Abstract
Background
Cardiomyopathy is the leading cause of death in Duchenne muscular dystrophy (DMD). Standard cardiac biomarkers are poor indicators of DMD cardiovascular disease. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) regulate collagen turnover. Given the cardiac fibrosis seen in DMD, we hypothesized that MMPs and TIMPs correlate with severity of DMD cardiomyopathy.
Methods and Results
Prospectively enrolled DMD subjects (n=42) underwent cardiac MRI for function and late gadolinium enhancement (LGE), including LGE severity from 0 (no LGE) to 4 (severe). Serum from DMD and healthy male controls (n=15) analyzed for MMP 1, 2, 3, 7, 9, 10 and TIMPs 1–4. MMP1, MMP7, and MMP10 were higher in DMD than control (median 5080pg/ml vs. 2120pg/ml, p=0.007; 2170pg/ml vs. 1420pg/ml, p<0.001; 216pg/ml vs. 140pg/ml, p=0.040); TIMP4 was lower in DMD (124pg/ml vs. 263pg/ml, p=0.046). Within DMD, MMP7 correlated inversely with left ventricular ejection fraction (r=−0.40, p=0.012) and directly with strain (r=0.54, p=0.001) and LGE severity (r=0.47, p=0.003). MMP7 was higher in DMD patients with LGE compared to those without LGE and controls (p<0.001).
Conclusions
Multiple MMPs are elevated in DMD compared with controls. MMP7 is related to DMD cardiac dysfunction and myocardial fibrosis, possibly through remodeling of the extracellular matrix.
Keywords: Duchenne muscular dystrophy, Biomarkers, Cardiomyopathy, Fibrosis
INTRODUCTION
Duchenne muscular dystrophy (DMD) is an X-linked disorder affecting 1 in 4700 male births.[1] Although perceived primarily as a skeletal myopathy, boys also develop insidious and progressive cardiomyopathy. In the current era, cardiomyopathy is the leading cause of mortality.[2] Because of skeletal muscle weakness, boys with cardiomyopathy are usually asymptomatic until they develop severe left ventricular (LV) dysfunction. Cardiac imaging is the primary modality for diagnosis of dysfunction. Unfortunately, standard heart failure biomarkers, such as brain natriuretic peptide (BNP), are only increased at end stage.[3, 4]
Therapeutic options for DMD cardiomyopathy are limited. Standard heart failure medications, including angiotensin converting enzyme inhibitors, beta-blockers, and aldosterone inhibitors, have demonstrated some level of efficacy.[5–8] However, therapeutic evaluation in DMD has been limited by small sample sizes and short duration of treatment and these medications only serve to delay the inevitable decline in function.[9] Given the differences in pathogenesis, disease-specific therapeutics are necessary.[10]
DMD cardiomyopathy appears to begin with diffuse myocardial fibrosis, followed by larger areas of focal fibrosis, and eventual overt myocardial dysfunction.[11, 12] A better understanding of the molecular effectors leading to DMD fibrosis may help identify novel biomarkers of disease progression or novel targets for drug therapy. These biomarkers could function as surrogate outcome measures or be used to monitor therapeutic response between cardiac MRIs. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) regulate collagen turnover in the myocardial extracellular matrix and may play a role in DMD fibrosis.[13] We hypothesized that MMPs and TIMPs would be elevated in DMD compared with control and would correlate with severity of DMD cardiomyopathy.
METHODS
Enrollment
This prospective study was approved by the Vanderbilt Institutional Review Board and the investigation conforms with the principles outlined in the Declaration of Helsinki. DMD subjects were enrolled from the neuromuscular cardiology clinic from 2012–2015. Informed consent was obtained from the subjects (or their guardians) and appropriate assents were obtained. Inclusion criteria were: 1) diagnosis of DMD with clinical phenotype and confirmation with either genetic testing or muscle biopsy; 2) blood obtained at time of cardiac MRI; 3) able to tolerate cardiac MRI without sedation or anesthesia; given difficulties with breath-holds in younger children, the youngest age enrolled was 7. In order to enroll a population with a broad range of cardiovascular disease severity, no upper age limit was used for DMD patients. Exclusion criteria were: 1) additional cardiac diagnoses that could confound biomarkers (one patient who, in addition to a DMD mutation, also had two known disease-causing mutations for hypertrophic cardiomyopathy), 2) inability to draw an adequate volume of blood for biomarker analysis. Pertinent clinical data were collected from patients and from the electronic medical record. Enrolled DMD subjects underwent: blood draw, cardiac MRI, and skeletal muscle strength assessment at a single time point.
Healthy, male pediatric patients aged 8–18 years old were enrolled as controls. These healthy children were recruited prior to treadmill testing for chest pain, syncope, palpitations, or tachycardia. Exclusion criteria were: 1) abnormal treadmill test, 2) presence or concern for structural or functional cardiovascular disease (congenital heart disease, cardiomyopathy, or any secondary cardiovascular disease), 3) abnormal echocardiogram, 4) arrhythmia or clinical concern for arrhythmia. All participants were determined to be healthy by their primary cardiologist after thorough evaluation as indicated by clinical presentation. All clinic notes and cardiac testing were reviewed by a study author (JHS) to ensure that all subjects met inclusion/exclusion criteria.
Biomarker Analysis
The Milliplex Map Human MMP Magnetic Bead Panels 1 and 2 and Human TIMP Magnetic Bead Panel 2 (EMD Millipore Corporation, Billerica, MA. Cat # HMMP1MAG-55K, HMMP2MAG-55K, and HTMP2MAG-54K) were used to detect serum MMP1, MMP2, MMP3, MMP7, MMP9, MMP10, TIMP1, TIMP2, TIMP3, and TIMP4 according to manufacturer’s instructions. The Milliplex Map Human Cytokine/Chemokine Magnetic Bead Panel (EMD Millipore Corporation, Billerica, MA. Cat # HCYTOMAG-60K) was used to detect serum interleukin 1 beta (IL-1b) and tumor necrosis factor alpha (TNFa). Seven working standards were generated by serial dilution (1:3) of the reconstituted standard provided in the kit. Two QC (Quality Control) samples were included in each plate run. Assay plate was read on Luminex 200 with XPONENT software using the parameters outlined in the assay kit instructions. The Milliplex Analyst 5.0 software was used for data analysis. The correlation efficiency (R) for the Standard Curve was greater than 0.99 for each assay. All assays were run in duplicate, and the average % coefficient of variation (CV) was less than 10%. Any individual samples with a CV greater than 25% were repeated twice and, if the subsequent CV was >25%, the results were removed from analysis - total DMD samples removed from analysis by biomarker: MMP1 (N=9), MMP3 (N=0), MMP7 (N=3), MMP9 (N=4), MMP10 (N=2), TIMP1 (N=4), TIMP2 (N=5), TIMP3 (N=0), TIMP4 (N=0). MMP2 was not considered further because of the large number of samples with CV >25%. We also conducted a sensitivity analysis where, instead of removing samples with CV > 25%, we used the median of all available values.
Cardiac Magnetic Resonance
Cardiac MRI was performed for DMD subjects using a 1.5 Tesla Siemens Avanto (Siemens Healthcare Sector, Erlangen, Germany). LV volume, mass, and function were calculated as previously described.[12] A peripheral intravenous line was used to administer Gd-DTPA contrast (gadopentate dimeglumine, Magnevist®, Bayer Healthcare Pharmaceuticals, Wayne, NJ, USA) at a dose of 0.2mmol/kg. Late gadolinium enhancement (LGE) was assessed using single shot and segmented inversion recovery bSSFP with optimized inversion recovery to null the signal from the myocardium, and phase sensitive inversion recovery bSSFP with an inversion time of 300ms.
Myocardial tagging was performed in the short axis at the level of the papillary muscles using a segmented k-space fast gradient echo sequence with ECG triggering. Grid tagging was performed with 9–13 phases. Typical imaging parameters included: slice thickness 6–8 mm, field of view 340 mm × 340 mm, matrix size 256 × 192, and minimum echo time and repetition time. The sequences were breath-holds and parallel imaging with GRAPPA (Siemens) and an acceleration factor of two was used. Analysis of myocardial tagged images was performed using harmonic phase (HARP) methodology (Diagnosoft Inc., Morrisville, NC) as previously described.[14] One reader performed the analysis blinded to pertinent clinical data. A mesh was created by manually contouring the endocardial and epicardial borders at end-systole. The software then calculated the peak global and segmental circumferential strain (εcc) values; segmental values were calculated in the 6 segments at the mid portion of the LV using the standard 17 segment model.[15]
The presence or absence of LGE, as well as location using the standard 17-segment model, was qualitatively assessed by one reader during initial CMR interpretation. LGE was confirmed by a second reader blinded to all clinical data. If the readers did not agree, the images were reviewed by both investigators and consensus was reached. Both readers assigned LGE severity using a modification of the global severity score reported by Menon, et al.[16] Each readers reviewed the complete short axis stack for all sequences performed (at least one single shot inversion recovery short axis stack, one set of segmented inversion recovery images in the short axis plane, and at least one phase sensitive inversion recovery short axis stack). The readers cross-referenced areas of LGE with the 4-chamber, 3-chamber, and 2-chamber views to insure that an accurate assessment of LGE severity was obtained. The score ranged from 0 (no LGE) to 4 (severe LGE) defined as the following: 0 - no LGE, 1 - LGE localized to basal or mid free wall, 2 - LGE involving free wall but less than 1/3 of free wall length, no or minimal septal involvement, 3 – LGE in multiple areas of LV free wall >1/3 and mild septal involvement or more extensive LV free wall involvement (>2/3) without septal involvement, 4 - extensive free wall and septal involvement. Percent LGE was calculated using Medis software and the full width half maximum (FWHM) technique by one reader blinded to all clinical data. For consistency and quality (some subjects had difficulty with with breath-holds for the segmented inversion recovery images), the single shot phase sensitive inversion recovery short axis stack was used for these calculations. The epicardial and endocardial borders were manually contoured, avoiding the LVOT in the basal slice and apical slices with significant through plane motion. A reference region of interest was placed in myocardium without visible LGE. The percent LGE was then calculated.
Quantitative Muscle Strength Testing
Quantitative muscle testing (QMT) is an objective, reproducible method for upper and lower extremity strength evaluation in DMD.[17–19] QMT was performed on DMD subjects using a handheld myometer by one investigator (WBB) as previously described.[20] Arm QMT score was calculated by adding flexion and extension values for the right and left elbows, leg QMT score was calculated by adding flexion and extension values for the right and left knees, and total QMT score was calculated by adding the total arm score and the total leg score. The QMT in normal children increases with age but often plateaus or decreases with age in patients with DMD, so we partially corrected for this by indexing the QMT to age.
Statistical Analysis
Demographic variables were compared using either a Wilcoxon rank-sum (continuous variables) or a Chi-square or Fisher’s exact test (categorical variables). Correlations between continuous variables were evaluated using Spearman’s rho. A Wilcoxon Rank Sum was used to evaluate the difference in continuous variables between two groups and a Kruskal Wallis was used to compare more than two groups.
LV dysfunction was defined as left ventricular ejection fraction (LVEF) < 55%. As the analyses were non-parametric, biomarker levels that were below (or above) detection levels were assumed to be tied and lower (or higher) than all values in the detectable range.
Analyses were performed using IBM SPSS statistics, version 24.0 (Armonk, NY: IBM Corp) and R studio 3.4.3 (online at http://www.rstudio.com/). Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Vanderbilt.[21]
RESULTS
Demographics
Forty-two DMD and 15 controls were enrolled. There was no significant difference in age between DMD and control (Table 1). As expected, DMD subjects were shorter and weighed less than controls.
Table 1:
Demographics
| Duchenne Muscular Dystrophy (N=42) |
Control (N=15) |
P-value | |
|---|---|---|---|
| Age (years) | 12.8 IQR(10.5,16.3) (range 8–27) |
14 (13,15) (range 8–17) |
0.663 |
| Height (cm) | 147 (131,159) | 168 (157,175) | 0.001 |
| Weight (kg) | 51 (36,59) | 63.5 (55,83) | 0.006 |
| Male gender | 100% | 100% | |
| Race | 0.178* | ||
| Caucasian | 88% (N=37) | 73% (N=11) | |
| African American | 5% (N=2) | 27% (N=4) | |
| Asian | 2% (N=1) | 0 | |
| Mixed | 5% (N=2) | 0 | |
| Ethnicity | 0.927 | ||
| Hispanic | 14% (N=6) | 13% (N=2) | |
| Current Medications | |||
| Glucocorticoids | 57% (N=24) | ||
| ACEi | 64% (N=27) | ||
| ARB | 12% (N=5) | ||
| Beta-Blocker | 41% (N=17) | ||
| Aldosterone inhibitor | 2% (N=1) | ||
| Ambulatory | 16% (N=9) |
Statistical analysis of race categories performed as a comparison of Caucasian vs Non-Caucasian.
DMD Cardiac Imaging
DMD subjects had a median (IQR) LVEF of 57% (47, 59) and an RVEF of 57% (52,61). Eighteen subjects (43%) had abnormal function, defined as an LVEF < 55%. Of 41 patients administered gadolinium contrast, 28 (68%) had at least one segment with LGE. The median global severity score was 2 (0,3), with 5 subjects having a global severity score of 4 (most severe). Only 3 subjects had symptoms of heart failure, which is not unexpected given the decreased activity level of boys with DMD. Three subjects had a documented arrhythmia prior to enrollment in the study, 2 with supraventricular tachycardia and 1 with ventricular tachycardia.
DMD Medications
A total of 24 DMD subjects (57%) were taking corticosteroids at the time of the study (Table 1). Additional medications included: 27 (64%) taking angiotensin converting enzyme inhibitors (ACEi), 5 (12%) taking angiotensin receptor blockers (ARB), 17 (41%) taking beta-blockers (BB), and 1 (2%) taking an aldosterone inhibitor.
Skeletal Muscle Strength Assessment
Only 9 patients were ambulatory at enrolment. The median arm QMT was 22.3 pounds IQR [11.3, 36.8], leg QMT was 40.0 pounds [24.4, 66.4], and total QMT was 64.5 pounds [40.8, 100.5]. As expected, indexed arm, leg, and total QMT were higher in ambulatory compared to non-ambulatory patients (3.5 [2.6, 4.2] vs 1.5 [0.5, 2.1] p=0.002, 7.3 [3.4, 8.3] vs 2.5 [1.4, 4.4] p=0.005, and 11.4 [6.0, 12.3] vs 4.0 [2.2, 6.8] p=0.004, respectively). Patients currently taking steroids had higher indexed arm, leg, and total QMT (2.4 [1.8, 3.7] vs 0.8 [0.3, 1.6], 5.0 [2.7, 7.7] vs 2.2 [0.7, 2.6], and 7.0 [4.9, 11.7] vs 2.8 [1.0, 4.3], p<0.001 for all), though this effect is likely at least partially related to patient age as patients not taking corticosteroids were significantly older (11 years [10, 12] vs 16 years [14, 20], p<0.001.
Biomarkers in DMD vs. Control
MMP1, MMP7, and MMP10 were elevated in DMD compared with control (median 5080 pg/ml [2890, 7900] vs. 2120 pg/ml [1470, 3380],p=0.007; 2170 pg/ml [1630, 4700] vs. 1420 pg/ml [862, 1630], p<0.001; and 216 pg/ml [120, 489] vs. 140 pg/ml [55.2, 170], p=0.040) (Table 2). TIMP4 was lowered compared with control (124 pg/ml [6.44, 335] vs. 263 pg/ml [87.2, 426], p=0.046).
Table 2:
Difference between matrix metalloproteinase (MMP) levels in Duchenne muscular dystrophy (DMD) and Control
| DMD (N=42) Median (IQR) |
Control (N=15) Median (IQR) |
P-value | |
|---|---|---|---|
| MMP1 (pg/ml) | 5080 (2890, 7900) | 2120 (1470, 3380) | 0.007 |
| MMP3 (pg/ml) | 8850 (2050, 37400) | 5090 (1930, 7410) | 0.071 |
| MMP7 (pg/ml) | 2170 (1640, 4700) | 1420 (862, 1630) | <0.001 |
| MMP9 (ng/ml) | 78.2 (46.9, 140) | 79.0 (37.8, 98.5) | 0.270 |
| MMP10 (pg/ml) | 216 (120, 289) | 140 (55.2, 170) | 0.040 |
| TIMP1 (pg/ml) | 94.5 (76.4, 131) | 95.8 (68.8, 126) | 0.752 |
| TIMP2 (pg/ml) | 45.5 (37.9, 57.0) | 45.2 (35.9, 55.6) | 0.834 |
| TIMP3 (pg/ml) | 771 (293, 1420) | 1010 (263, 2790) | 0.235 |
| TIMP4 (pg/ml) | 124 (6.44, 335) | 263 (87.2, 426) | 0.046 |
| MMP1/TIMP1 | 37.1 (22.9, 95.6) | 32.7 (18.8, 37.7) | 0.155 |
Biomarkers and DMD Skeletal Muscle Strength
MMP7 correlated inversely with total indexed QMT (rho=−0.43 p=0.010, respectively) (Table 3). MMP1 and the MMP1/TIMP1 ratio also correlated inversely with total indexed QMT (rho=−0.44, p=0.016 and rho=−0.55, p=0.002) while TIMP2 correlated directly with total indexed QMT (rho=0.44, p=0.009). Results for indexed arm and leg QMT and absolute (non-indexed) QMT were similar except that MMP1 did not correlate with total absolute QMT. MMP7 was significantly higher in non-ambulatory subjects (2420pg/ml [1710,4910] vs 1410pg/ml [860,1620], p=0.007) but there were no other significant differences. A subset analysis in the 9 subjects who were ambulatory revealed only a correlation between TIMP4 and arm QMT (rho=0.73, p=0.039), likely due to the small sample size.
Table 3:
Correlations between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) and markers of cardiovascular disease and skeletal muscle strength
| LVEF Rho p-value |
Global LGE Rho p-value |
FWHM Rho p-value |
εcc
Rho p-value |
QMT arm Rho p-value |
QMT leg Rho p-value |
Total QMT Rho p-value |
|
|---|---|---|---|---|---|---|---|
| MMP1 (pg/ml) | −0.3 0.09 |
0.36 0.043 |
0.34 0.060 |
0.13 0.495 |
−0.26 0.168 |
−0.35 0.056 |
−0.32 0.09 |
| MMP3 (pg/ml) | −0.07 0.672 |
−0.01 0.944 |
0.21 0.179 |
0.16 0.329 |
0.2 0.229 |
0.32 0.048 |
0.28 0.089 |
| MMP7 (pg/ml) | −0.4 0.012 |
0.47 0.003 |
0.44 0.006 |
0.54 0.001 |
−0.39 0.019 |
−0.36 0.036 |
−0.38 0.024 |
| MMP9 (ng/ml) | 0.02 0.913 |
0.03 0.856 |
0.13 0.448 |
−0.08 0.626 |
−0.11 0.529 |
−0.07 0.705 |
−0.09 0.597 |
| MMP10 (pg/ml) | 0.17 0.281 |
−0.26 0.114 |
−0.20 0.220 |
0.03 0.845 |
−0.39 0.02 |
−0.35 0.034 |
−0.37 0.027 |
| TIMP1 (pg/ml) | 0.24 0.146 |
−0.07 0.669 |
0.12 0.49 |
0.13 0.451 |
0.05 0.78 |
0.16 0.349 |
0.13 0.425 |
| TIMP2 (pg/ml) | 0.06 0.709 |
−0.29 0.078 |
−0.32 0.855 |
−0.13 0.447 |
0.35 0.037 |
0.49 0.003 |
0.45 0.006 |
| TIMP3 (pg/ml) | −.07 0.640 |
0.07 0.644 |
0.10 0.53 |
0.06 0.724 |
0.09 0.58 |
0.01 0.951 |
0.03 0.839 |
| TIMP4 (pg/ml) | −0.12 0.450 |
0.35 0.026 |
0.28 0.076 |
0.26 0.099 |
−0.22 0.177 |
−0.3 0.068 |
−0.26 0.11 |
| MMP1/TIMP1 | −0.39 0.025 |
0.33 0.068 |
0.21 0.244 |
0.039 0.827 |
−0.38 0.038 |
−0.48 0.007 |
−0.45 0.012 |
LVEF – left ventricular ejection fraction
LGE – late gadolinium enhancement
FWHM – percent LGE measured using full width half maximum
εcc – circumferential myocardial strain
QMT – quantitative muscle testing
Biomarkers and Current Medications
MMP1, TIMP4, and the MMP1/TIMP1 ratio were lowered in patients presently on corticosteroids (3560 pg/ml [2210, 7420] vs. 7530 pg/ml [4190, 12800] p=0.017; 13.7 pg/ml [6.44, 165] vs. 285 pg/ml [107, 667] p=0.004; 31.5 [18.0, 55.1] vs. 113 [36.2, 162] p=0.007); there was a trend towards lower MMP7 in patients on corticosteroids (p=0.066). MMP3 and TIMP2 were greater in patients presently on corticosteroids (29600 pg/ml [3630, 57900] vs. 6200 pg/ml [1750, 10600] p=0.031 and 51.0 pg/ml [45.3, 62.1] vs. 42.2 [35.0, 49.5] p=0.022). There were no significant differences in MMPs or TIMPs in patients taking ACEi, ARB, BB, or aldosterone inhibitors.
Biomarkers and DMD Cardiovascular Disease
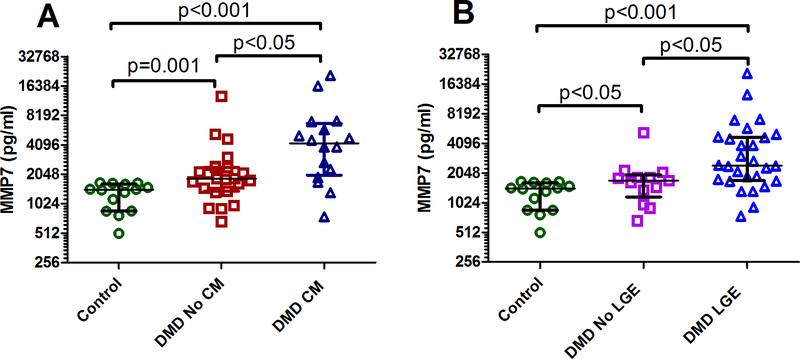
A comparison between DMD without CM, DMD with CM, and control demonstrated a significant difference between groups for MMP1 (p=0.003), MMP7 (p<0.001), TIMP4 (p=0.012), and MMP/TIMP1 (p=0.026) (Table 4). When considering the relationship with cardiomyopathy, the most notable biomarker was MMP7. DMD subjects with abnormal function (defined as LVEF < 55%) had elevated MMP7 compared to those with normal function and controls (Table 3, Figure 1A). DMD subjects with LGE had higher MMP7 compared with those without LGE and compared with controls (p<0.001 for Kruskal Wallis, Figure 1B demonstrates individual comparisons); DMD subjects without LGE also had higher MMP7 compared with controls (Figure 1).
Table 4:
Difference between MMP levels in Duchenne muscular dystrophy (DMD) patients with and without left ventricular (LV) dysfunction and controls
| A | B | C | |||||
|---|---|---|---|---|---|---|---|
| Control (N=15) Median (IQR) |
DMD without LV dysfunction (N=24) Median (IQR) |
DMD with LV dysfunction (N=18) Median (IQR) |
P-value Kruskal Wallis |
P-value A vs B |
P-value A vs C |
P-value B vs C |
|
| MMP1 (pg/ml) | 2120 (1470, 3380) | 3960 (2350, 7420) | 7530 (4430, 12400) | 0.003 | 0.076 | 0.001 | 0.041 |
| MMP3 (pg/ml) | 5090 (1930, 7400) | 14790 (1840, 57900) | 8850 (5070, 13000) | 0.194 | |||
| MMP7 (pg/ml) | 1420 (860, 1630) | 1850 (1370, 2420) | 4240 (2000, 6760) | <0.001 | 0.004 | <0.001 | 0.009 |
| MMP9 (ng/ml) | 79.0 (37.8, 98.5) | 73.2 (43.0, 141) | 100 (48.2, 140) | 0.498 | |||
| MMP10 (pg/ml) | 140 (55.2, 170) | 219 (149, 289) | 159 (111, 319) | 0.071 | |||
| TIMP1 (pg/ml) | 95.8 (68.8, 126) | 100 (74.7, 131) | 89.2 (74.7, 145) | 0.855 | |||
| TIMP2 (pg/ml) | 45.2 (35.9, 55.6) | 51.0 (41.9, 57.4) | 44.0 (35.0, 54.8) | 0.373 | |||
| TIMP3 (pg/ml) | 1010 (263, 2790) | 515 (179, 1410) | 870 (552, 1470) | 0.348 | |||
| TIMP4 (pg/ml) | 263 (87.2, 426) | 13.7 (6.44, 247) | 208 (29.8, 667) | 0.012 | 0.004 | 0.664 | 0.034 |
| MMP1/TIMP1 | 32.7 (18.8, 37.7) | 33.1 (17.9, 66.2) | 59.3 (35.0, 162) | 0.026 | 0.741 | 0.010 | 0.030 |
Figure 1:
(A) Comparison of matrix metalloproteinase (MMP)7 levels in controls (n=14), Duchenne muscular dystrophy (DMD) patients without cardiomyopathy (CM) (n=23), and DMD patients with CM (n=16). (B) Comparison of MMP7 levels in controls (n=14), DMD patients without late gadolinium enhancement (LGE) (n=13), and DMD patients with LGE (n=25). Overall p<0.001. P-values for individual comparisons shown on figure. MMP7 plotted on a Log2 scale. The horizontal lines (from top to bottom) represent the upper quartile, median and lower quartile.
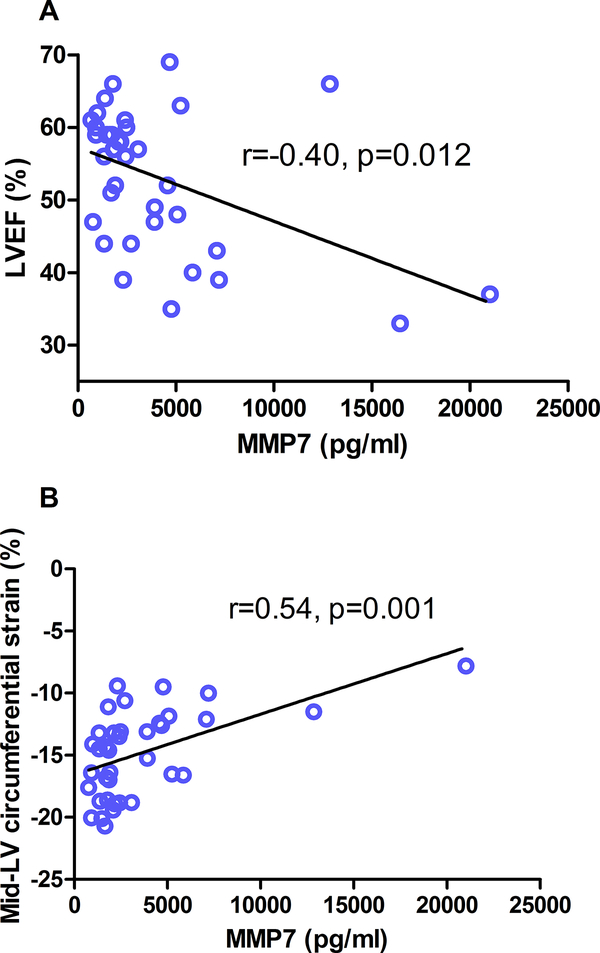
Within DMD, MMP7 levels correlated inversely with LVEF (Figure 2A, rho=−0.40, p=0.012), and directly with LGE global severity score and FWHM (rho=0.47, p=0.003, and rho=0.44, p=0.006) (Table 3). MMP7 also correlated with εcc in the mid-LV slice (rho=0.54, p=0.001) (Figure 2B). MMP7 correlated with indexed LV end systolic volumes (rho=0.38, p=0.012) and had a trend for correlation with LV end diastolic volumes (rho=0.28, p=0.07).
Figure 2:
(A) Correlation between matrix metalloproteinase (MMP)7 and left ventricular ejection fraction (LVEF) (n=39) and (B) circumferential strain at the level of the papillary muscles (n=37).
MMP1 and the MMP1/TIMP1 ratio were greater in DMD patients with abnormal LVEF compared to those with normal function (3960 pg/ml [2350, 7420] vs. 7530 pg/ml [4430, 12400] p=0.041, 33.1 [18.0, 66.2] vs. 59.3 [35.0, 162] p=0.034). TIMP4 was elevated in DMD subjects with abnormal function and with LGE compared to those with normal function and without LGE (13.7 pg/ml [6.44, 247] vs. 208 pg/ml [29.8, 667] p=0.034 and 174 pg/ml [6.44, 381] vs. 6.44 pg/ml [6.44, 184], p=0.047). TIMP4 correlated with LGE global severity and FWHM (rho=0.35, p=0.026).
Inflammatory markers
Given that the role of MMPs in regulating inflammation, TNFa and IL1-b were also analyzed. TNFa and IL1-b were not increased significantly in DMD compared with control. Levels of TNFa and IL1-b did not correlate with any of the MMPs or TIMPs analyzed. Moreover, TNFa and IL1-b did not correlate with QMT or measures of ventricular function or myocardial fibrosis.
Sensitivity Analysis
When median values were substituted for levels that were removed for increased duplicate %CV, MMP1 and MMP1/TIMP1 ratio were no longer significantly elevated in DMD patients with abnormal LVEF, though MMP1 remained elevated in DMD vs. control. The remainder of the analyses were unchanged.
DISCUSSION
Our data demonstrate significant differences in MMPs and TIMPS between DMD and controls. In addition, multiple MMPs, MMP7 in particular, correlate with cardiovascular disease severity in DMD. These findings are important as they suggest abnormalities in extracellular matrix remodeling as an underlying cause of DMD cardiovascular disease progression. Given the dearth of DMD cardiovascular biomarkers, MMP7 has potential as a biomarker of cardiovascular disease severity.
While standard biomarkers do not accurately reflect severity of DMD cardiovascular disease, recent investigations suggest the potential of interleukin 1 receptor-like 1 (ST2), brain derived neurotrophic factor, and osteopontin to serve as DMD cardiovascular biomarkers.[22, 23] Our data suggest that MMPs, particularly MMP7, could play a role in assessing severity of disease. Whether these laboratory values can predict disease progression or mortality will require further study. It also must be noted that MMP7 correlates with QMT, suggesting that it is related to both cardiac and skeletal muscle fibrosis. This may limit cardiac specificity. In addition, it is likely that these correlations reflect the generalized inflammatory response known to occur in DMD, though this would not diminish the significance of the correlation with cardiovascular disease severity, and could provide a basis for assessment of therapeutics for both cardiac and skeletal muscle disease. Of note, the lack of correlation with TNFa and IL1-b decreases the likelihood that MMPs are acting through inflammatory pathways but does not eliminate this possibility as there are multiple other molecules through which MMPs could be signaling. A subset analysis in the 18 subjects not taking corticosteroids only demonstrated a significant correlation between MMP7 and LVEF (rho=−0.63, p=0.005) but not measures of fibrosis. In patients taking corticosteroids, there were no significant correlations between MMP7 and LVEF or measures of fibrosis. Though these data could suggest the hypothesis that corticosteroids are acting through modulation of MMP pathways, this analysis was underpowered and further evaluation in a larger cohort is warranted before mechanistic studies are conducted.
MMPs and TIMPs regulate remodeling of the extracellular matrix and inflammatory response.[24–26] MMP7 is localized primarily in endothelial cells and vascular smooth muscle cells. There are limited reports evaluating MMP7 in DMD patients or in patients with cardiovascular disease. A previous study demonstrated elevated circulating MMP7 in DMD and in vitro studies demonstrate elevated MMP7 in DMD fibroblasts.[27, 28] Animal studies demonstrate improved survival after MI in mice with MMP7 gene deletion.[29] MMP7 levels also predict progression of lung disease in patients with idiopathic pulmonary fibrosis, suggesting the importance of MMP7 in multiple fibrotic disease processes.[30]
MMP1 levels are elevated in the setting of inflammation.[25] Given that inflammation is felt to be a major component of DMD progression, it is not surprising that we found elevated MMP1 compared with control. Interestingly, previous in vitro data suggest downregulation of MMP1 in DMD fibroblasts.[27] We suspect this discrepancy is secondary to our measuring circulating MMP levels, not fibroblast expression. Indeed, other studies have suggested a downregulation of MMP1 at the tissue level but an upregulation in circulating MMP1.[24] Studies in adults with hypertension demonstrate elevated MMP1 associated with LV dilatation and dysfunction.[31] Adults with dilated cardiomyopathy also have higher MMP1, as well as higher TIMP1, and MMP1/TIMP1 ratio, suggesting the important role of collagen homeostasis.[32]
Previous studies have demonstrated elevated MMP9 in DMD compared with controls and a progressive increase in MMP9 as DMD patients age.[22, 33] It is notable that our data demonstrated nearly identical median values (Table 2) but a trend to elevated mean MMP9 in DMD that did not reach significance (104ng/ml +/− 70 vs. 74ng/ml +/− 41, p=0.07). The MMP9 discrepancy may be partially explained by differences in cohorts, including age and severity of skeletal muscle or cardiovascular disease, or differences in assays. Of note, ours is not the only study that has failed to detect a difference in MMP9 between DMD and control.[34]
Determining factors involved in the pathogenesis of DMD cardiomyopathy may help explain the mechanisms of current medications and help develop novel therapeutics. Our data suggest that corticosteroids modulate MMPs and TIMPS. Corticosteroids are one of the few therapies to benefit the cardiac and skeletal myopathy in DMD, but their mechanism of action remains unclear.[35–37] It is possible that this modulation of MMPs and TIMPs is one of the reasons for corticosteroid efficacy. MMP inhibitors have also been studied for multiple disease processes, primarily oncologic. Batimistat inhibition of MMPs improved pathologic changes in skeletal muscle of mdx mice.[38] Nonspecific inhibition using tetracyclines has also demonstrated promise in animal models.[39] However, most human trials of MMP inhibitors to this point have been unsuccessful due to poor efficacy and side effects.[28, 40] Newer and more specific medications hold promise for future studies.[41] Whether DMD patients would benefit from MMP inhibition, and which MMP should be inhibited, is unclear but these data suggest further study should be undertaken.
Limitations
Medication therapy may have modified the difference between DMD and control. Unfortunately, given the early adoption of medication therapy by families of boys with DMD, it would be extremely difficult to enroll medication naïve patients with a broad range of cardiovascular disease severity. Given the sample size limitations, we did not adjust for current or previous medications, though our data suggest that only corticosteroids led to a difference in levels. Except for MMP3 and TIMP2, most MMP/TIMP levels were lowered in DMD patients on corticosteroids, suggesting that therapy would decrease the difference between DMD subjects and controls. Aldosterone inhibitors are the most likely additional medication to modulate fibrosis, but only 5 patients were taking aldosterone inhibitors, making assessment of the effect of aldosterone inhibition on biomarker levels difficult. The effects of medications should be addressed in future, multi-center studies.
These data evaluate the relationship of biomarkers to cardiac and skeletal muscle function at one point in time. While these results are an important first step in identifying biomarkers for use in DMD, longitudinal analysis and evaluation of biomarkers in relation to future outcomes will be important before adopting these biomarkers in routine clinical practice. Due to the smaller sample size, multivariable analysis could not be performed. Some samples were eliminated from analysis due to suboptimal coefficients of variability, despite multiple repeat assays. This suggests that, in addition to research in a larger sample size, further optimization of the assay is necessary. Although there were multiple analyses performed in this manuscript, all of the analyses were pre-specified. However, some of the significant results may be due to a type I error. The conservative Bonferonni correction assumes that hypothesis tests are independent, but we suspect that our tests have varying degrees of correlation. Rather than make a formal adjustment for multiple comparisons, we have included the results of all comparisons in Table 3.
Conclusions
MMPs and TIMPs, particularly MMP7, are related to DMD cardiac dysfunction and myocardial fibrosis, likely through their role in fibrosis and inflammation. MMP7 has potential as a biomarker of cardiovascular disease severity in DMD.
ACKNOWLEDGEMENTS:
Funding: This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K23HL123938 (Bethesda, MD) (Soslow). It was also supported by CTSA award number UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Heart, Lung, and Blood Institute, National Center for Advancing Translational Sciences, or the National Institutes of Health.
This project was supported by the Fighting Duchenne Foundation and the Fight DMD/Jonah & Emory Discovery Grant (Nashville, TN) (Markham).
The sponsors and funders had no role in the design and conduct of the study or in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
ABBREVIATIONS
- DMD
Duchenne muscular dystrophy
- LV
Left ventricular
- BNP
Brain natriuretic peptide
- MMPs
Matrix metalloproteinases
- TIMPs
Tissue inhibitors of metalloproteinases
- LGE
Late gadolinium enhancement
- εcc
Circumferential strain
- QMT
Quantitative muscle testing
- LVEF
Left ventricular ejection fraction
- ACEi
Angiotensin converting enzyme inhibitors
- ARB
Angiotensin receptor blockers
- BB
Beta-blockers
Footnotes
DISCLOSURES: The authors have no conflicts of interest.
REFERENCES:
- [1].Dooley J, Gordon KE, Dodds L, MacSween J. Duchenne muscular dystrophy: a 30-year population-based incidence study. Clin Pediatr (Phila). 2010;49:177–9. [DOI] [PubMed] [Google Scholar]
- [2].Bach JR, Martinez D. Duchenne muscular dystrophy: continuous noninvasive ventilatory support prolongs survival. Respir Care. 2011;56:744–50. [DOI] [PubMed] [Google Scholar]
- [3].Mori K, Manabe T, Nii M, Hayabuchi Y, Kuroda Y, Tatara K. Plasma levels of natriuretic peptide and echocardiographic parameters in patients with Duchenne’s progressive muscular dystrophy. Pediatr Cardiol. 2002;23:160–6. [DOI] [PubMed] [Google Scholar]
- [4].Mohyuddin T, Jacobs IB, Bahler RC. B-type natriuretic peptide and cardiac dysfunction in Duchenne muscular dystrophy. International journal of cardiology. 2007;119:389–91. [DOI] [PubMed] [Google Scholar]
- [5].Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Becane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45:855–7. [DOI] [PubMed] [Google Scholar]
- [6].Duboc D, Meune C, Pierre B, Wahbi K, Eymard B, Toutain A, et al. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow-up. Am Heart J. 2007;154:596–602. [DOI] [PubMed] [Google Scholar]
- [7].Kajimoto H, Ishigaki K, Okumura K, Tomimatsu H, Nakazawa M, Saito K, et al. Beta-blocker therapy for cardiac dysfunction in patients with muscular dystrophy. Circ J. 2006;70:991–4. [DOI] [PubMed] [Google Scholar]
- [8].Raman SV, Hor KN, Mazur W, Halnon NJ, Kissel JT, He X, et al. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial. Lancet neurology. 2015;14:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hor KN, Mazur W, Taylor MD, Al-Khalidi HR, Cripe LH, Jefferies JL, et al. Effects of steroids and angiotensin converting enzyme inhibition on circumferential strain in boys with Duchenne muscular dystrophy: a cross-sectional and longitudinal study utilizing cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance. 2011;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Connuck DM, Sleeper LA, Colan SD, Cox GF, Towbin JA, Lowe AM, et al. Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: a comparative study from the Pediatric Cardiomyopathy Registry. Am Heart J. 2008;155:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Frankel KA, Rosser RJ. The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol. 1976;7:375–86. [DOI] [PubMed] [Google Scholar]
- [12].Soslow JH, Damon SM, Crum K, Lawson MA, Slaughter JC, Xu M, et al. Increased myocardial native T1 and extracellular volume in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson. 2016;18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Passino C, Barison A, Vergaro G, Gabutti A, Borrelli C, Emdin M, et al. Markers of fibrosis, inflammation, and remodeling pathways in heart failure. Clin Chim Acta. 2015;443:29–38. [DOI] [PubMed] [Google Scholar]
- [14].Simpson SA, Field SL, Xu M, Saville BR, Parra DA, Soslow JH. Effect of Weight Extremes on Ventricular Volumes and Myocardial Strain in Repaired Tetralogy of Fallot as Measured by CMR. Pediatr Cardiol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. [DOI] [PubMed] [Google Scholar]
- [16].Menon SC, Etheridge SP, Liesemer KN, Williams RV, Bardsley T, Heywood MC, et al. Predictive value of myocardial delayed enhancement in Duchenne muscular dystrophy. Pediatr Cardiol. 2014;35:1279–85. [DOI] [PubMed] [Google Scholar]
- [17].Mathur S, Lott DJ, Senesac C, Germain SA, Vohra RS, Sweeney HL, et al. Age-related differences in lower-limb muscle cross-sectional area and torque production in boys with Duchenne muscular dystrophy. Arch Phys Med Rehabil. 2010;91:1051–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lerario A, Bonfiglio S, Sormani M, Tettamanti A, Marktel S, Napolitano S, et al. Quantitative muscle strength assessment in duchenne muscular dystrophy: longitudinal study and correlation with functional measures. BMC Neurol. 2012;12:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Connolly AM, Malkus EC, Mendell JR, Flanigan KM, Miller JP, Schierbecker JR, et al. Outcome reliability in non-ambulatory boys/men with Duchenne muscular dystrophy. Muscle Nerve. 2015;51:522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Posner ADS JH; Burnette WB; Bian A; Shintani A; Sawyer DB; Markham LW The Correlation of Skeletal and Cardiac Muscle Dysfunction in Duchenne Muscular Dystrophy. Journal of Neuromuscular Diseases. 2016;3:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Anderson J, Seol H, Gordish-Dressman H, Hathout Y, Spurney CF, Investigators C. Interleukin 1 Receptor-Like 1 Protein (ST2) is a Potential Biomarker for Cardiomyopathy in Duchenne Muscular Dystrophy. Pediatr Cardiol. 2017;38:1606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Galindo CL, Soslow JH, Brinkmeyer-Langford CL, Gupte M, Smith HM, Sengsayadeth S, et al. Translating golden retriever muscular dystrophy microarray findings to novel biomarkers for cardiac/skeletal muscle function in Duchenne muscular dystrophy. Pediatr Res. 2016;79:629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].de Jong S, van Veen TA, de Bakker JM, Vos MA, van Rijen HV. Biomarkers of myocardial fibrosis. J Cardiovasc Pharmacol. 2011;57:522–35. [DOI] [PubMed] [Google Scholar]
- [25].Mittal R, Patel AP, Debs LH, Nguyen D, Patel K, Grati M, et al. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J Cell Physiol. 2016;231:2599–621. [DOI] [PubMed] [Google Scholar]
- [26].Masciantonio MG, Lee CKS, Arpino V, Mehta S, Gill SE. The Balance Between Metalloproteinases and TIMPs: Critical Regulator of Microvascular Endothelial Cell Function in Health and Disease. Prog Mol Biol Transl Sci. 2017;147:101–31. [DOI] [PubMed] [Google Scholar]
- [27].Zanotti S, Gibertini S, Mora M. Altered production of extra-cellular matrix components by muscle-derived Duchenne muscular dystrophy fibroblasts before and after TGF-beta1 treatment. Cell Tissue Res. 2010;339:397–410. [DOI] [PubMed] [Google Scholar]
- [28].Ogura Y, Tajrishi MM, Sato S, Hindi SM, Kumar A. Therapeutic potential of matrix metalloproteinases in Duchenne muscular dystrophy. Front Cell Dev Biol. 2014;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, et al. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation. 2006;113:2919–28. [DOI] [PubMed] [Google Scholar]
- [30].Bauer Y, White ES, de Bernard S, Cornelisse P, Leconte I, Morganti A, et al. MMP-7 is a predictive biomarker of disease progression in patients with idiopathic pulmonary fibrosis. ERJ Open Res. 2017;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lopez B, Gonzalez A, Querejeta R, Larman M, Diez J. Alterations in the pattern of collagen deposition may contribute to the deterioration of systolic function in hypertensive patients with heart failure. J Am Coll Cardiol. 2006;48:89–96. [DOI] [PubMed] [Google Scholar]
- [32].Schwartzkopff B, Fassbach M, Pelzer B, Brehm M, Strauer BE. Elevated serum markers of collagen degradation in patients with mild to moderate dilated cardiomyopathy. Eur J Heart Fail. 2002;4:439–4. [DOI] [PubMed] [Google Scholar]
- [33].Nadarajah VD, van Putten M, Chaouch A, Garrood P, Straub V, Lochmuller H, et al. Serum matrix metalloproteinase-9 (MMP-9) as a biomarker for monitoring disease progression in Duchenne muscular dystrophy (DMD). Neuromuscul Disord. 2011;21:569–78. [DOI] [PubMed] [Google Scholar]
- [34].Zocevic A, Rouillon J, Wong B, Servais L, Voit T, Svinartchouk F. Evaluation of the serum matrix metalloproteinase-9 as a biomarker for monitoring disease progression in Duchenne muscular dystrophy. Neuromuscul Disord. 2015;25:444–6. [DOI] [PubMed] [Google Scholar]
- [35].Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, et al. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. The New England journal of medicine. 1989;320:1592–7. [DOI] [PubMed] [Google Scholar]
- [36].Schram G, Fournier A, Leduc H, Dahdah N, Therien J, Vanasse M, et al. All-cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. J Am Coll Cardiol. 2013;61:948–54. [DOI] [PubMed] [Google Scholar]
- [37].Markham LW, Kinnett K, Wong BL, Woodrow Benson D, Cripe LH. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord. 2008;18:365–70. [DOI] [PubMed] [Google Scholar]
- [38].Kumar A, Bhatnagar S, Kumar A. Matrix metalloproteinase inhibitor batimastat alleviates pathology and improves skeletal muscle function in dystrophin-deficient mdx mice. Am J Pathol. 2010;177:248–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pereira JA, Taniguti AP, Matsumura C, Marques MJ, Neto HS. Doxycycline ameliorates the dystrophic phenotype of skeletal and cardiac muscles in mdx mice. Muscle Nerve. 2012;46:400–6. [DOI] [PubMed] [Google Scholar]
- [40].Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov. 2014;13:904–27. [DOI] [PubMed] [Google Scholar]
- [41].Gossage DL, Cieslarova B, Ap S, Zheng H, Xin Y, Lal P, et al. Phase 1b Study of the Safety, Pharmacokinetics, and Disease-Related Outcomes of the Matrix Metalloproteinase-9 Inhibitor Andecaliximab in Patients With Rheumatoid Arthritis. Clin Ther. 2017. [DOI] [PubMed] [Google Scholar]