Abstract
Objectives:
The purpose of this systematic review was to determine the diagnostic capability of ultrasound to assess TMJ alterations as disc displacement (DD), joint effusion (JE) and condylar changes (CC) using 3D imaging modalities as reference standard.
Methods:
Studies were gathered by searching several electronic databases and partial grey literature up to January eighth, 2018 without restrictions of language and time. The risk of bias was evaluated using the second version of Quality Assessment Tool for Diagnostic of Accuracy Studies-2 (QUADAS-2). The grading of Recommendation, Assessment, Development and Evaluation (GRADEpro system) instrument was applied to assess the level of evidence across the studies.
Results:
After applying the eligibility criteria, 28 studies were identified and synthesized. All studies were methodologically acceptable presenting low applicability concerns, although none of them fulfilled all QUADAS-2 criteria. The quantitative analysis included 22 studies, 2829 joints in total. The quality of the evidence evaluated by GRADE system suggested moderate confidence in estimating the outcomes.
Conclusion:
This systematic review demonstrated the ultrasound has acceptable capability to screen for DD and JE in TMD patients. For screening of condylar changes, ultrasound needs further studies using CT or CBCT as reference standard to support its use. More advanced imaging such as MRI can thereafter be used to confirm the diagnosis if deemed necessary.
Keywords: ultrasound, temporomandibular joint, temporomandibular joint disorder, systematic review
Introduction
The temporomandibular joint (TMJ) is a synovial articulation between the mandibular condyle and the glenoid fossa in the temporal bone. Temporomandibular joint disorders (TMD) constitute structural and/or functional disorders that affect TMJ, masticatory muscles and related structures. These disorders may present with clinical signs such as articular noises, TMJ pain and/or limitation in opening and closing mouth.1
Diagnostic imaging is an essential part of the TMD evaluation.2 In the last two decades, several techniques have been described in the literature to assess bony and soft TMJ tissues.3 Magnetic resonance imaging (MRI) is accepted as the reference standard for imaging diagnosis of TMD.4 MRI has ability to evaluate soft tissue areas and inflammatory conditions; however, it has limited value to accurately diagnose osseous alterations.5 Although MRI uses non-ionizing radiation, its downside as a TMD screening tool is related to the high cost, time-consuming procedure and relatively low availability. Computed tomography (CT) has been the method of choice to evaluate the contours of the cortical bone and TMD osseous alterations. A variation of CT, Cone-beam CT (CBCT), has a diagnostic accuracy comparable with CT for detecting TMD osseous changes and has the advantage of lower ionizing radiation exposure.6,7 However, CT and CBCT poorly assess TMJ soft tissues such as the articular disk.7,8
Several studies have assessed ultrasound (US) to evaluate TMJ alterations.9–13 The high-frequency source pulse emitted, and the echoes detected are accomplished by a transducer placed in contact to the patient skin acquiring the image in real time. The ultrasound frequency usually ranges from 2 MHz to 15 MHz depending on the anatomic region depth to be evaluated. For TMJ, the imaging protocol includes longitudinal and transverses scans using probes with frequencies ranging from 7.5MHz to 20MHz. As an option, static and dynamic evaluations can be performed while the mouth is closed or open. This non-ionizing imaging method is less expensive, transportable, more comfortable to the patient, and could be easily used in a dental setting.10–14 While not in general clinical use, there are studies reporting that ultrasound has acceptable diagnostic efficacy to detect disk displacement.15,16
Although ultrasound assessment of TMJ disorders has been reviewed previously,15–17 these studies only assessed disk displacement in patients without systemic diseases using MRI or arthrography as reference standard. Therefore, the purpose of this review is to systematically analyze the capability of ultrasound to detect TMJ alterations, specifically disk displacement (DD), joint effusion (JE) and condylar changes (CC) using 3D appropriate imaging modalities (MRI, CT and/or CBCT) as the reference standard.
Methods and materials
This systematic review adhered to the Preferred Reporting Items for a Systematic Reviews and Meta-Analysis of Diagnostic Test Accuracy Studies, PRISMA-DTA Checklist.18
Protocol and registration
This protocol was registered at PROSPERO–International Prospective Register of Systematic Reviews–under number CRD42017078836.
Study design
A systematic review of human studies was undertaken to answer the research question “For patients with TMD with or without systemic diseases, does ultrasound imaging have similar diagnostic performance as CT/CBCT or MRI to identify TMJ pathology including disk displacement, joint effusion and bony structural changes?”.
Eligibility criteria
Inclusion criteria
Diagnostic studies in which the primary objective was to evaluate the diagnostic capability of 2D or 3D ultrasound imaging in assessing adults or children with TMD were included. Patients with or without systemic diseases that affected TMJ were considered. The reference standard imaging was established 3D imaging modalities (MRI, CT or CBCT). No language or time restrictions were set.
Exclusion criteria
The following exclusion criteria were applied: (1) Reviews, letters, personal opinions, book chapters, and conference abstracts; (2) studies involving in vitro with phantom or in vivo animal models; (3) studies without the reference standard comparison (MRI, CT or CBCT); (4) studies that did not provide accuracy outcome variables such as sensitivity and specificity or ROC curve.
Information sources and search strategy
Detailed individual search strategies for each of the following electronic database were performed: Cochrane, Embase, Medline, PubMed and Web of Science. A partial gray literature was accessed using Google Scholar by screening the abstracts for the first 100 results (filtered by “relevance”). The end search day, across all databases, was January 8, 2018. In addition to the electronic search, a hand search and experts’ consultations were implemented, and the reference lists of the selected articles screened.
Appropriate truncation and word combinations were selected and adapted to each database search (Supplementary Material 1) using the expertise of a health sciences librarian. All references were managed by reference manager software (Refworks-COS, ProQuest, Bethesda, MD) and duplicate papers were removed.
Study selection
A two-phase selection of articles was conducted. In Phase 1, two authors (FTA and CP-P) reviewed the titles and abstracts of all the references independently. These authors selected articles that appeared to meet the inclusion criteria based on their titles and abstracts. In Phase 2, the same authors assessed the full text of all screened articles and excluded studies that did not meet the inclusion criteria. We used in this phase the Rayyan Application (Qatar Computing Research Institute, Doha, Qatar),19 a specific tool for systematic review screening process, available at https://rayyan.qcri.org/. Disagreements between the two authors were initially resolved by consensus. The final selections were always based on the full text of the publication.
Data collection process and data extraction
One author (FTA) collected the required information from the included articles and a second author (CP-P) crosschecked all the collected data. Once again, disagreements between them were resolved by consensus.
For all included studies, the following information was extracted: study characteristics (author, year and country), sample characteristics (population studied, age range), intervention characteristics (reference standard, index test, transducer frequency, target) and outcome (sensitivity, specificity, ROC values). If the required data were not complete, attempts were made to contact the authors to retrieve the missing information.
Risk of bias and applicability
To assess the methodological quality and applicability of the included studies, the Quality Assessment Tool for Diagnostic Accuracy Studies-2 (QUADAS-2) was applied.20 One author (FTA) and one collaborator (SC) independently evaluated the quality of each included study and scored each item as “yes”, “no” or “unclear”. A third author (CP-P) joined the discussion when disagreements arose.
Diagnostic accuracy measures
Sensitivity and specificity of 2D or 3D US as diagnostic tests against MRI, CT or CBCT were considered as the primary outcome measures. Positive predictive values (PPV), negative predictive values (NPV) as well as the cut off values provided by ROC curves were considered as secondary outcomes. Confidence interval at 95% was considered. Other diagnostic measures were also considered: positive likelihood ratio (LR+), negative likelihood ratio (LR-) and diagnostic odds ratio (DOR).
The diagnostic test accuracy (DTA) was evaluated based on sensitivity, specificity, LR+, LR- and DOR values. The DTA was considered excellent with LR +>10/LR–<0.1 and acceptable with LR +>3/LR–<0.3.21 High DOR values indicated better test performance.22 We rated sensitivity/specificity as acceptable (70–80%/80–90%) and excellent (>80%/>90%).23
Synthesis of results
The capability of the ultrasound to identify TMJ alterations was evaluated by diagnostic accuracy measures following the appropriate Cochrane Guidelines.24 We generated estimates of sensitivity, specificity and their 95% confidence intervals in forest plots and hierarchical receiver operating characteristic (ROC) curve using Review Manager 5.3 (Rev-Man 5.3, The Nordic Cochrane Centre, Copenhagen, Denmark) and Stata 13.0 (StataCorp. LP 2013, Stata Statistical Software, Release 13. College Station, TX). For this quantitative analysis, we extracted the data for the true positive, true negative, false positive and false negative values for index test in each included study. In addition, these studies were clustered according to the target investigated. (Group 1) studies which assessed disk displacement (DD), (Group 2) studies which assessed condylar changes (CC) and (Group 3) studies which assessed joint effusion (JE). The studies were quantitatively analyzed by target investigated due to the diagnostic process for each TMD (DD, JE, CC).
Studies that did not provide separate data were not included in the quantitative analysis. For data not being suitable for meta-analysis a qualitative analysis was pursued.
Investigation of heterogeneity
We assessed heterogeneity by visually examining forest plots of sensitivities and specificities and ROC space for index test in all target investigated. In addition, it was considered the heterogeneity values (I2) presented in the forest plots. From the results of I2, the Cochrane handbook parameters were followed for interpretation as follows: 0 to 40%: might not be important; 30 to 60%: representing moderate heterogeneity; 50 to 90%: representing substantial heterogeneity; 75 to 100%: considerable heterogeneity.
Assessment of reporting bias
A funnel plot to investigate reporting bias using the statistical method suggested by Deeks et al24 was created. Significant asymmetry (p < 0.10) indicates the presence of publication bias in the data.
Level of evidence
The grading of Recommendation, Assessment, Development and Evaluation (GRADEpro system) instrument recommended by Cochrane guidelines25 was used to assess the evidence level across the studies. The quality of evidence was assessed based on the study design, risk of bias (RoB), inconsistency, indirectness, imprecision, and publication bias at the outcome level. The Grade was applied in studies with TMD patients separated by target. The quality of evidence was characterized as high, moderate, low, or very low.26 The GRADE was assessed using the website http://gradepro.org
Results
Study selection
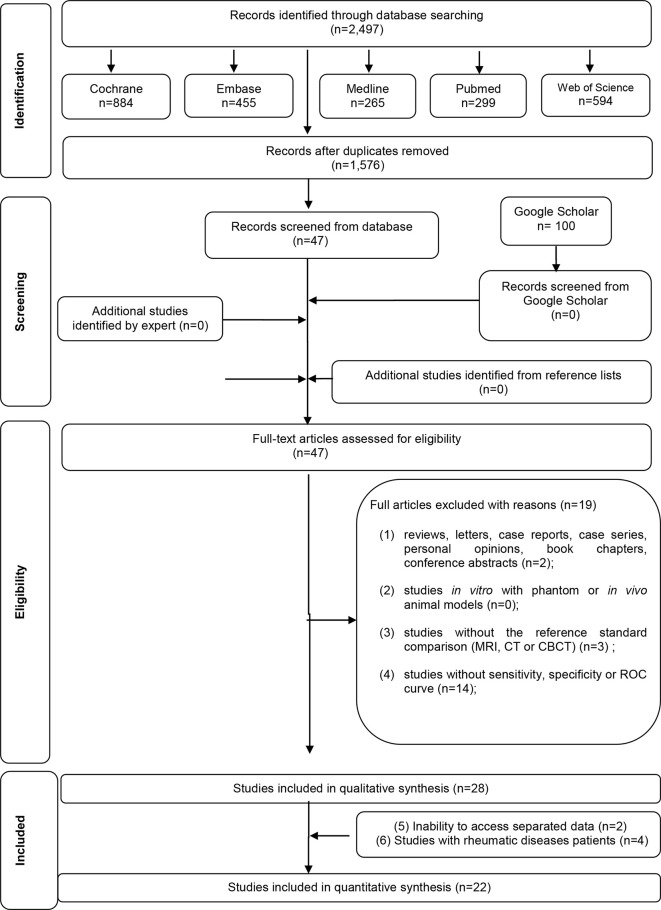
A flow diagram detailing the process of identification, inclusion and exclusion of the studies is shown in Figure 1. A full-text analysis was conducted on the 47 articles retrieved from the first phase of the selection process. This process led to the exclusion of 19 studies presented in Supplementary Material 2. Finally, 28 studies satisfied the inclusion criteria of this review and were selected for the qualitative synthesis..9–14,27–48 22 studies were quantitatively divided in groups and analyzed by a meta-analysis.10–14,27,28,30–40,43,46–48
Figure 1.
Flow Diagram of literature search and selection criteria adapted from PRISMA.
Study characteristics
The included studies were published from 1997 to 2016. All articles were written in English, except one in Chinese48 and one in Korean.34 The studies were conducted in 14 different countries (Austria, Japan, Italy, Germany, Turkey, France, China, Egypt, Norway, Korea, India, Brazil, Israel and Switzerland).
27 studies were cohort studies 10–14,27,28,31–40,43,46–48 and one was case-control design.30 Sample size ranged from 3 to 100 patients with TMD. Those sample have patients with juvenile idiopathic arthritis (JIA),9,44 rheumatoid arthritis (RA) or psoriatic arthritis (PsA).41,42 DD was assessed in 22 studies,10–14,27,29–38,41–43,45,47,48 JE in 9,9,36,37,40–44,46 and CC in 11 studies,10,12,13,28,34,36,39,41,43–45 In total, 11 studies assessed more than one TMJ alteration.10,12,13,34,36,37,41–45
MRI was used as reference standard in 27 studies.9–14,27–33,35–48 One of them used MRI and/or CT.35 Only one study used CT alone34 and none used CBCT as reference standard.
Regarding the index test, 25 studies appraised 2D US,9–11,13,14,27–37,40–48 two-studies assessed 3D US38,39 and one study evaluated 2D and 3D US.12 All of these studies used extra oral ultrasound approach to evaluate TMJ. The ultrasound transducer frequency used in the studies ranged from 5 to 20 megahertz (MHz). A summary of the descriptive characteristics of the included article is given in Table 1.
Table 1.
Summary of descriptive characteristics of included studies
| Study characteristics | Sample characteristics | Intervention characteristics | Outcomes | ||||||
| Study/Year | Country | Population studied (N = patients) |
Age range (y) |
Reference standard | Index test (US) |
Transducer frequency (MHz) | Target | Sensitivity (%) |
Specificity (%) |
| Emshoff et al., 1997 | Austria | TMD (17) | 16–60 | MRI | 2D static/dynamic | 7.5 | DD | Static CM/OM: 50/13 Dynamic CM/OM: 39/13 |
71/70 100/95 |
| Hayashi et al., 2001 |
Japan | TMD (18) | 8–12 | MRI and/or CT | 2D dynamic | 8 or 10 | DD | 83 | 96 |
| Jank et al., 2001 |
Austria | TMD (66) | 13–78 | MRI | 2D static | 12 | DD | CM: 78 OM: 61 |
CM: 78 OM: 95 |
| Emshoff et al., 2002 a | Austria | TMD (64) | 17–65 | MRI | 2D dynamic | 12 | DD | 82 | 95 |
| Emshoff et al., 2002 b | Austria | TMD (29) | 19–62 | MRI | 2D static | 12 | DD | CM: 90 OM: 95 |
CM: 94 OM: 91 |
| Brandlmaier et al., 2003 a | Austria | TMD (48) | 17–67 | MRI | 2D static | 12.5 | DD | 82 | 85 |
| Tognini et al., 2005 |
Italy | TMD (41) | ND | MRI | 2D static/dynamic | 8–20 | DD | 65 | 80 |
| Landes et al., 2006 a |
Germany | TMD (53) | 14–77 | MRI | 3D static | 8–12.5 | DD | 53 | 74 |
| Cakir-Ozkan et al., 2010 | Turkey | TMD (28) | 16–51 | MRI | 2D static/dynamic | 12 | DD | CM: 57 OM: 64 |
CM: 78 OM: 71 |
| Dupuy-Bonafe et al.,2012 | France | TMD (40) controls (20) | 21–59 21–29 |
MRI | 2D static/dynamic | 5–12 | DD | CM: 22 OM: 0 |
CM; 96 OM: 98 |
| Yang et al., 2012 |
China | TMD (35) | 37.3 mean |
MRI | 2D static | 12 | DD | 82 | 94 |
| Razek et al., 2015 |
Egypt | TMD (20) | 15–57 | MRI | 2D static/dynamic | 12 | DD | 79 | 72 |
| Manfredini et al., 2003 |
Italy | TMD (69) | ND | MRI | 2D static/dynamic | 8–20 | JE | 83 | 73 |
| Tognini et al., 2003 |
Italy | TMD (44) | ND | MRI | 2D static/dynamic | 8–15 | JE | 75 | 76 |
| Kirkhus et al., 2016 |
Norway | JIA (55) | <18 | MRI | 2D static | 12–18 | JE | 72 | 70 |
| Brandlamaier et al., 2003 b |
Austria | TMD (40) | 16–78 | MRI | 2D static | 12.5 | CC | 87 | 20 |
| Landes et al., 2006 b |
Germany | TMD (53) | 14–77 | MRI | 3D static | 8–12.5 | CC | 70 | 76 |
| Melchiorre et al., 2003 |
Italy | RA (22), PsA (11) | 30–81 | MRI | 2D static/dynamic | 7.5 | DD JE |
69 70 |
30 75 |
| Kaya et al., 2010 |
Turkey | TMD (52) | 28.3 mean |
MRI | 2D static/dynamic | 7.5 | DD JE |
91 53 |
16 63 |
| Gook et al., 2008 |
Korean | TMD (20) | ND | CT | 2D static | 12 | DD CC |
95 87 |
90 62 |
| Sinha et al., 2012 |
India | TMD (3) | ND | MRI | 2D dynamic | 10 | DD CC |
33 0 |
100 100 |
| Emshoff et al., 2003 |
Austria | TMD (48) | 15–72 | MRI | 2D dynamic | 12 | DD CC |
95 83 |
91 63 |
| Habashi et al., 2015 |
Israel | TMD (39) | 18–77 | MRI | 2D static/dynamic | 5–17 | DD CC |
74 36 |
84 83 |
| Landes et al., 2007 |
Germany | TMD (33) | 14–77 | MRI | 2D/3D static | 8–12 | DD CC |
2D: 58 3D: 60 2D: 69 3D: 69 |
63 68 74 78 |
| Muller et al., 2009 |
Switzerland | JIA (30) | 2–16 | MRI | 2D static/dynamic | 12 | JE CC |
33* | 82* |
| Jank et al., 2005 |
Austria | TMD (100) | ≥16 | MRI | 2D dynamic | 12 | DD JE CC |
CM/OM: 92/86 81 94 |
92/91 100 100 |
| Manfredini et al.,2005 | Italy | TMD/RA/PsA (68) | 43.4 mean age |
MRI | 2D static/dynamic | 8–20 | DD JE CC |
56 85 67 |
73 66 26 |
| Mello et al., 2011 |
Brazil | TMD (38) | 16–65 | MRI | 2D static | 12.5 | DD JE CC |
CM/OM: 83/0 20 15 |
100/100 100 87 |
AC, accuracy; CC, condylar change; CM, closed mouth; CT, computed tomography; DD, disk displacement; JE, joint effusion; JIA, juvenile idiopathic arthritis; MHz, megahertz; MRI, magnetic resonance imaging; ND, not described; OM, open mouth; PsA, psoriatic arthritis; RA, rheumatoid arthritis; TMD, temporomandibular joint disorder; US, ultrasound; y, year; 2D, two dimensional; 3D, three dimensional; CM, closed mouth;
*separate data not available.
Risk of bias (RoB) and applicability
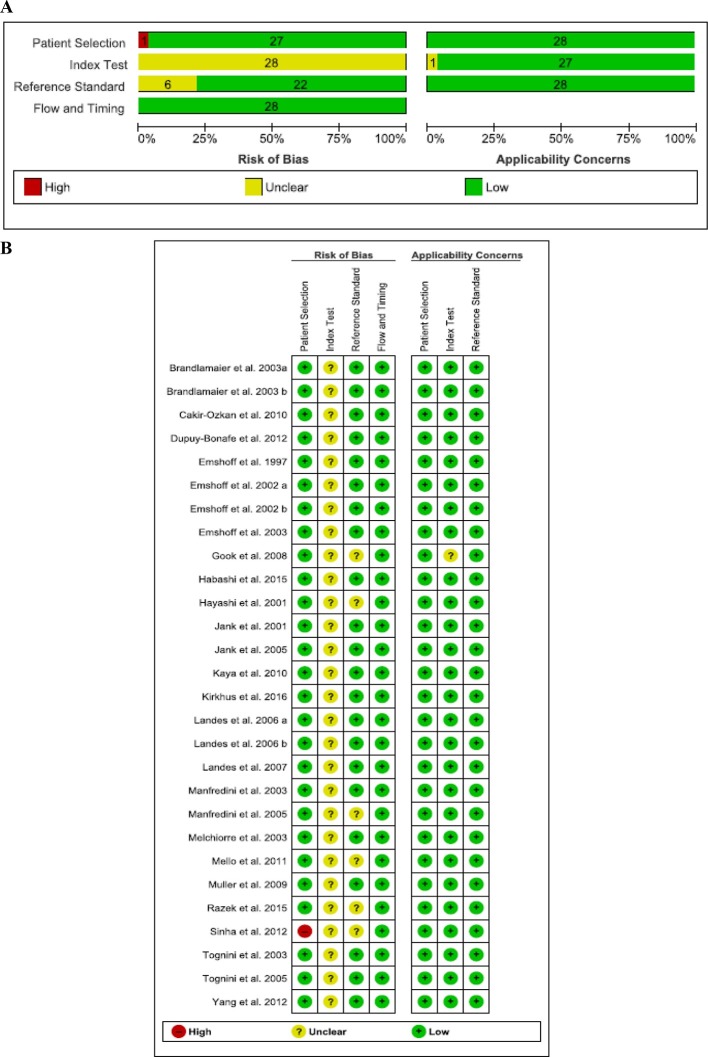
None of the studies fulfilled all of the methodological quality criteria (Figure 2). The RoB of index test was scored as “unclear” for all studies due to the lack of information on the used threshold. Six studies11,34,35,41,43,45 were evaluated as “unclear” in reference standard RoB domain. 27 studies presented low applicability concerns in all domains. Supplementary Material 3 shows the QUADAS-2 criteria for each included study.
Figure 2.
Risk of bias and applicability concerns graph: review authors’ judgments about each domain presented as percentages across included studies. (A) Risk of bias graph; (B) Risk of bias summary.
Results of individual studies
Four studies assessed TMJ alterations in rheumatic diseases patients (JIA, RA and/or PsA)9,41,42,44 and the results from these studies were analyzed separately from those in TMD patients. Melchiorre et al42 and Manfredini et al41 assessed DD with 2D US. The sensitivity/specificity were 69%/30 and 56%/73% respectively. All four studies evaluated JE using 2D US. Two of these studies assessed JE indirectly using the capsular distention measurements to discriminate joints with effusion from normal joints.9,41 The highest sensitivity (85%) for JE assessment was seen in Manfredini et al41 and Khirkus et al9 described the highest specificity (70%) using a cut-off value of 1.2 mm for capsular width. The CC was evaluated by two studies.41,44 The highest values were 67% sensitivity and 26% specificity.41
24 studies evaluated DD, JE and/or CC in TMD patients.10–14,27–40,43,45–48 To improve our interpretation of the results, these studies were clustered in groups by target assessed (DD, JE and CC).
US to DD assessment
In those studies, evaluating the ability for ultrasound to detect DD in TMD patients (n = 20), a very wide range of sensitivity (from 22 to 95%) and specificity (from 16 to 100%) was present (Table 1).
Seven studies reported excellent sensitivity (>80%) and specificity (>90%).13,32–36,48 Using 2D dynamic US with 12 MHz in 100 TMD patients (n = 200 joints), Jank et al36 reported a sensitivity and specificity of 92% in closed-mouth evaluation. Emshoff et al investigated 2D static and dynamic US (12MHz) in three studies, they found a sensitivity ranging from 82 to 95% and specificity ranging from 92 to 96% in closed and opened-mouth.13,32,33
The capability of 3D US to assess DD was tested by two studies.12,38 Landes et al reported a sonographic evaluation using 8–12.5 MHz with 53% sensitivity and 74% specificity.38 They emphasized that 3D sonography could provide better results by using automated image enhancement and higher transducer frequency.
US to JE assessment
Five studies addressed the ultrasound capability to evaluate JE in TMD patients.36,37,40,43,46 Using direct evaluation of the articular space to detect effusion, Jank et al36 described excellent sensitivity (83%) and specificity (100%) and Mello et al43 found 100% of specificity and 20% of sensitivity. Manfredini et al studied the cut-off values of the capsular distention to evaluate effusion. Ultrasound sensitivity was high (83.9%) with cut-off values less than 1.9 mm values while US specificity was high (88%) presenting cut-off greater than 2.1 mm.40
US to CC assessment
Nine studies assessed CC in TMD patients and the results ranged from 0 to 94% for sensitivity and from 20 to 100% for specificity.10,12,13,28,34,36,39,43,45 In these studies, CC were considered by the presence of bone erosion,10,12,13,28,34,36,39,44 flattening12,28,34,39,45 and/or osteophyte12,28,39,45 Only Gook et al34 used CT as a reference standard for CC evaluation and the sensitivity was 87% and specificity 62%. With ultrasound showing a sensitivity of 87% and specificity of 20%, Brandlamaier et al presented that US is valuable in diagnosing the presence but insufficient in diagnosing the absence of bone erosion, flattening and/or osteophyte signs.28 On the other hand, a sensitivity of 0% was found by other study evaluating flattening and erosion in three patients with TMD.45
Landes et al evaluated CC with 3D US (8–12.5 Mhz). This index test had 75% accuracy (sensitivity 70%/specificity 76%), PPV 44% and NPV 90% and concluded that 3D US is more reliable than 2D US for CC exclusion.39
Synthesis of results–quantitative analysis
DTA tables were constructed by target for the studies with TMD patients using the data extracted from each article (sensitivity, specificity, PPV, NPV, LR+, LR- and DOR) (Tables 2–4). Two out of 24 studies eligible for those analysis did not provide separated data to calculate DTA values.42,48 29,45 These authors were contacted without success.
Table 2.
Diagnostic test accuracy, measurements for US in DD assessment of TMD patients
| Author, Year | Sample size (N joints) |
Prevalence (%) a | Sensitivity (%) | Specificity (%) | PPV (%) a | NPV (%) a | LR+ a | LR– a | DOR a |
| Emshoff et al,1997 | 33 | 78 | 50 | 71 | 86 | 27 | 1.75 | 0.70 | 2.45 |
| Hayashi et al, 2001 | 36 | 33 | 83 | 95 | 90 | 92 | 20.0 | 0.17 | 117.6 |
| Jank et al, 2001 | 132 | 65 | 78 | 77 | 87 | 64 | 3.5 | 0.28 | 12.5 |
| Emshoff et al, 2002a | 128 | 21 | 81 | 95 | 81 | 95 | 16.45 | 0.19 | 81.0 |
| Emshoff et al, 2002b | 116 | 54 | 92 | 92 | 93 | 90 | 12.1 | 0.08 | 151.2 |
| Emshoff et al, 2003 | 96 | 44 | 95 | 90 | 89 | 96 | 10.1 | 0.05 | 202.0 |
| Brandlmaier et al, 2003a | 192 | 46 | 82 | 85 | 82 | 85 | 5.2 | 0.21 | 25.33 |
| Tognini et al, 2005 | 82 | 50 | 65 | 80 | 77 | 70 | 3.37 | 0.42 | 7.97 |
| Jank et al, 2005 | 200 | 69 | 92 | 92 | 96 | 83 | 11.4 | 0.08 | 142.5 |
| Landes et al, 2006a | 105 | 44 | 61 | 62 | 58 | 66 | 1.62 | 0.61 | 2.63 |
| Landes et al, 2007 | 66 | 45 | 63 | 47 | 50 | 60 | 1.2 | 0.7 | 1.52 |
| Gook et al, 2008 | 40 | 50 | 95 | 90 | 90 | 94 | 9.5 | 0.05 | 190 |
| Kaya et al,2010 | 52 | 88 | 91 | 16 | 89 | 20 | 1.09 | 0.52 | 2.08 |
| Dupuy-Bonafe et al, 2012 | 98 | 40 | 22 | 96 | 81 | 64 | 6.52 | 0.80 | 8.02 |
| Yang et al, 2012 | 40 | 57 | 82 | 94 | 95 | 80 | 14.04 | 0.18 | 78 |
| Razek et al, 2015 | 39 | 71 | 78 | 72 | 88 | 57 | 2.88 | 0.29 | 9.93 |
| Habashi et al, 2015 | 78 | 38 | 74 | 83 | 73 | 83 | 4.4 | 0.31 | 13.9 |
DOR, diagnostic odds ratio; LR-, negative likelihood ratio; LR+, positive likelihood ratio; NPV, negative predictive value; PPV, positive predictive value;
when the data is not available, the authors calculated data from information available in the article.
Table 3. .
Diagnostic test accuracy, measurements for US in JE assessment of TMD patients.
| Author, Year | Sample Size (N joints) | Prevalence %) a | Sensitivity (%) | Specificity (%) | PPV (%) a | NPV (%) a | LR+ a | LR– a | DOR a |
| Manfredini et al, 2003 | 138 | 44 | 83 | 73 | 59 | 80 | 1.7 | 0.3 | 5.67 |
| Tognini et al, 2003 | 88 | 46 | 75 | 76 | 73 | 78 | 3.2 | 0.3 | 9.50 |
| Jank et al,2005 | 200 | 29 | 83 | 100 | 100 | 93 | ∞ | 0.18 | ∞ |
| Kaya et al, 2010 | 52 | 57 | 53 | 63 | 66 | 50 | 1.4 | 0.73 | 1.92 |
| Mello et al, 2011 | 76 | 6 | 20 | 100 | 100 | 94 | ∞ | 0.8 | ∞ |
DOR, diagnostic odds ratio; LR+, positive likelihood ratio; LR–, negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value;
when the data is not available, the authors calculated data from information available in the article
Table 4. .
Diagnostic test accuracy, measurements for US in CC assessment of TMD patients
| Author, Year | Sample Size (N joints) | Prevalence (%) a | Sensitivity (%) | Specificity (%) | PPV (%) a | NPV (%) a | LR+ a | LR– a | DOR a |
| Emshoffet al 2003 | 48 | 18 | 83 | 62 | 34 | 94 | 2.24 | 0.26 | 8.62 |
| Brandlmaier et al, 2003b | 80 | 87 | 87 | 20 | 88 | 18 | 1.08 | 0.64 | 1.69 |
| Jank et al, 2005 | 200 | 95 | 94 | 100 | 100 | 45 | ∞ | 0.06 | ∞ |
| Landes et al, 2006b | 106 | 21 | 69 | 75 | 44 | 90 | 2.8 | 0.4 | 6.68 |
| Landes et al, 2007 | 66 | 24 | 68 | 75 | 45 | 88 | 2.6 | 0.4 | 6.19 |
| Gook et al, 2008 | 40 | 20 | 87 | 62 | 36 | 95 | 2.33 | 0.20 | 10.92 |
| Mello et al, 2011 | 76 | 17 | 15 | 87 | 20 | 83 | 1.2 | 0.96 | 1.18 |
| Habashi et al, 2015 | 78 | 50 | 36 | 82 | 66 | 56 | 2.0 | 0.78 | 2.56 |
DOR, diagnostic odds ratio; LR+, positive likelihood ratio; LR-, negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value;
when the data is not available, the authors calculated data from information available in the study.
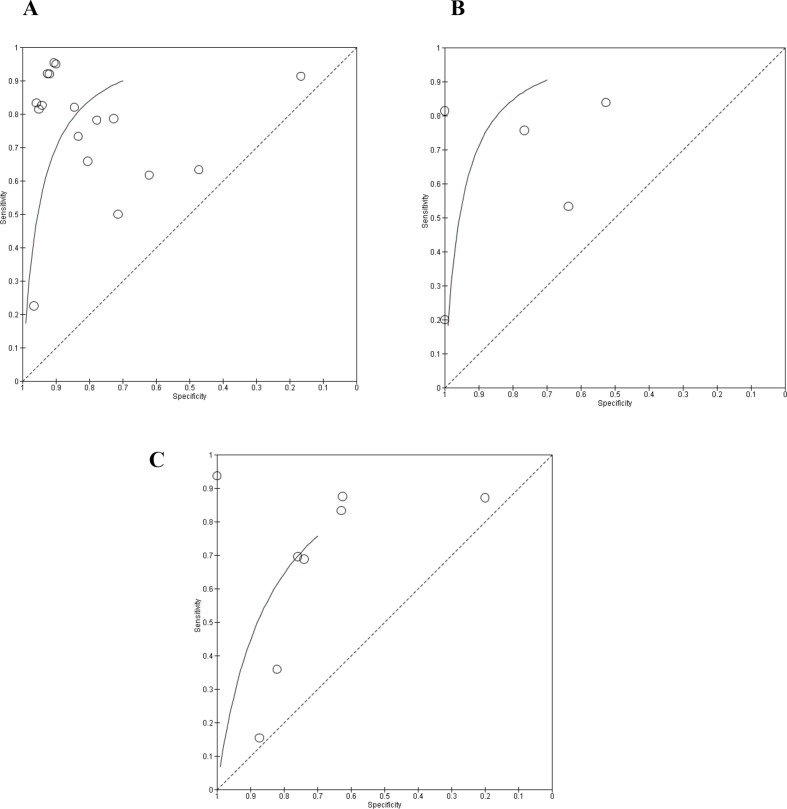
Our quantitative analysis included 2829 joints (1533 in DD, 554 in JE and 742 in CC assessment). Measures of the diagnostic test accuracy such as sensitivity and specificity of each included study in the quantitative analysis and summary sensitivity/specificity for each TMD are shown in Figure 3. In general, ultrasound sensitivity and specificity varied substantially, from 22 to 95% and 16 to 100%, respectively. Figure 4 shows the ROC curve for each group.
Figure 3.
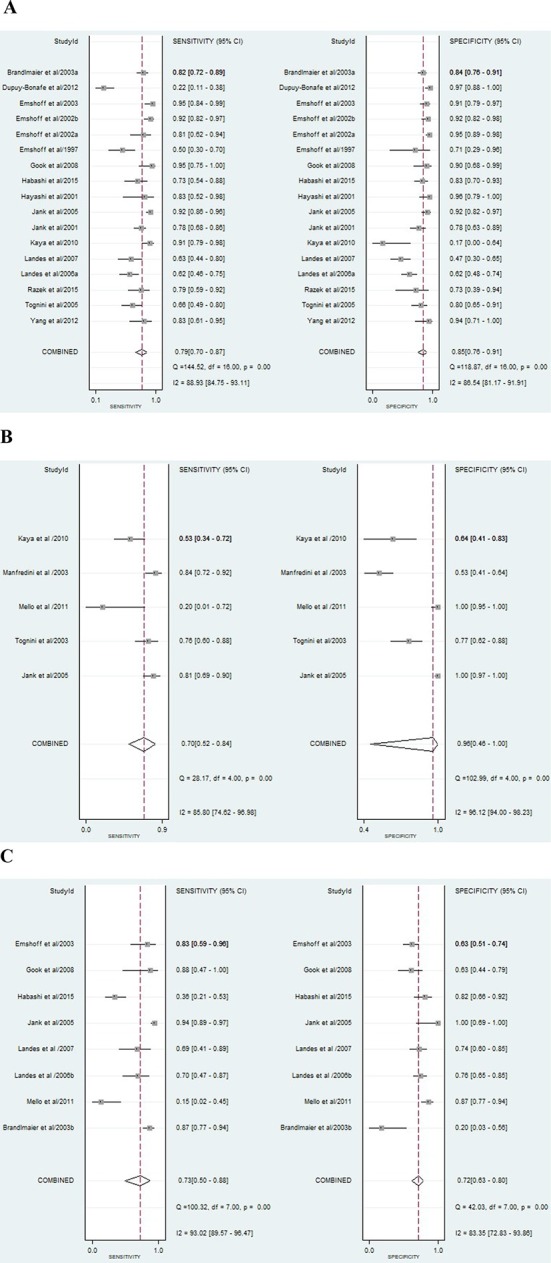
Forest plot with the diagnostic test accuracy (DTA) of each study (sensitivity, specificity and 95% confidence interval) and heterogeneity assessment. (A) Forest plot with the diagnostic test accuracy (DTA) for US to DD assessment; (B) Forest plot with the diagnostic test accuracy (DTA) for US to JE assessment; (C) Forest plot with the diagnostic test accuracy (DTA) for US to CC assessment.
Figure 4.
Receiver operating characteristic (ROC) curves for each group. (A) ROC curve for US to DD assessment; (B) ROC curve for US to JE assessment; (C): ROC curve for US to CC assessment.
For DD assessment in TMD patients (n = 17), 70% of the included studies presented DTA values considered excellent or acceptable10,13,14,27,30,32–36,47,48 and 30% reported poor values.11,12,31,37,38 Regarding PPV and NPV, the studies from Jank et al36 and Emshoff et al13 reported the highest values respectively. Additionally, the highest DOR values were observed in these two studies (Table 2).
For JE assessment, the additional analyses were done in five studies.36,37,40,43,46 Jank et al36 and Mello et al43 described excellent LR+, LR- and DOR values (Table 3). Also, from eight studies used for CC assessment additional analyses,10,12,13,28,34,36,38,39,43 only one36 reported LR values (LR+ ∞, LR- 0.06) and DOR (∞) excellent for DTA. This study provided the highest PPV (100%) value and the best NPV (95%) was seen in Gook et al article34 (Table 4).
High heterogeneity (I2 ranging from 83.35 to 96.12) was observed between the studies included in the meta-analysis (Figure 3).
Risk of bias across studies
The main methodological limitations were related to the lack of clear information in reporting QUADAS domain 2 item 2 addressing/reporting the use of a threshold. The potential bias is related to the fact that the threshold may influence the interpretation of the index test results. Additionally, QUADAS domain 4 item 1, exploring the interval between index test(s) and reference standard, were scored as “unclear” as no information on the timing between the examinations reported in some studies. Figure 2 details RoB and applicability concerns across included studies.
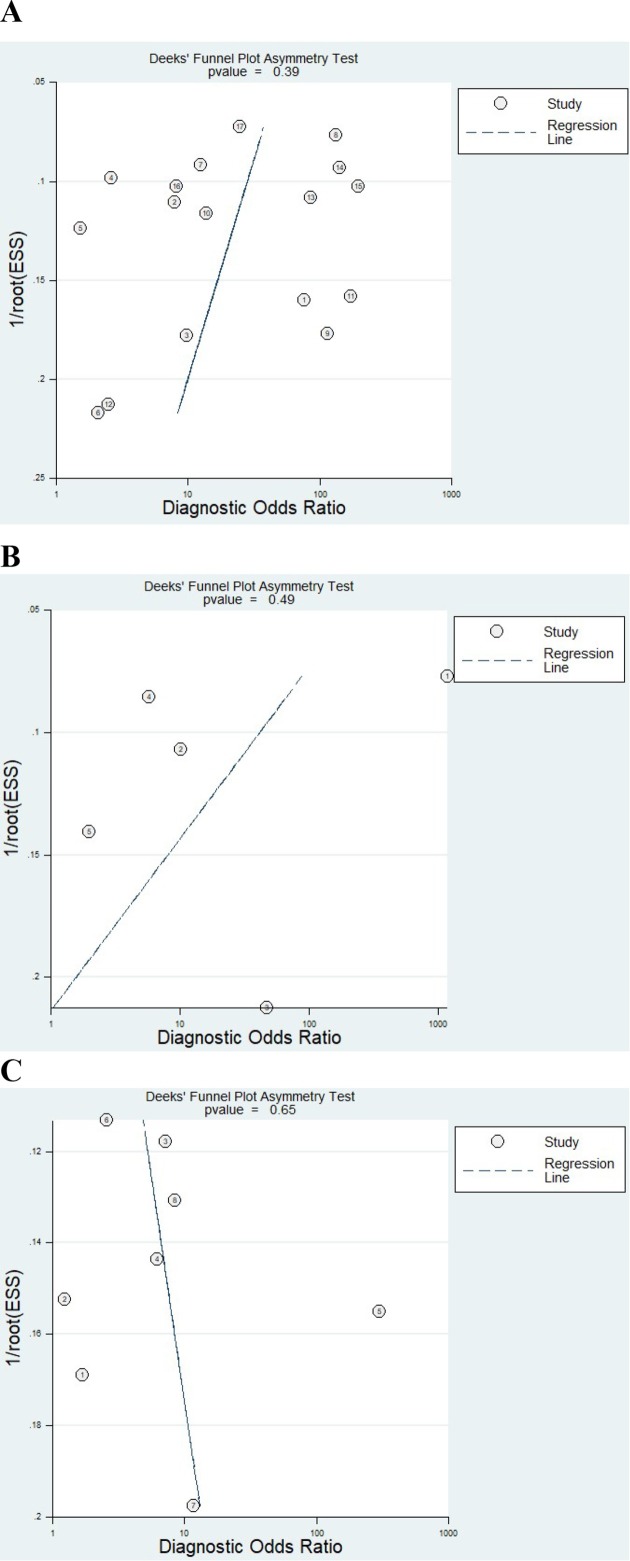
The Deeks funnel plot showed p-value = 0.39 for DD, 0.49 for JE and 0.65 for CC (Figure 5). This high asymmetry in the data suggests possible publication bias.
Figure 5.
Publication bias assessment. Deeks’ funnel plot with superimposed regression line. (A) Deeks’ funnel plot for US to DD assessment; (B) Deeks’ funnel plot for US to JE assessment; (C) Deeks’ funnel plot for US to CC assessment.
Additional analysis–level of evidence
Overall, the quality of the evidence evaluated by GRADE system was determined to be moderate. It suggested moderate confidence in estimating the outcomes. The indirectness factor was judged as serious due to the different parameters used in the index test studied and unclear information regarding images interpretation expertise (Supplementary Material 4).
Discussion
The number of affected people with TMJ alterations or TMD has been growing. A recent meta-analysis showed that one in 6 children and adolescents have clinical signs of TMD.49 Some studies suggested that the prevalence of TMD in adults range from 1 to 75% and approximately 33% of the adults have at least one symptom.50–52 There is evidence that the TMD signs or symptoms could be more common in the adult population than it is reported.52 However, TMD prevalence in the adult population is a subject under debate, due to the heterogeneity in the diagnostic criteria used and the modality of patients’ recruitment. Recently, the Research Diagnostic Criteria for TMD (RDC/TMD) was reviewed and a new Diagnostic Criteria (DC/TMD) was proposed.2 In this context, the diagnostic imaging plays an important role in improving TMD detection.
This systematic review investigated the available evidence on the diagnostic capability of ultrasound to assess TMJ alterations (DD, JE and CC). It is important to emphasize that multiple imaging modalities such as panoramic radiography, CBCT, CT and MRI have been used, with some limitations, to assess those TMJ alterations. Conventional imaging techniques as panoramic radiography are not useful to detect the first stages of the alterations just providing two-dimensional images.3,4 CT and CBCT are not used as screening method due to ionizing radiation and they are not able to detect articular disk alterations.8 Currently, although MRI is the method of choice to TMD evaluation, its limited accessibility, somewhat limited assessment of osseous changes, high-cost, and there are limitations related to claustrophobic patients and patients using metal devices with ferromagnetic properties.3 Moreover, dynamic MRI is hard to be acquired and have lower image quality than static images. Therefore, as ultrasound has merits such as low-cost, accessibility due to mobile units, non-invasive and non-ionizing radiation, it could be considered a screening method.15 For this reason, it is important to investigate its diagnostic capability.
Disc displacement
The most data about DTA of ultrasound from this study was provided by studies that assessed DD. Regarding this TMD, 70% of the studies showed excellent or acceptable DTA of ultrasound. These studies used different methodologies to evaluate DD and the disc in ultrasound images ranged from hyperechoic to hypoechoic. Most likely, because the challenge of viewing the disk due to the surrounding bone structures, the variation in ultrasound technical characteristics and the absence of a standardized US protocol for TMJ evaluation.53 Ultrasound was tested using different frequencies (ranging from 7.5 to 20MHz) and dynamic and static imaging were investigated in closed and/or open-mouth. In our review, excellent and acceptable DTA values for DD assessment were found in studies using dynamic and/or static US. The meta-analysis of Su et al found higher diagnostic values of the combined static and dynamic examinations than static examination alone.17 Other systematic review addressing this subject applied meta-regression to determine if the clinical heterogeneity could influence diagnostic accuracy. The influence of the different types of ultrasound on diagnostic efficacy was minimal. However, it is relevant to question the meta-regression statistical power due to few studies using dynamic and 3D US.15
The majority of studies addressed 2D US and a wide variation in accuracy values was observed in our review. A disadvantage of the 2D US is that an incorrect transducer angulation could easily cause the correctly positioned disk to disappear from the sonographic picture and could be the main reason for false-negative results. Moreover, one of the major shortcomings of the 2D US is the technique limitation to detect DD laterally or medially.29,54,55
Effusion
ultrasound has been used to detect synovitis and effusion in several joints. It is accurate to detect intra articular fluids in larger joints.56 With the technologies progress in the last years, attempts are made to study effusion in small joints using the ultrasound. The hallmark of JE in large joints is the distention of the joint capsule and due to the lack of literature on this specific issue in TMJ, the diagnostic criteria is not well established. The presence of effusion using MRI is depicted as hyperintense signal within articular space. In ultrasound evaluation, effusion may be detected by direct visualization as a hypoechoic area within the articular space or by indirect measurement of the capsular distention. In our results, just five studies investigated effusion in TMD patients, while three of them presented acceptable or excellent DTA values..36,43,46 Manfredini et al, 40 using indirect visualization of effusion, showed higher sensitivity (83%) and specificity (73.7%) with 1.9 mm of capsular distention. Jank et al36 studied 200 joints and found the same sensitivity (83%) presented by Manfredini et al40 and higher values for specificity (100%). The threshold used to evaluate JE was not detailed in Jank et al study. Standardized parameters to detect JE was further encouraged by all those studies and are reinforced by our results. At the moment, as there is no clarified classification system for effusion diagnosis by ultrasound, the comparison between the reference standard MRI and US could be not reliable.
Condylar changes
The ultrasound capability to assess hard tissues is a subject under controversy in the literature.10,13,28 Only one study out of eight included in the DTA table showed an excellent capability to assess condylar erosion reporting 94% of sensitivity and 100% of specificity.36 It is important to discuss that ten of the studies addressing CC in this systematic review used MRI as the reference standard. Since MRI has limitations to assess bone alterations as erosion, flattening and osteophyte, the results of these studies could have been misinterpreted. Brandlmaier discussed in his study that many of CC diagnosed with ultrasound could be not visible in MRI images.28 Using CT as the reference standard and 2D static US, Gook et al found 87% for US sensitivity and 62% to specificity when evaluating condyle flattening and erosion.34 These results allow us to affirm that the diagnostic capability of US to assess CC should be more explored using CT or CBCT as the reference standard.
Overall, there is a very wide variation in sensitivity and specificity of ultrasound for each type of pathology across a variety of included studies. This variation could be related to the parameters established to diagnose the presence or absence of the TMD such as diagnostic criteria for disc displacement. On the other hand, some of this variability could be due to technical factors such as the transducer frequency used, probe design, methods of examination and the diagnostic ability of the sonographer and image reader. High-resolution transducers (≥12 MHz) have shown a better visualization of the TMJ structures with better results than low-resolution devices.10,33 Ultrasound requires an experienced reader for image interpretation.57 Technical factors such as the type of gel or standoff pad used to scan the patient, contact pressure and probe angle could influence the quality of US image and its interpretation. In general, the included studies in this review did not provide detailed and replicable information about these parameters. Also, the visualization of the disc, articular space and condyle with US could be challenging due to the anatomic configuration of the TMJ, especially the medial part of the joint. The extraoral ultrasound approach used by the included studies in the current systematic review presented challenges to acquire the images externally through the zygomatic process and temporal bone. Recently, Katzberg et al described the first trans-oral ultrasound approach using an intraoral probe and images were acquired with adequate anatomic depth. The condyle and sub condylar space were visible in 100% of the joints and the disc was visible in 70%.53 For researches, could be a promising approach to improving the limitations caused by the difficulty of imaging structures behind bone and air.
3D US
On its acquisition process, the tissue block is obtained and slicing in several plans which allow multiplanar views (sagittal, coronal and axial) of a joint portion and favor the interpretation. In our study, three papers from the same group tested the performance of 3D US to assess TMD.12,38,39 One of them, compared 2D vs 3D US and the 3D one presented better results, maybe due to the better viewing.12 All those papers discussed that the main advantage of using 3D US was to obtain a complete overview of the articular disk and condyle. They also discussed that the 3D US performance could be improved by applying automated imaging enhancement. 3D ultrasound have been currently used in some areas of medicine and new technologies to improve those image analyses have been tested.58,59 Hareendranathan et al proposed a technique for semiautomatic segmentation of echogenic structures from 3D US applied to hip dysplasia. The study showed that it is a fast and reliable method to delineate the surface and shape of the structures aiding in more accurate diagnosis of the evaluated area. They emphasized the technique could be applied for any 3D US.58 As the current approach to evaluate TMD by ultrasound is a challenging, this type of technology applied to the image could be an opportunity for further studies.
Inflammatory arthritis
We found just four papers addressing ultrasound for TMJ alterations in rheumatic disease population,9,41,42,44 too few to construct a meaningful DTA table, forest plot or ROC curve and to draw conclusions. Besides that, these papers studied different rheumatic diseases with their own prevalence and TMD etiopathogenesis, which could lead to misinterpretation if combined. A recent review investigated the performance of US compared to MRI in JIA patients and found a wide variation in the sensitivity (0–72%) and specificity ranging from 70 to 83%. Furthermore, they found that in JIA patients, dynamic US improves sensitivity and specificity compared to static US.60
Summary of evidence
Based on this review results, the diagnostic ability for ultrasound to diagnose TMJ pathology varied widely from poor to excellent, depending on factors which are not clearly delineated in the various reports. In summary, the present synthesis showed US has a moderate diagnostic capability to assess DD and JE, which justify its use in clinical practice for these purposes. We cannot fully assess its diagnostic capability to CC assessment since only one study presented acceptable DTA values. Overall, the specificity and NPV of ultrasound seemed slightly higher than sensitivity and PPV values for each type of TMJ alteration. Clinically, these indicated that ultrasound is better to exclude than to confirm TMD. In this case, MRI should be used to confirm TMD detected by US. Therefore, ultrasound may contribute to clinical examination as an initial screening tool as has already been proposed in some medical areas. More advanced imaging can follow. That way the expensive resources are used more efficiently.
For the future studies, a 3D US dynamic approach with high-resolution transducer should be considered to verify if the reliability of the examination would increase. To DD assessment, the clinical significance of the disc movement patterns and its position abnormalities should be taken into account in further studies. Also, researchers are encouraged to address the diagnostic capability of US to CC assessment using appropriate reference standard. Despite the large studies in this field and the improvement of the ultrasound imaging in the last years, it is still necessary to standardize the TMD assessment by ultrasound. Possibly, studies stablishing the normal parameters of ultrasound TMJ evaluation could contribute to the standardization.
Limitations
Some limitations of this review should be considered. First, some papers did not describe the thresholds to determine the presence of DD, JE or CC. Second, six papers did not provide information about the blind interpretation of the reference standard results. These two limitations may influence the test performance estimation and DTA values presenting here could be affected.
Despite the summary of sensitivity/specificity were provided by the presented forest plots, caution should be exercised while interpreting these pooled results. First, positive and negative predictive values may vary with disease prevalence. Second, high heterogeneity and potential biases inherent to the included studies could have magnified pooled accuracy. Finally, the combined approach does not take into account the possibility of different test thresholds in the studies.
The forest plot shows a large range of accuracy values, this could be due to the studies criteria to select participants or the criteria used to acquire and to evaluate the images. There was a large number of studies from the same group. 16 studies belong to three research groups (Frankfurt University-Germany, University of Pisa-Italy and University of Innsbruck-Austria).12–14,27,28,31–33,36,38–42,46,47 Despite this, the results vary between the studies of the same group and no standardization or protocol are observed for TMD assessment by US.
Conclusion
This systematic review suggests that ultrasound may have clinically acceptable capability to screen for disk displacement and joint effusion in TMD patients. For screening of condylar changes, ultrasound needs further studies using CT or CBCT as the reference standard to support its use. More advanced imaging such as MRI can be used after a positive screening with ultrasound to confirm the TMD diagnosis if deemed necessary.
Footnotes
Acknowledgment: We thank Silvia Capenakas (SC) for collaboration on the methodological quality assessment of the studies and Isabella Porto de Toledo for the meta-analysis assistance.
Conflict of interest: The authors declare that they have no conflict of interest.
Contributor Information
Fabiana Tolentino Almeida, Email: fabiana@ualberta.ca.
Camila Pacheco-Pereira, Email: cppereir@ualberta.ca.
Carlos Flores-Mir, Email: cf1@ualberta.ca.
Lawrence H. Le, Email: lel@ualberta.ca.
Jacob L. Jaremko, Email: jjaremko@ualberta.ca.
Paul W. Major, Email: major@ualberta.ca.
REFERENCES
- 1. Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 1992; 6: 301–55. [PubMed] [Google Scholar]
- 2. Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014; 28: 6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bag AK, Gaddikeri S, Singhal A, Hardin S, Tran BD, Medina JA, et al. Imaging of the temporomandibular joint: An update. World J Radiol 2014; 6: 567–82. doi: 10.4329/wjr.v6.i8.567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hunter A, Kalathingal S. Diagnostic imaging for temporomandibular disorders and orofacial pain. Dent Clin North Am 2013; 57: 405–18. doi: 10.1016/j.cden.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 5. Alkhader M, Ohbayashi N, Tetsumura A, Nakamura S, Okochi K, Momin MA, et al. Diagnostic performance of magnetic resonance imaging for detecting osseous abnormalities of the temporomandibular joint and its correlation with cone beam computed tomography. Dentomaxillofac Radiol 2010; 39: 270–6. doi: 10.1259/dmfr/25151578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zain-Alabdeen EH, Alsadhan RI. A comparative study of accuracy of detection of surface osseous changes in the temporomandibular joint using multidetector CT and cone beam CT. Dentomaxillofac Radiol 2012; 41: 185–91. doi: 10.1259/dmfr/24985971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Honda K, Larheim TA, Maruhashi K, Matsumoto K, Iwai K. Osseous abnormalities of the mandibular condyle: diagnostic reliability of cone beam computed tomography compared with helical computed tomography based on an autopsy material. Dentomaxillofac Radiol 2006; 35: 152–7. doi: 10.1259/dmfr/15831361 [DOI] [PubMed] [Google Scholar]
- 8. Larheim TA, Abrahamsson AK, Kristensen M, Arvidsson LZ. Temporomandibular joint diagnostics using CBCT. Dentomaxillofac Radiol 2015; 44: 20140235. doi: 10.1259/dmfr.20140235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirkhus E, Gunderson RB, Smith HJ, Flatø B, Hetlevik SO, Larheim TA, et al. Temporomandibular joint involvement in childhood arthritis: comparison of ultrasonography-assessed capsular width and MRI-assessed synovitis. Dentomaxillofac Radiol 2016; 45: 20160195. doi: 10.1259/dmfr.20160195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Habashi H, Eran A, Blumenfeld I, Gaitini D. Dynamic high-resolution sonography compared to magnetic resonance imaging for diagnosis of temporomandibular joint disk displacement. J Ultrasound Med 2015; 34: 75–82. doi: 10.7863/ultra.34.1.75 [DOI] [PubMed] [Google Scholar]
- 11. Razek AA, Al Mahdy Al Belasy F, Ahmed WM, Haggag MA. Assessment of articular disc displacement of temporomandibular joint with ultrasound. J Ultrasound 2015; 18: 159–63. doi: 10.1007/s40477-014-0133-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Landes CA, Goral WA, Sader R, Mack MG. Three-dimensional versus two-dimensional sonography of the temporomandibular joint in comparison to MRI. Eur J Radiol 2007; 61: 235–44. doi: 10.1016/j.ejrad.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 13. Emshoff R, Brandlmaier I, Bodner G, Rudisch A. Condylar erosion and disc displacement: detection with high-resolution ultrasonography. J Oral Maxillofac Surg 2003; 61: 877–81. doi: 10.1016/S0278-2391(03)00247-7 [DOI] [PubMed] [Google Scholar]
- 14. Jank S, Rudisch A, Bodner G, Brandlmaier I, Gerhard S, Emshoff R. High-resolution ultrasonography of the TMJ: helpful diagnostic approach for patients with TMJ disorders ? J Craniomaxillofac Surg 2001; 29: 366–71. doi: 10.1054/jcms.2001.0252 [DOI] [PubMed] [Google Scholar]
- 15. Li C, Su N, Yang X, Yang X, Shi Z, Li L. Ultrasonography for detection of disc displacement of temporomandibular joint: a systematic review and meta-analysis. J Oral Maxillofac Surg 2012; 70: 1300–9. doi: 10.1016/j.joms.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 16. Dong XY, He S, Zhu L, Dong TY, Pan SS, Tang LJ, et al. The diagnostic value of high-resolution ultrasonography for the detection of anterior disc displacement of the temporomandibular joint: a meta-analysis employing the HSROC statistical model. Int J Oral Maxillofac Surg 2015; 44: 852–8. doi: 10.1016/j.ijom.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 17. Su N, van Wijk AJ, Visscher CM, Lobbezoo F, van der Heijden G. Diagnostic value of ultrasonography for the detection of disc displacements in the temporomandibular joint: a systematic review and meta-analysis. Clin Oral Investig 2018; 22: 2599–614. doi: 10.1007/s00784-018-2359-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, , et al.the PRISMA-DTA Group . Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA 2018; 319: 388–96. doi: 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 19. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–36. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 21. Hayden SR, Brown MD. Likelihood ratio: A powerful tool for incorporating the results of a diagnostic test into clinical decisionmaking. Ann Emerg Med 1999; 33: 575–80. doi: 10.1016/S0196-0644(99)70346-X [DOI] [PubMed] [Google Scholar]
- 22. Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003; 56: 1129–35. doi: 10.1016/S0895-4356(03)00177-X [DOI] [PubMed] [Google Scholar]
- 23. Akobeng AK. Understanding diagnostic tests 1: sensitivity, specificity and predictive values. Acta Paediatr 2007; 96: 338–41. doi: 10.1111/j.1651-2227.2006.00180.x [DOI] [PubMed] [Google Scholar]
- 24. Deeks JJ Bossuyt P, Gatsonis C, eds. Cochrane handbook for systematic reviews of diagnostic test accuracy Version 1.0. The Cochrane collaboration 2010;. [Google Scholar]
- 25. Deeks J, Higgins JPT, Altman D, Green S. Chapter 12.2.1: The GRADE Approach. In: Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration 2011;. [Google Scholar]
- 26. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–6. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 27. Brandlmaier I, Rudisch A, Bodner G, Bertram S, Emshoff R. Temporomandibular joint internal derangement: detection with 12.5 MHz ultrasonography. J Oral Rehabil 2003; 30: 796–801. doi: 10.1046/j.1365-2842.2003.01063.x [DOI] [PubMed] [Google Scholar]
- 28. Brandlmaier I, Bertram S, Rudisch A, Bodner G, Emshoff R. Temporomandibular joint osteoarthrosis diagnosed with high resolution ultrasonography versus magnetic resonance imaging: how reliable is high resolution ultrasonography? J Oral Rehabil 2003; 30: 812–7. doi: 10.1046/j.1365-2842.2003.01125.x [DOI] [PubMed] [Google Scholar]
- 29. Cakir-Ozkan N, Sarikaya B, Erkorkmaz U, Aktürk Y. Ultrasonographic evaluation of disc displacement of the temporomandibular joint compared with magnetic resonance imaging. J Oral Maxillofac Surg 2010; 68: 1075–80. doi: 10.1016/j.joms.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 30. Dupuy-Bonafé I, Picot MC, Maldonado IL, Lachiche V, Granier I, Bonafé A. Internal derangement of the temporomandibular joint: is there still a place for ultrasound? Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113: 832–40. doi: 10.1016/j.oooo.2011.11.017 [DOI] [PubMed] [Google Scholar]
- 31. Emshoff R, Bertram S, Rudisch A, Gassner R. The diagnostic value of ultrasonography to determine the temporomandibular joint disk position. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997; 84: 688–96. doi: 10.1016/S1079-2104(97)90374-7 [DOI] [PubMed] [Google Scholar]
- 32. Emshoff R, Jank S, Rudisch A, Bodner G. Are high-resolution ultrasonographic signs of disc displacement valid? J Oral Maxillofac Surg 2002; 60: 623–8. doi: 10.1053/joms.2002.33105 [DOI] [PubMed] [Google Scholar]
- 33. Emshoff R, Jank S, Bertram S, Rudisch A, Bodner G. Disk displacement of the temporomandibular joint: sonography versus MR imaging. AJR Am J Roentgenol 2002; 178: 1557–62. doi: 10.2214/ajr.178.6.1781557 [DOI] [PubMed] [Google Scholar]
- 34. Gook KB. Ultrasounds Image on the Disorders of the Ligaments Surrounding Temporomandibular Joints. Journal of Oral Med Pain 2008; 33: 387–94. [Google Scholar]
- 35. Hayashi T, Ito J, Koyama J, Yamada K. The accuracy of sonography for evaluation of internal derangement of the temporomandibular joint in asymptomatic elementary school children: comparison with MR and CT. AJNR Am J Neuroradiol 2001; 22: 728–34. [PMC free article] [PubMed] [Google Scholar]
- 36. Jank S, Emshoff R, Norer B, Missmann M, Nicasi A, Strobl H, et al. Diagnostic quality of dynamic high-resolution ultrasonography of the TMJ--a pilot study. Int J Oral Maxillofac Surg 2005; 34: 132–7. doi: 10.1016/j.ijom.2004.03.014 [DOI] [PubMed] [Google Scholar]
- 37. Kaya K, Dulgeroglu D, Unsal-Delialioglu S, Babadag M, Tacal T, Barlak A, et al. Diagnostic value of ultrasonography in the evaluation of the temporomandibular joint anterior disc displacement. J Craniomaxillofac Surg 2010; 38: 391–5. doi: 10.1016/j.jcms.2009.10.017 [DOI] [PubMed] [Google Scholar]
- 38. Landes CA, Goral WA, Sader R, Mack MG. 3-D sonography for diagnosis of disk dislocation of the temporomandibular joint compared with MRI. Ultrasound Med Biol 2006; 32: 633–9. doi: 10.1016/j.ultrasmedbio.2006.02.1401 [DOI] [PubMed] [Google Scholar]
- 39. Landes CA, Goral W, Mack MG, Sader R. 3-D sonography for diagnosis of osteoarthrosis and disk degeneration of the temporomandibular joint, compared with MRI. Ultrasound Med Biol 2006; 32: 627–32. doi: 10.1016/j.ultrasmedbio.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 40. Manfredini D, Tognini F, Melchiorre D, Zampa V, Bosco M. Ultrasound assessment of increased capsular width as a predictor of temporomandibular joint effusion. Dentomaxillofac Radiol 2003; 32: 359–64. doi: 10.1259/dmfr/25091144 [DOI] [PubMed] [Google Scholar]
- 41. Manfredini D, Tognini F, Melchiorre D, Bazzichi L, Bosco M. Ultrasonography of the temporomandibular joint: comparison of findings in patients with rheumatic diseases and temporomandibular disorders. A preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 100: 481–5. doi: 10.1016/j.tripleo.2005.02.071 [DOI] [PubMed] [Google Scholar]
- 42. Melchiorre D, Calderazzi A, Maddali Bongi S, Cristofani R, Bazzichi L, Eligi C, et al. A comparison of ultrasonography and magnetic resonance imaging in the evaluation of temporomandibular joint involvement in rheumatoid arthritis and psoriatic arthritis. Rheumatology 2003; 42: 673–6. doi: 10.1093/rheumatology/keg181 [DOI] [PubMed] [Google Scholar]
- 43. Mello Junior C, Saito O, Guimarães Filho H. Sonographic evaluation of temporomandibular joint internal disorders. Radiol Bras 2011; 44: 355–9. [Google Scholar]
- 44. Müller L, Kellenberger CJ, Cannizzaro E, Ettlin D, Schraner T, Bolt IB, et al. Early diagnosis of temporomandibular joint involvement in juvenile idiopathic arthritis: a pilot study comparing clinical examination and ultrasound to magnetic resonance imaging. Rheumatology 2009; 48: 680–5. doi: 10.1093/rheumatology/kep068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sinha VP, Pradhan H, Gupta H, Mohammad S, Singh RK, Mehrotra D, et al. Efficacy of plain radiographs, CT scan, MRI and ultra sonography in temporomandibular joint disorders. Natl J Maxillofac Surg 2012; 3: 2–9. doi: 10.4103/0975-5950.102138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tognini F, Manfredini D, Melchiorre D, Zampa V, Bosco M. Ultrasonographic vs magnetic resonance imaging findings of temporomandibular joint effusion. Minerva Stomatol 2003; 52(7-8): 365–70. [PubMed] [Google Scholar]
- 47. Tognini F, Manfredini D, Melchiorre D, Bosco M. Comparison of ultrasonography and magnetic resonance imaging in the evaluation of temporomandibular joint disc displacement. J Oral Rehabil 2005; 32: 248–53. doi: 10.1111/j.1365-2842.2004.01410.x [DOI] [PubMed] [Google Scholar]
- 48. Yang J, Liu W, Zhong Y, Zhao H. The diagnostic value of high-resolution ultrasonography for detecting anterior disc displacement without reduction of temporomandibular joint. Hua Xi Kou Qiang Yi Xue Za Zhi 2012; 30: 632–40. [PubMed] [Google Scholar]
- 49. da Silva CG, Pachêco-Pereira C, Porporatti AL, Savi MG, Peres MA, Flores-Mir C, et al. Prevalence of clinical signs of intra-articular temporomandibular disorders in children and adolescents: A systematic review and meta-analysis. J Am Dent Assoc 2016; 147: 10–18. doi: 10.1016/j.adaj.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 50. Casanova-Rosado JF, Medina-Solís CE, Vallejos-Sánchez AA, Casanova-Rosado AJ, Hernández-Prado B, Avila-Burgos L. Prevalence and associated factors for temporomandibular disorders in a group of Mexican adolescents and youth adults. Clin Oral Investig 2006; 10: 42–9. doi: 10.1007/s00784-005-0021-4 [DOI] [PubMed] [Google Scholar]
- 51. Nassif NJ, Al-Salleeh F, Al-Admawi M. The prevalence and treatment needs of symptoms and signs of temporomandibular disorders among young adult males. J Oral Rehabil 2003; 30: 944–50. doi: 10.1046/j.1365-2842.2003.01143.x [DOI] [PubMed] [Google Scholar]
- 52. Rutkiewicz T, Könönen M, Suominen-Taipale L, Nordblad A, Alanen P. Occurrence of clinical signs of temporomandibular disorders in adult Finns. J Orofac Pain 2006; 20: 208–17. [PubMed] [Google Scholar]
- 53. Katzberg RW, Conway WF, Ackerman SJ, Gonzales TS, Kheyfits V, Cronan MS. Pilot study to show the feasibility of high-resolution sagittal ultrasound imaging of the temporomandibular joint. J Oral Maxillofac Surg 2017; 75: 1151–62. doi: 10.1016/j.joms.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 54. Bas B, Yılmaz N, Gökce E, Akan H. Diagnostic value of ultrasonography in temporomandibular disorders. J Oral Maxillofac Surg 2011; 69: 1304–10. doi: 10.1016/j.joms.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 55. Emshoff R, Jank S, Rudisch A, Walch C, Bodner G. Error patterns and observer variations in the high-resolution ultrasonography imaging evaluation of the disk position of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002; 93: 369–75. doi: 10.1067/moe.2002.121432 [DOI] [PubMed] [Google Scholar]
- 56. Iagnocco A, Ceccarelli F, Rizzo C, Truglia S, Massaro L, Spinelli FR, et al. Ultrasound evaluation of hand, wrist and foot joint synovitis in systemic lupus erythematosus. Rheumatology 2014; 53: 465–72. doi: 10.1093/rheumatology/ket376 [DOI] [PubMed] [Google Scholar]
- 57. Kundu H, Basavaraj P, Kote S, Singla A, Singh S. Assessment of TMJ Disorders Using Ultrasonography as a Diagnostic Tool: A Review. J Clin Diagn Res 2013; 7: 3116–20. doi: 10.7860/JCDR/2013/6678.3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hareendranathan AR, Mabee M, Punithakumar K, Noga M, Jaremko JL. A technique for semiautomatic segmentation of echogenic structures in 3D ultrasound, applied to infant hip dysplasia. Int J Comput Assist Radiol Surg 2016; 11: 31–42. doi: 10.1007/s11548-015-1239-5 [DOI] [PubMed] [Google Scholar]
- 59. Noble JA, Boukerroui D. Ultrasound image segmentation: a survey. IEEE Trans Med Imaging 2006; 25: 987–1010. doi: 10.1109/TMI.2006.877092 [DOI] [PubMed] [Google Scholar]
- 60. Hechler BL, Phero JA, Van Mater H, Matthews NS. Ultrasound versus magnetic resonance imaging of the temporomandibular joint in juvenile idiopathic arthritis: a systematic review. Int J Oral Maxillofac Surg 2018; 47: 83–9. doi: 10.1016/j.ijom.2017.07.014 [DOI] [PubMed] [Google Scholar]