Abstract
Objectives:
To examine the effectiveness of terahertz (THz) pulsed imaging (TPI) in comparison to intraoral photostimulable phosphor late (PSP) and cone beam CT (CBCT) for the detection of dental caries ex vivo.
Methods:
Newly extracted 32 human permanent teeth surfaces (16 with caries and 16 without caries) were serially sectioned mesiodistally and imaged by using four image sets as follows: (1) CBCT; (2) PSP; (3) THz movie video; and (4) THz static images. All images were evaluated twice separately by two calibrated observers for the presence/absence of caries using a 5-grade rating/confidence scale. Weighted κ coefficients were calculated. Different image sets were compared with the histological gold-standard using the receiver operating characteristic and area under curves for each image set were compared using χ2 tests, with a significance level of α = 0.05.
Results:
Intra- and interobserverκ-values for all image sets were almost excellent ranging between 0.777 and 0.988. Mean Az values of observers and readings were 0.898 for CBCT, 0.888 for PSP images, 0.853 for THz static images and 0.781 for THz video movie. No statistically significant differences (p > 0.05) were found between Az values for the different image sets. When observer scores were evaluated according to caries location again no statistically significant differences (p > 0.05) were found between Az values for the occlusal and proximal caries for the four image sets.
Conclusions:
Terahertz pulsed imaging was found to be successful for the detection of dental caries ex vivo.
Keywords: caries detection, radiology, THz imaging
Introduction
Diagnosis of dental caries is one of the major and common issues for the clinician. In order to enable better diagnosis of caries, most common methods like probing and visual examination can be used in combination with X-rays, electrical impedance measurements, ultrasound and fluorescence-based methods.1–3 The difficulty of plaque removal from occlusal surfaces along with their unique morphology makes them the most common sites of caries in adults. Visual examination alone is not always sufficient for diagnosing occlusal caries, and therefore it may progress without a visible breakdown of the enamel structure. For these reasons, it is crucial to identify non-traumatic, non-invasive techniques that can accurately diagnose occlusal caries.1–3 On the other hand, interproximal caries lesions develop between the contacting proximal surfaces of two adjacent teeth. They first appear clinically as opaque regions, which occur due to loss of enamel translucency at the outermost enamel between the contact point and the top of the free gingival margin. Proximal caries on posterior teeth are usually difficult to identify on radiographs and early detection of a proximal caries lesion enables immediate operative therapy, thereby preventing extensive tooth loss.4 Two- or three-dimensional X-ray imaging causes an ionizing radiation with some detrimental effects to human health. Therefore, recent technological advances have supported the exploration of additional strategies for caries detection with a particular emphasis on diagnosis at an earlier stage of formation without using ionizing radiation.5
In the late 1980s with the work of a few research groups, THz time-domain spectroscopy was developed and now it has gained remarkable importance for several application areas. Due to its specific region in the electromagnetic spectrum which is between microwaves and the infrared, terahertz (THz) pulsed imaging (TPI) technique is a non-ionizing and non-invasive imaging method.6,7 Therefore, this imaging modality does not cause any harm to biological tissues when given in moderate quantities through the low photon energy of THz radiation. Biomedical diagnostics for cancer and dermatologic diseases with THz radiation have been reported. Although the penetration depth of THz waves into biological tissues is limited because of the high attenuation of water in THz frequencies, three dimensional investigation of biological structures such as teeth are possible by using reflection-based THz imaging systems. In this type of imaging, the depth profile of the tooth samples can be extracted from the reflective surfaces of different layers and the raster scanning of the samples through the THz beam provides the information required to successfully reconstruct their three-dimensional profiles. Diagnostic capability of THz imaging is one of the main advantages of this non-ionizing imaging modality. Typically, caries are detected by examining changes in refractive index, loss and scattering of the THz signal reflected from the tissue. In this regard, the measurements can provide false positives if there are structural changes in the tooth unrelated to caries formation. However, dental caries is a consequence of mineral loss in enamel and dentine, thus frequency-dependent THz spectra offer the possibility of investigating mineralization level in this tissue. Small refractive index variations which occur as a result of these structural changes can also be monitored and changes in mineral density of the media can be accurately detected.8–10
THz imaging has the potential to be used in assessing tooth and periodontal structures without the risks of ionizing the surrounding tissue. Authors of a previous study, showed that TPI could quantitatively determine the extent of lesions formed by acid gel ex vivo.9 Recent findings regarding characterization of THz imaging by using extracted teeth samples revealed that it had the potential to be used in assessing dental caries.11 Therefore, in the present research, a TPI system operating in reflection mode was constructed in order to investigate various teeth samples in three dimensions. Our objective was to investigate the potential of this system in the detection of structural variations due to mineral content change in teeth samples which result in dental caries. THz imaging was also compared with intraoral photostimulable phosphor (PSP) plate images and cone beam CT (CBCT) images in order to assess the validity and effectiveness of this innovative technique.
Methods and materials
Sample selection and preparation
Ethical approval was obtained from the Ethical Committee of the Faculty of Dentistry of Ankara University (36290600/105). Newly extracted 32 human permanent teeth surfaces were cleaned and stored in distilled water. Each tooth was serially sectioned mesiodistally in parallel to the long axis of the crown using a water cooled diamond saw at low speed so that THz radiation could probe the layered structure of the sample. Thereafter, both sides of each section were examined under a stereomicroscope (x10) (Stemi 2000; Carl Zeiss, Jena, Germany) by one of the authors. Teeth were recorded as either sound or as having a caries lesion, which was defined as a demineralized white or yellowish-brown discolored area in the enamel or dentine. Examination of the 32 teeth surfaces revealed no caries in 16 and dentine caries in 16 teeth (8 occlusal and 8 proximal).
Experimental set up
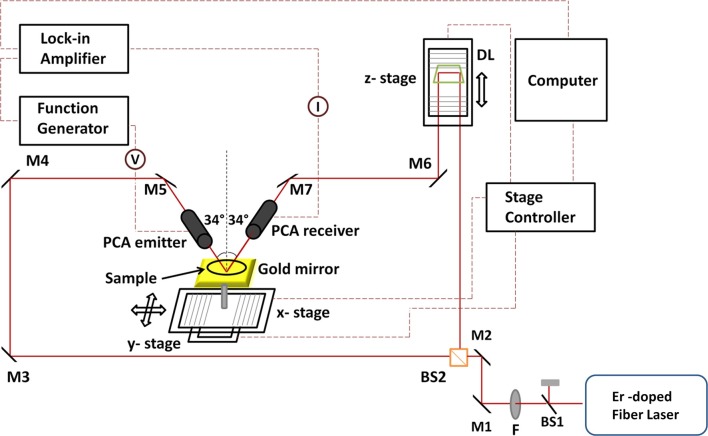
The TPI system in a reflection mode is presented in Figure 1. An ultrafast mode-locked Erbium (Er) doped fiber laser which generates pulses at a center wavelength of 1550 nm with a repetition rate of 89 MHz was employed for optical excitation. The output power of the source was 160 mW. The output beam of the laser was separated into a pump beam which was used to generate THz pulse and into a probe beam which was used to detect the reflected THz pulse from tooth samples. The generated THz beam was focused on the tooth samples which were placed on the focal point of the THz pulse with an incidence angle of 34° to the normal. Reflected pulses from these samples interacted with the probe beam within the detector and electric field amplitude of the THz pulse was determined by the delay unit which provided THz waveforms as a function of time. Two-dimensional THz images of teeth were obtained by the movement of these samples through the THz beam focus using x- and y-stages as shown in Figure 1. The z-stage, however, provided THz waveforms by raster scanning and gave depth information. In this way, three-dimensional images of teeth samples were obtained. When studying thick samples, the reflected pulses from the bottom of these samples were analyzed for image construction.
Figure 1.
The layout of the three-dimensional THz pulsed imaging system. (BS1,2, beam splitters; F, filter; M1-7, mirrors; PCA, photoconductive antenna; DL, delay line).
The analyses of reflected THz pulses from samples in time and frequency domains were performed using MATLAB (MathWorks, Natick, MA). In order to obtain video movie images, a complex THz time domain signal was obtained using Hilbert transform and envelope of the THz signal for each pixel was calculated and plotted. Each two-dimensional (2D) image corresponded to depth slices at different delay times. Power spectrum was also calculated for each pixel and results were shown in specific frequencies and these images were linear plots. In addition, images of the same samples were taken by using CBCT unit and intraoral digital radiography after embedding them in wax blocks. Images of each tooth were obtained using ProMax® 3D Max CBCT (Planmeca, Helsinki, Finland) with a flat panel sensor, using low artifact reduction mode operating at 96 kVp, 1 mA, normal resolution 0.2 mm voxel size and, 55 x 50 mm field of view. The exposure time ranged between 13.481 and 13.503 s. Images of each tooth were also taken with an X-ray generator, Evolution X3000 2C/1 New Life (New Life Radiology, Grugliasco, TO, Italy) operated at 70 kVp and 8 mA. Images were recorded using a Digora Optime DXR-50 PSP digital intraoral system (Soredex, Tuusula, Finland), which includes a feature that automatically erases residual image signals. Image recording was set at a 40 µm (super) pixel size, 14-bit grayscale, and 12.5 lp/mm spatial resolution. A size 2 imaging plate was used. Ex vivo imaging was performed using standardized periapical paralleling technique equipment (Rinn Manufacturing Company, Elgin, IL) with a focus-receptor distance of 40 cm and an image-exposure time of 0.20 s. Exposure time was determined by consensus. Pulpal root canal, dentine, and enamel visibility were used as indicators of optimal image quality.
Finally, a total of four image sets were obtained as follows: (1) CBCT; (2) PSP; (3) THz video movie; and (4) THz static images. First of all, a pilot viewing session using a tooth with both occlusal and interproximal caries that was not included in the study was performed. Observers scrolled through THz video movie, reconstructed static THz images and X-ray images in order to become familiar with assessing dental caries. All images were evaluated separately by two calibrated observers on a 22″ NEC MD213MG LCD monitor (NEC, Tokyo, Japan), at a screen resolution of 2048 × 1536 pixel and 32-bit color depth. Image sets were viewed at 1-week intervals, and repetitions performed 1 month after the initial viewings. No time restriction was placed on the observers. By using reconstructed THz images and THz video movies and dedicated software of CBCT Romexis (Planmeca, Helsinki, Finland) and PSP Digora (Soredex, Tuusula, Finland) systems and built in enhancement tools, all teeth were randomly evaluated for the presence/absence of caries and were scored using a 5-grade rating/confidence scale as follows: 1 = caries definitely present; 2 = caries probably present; 3 = uncertain-unable to tell; 4 = caries probably not present; and 5 = caries definitely not present.
Statistical analysis
Weighted κ coefficients were calculated to assess the intra- and interobserver agreement for each set-up. κ-values were interpreted according to the following criteria: 0.10: no agreement; 0.10–0.40: poor agreement; 0.41 0.60: moderate agreement; 0.61–0.80: strong agreement; and 0.81–1.00: excellent agreement.12 Scores obtained from different image sets (CBCT, Digora, THz Video Movie, THz Static Images) were compared with the gold-standard using the receiver operating characteristic analysis to evaluate the ability of observers for differentiating between teeth with and without caries. Sensitivity and specificity values with confidence intervals were calculated using a two-category classification constructed by considering a score of 3 or greater as positive. The areas under the receiver operating characteristic curves (Az) with standard error and 95% confidence intervals were calculated, and area under curves for each image set were compared using chi-square tests, with a significance level of α = 0.05. All calculations were carried out using STATA 12/MP4 statistical software (StataCorp LP., College Station, TX).
Results
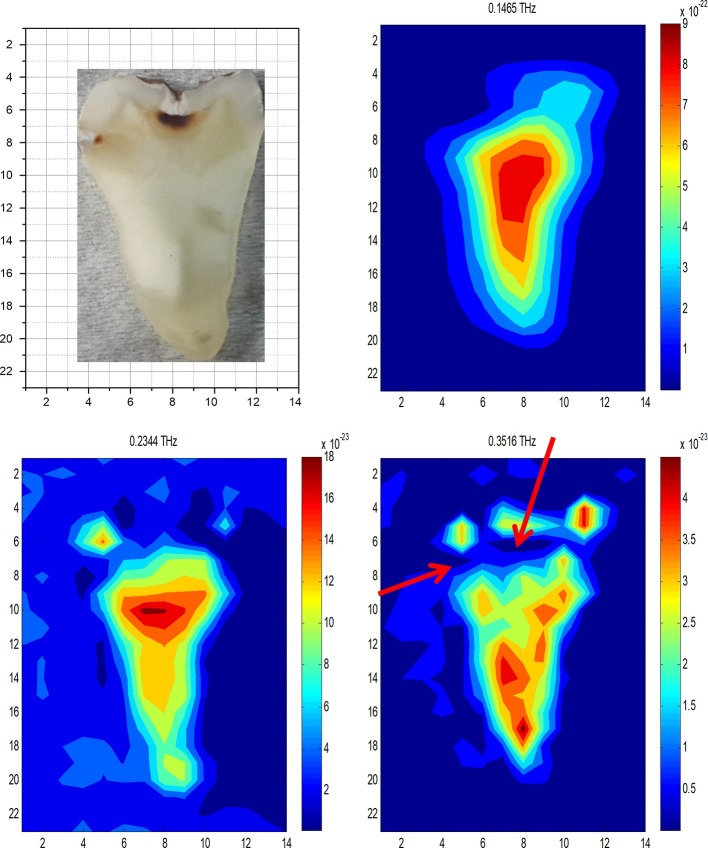
After reflecting from the lower surfaces of the tooth slices, three-dimensional THz data sets were investigated in both time and frequency domains for each sample. In the time domain analyses, changes in the THz peak amplitude in waveforms show distinctions between zones with different health statues. In our frequency domain analyses, THz power data were used to construct 2D images of samples at specific frequency values. In these analyses, distinctions between healthy and unhealthy zones could be observable due to the variations in the transmitted THz frequencies through these points. Frequency domain outcomes for the pilot tooth are shown in Figure 2. The contrast between different zones of samples in THz images resulted from the absorption of an incident THz beam in different proportions. These highly absorbing regions were observed dark blue in THz images and variations in the sample structure were explicit. These variations in the absorbance were more explicit when the power spectra obtained with single pixel investigation of healthy and unhealthy points were compared. The positions of these highly absorbing zones for different tooth slices showed similarities with the locations of caries points detected in these slices. Figure 3 shows images of the pilot tooth shown in Figure 2 with occlusal and interproximal caries obtained by using reconstructed THz static images constructed by using frequency domain; THz video movie constructed by using time domain THz data; CBCT images obtained at 0.2 mm voxel size; and PSP sensor. In THz images, healthy parts appear as lighter regions (red and yellow colors) compared to caries regions (dark blue color) because of the strongly reflected THz pulse. A significant contrast in the reflectivity between the unhealthy and healthy regions was clear.
Figure 2.
After reflecting from the lower surface of the tooth slice the THz images were reconstructed using the power absorption spectral data at specific frequency values. Highly absorbing regions were observed as dark blue in THz images and indicated structural variations in the sample and their locations showed similarities with the position of caries observed in tooth slices. Arrows show caries regions.
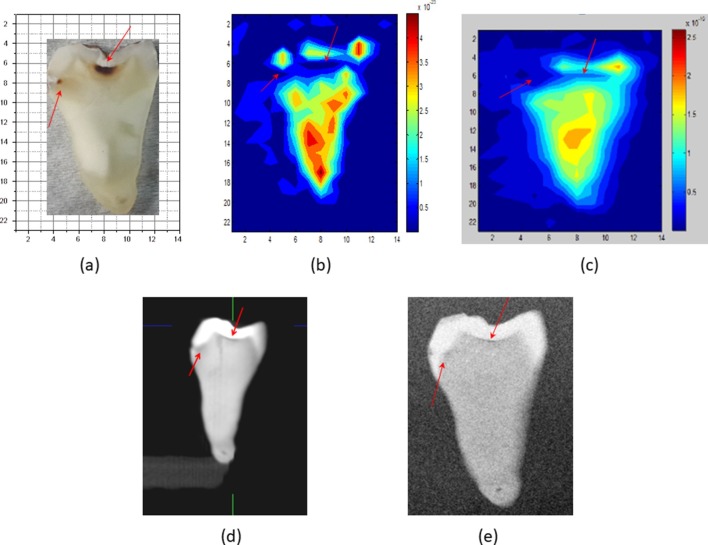
Figure 3.
(a) Tooth sample in Figure 2, (b) reconstructed THz static image at 0.35 THz, (c) THz video movie, (d) CBCT image and (e) PSP image of tooth sample in Figure 2. Arrows show carious regions.
Intra- and interobserver κ coefficients calculated by image type are shown in Tables 1 and 2, respectively. Intra- and interobserver κ values for all image sets were almost excellent ranging between 0.777 and 0.988. Given the overall excellent intra- and interobserver agreement, mean Az values of observers and readings were calculated in order to determine the comparison of general performance (Table 3). Mean Az values of observers and readings were 0.898 for CBCT, 0.888 for PSP images, 0.853 for THz static images and 0.781 for THz video movie. CBCT and PSP images were found to have the highest Az values followed by the THz static images, whereas the THz video movie images were found to have the lowest Az values. No statistically significant differences (p > 0.05) were found between Az values for the different image sets. When observer scores were evaluated according to caries location again no statistically significant differences (p > 0.05) were found between Az values for the occlusal and proximal caries for the four image sets. Table 4 shows the sensitivity, specificity, positive-predictive value and negative-predictive value calculated for both observers.
Table 1.
Intraobserver κ coefficients calculated by image type
| Observer 1 | Observer 2 | |
| Weighted κ-Se | Weighted κ-Se | |
| CBCT | 0.988–0.215 | 0.986–0.212 |
| Digora PSP | 0.976–0.211 | 0.967 –0.213 |
| Thz video | 0.884–0.214 | 0.777–0.213 |
| Thz static | 0.856–0.209 | 0.883–0.212 |
| General | 0.926–0.108 | 0.903–0.106 |
CBCT, cone beam CT; PSP, photostimulable phosphor;
Table 2.
Interobserver κ coefficients calculated by image type
| First readings | Second readings | |
| Obs1–Obs2 | Obs1–Obs 2 | |
| Weighted κ-Se | Weighted κ-Se | |
| CBCT | 0.988–0.215 | 0.978–0.211 |
| Digora PSP | 0.987–0.213 | 0.974–0.211 |
| Thz video | 0.870–0.212 | 0.872–0.213 |
| Thz static | 0.956–0.212 | 0.789–0.212 |
| General | 0.950–0.109 | 0.903–0.106 |
CBCT, cone beam CT; PSP, photostimulable phosphor;
Table 3.
Mean Az values of observers and readings showing comparison of general performance
| ROC | Asymptotic normal | ||||
| Obs | Area | Std. err. | [95% conf. interval] | ||
| CBCT | 32 | 0.8983 | 0.0651 | 0.78077 | 0.998 |
| Digora | 32 | 0.8883 | 0.0651 | 0.78077 | 0.989 |
| Thz video | 32 | 0.7811 | 0.0844 | 0.60754 | 0.976 |
| Thz static | 32 | 0.8533 | 0.0755 | 0.66370 | 0.988 |
CBCT, cone beam CT; ROC, receiver operating characteristic;
Ho: area (CBCT) =area (digora) = area(Thz video) = area (Thz static) chi2(2) = 2.63Prob>chi2 = 0.2682
Table 4.
Sensitivity, specificity, PPV and NPV for both observers
| Image type | Sensitivity | [95% conf. interval] | Specificity | [95% conf. interval] | PPV | [95% conf.interval] | NPV | [95% conf. interval] | Accuracy |
| CBCT | 0.891 | 0.646–0.889 | 0.899 | 0.596–0.982 | 0.897 | 0.625–0.885 | 0.899 | 0.591–0.882 | 0.819 |
| Digora | 0.881 | 0.646–0.899 | 0.893 | 0.596–0.982 | 0.891 | 0.616–0.885 | 0.891 | 0.586–0.882 | 0.809 |
| Thz video | 0.749 | 0.468–0.888 | 0.759 | 0.397–0.892 | 0.758 | 0.469–0.911 | 0.756 | 0.397–0.812 | 0.747 |
| Thz static | 0.799 | 0.513–0.889 | 0.777 | 0.434–0.903 | 0.755 | 0.457–0.911 | 0.801 | 0.491–0.843 | 0.783 |
CBCT, cone beam CT; NPV, negative-predictive value; PPV, positive-predictive value;
Discussion
Recent technological advances have supported the exploration of additional strategies for caries detection with a particular emphasis on diagnosis at an earlier stage of formation without using ionizing radiation. A previous study,13 assessed the effectiveness and reliability of a high resolution ultrasonography device by comparing its findings with those of clinically available radiographic techniques.13 Similarly, in order to assess the validity and effectiveness of the innovative technique presented here, we compared THz imaging with common radiological techniques such as; intraoral periapical images and CBCT images. The present study was designed to evaluate the imaging capacity of THz waves by using reflection measurements. To our knowledge, this study is the first to assess a new diagnostic technique “terahertz imaging in dentistry” obtained by the TPI system in the reflection mode in comparison to 2D and three-dimensional X-ray imaging. Through this new process, it is possible to obtain ex vivo images similar to that of reconstructed tomographic image slices while also showing video movie of the teeth in question. This approach is claimed to be helpful in the future especially for pediatric and pregnant patients due to its non-ionizing nature. The visibility of caries in intraoral radiography may depend on various factors such as caries’ depth, tooth position in the jaw, angulation of the X-ray beam, superimposition of adjacent structures, artifacts, and patient-related factors. In the present study, different extracted teeth were appropriately placed in the system and then images were obtained. Therefore, it was not possible to produce a real clinical situation and images obtained without a soft tissue equivalent might increase the observer’s ability to detect caries. We studied different human teeth samples with and without localized caries. Three-dimensional THz images of these samples were obtained and these data sets were also investigated in the frequency domain. Previous efforts were concentrated on reconstructing the images of the samples in the time domain, by examining the change in amplitude of the THz peak in the waveform after applying appropriate filters. In order to detect the presence of caries and distinguish them from the surrounding similar media, a frequency-dependent approach in the analysis was thought to be more appropriate. In contrast to radiography, the ability of performing spectroscopic measurements of each pixel in a sample is one of the advantages of TPI. This spectroscopic assessment offers a spectrum of different frequencies and hence frequency-dependent THz spectra enable investigation of mineral content.14
Considering the fact that we found almost excellent agreement within and between observers for all image sets, it can be said that THz imaging showed very high repeatability and reliability in the detection of caries in an ex vivo setting. After a full training session, observers became familiar with the THz system and diagnosed the regions with caries and managed to differentiate them from healthy structures. Although CBCT and PSP images were found to have higher Az values when compared to THz images without statistical significance, THz images also revealed high Az values suggesting that it is an effective and reliable tool for the detection of caries. THz video movie images were found to have the lowest Az values. This could be due to the fact that observers had to follow the THz video movie carefully each time in order to detect the dark blue caries region with high absorption and scroll through video movie images in order to figure out the accurate section. However, observer performance increased when assessing reconstructed serial sections of THz static images, which were similar to cross-sectional tomographic images. Obviously, observer performance is expected to increase with experience in using the system in future research. We found no statistically significant differences between Az values for the occlusal and proximal caries for all image sets. However, with THz images, we found higher Az values for occlusal caries when compared to proximal caries without statistical significance. It is possible that newer studies with larger sample size and different settings may reveal different results. It should be also noted that the terahertz region lies between the microwave and infrared regions of the electromagnetic spectrum, so it is strongly attenuated by water and very sensitive to water content. In real clinical situation, the tooth surface is always in contact with water and the thickness of the water layer is not constant over the whole tooth. The terahertz images obtained in this environment may have an influence on the observer's ability to detect caries.
Authors of a previous study,15 compared TPI with transverse microradiography and microindentation to measure remineralization of artificial caries lesions which were formed in bovine enamel using a solution of lactic acid. The time-domain data were used to calculate the refractive index and the differences in the integrated areas between the baseline and post-treatment profiles were calculated. In addition, the change from baseline in both the lesion depth and the intensity of the reflected pulse from the air/enamel interface was determined. TPI and transverse micro radiography/microindentation highly correlated with each other suggesting that TPI had potential as a research tool for hard tissue imaging.15 THz is strongly attenuated by water and is very sensitive to water content. In real clinical conditions, the tooth surface is always in contact with water and thickness of the water layer is not constant over the whole tooth. The THz images obtained in real clinical situations may influence observer ability to detect caries. We used natural carious teeth in order to obtain more realistic diagnostic results. Caries activity and severity were out of the scope of the present research. Our efforts will focus on THz imaging of tooth structures in terms of caries extension and activity.
We utilized digital periapical images for the comparison of THz images since clinical examination along with X-ray-based periapical radiography is the most conventional and accepted technique used to diagnose dental caries in routine dental practice. Due to their 2D nature, intraoral techniques may fail to provide enough information for caries detection. Regardless of the intraoral radiographic system used, the nature of 2D images, limits the data obtained and their diagnostic value is dependent on beam angulation, superimposition of anatomical structures and factors related with patients. Therefore the search for non-invasive, harmless and high-resolution techniques in the detection of caries will continue in the future years. Digital radiographs also offer the potential for image enhancement by applying some algorithms and can change the white end of the grayscale (Rayleigh and hyperbolic logarithmic probability) and others the black end (hyperbolic cube root function).16 When comparing images obtained using a PSP sensor (Digora Optime, Helsinki, Finland) from the same tooth under both in vivo and in vitro conditions for the detection of occlusal caries lesion revealed quite comparable results. Moreover, in vivo time efficiency was found to be very good for single radiographs of mandibular third molars.17 Under in vitro conditions; the diagnostic ability of visual inspection, conventional film, charge-coupled device (CCD) sensor, and PSP sensor were found similar in the diagnosis of proximal lesions in posterior teeth when compared with the histological gold-standard.4 In order to obtain optimum image quality, an intraoral radiographic examination should be used with aiming equipment. It would be useful to compare the diagnostic accuracy of images obtained with different intraoral sensors and THz images.
Moreover, we compared THz images with those of CBCT images, since earlier studies suggested that available CBCT images taken for different purposes could be used to diagnose caries lesions. However, due to radiation risk CBCT must not be routinely used for caries diagnosis. A previous study assessed the in vitro diagnostic ability of CBCT images using different display types in the detection of recurrent caries under restorations. Mentioned study revealed generally rather low Az values as well as a low interrater agreement. Higher Az values were obtained with medical grayscale monitors when used with dedicated software than with other monitor types, which had similar Az results.18 Therefore, in the present study, we utilized dedicated softwares of the available techniques for the detection of caries. Also, we reconstructed the THz images by using specific software and obtained three-dimensional images similar to those of CBCT slices.
The development of techniques which utilize terahertz waves for applications in dentistry is growing evermore rapidly with the development of instrumentation. Terahertz technologies are developing rapidly and evermore tools are being developed to assess the health of tissues for in vivo applications. For example, while the measurements done here have focused on ex vivo measurements due to the limitations in the instrumentation, this technique was successfully demonstrated for in vivo measurements of the oral cavity wall using optical fiber coupled delivery systems.10 In our study, observations show that one can differentiate caries regions from healthy zones using both time and frequency domain analyses. The absorption of THz beam in distinct regions of samples showed differences with higher absorption coefficient in caries regions. The measurements performed and images obtained by terahertz waves showed that there were differences between carious and healthy teeth. Our THz imaging results also showed similarities with X-ray imaging results. These ex vivo measurements can be further improved upon by developing the system to perform in vivo studies. Our future work will also focus on the imaging of various dental structures and materials by using TPI. In our notion, possible clinical application areas which may be useful for diagnostic dentistry are caries detection, diagnosis of resorptive defects, periodontal imaging, soft tissue imaging, peri-implant imaging, assessment of restorative materials and differentiation of oral lesions.19 While the studies here were done ex vivo using table-top systems, the technology to create and detect THz waves is developing rapidly, and one company now offers a handheld THz diagnostic tool which is more suitable for patient use. For this reason, we believe studies like ours will aid in the further rapid development of these technologies for use by medical professionals.
Conclusion
A TPI system operating in reflection geometry was constructed to investigate tooth samples in three dimensions and observer performance was compared with those of PSP and CBCT images for the detection of caries. Given the limitations of this ex vivo research, we found excellent within and between observer agreements along with comparable performance in the detection of caries for all image sets utilized. Nevertheless, these results show that TPI methods may be useful in the detection of dental caries.
Footnotes
Acknowledgment: Burcu Karagoz and Hakan Altan acknowledge support from Turkish Academy of Sciences in the framework of the Young Scientist Award Program (TUBA-GEBIP). Hakan Altan also acknowledges support from BAGEP Award of the Science Academy in Turkey.
REFERENCES
- 1. Kamburoğlu K, Kurt H, Kolsuz E, Öztaş B, Tatar I, Çelik HH. Occlusal caries depth measurements obtained by five different imaging modalities. J Digit Imaging 2011; 24: 804–13. doi: 10.1007/s10278-010-9355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kamburoğlu K, Murat S, Yüksel SP, Cebeci AR, Paksoy CS. Occlusal caries detection by using a cone-beam CT with different voxel resolutions and a digital intraoral sensor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109: e63–e69. doi: 10.1016/j.tripleo.2009.12.048 [DOI] [PubMed] [Google Scholar]
- 3. Torres MG, Santos AS, Neves FS, Arriaga ML, Campos PS, Crusoé-Rebello I. Assessment of enamel-dentin caries lesions detection using bitewing PSP digital images. J Appl Oral Sci 2011; 19: 462–8. doi: 10.1590/S1678-77572011000500005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Senel B, Kamburoglu K, Uçok O, Yüksel SP, Ozen T, Avsever H. Diagnostic accuracy of different imaging modalities in detection of proximal caries. Dentomaxillofac Radiol 2010; 39: 501–11. doi: 10.1259/dmfr/28628723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stookey GK, González-Cabezas C. Emerging methods of caries diagnosis. J Dent Educ 2001; 65: 1001–6. [PubMed] [Google Scholar]
- 6. Smye SW, Chamberlain JM, Fitzgerald AJ, Berry E. The interaction between terahertz radiation and biological tissue. Phys Med Biol 2001; 46: R101–R112. doi: 10.1088/0031-9155/46/9/201 [DOI] [PubMed] [Google Scholar]
- 7. Shen Y-C, Taday PF. Development and application of terahertz pulsed imaging for nondestructive inspection of pharmaceutical tablet. IEEE J Select Top Quant Elect 2008; 14: 407–15. doi: 10.1109/JSTQE.2007.911309 [DOI] [Google Scholar]
- 8. Beard MC, Turner GM, Schmuttenmaer CA. Terahertz spectroscopy. J Phys Chem B 2002; 106: 7146–59. doi: 10.1021/jp020579i [DOI] [Google Scholar]
- 9. Pickwell E, Wallace VP, Cole BE, Ali S, Longbottom C, Lynch RJ, et al. . A comparison of terahertz pulsed imaging with transmission microradiography for depth measurement of enamel demineralisation in vitro. Caries Res 2007; 41: 49–55. doi: 10.1159/000096105 [DOI] [PubMed] [Google Scholar]
- 10. Ji YB, Lee ES, Kim SH, Son JH, Jeon TI. A miniaturized fiber-coupled terahertz endoscope system. Opt Express 2009; 17: 17082–7. [DOI] [PubMed] [Google Scholar]
- 11. Kamburoğlu K, Yetimoĝlu NÖ, Altan H. Characterization of primary and permanent teeth using terahertz spectroscopy. Dentomaxillofac Radiol 2014; 43: 20130404. doi: 10.1259/dmfr.20130404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. doi: 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 13. Nguyen KT, Le LH, Kaipatur NR, Zheng R, Lou EH, Major PW. High-resolution ultrasonic imaging of dento-periodontal tissues using a multi-element phased array system. Ann Biomed Eng 2016; 44: 2874–86. doi: 10.1007/s10439-016-1634-2 [DOI] [PubMed] [Google Scholar]
- 14. Crawley D, Longbottom C, Wallace VP, Cole B, Arnone D, Pepper M. Three-dimensional terahertz pulse imaging of dental tissue. J Biomed Opt 2003; 8: 303–7. doi: 10.1117/1.1559059 [DOI] [PubMed] [Google Scholar]
- 15. Churchley D, Lynch RJ, Lippert F, Eder JS, Alton J, Gonzalez-Cabezas C. Terahertz pulsed imaging study to assess remineralization of artificial caries lesions. J Biomed Opt 2011; 16: 026001. doi: 10.1117/1.3540277 [DOI] [PubMed] [Google Scholar]
- 16. White SC, Pharoah MJ. The evolution and application of dental maxillofacial imaging modalities. Dent Clin North Am 2008; 52: 689–705. doi: 10.1016/j.cden.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 17. Kamburoglu K, Senel B, Yüksel SP, Ozen T. A comparison of the diagnostic accuracy of in vivo and in vitro photostimulable phosphor digital images in the detection of occlusal caries lesions. Dentomaxillofac Radiol 2010; 39: 17–22. doi: 10.1259/dmfr/91657756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baltacıoĝlu İH, Eren H, Yavuz Y, Kamburoğlu K. Diagnostic accuracy of different display types in detection of recurrent caries under restorations by using CBCT. Dentomaxillofac Radiol 2016; 45: 20160099. doi: 10.1259/dmfr.20160099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamburoğlu K, Yetimoĝlu NO. Applications of terahertz imaging in medicine. OMICS J Radiol 2014; 3: e127. [Google Scholar]