: a histomorphometric study . Exp Gerontol 2000. ; 35 : 85 – 93 . doi: 10.1016/S0531-5565(99)00079-0 [DOI] [PubMed] [Google Scholar]
- 23. Scott J , Flower EA , Burns J . A quantitative study of histological changes in the human parotid gland occurring with adult age . J Oral Pathol 1987. ; 16 : 505 – 10 . doi: 10.1111/j.1600-0714.1987.tb00681.x [DOI] [PubMed] [Google Scholar]
- 24. Takashima H , Takebayashi T , Ogon I , Yoshimoto M , Terashima Y , Imamura R , et al. . Evaluation of intramyocellular and extramyocellular lipids in the paraspinal muscle in patients with chronic low back pain using MR spectroscopy: preliminary results . Br J Radiol 2016. ; 89 : 20160136 . doi: 10.1259/bjr.20160136 [DOI] [PMC free article] [PubMed] [Google Scholar]