Abstract
The blood-brain barrier (BBB) serves as a gateway for passage of drugs, chemicals, nutrients, metabolites and hormones between vascular and neural compartments in the brain. Here, we review BBB development with regard to the microphysiology of the neurovascular unit (NVU) and the impact of BBB disruption on brain development. Our focus is on modeling these complex systems. Extant in silico models are available as tools to predict the probability of drug/chemical passage across the BBB; in vitro platforms for high-throughput screening and high-content imaging provide novel data streams for profiling chemical-biological interactions; and engineered human cell-based microphysiological systems provide empirical models with which to investigate the dynamics of NVU function. Computational models are needed that bring together kinetic and dynamic aspects of NVU function across gestation and under various physiological and toxicological scenarios. This integration will inform adverse outcome pathways to reduce uncertainty in translating in vitro data and in silico models for use in risk assessments that aim to protect neurodevelopmental health.
Keywords: predictive toxicology, children’s environmental health, neurovascular development, blood-brain barrier
1. Introduction
Specialized endothelial cells with unique barrier characteristics distinguish the brain microvasculature, from capillaries in other parts of the body based on the presence of tight junctions and molecular transporters, coupled with the absence of fenestrations (Obermeier et al., 2013). Together, these specializations serve to maintain a selective blood-brain barrier (BBB) that prevents the influx of many hydrophilic substances, moderates the efflux of xenobiotics from the neural compartment, and readily accommodates appropriate nutrient and hormone influx into the brain (Abbott et al., 2010). Additional BBB functions include nutrient metabolism (e.g., glycolysis) (Pardridge, 1983) and xenobiotic metabolism (Shawahna et al., 2011). At least five cell types within the neurovascular unit (NVU) participate in BBB formation: endothelial cells, pericytes, astrocytes, neurons, and microglia (Figure 1A). However, despite an abundant body of research on adult BBB functionality and effectiveness (Abbott, 2013; Pardridge, 2012; Scherrmann, 2002), the developing BBB remains understudied, and is often considered functionally immature. As pointed out by (Ek et al., 2012), this mischaracterization derives partly because physical barrier characteristics (i.e., molecular influx) are commonly-used indicators of BBB integrity, whereas physiological properties such as metabolism and efflux are potentially overlooked (Ek et al., 2012). A more complete understanding of BBB development would advance scientific understanding of developmental neurotoxicity (DNT) at a systems biology level, providing information on the neurodevelopmental impact of xenobiotic exposure (Aschner et al., 2017; Bal-Price et al., 2015; Corada et al., 2010; Grandjean and Landrigan, 2014; Mundy et al., 2015; Tsuji and Crofton, 2012).
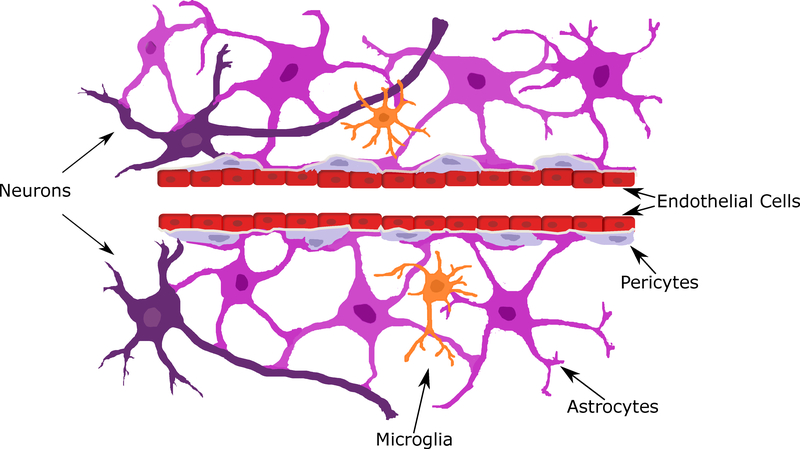
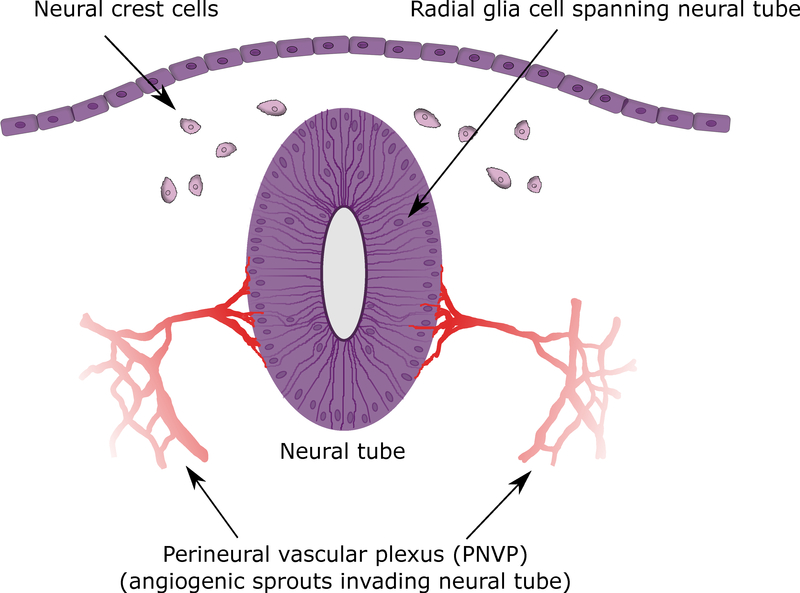
Figure 1: Neurovascular unit (NVU).
A) NVU showing endothelial cells (red), pericytes (light purple), astrocytes (bright purple), neurons (dark purple), and microglia (orange); color coded to match Figure 3. B) Neural tube, perineural vascular plexus (PNVP), neural crest, and neural ectoderm. Transverse section at mouse Thieler Stage (TS) 16 (E10).
An important area of children’s environmental health research focuses on gene-environment interactions during vulnerable developmental periods (Wright and Christiani, 2010). These interactions may take place at any location in the brain, including the BBB, whereby chemical-induced disruptions of molecular events could lead to DNT. While reference lists of known developmental neurotoxicants have been established (Aschner et al., 2017; Kadereit et al., 2012; Mundy et al., 2015), the role of the BBB in mediating DNT effects has not been adequately explored. Two broad routes by which developmental BBB disruption could lead to DNT are: 1) toxicokinetics (what the BBB does to a chemical, which may include transport and/or bioactivation to a more toxic metabolite); and 2) toxicodynamics (what the chemical does to the BBB (e.g., altering transport of key nutrients or promoting neuroinflammation). Hypothetically, both processes could also exacerbate a chemical’s toxic effects. For example, the BBB could bioactivate a parent compound to a form that causes neuroinflammation and loss of BBB integrity, thereby allowing more of the toxicant (or co-occurring DNT compounds) into the brain leading to adverse neurodevelopmental outcomes.
To assess whether environmental exposure to select xenobiotics poses a risk to the developing brain, an often asked question is whether a chemical crosses the BBB to damage neurons (primary effects), leaving another critical question unaddressed: does a chemical impact the BBB (or cell types of the NVU), itself? Viewing the developing BBB as a potential target of xenobiotics is a useful approach for identifying neurotoxicants that may impact brain development and function through primary (e.g., altered transporter function) or secondary effects (e.g., localized inflammation) associated with NVU disruption. Knowledge of the complex cellular networks within the NVU allows us to ask more specific questions about a chemical’s potential DNT, which may be useful in predicting adverse neurobehavioral outcomes (Elsabbagh et al., 2012; Heyer and Meredith, 2017). In order to identify potential developmental neurotoxicants, chemical screening integrated with an adverse outcome pathway (AOP) approach is helpful. AOPs are linear ‘maps’ that include molecular initiating events (MIEs), key events (KEs), and adverse outcomes (AOs) such as DNT that are useful for tracing the molecular targets of putative DNT compounds (Villeneuve et al., 2014a, b). First-tier testing to identify DNT compounds requires an NVU model amenable to rapid, high-throughput screening (HTS).
Although whole animal models have advanced the field of developmental BBB research, in vivo tests are resource and time-consuming and do not align with toxicity testing in the 21st century (NRC, 2007) as a screening approach for identifying the molecular targets of chemicals. Furthermore, alternative testing methods that utilize human cells and in silico methods are in line with the amended Toxic Substances Control Act (TSCA) (i.e., Frank R. Lautenberg Chemical Safety for the 21st Century Act of 2016), which promotes replacing animal use in toxicity testing (Congress, 2016). Applications of human cell-based in vitro models, including complex co-cultures, engineered micro-tissues, and more recently microphysiological systems, provide new ways to study the human NVU at multiple levels of biological organization from molecular to physiological. These models help reveal the underlying complexity of diverse cellular interactions that comprise an integrated system. In order to integrate the findings from these models with meaningful predictions, establishing a foundation of molecular signaling underlying BBB development is warranted.
Here, we address two perspectives of BBB development and DNT: 1) embryological (formation of brain microvasculature through angiogenesis); and 2) teratological (AOPs for developmental disruption). We limit this review to the molecular biology underlying BBB formation and approaches for assessing chemical effects on human BBB development and function. For reviews on BBB formation and maintenance, see (Ek et al., 2012; Engelhardt and Liebner, 2014; Obermeier et al., 2013).
2. BBB formation
BBB development is evolutionarily conserved in chordates (Bundgaard and Abbott, 2008), therefore providing a window into human development through animal models (Figure 2). In mammals, BBB formation and differentiation begins during the early embryonic period, and although it is functional shortly after it is formed (Daneman et al., 2010; Ek et al., 2012; Saunders et al., 2009), mature cell types such as astrocytes and myelinated neurons do not appear until soon after birth (Obermeier et al., 2013) (Figure 3). Embryologically, the BBB originates from the perineural vascular plexus (PNVP) surrounding the neural tube (Figure 1B). Its foundation advances in a multi-step process that is orchestrated by cellular interactions within the developing NVU and closely integrated with the nascent central nervous system (CNS). Here, we focus on the developing BBB beginning with an evolutionary perspective, then dive into the cellular and molecular underpinnings of each stage of BBB formation and maturation. An understanding of this complex biological process informs hypothesis development for BBB-specific chemical disruption.
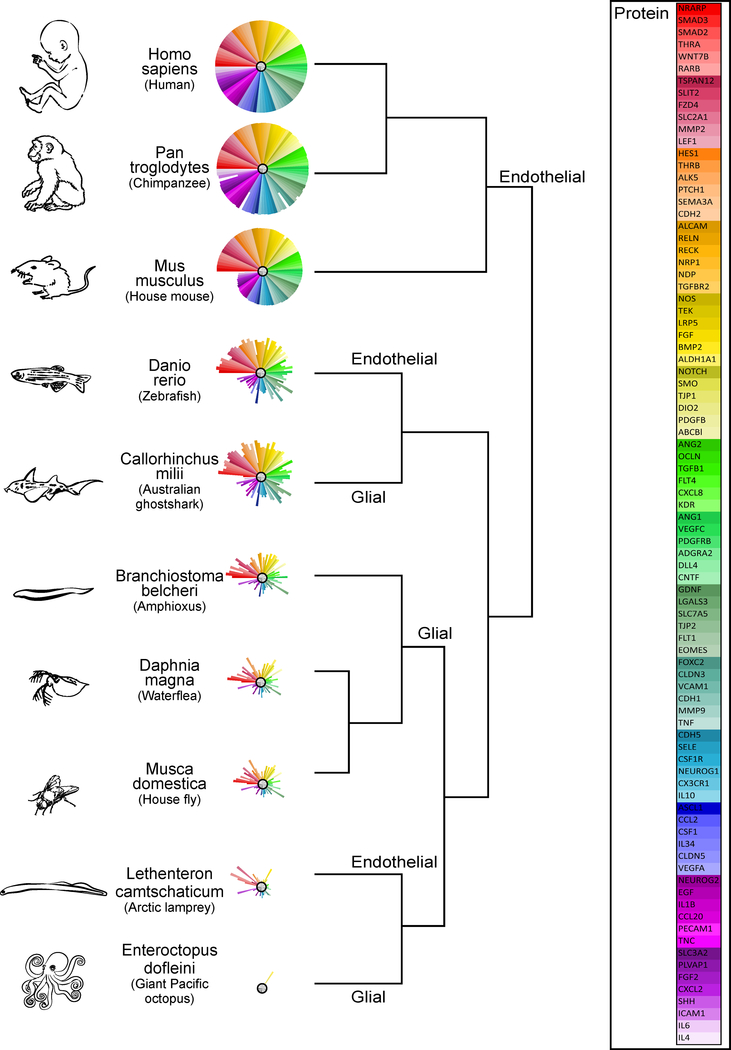
Figure 2: Phylogeny of the BBB.
Primary amino acid sequence similarity for 86 proteins implicated in BBB development (see figure 4) was determined for species representing classes that appear at different times throughout evolutionary history: (Mammalia: Homo sapiens, Pan troglodytes, Mus musculus; Actinopterygii (ray-finned fish): Danio rerio; Chondrichthyes (cartilaginous fish): Callorhinchus milii; Leptocardii (earliest vertebrates): Branchiostoma belcheri; Branchiopoda (crustaceans): Daphnia magna; Insecta: Musca domestica; Hyperoartia (invertebrate chordate): Lethenteron camtschaticum; Cephalopoda: Eteroctopus dofleini). Comparisons to the human protein (100%) were made by an amino acid sequence query using the Basic Local Alignment Search Tool for proteins (BLASTp) of the National Center for Biotechnology Information (NCBI) protein database using SeqAPASS v2.0 software (https://seqapass.epa.gov/seqapass) (LaLone et al., 2016), which facilitates consistent comparisons of amino acid sequence similarity across multiple species. For proteins with multiple isoforms, SeqAPASS best practices were used to select the most representative NCBI protein accession number for the BLAST; and full sequence lengths (rather than functional domains) were used. A beta testing version of ToxPi software v2.0 provided by David Reif (NCSU) was used to produce the sequence similarity ToxPis using the ‘single’ clustering algorithm (Reif et al., 2010). For each protein, the % similarity to the human protein (100%) is represented by the length of pie slice (i.e., shorter pie slices represent lower similarity; or absence of a slice indicates no sequence similarity). Slices are arranged from highest to lowest % similarity in the mouse compared to humans (i.e., red slices have the highest similarity and light purple slices represent the lowest similarity). The type of BBB (glial or endothelial) is indicated on the dendrogram at the node representing a common ancestor with the same type of BBB. The symbols listed in the ‘protein’ key correspond to standard NCBI gene names. NCBI protein database accessed between 6/27/17 and 7/27/17.
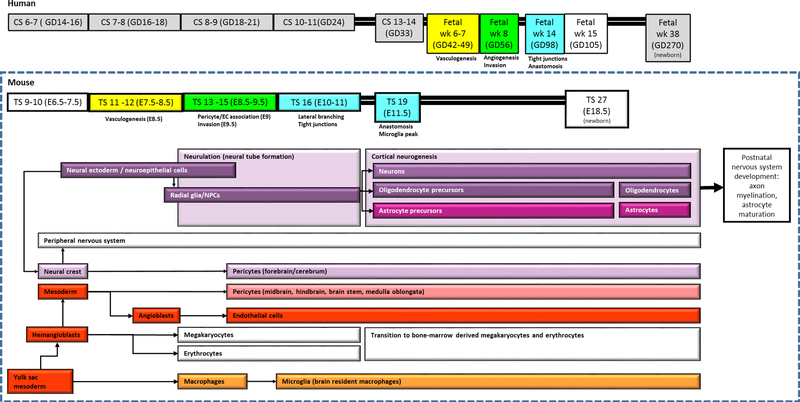
Figure 3: Timeline of NVU development.
The boxed area indicates mouse Thieler Stages (TS) 9 (E6.5) to 27 (newborn). The section above the box roughly indicates the corresponding human Carnegie Stages (CS) and gestational days (GD) beginning at human CS 6–7 (GD14 −16). Neurons, oligodendrocytes, astrocytes, and forebrain pericytes derive from neural ectoderm (purple); while endothelial cells, mid- and hindbrain pericytes, and microglia derive from mesoderm (red). Vasculogenesis begins during mouse TS 12 (E8.5), angiogenesis/invasion occurs at TS 15 (E9.5), lateral branching and tight junctions are present by TS 16 (E10), and anastomosis and microglia peak at TS 19 (E11.5).
2.1. The BBB is evolutionarily conserved
An evolutionary perspective offers several insights into human BBB development: the selective advantage of a barrier system to support increasingly complex CNS development; the stepwise advancement of an increasingly complex NVU architecture; and the nature of signaling systems that coordinate its morphogenesis and differentiation (Figure 2).
The earliest anatomical notion of a structural BBB appears in Cephalopods (e.g., cuttlefish, octopus, squid), the only members of the phylum Mollusca that have a closed circulatory system (Abbott et al., 1992) (Figure 2). While it might be tempting to speculate that a closed circulatory system is the major selective pressure underlying microvascular specialization in the primitive brain, this is not a prerequisite. Insects, for example, lack microvasculature extending into the brain but possess a layer of perineurial and subperineurial glial cells in an extracellular matrix that encapsulates the entire nervous system, forming a physiological barrier with the hemolymph (Hindle and Bainton, 2014). In Drosophila, ectoderm-derived subperineurial glia are the primary contributors to the barrier function of the microvasculature by the time rudimentary organ systems have formed (Hindle and Bainton, 2014). Similarly, ectoderm-derived glial cells likely formed the primitive BBB in an archetypical chordate species, which has been maintained in modern cartilaginous fish (i.e., sharks, skates, and rays; class Chondrichthyes) and vertebrate fish (i.e., sturgeon; class Actinopterygii) (Bundgaard and Abbott, 2008). In sturgeon, for example, multiple layers of glia joined by gap junctions surround the brain microvasculature as a physiological barrier to the passive diffusion of substances ordinarily able to cross endothelial cells, which lack tight junctions (Bundgaard and Abbott, 2008).
Interestingly, the most primitive vertebrates (i.e., lamprey and hagfish; class Agnatha) have endothelial cell tight junctions that form a physiological barrier similar to the BBB found in higher vertebrates. This observation led Bundgaard and Abbott (2008) to propose that a mesoderm-derived BBB provided enough selective advantage to have arisen independently multiple times throughout evolution (convergent evolution) (Bundgaard and Abbott, 2008) resulting in the Vertebrate (with the exception of elasmobranchs and sturgeon) BBB that is characterized by tight junctions between endothelial cells in capillaries feeding the brain (Bundgaard, 1982; Bundgaard and van Deurs, 1982; Fleming et al., 2013). Notably, the appearance of this version of the BBB in Teleosts coincided with evolutionary arrival of T and B lymphoid cells, which may reflect a selective pressure to restrict these potentially harmful cells from accessing the CNS (Zhu et al., 2014). Moreover, this shared biology supports the relevance of using vertebrates including rodents and zebrafish to study the potential effects of chemical exposures on human BBB development. Indeed, these animal models have homologous counterparts for most of the proteins important in BBB development (Figures 2 and 4).
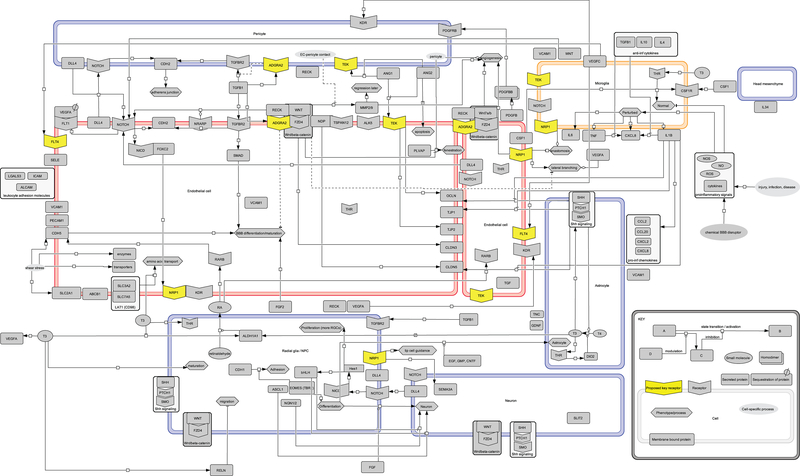
Figure 4: Control network for neurovascular development.
Biowiring diagram with representative signaling pathways, receptors, and ligands that are important for blood-brain barrier (BBB) development. Large rectangles are cells (color-coded to match Figures 1 and 2): stalk and tip endothelial cells (red); pericyte, radial glia/NPC, neuron, astrocyte, head mesenchyme (purple); microglia (orange). Yellow highlights represent key receptors hypothesized to mediate developmental NVU toxicity. Molecule abbreviations are standard human NCBI gene symbols (see Table 1 for names).
2.2. BBB ontogeny begins during neurulation
Vasculogenesis, or de novo vasculature formation from angioblast precursors, sets the stage for BBB development by establishing the PNVP in the head mesenchyme surrounding the neural tube (Hogan et al., 2004). Once a PNVP is established, the distinct process of angiogenesis, blood vessel sprouting from established vessels, is responsible for BBB capillary formation and invasion of the rudimentary brain (Hogan et al., 2004). The nutrient supply provided by these microvessels facilitates brain growth through the proliferation and migration of neuroprogenitor cells (NPCs) in the neural tube.
2.2.1. Vasculogenesis establishes the PNVP
The primitive vasculature derives from yolk sac blood islands, which form on mouse embryonic day 7.5 (E7.5) (Palis et al., 1995) and harbor hemangioblasts (angioblast precursors) and megakaryocytes (Baron et al., 2012) (Figure 3). Blood vessels of the PNVP form by E8.7 in mouse (Duan et al., 2003) when endothelial cell precursors that express VEGFR2 (KDR) are recruited from the adjacent lateral plate and pre-somitic mesoderm via a hypoxia-induced VEGFA gradient generated within the neuroepithelium (Hogan et al., 2004). Notably, the ectoderm-derived VEGFA signal is somewhat unique, as vasculogenesis is induced by endoderm-originated signals in other parts of the body (Goldie et al., 2008; Pardanaud et al., 1989). By E8.5 - E9.5, endothelial cells are present (Palis et al., 1995) and circulation has begun (Baron et al., 2012). Altogether, the developmental period encompassing the very first events of neural cell fate determination concomitant with PNVP formation takes place from E7.5 to E9.5 in the mouse (Baron et al., 2012). Although the corresponding period of human development based on physical landmarks is days 17–21 (GD17–21 (Hill, 2016), the formation of the human PNVP (or PCAP; pial capillary anastomotic plexus) is in place by human gestational weeks 6 to 7 (Marin-Padilla, 2012) (Figure 3).For a more detailed review of vasculogenesis, see (Coultas et al., 2005).
2.2.2. Angiogenesis gives rise to the BBB
Angiogenesis is the process that establishes the BBB as specialized endothelial cells of the newly formed brain microvasculature. This process begins at gestational week 8 in humans (Marin-Padilla, 2012)) and peaks at gestational week 35 in humans (Mito et al., 1991). In contrast, the process begins at E9.75 in mice (Fantin et al., 2013) and peaks shortly after birth in rodents (Robertson et al., 1985). Similar to vasculogenesis, angiogenesis proceeds when a hypoxic microenvironment causes the closing neural tube to express VEGFA in a gradient that induces the growth of capillaries toward the neuroectoderm (Raab et al., 2004).
Consistent with the brain’s anteroposterior patterning, capillary growth and invasion of the nearby neural tissue is directed in a predictable spatial pattern driven by the interplay of several signaling pathways, including Wnt/β-catenin signaling, Notch, and VEGF (Hellstrom et al., 2007; James et al., 2009; Stenman et al., 2008). The locations at which capillaries arise and enter the rudimentary brain are conserved and capillaries invade the hindbrain before colonizing the forebrain (James et al., 2009; Raab et al., 2004). This pattern is consistent with concentrations of growth signals (e.g., Wnt, BMP, FGF), which are generally higher in the hindbrain (Gilbert, 2013). Following hindbrain invasion, growing capillaries ingress at lateral and ventral regions of the neural tube following a VEGF (specifically, heparin binding isoforms VEGF164 or VEGF188) gradient in areas specified by the absence of suppressive signals such as VEGFR1 (FLT1) (James et al., 2009). Dorsal sites are subsequently populated by migrating angioblasts (James et al., 2009). Upon endothelial cell invasion into the developing brain through angiogenesis, the BBB is found to be a functional unit able to selectively transport molecules into the developing brain (Morris et al., 2017). This occurs due to the interaction of all cell types of the NVU. Regardless of the cell types present in the developing neuroepithelium, the initiation of circulation is critical in the mammalian embryo as nascent capillaries formed through angiogenesis deliver oxygen, nutrients, and hormones to allow continued growth of the embryonic brain.
2.3. Cell biology informs NVU models
Endothelial cells forming the microvasculature of the brain acquire their unique barrier characteristics through interactions and molecular signaling events originating from additional cell types of the NVU. These barrier characteristics distinguish endothelial cells of the brain from vascular endothelium in the rest of the body and include tight junctions, absence of fenestrations, expression of specialized transporters, and close association with other cell types comprising the NVU (Obermeier et al., 2013). Molecular markers of endothelial cell barrier properties include tight junction markers: occludin (OCLN), claudins (CLDN), and ZO-1 (TJP1); adherens junction markers: PECAM and VE-cadherin (CDH5); and the transporter, GLUT1 (SLC2A1) (Daneman et al., 2009) (Figure 4). Barriergenesis, or the establishment of barrier characteristics within or between endothelial cells, generally proceeds in three overlapping phases in pace with neurogenesis and brain growth: angiogenesis, differentiation, and maturation. These phases not only overlap temporally within the brain as a whole, but also spatially at the cell level and physiologically at the molecular level. Although some cells of the NVU do not appear in a mature form (e.g., astrocytes) until after birth, the interaction of these cell types during neural angiogenesis gives rise to a BBB that is functional nearly as soon as it is formed (Ek et al., 2012; Saunders et al., 2009) based on the establishment of tight junctions by mouse E11 (rat E12) (Daneman et al., 2010 ). Although the human gestational age that corresponds to this stage of rodent development is approximately 33 days (Carnegie stage 13–14; (Hill, 2016)), rodent and human developmental trajectories are not identical (Xue et al., 2013). In contrast to rodents, tight junctions definitively appear later in human fetal brains at 14 weeks of gestation (Virgintino et al., 2004) (Figure 3).
The dynamic events of barriergenesis are interwoven, wherein certain cell types and molecular events emerge more prominently at different phases of BBB development. With this in mind, key BBB-specific molecular events existing within or between the five NVU cell types are discussed below with each cell listed under the phase during which it has the most prominent role. This approach is for clarity and the reader should keep in mind that each cell of the NVU is involved to some extent in all phases of barrier formation. Additionally, the presentation of the cells and molecular events in the current section allows for their inclusion in the in vitro and in silico models being built for toxicity screening applications.
2.3.1. Endothelial cells
Brain angiogenesis commences around mouse E9.5 (in humans, this process does not begin until fetal week 8 (Marin-Padilla, 2012)) when specialized endothelial cells (ECs) branch off from vessels of the PNVP to form capillaries that invade nearby burgeoning neural tissue (Raab et al., 2004). The growing vessel is composed of EC-tip cells located at the leading edge and EC-stalk cells that form the vessel body. EC-tip cells are distinguished by PDGFB and PECAM1 expression (Gerhardt and Betsholtz, 2003), and filopodia rich in VEGFR2 that lead the growing capillary bud by extending and migrating along a VEGFA gradient produced by the neuroectoderm (neuroepithelial cells) in the ventricular zone of the neural tube (Figure 4) (Gerhardt et al., 2003; Raab et al., 2004). The function of the EC-tip cells is to lead vessel growth, which is driven by EC-stalk cell replication (Gerhardt et al., 2003).
EC-tip cell phenotype is controlled by communication with EC-stalk cells via a Notch intracellular domain (NICD)-mediated feedback loop that directs crosstalk between the VEGF and Dll4/Notch signaling pathways (Hellstrom et al., 2007) as a reversible cell-fate switch (Hayward et al., 2008). During vessel outgrowth, EC-tip cells with low NOTCH levels sustain their phenotype by producing more VEGFR2 in response to VEGFA. Meanwhile, stalk cells maintain their phenotype when NOTCH receptors bind DLL4 ligand on EC-tip cells resulting in suppressed VEGFR2 or increased secretion of the soluble decoy receptor, VEGFR1 (Hayward et al., 2008). Taken together, the key role of VEGFR2 in directing tip and stalk cell responses positions it as a pivotal receptor whose expression can be modulated to control the direction of capillary growth and facilitate arborization and refinement of the nascent microvessels (Figure 4).
While VEGF and Notch are key signaling pathways driving endothelial cell phenotype, several other proteins expressed or secreted by endothelial cells are also critical to angiogenesis, including NRP1, TIE2 (TEK), and matrix metallopeptidases such as MMP9. NRP1 is a VEGFR2 co-receptor that is not only expressed in tip and stalk cells, but also neuroprogenitor cells (NPCs), radial glia, and macrophages (Gerhardt et al., 2004; Kawasaki et al., 1999). NRP1 is required for lateral capillary branching and mediates macrophage-associated anastomosis of capillaries beginning at E10 in mice (Fantin et al., 2010; Gerhardt et al., 2004). Importantly, NRP1 expression by ECs also drives NPC differentiation (Tata et al., 2016). TIE2 is another receptor tyrosine kinase that is not only expressed by stalk cells, but also macrophages (Fantin et al., 2010). TIE2 differentially regulates angiogenesis in response to angiopoietins secreted by endothelial cells or pericytes (Aguilera and Brekken, 2014; Jeltsch et al., 2013). Generally, ANG1 is anti-inflammatory and stimulates sprouting, while ANG2 is pro-inflammatory and represses sprouting (Augustin et al., 2009). In addition to receptor expression, ECs also secrete MMP9, which not only promotes extracellular matrix remodeling to facilitate vessel outgrowth, but also recruits NPCs to sites of injury or inflammation (Wang, L. et al., 2006) and reduces pericyte recruitment to endothelial cells (Aguilera and Brekken, 2014). Taken together, NRP1, TIE2, and MMP9 have key roles in BBB development.
2.3.2. Microglia
Macrophages are well-known for their role as inflammatory cells of the innate immune system that support tissue development, homeostasis, repair and immunity (Wynn et al., 2013). Microglia are a unique population of CNS-resident macrophages in terms of their developmental origins and in some species, migration of these cells to the neuroepithelium coincides with the maturing circulatory system. As reviewed below, these specialized macrophages of the CNS contribute to BBB formation through vascular remodeling of invading blood vessels and through local surveillance at the site of the NVU in the brain.
2.3.2.a. Microglial cell lineage arises from the yolk sac
Microglia originate as primitive macrophages from early erythro-myeloid progenitors generated in the yolk sac of mice from E8.0 (for review, see (Hoeffel and Ginhoux, 2015)). These cells infiltrate the mouse brain from E8.5 to E9.5 at the time circulation commences (Ginhoux et al., 2010) and populate the mesenchyme and neuroepithelium by E10.5, which corresponds with brain infiltration as early as gestational week 4.5 in humans (Ginhoux et al., 2010; Monier et al., 2007). In zebrafish, microglia that originate in the rostral blood island (RBI; a region analogous to the mouse yolk sac) (Xu et al., 2015) and differentiate in the yolk sac (Herbomel et al., 1999) enter the brain by 2.5 days post fertilization (dpf) from the ventral surface and follow lateral or midline paths towards the optic tectum (Xu et al., 2016) in a pattern similar to blood vessel invasion of the neuroepithelim described above. However, in this model, initial recruitment signals arise from apoptotic neurons in a circulation-independent manner. This is inconsistent with observations in the mouse model showing a link between microglia invasion at E9.5 and the beginning of circulation (Ginhoux et al., 2010), but may reflect differences in developmental timing and pathways between the two species (Xu et al., 2016).
Importantly, their yolk sac origin distinguishes these microglia from the circulating myelomonocytic macrophages from fetal liver or bone marrow-derived microglia that may infiltrate the brain at later stages upon inflammation (Ginhoux et al., 2010; Ginhoux et al., 2013) (for review, see (Ginhoux et al., 2016)). Monier et al. (2007) used human histopathology data to detect microglia in the cerebral cortex at the earliest age represented, 4.5 weeks gestation. These authors also demonstrated that macrophages first enter the developing brain near the ventricular zone where they are most abundant in the area of rapid neurogenesis around gestational weeks 9–11 before migrating peripherally by 12–13 weeks. At 10.5 weeks, microglia accumulated between the cortical plate and subplate where they appeared to proliferate. Although no contact was evident between microglia and endothelial cells at 11 weeks, they were closely associated by embryonic week 23.5 (Monier et al., 2007).
2.3.2.b. Microglia have a role in microvascularization of the neuroepithelium
Microglia colonization coincides with CNS vascularization during embryonic development (Earle and Mitrofanis, 1998), and microglia release soluble factors that stimulate the growth of blood vessels (Rymo et al., 2011). Embryonic microglia that are critical for BBB angiogenesis are characterized by TIE2 and NRP1 (Fantin et al., 2010) and express known macrophage markers such as CX3CR1, CD11b/c, and CD45 by E11.5 (Dudvarski Stankovic et al., 2016). Fantin et al. (2010) observed in mice that TIE2-expressing monocytes/macrophages in the brain peak in numbers at E11.5 concomitant with the fusing of sprouted capillaries (i.e., anastomosis) to form the subventricular vascular plexus (Fantin et al., 2010). During this time, macrophages associate closely with the filopodia of EC-tip cells and mediate anastomosis, as demonstrated by a significantly reduced number of intersected vessels in macrophage-deficient mutants. Macrophages that modulate anastomosis express CSF1-R and are recruited from the head mesenchyme by CSF1, a macrophage chemoattractant that is expressed in the hindbrain by mesenchymal cells at this time (Fantin et al., 2010). Notably, CSF1 is the primary chemoattractant that induces macrophage infiltration into the brain, in contrast to VEGF164, which induces chemotaxis of macrophages from a monocyte lineage (Barleon et al., 1996). Although CSF1 is the primary chemoattractant for brain infiltration, it is not the only identified CSF1-R ligand. For example, IL34 is a CSF1-R ligand that is more abundant than CSF1 in the brain (Ginhoux et al., 2010; Greter et al., 2012; Wei et al., 2010), and may have a role in microglia differentiation (Wang et al., 2012b) and maintenance (Greter et al., 2012).
While CSF1 has a role, the molecular signals underlying macrophage-endothelial interactions are not fully characterized. Rymo et al. (2011) observed bidirectional communication between blood vessels and microglia in a mouse retina model; namely, microglia were attracted to growing blood vessels and in turn promoted angiogenesis via an unidentified soluble factor other than VEGFA in a contact-independent manner (Rymo et al., 2011). Thus, direct cell-cell contact may not be required for microglia-mediated anastomosis. Additional studies in the retina model indicate that VEGFC produced by macrophages may enhance Notch signaling to promote branching by VEGFR3 (FLT4)-expressing tip cells (Tammela et al., 2011). Microglia may also secrete soluble VEGFR1, the VEGFA decoy receptor that stalk cells produce to maintain their phenotype and direct vessel growth; however, this is based on CD11b+ monocytes in a retina model (Stefater et al., 2011).
2.3.2.c. Microglial activation is a response to the local environment
Microglia respond to environmental stimuli by adopting physical states ranging from a ramified ‘surveying’ phenotype (expressing long, branching processes) to an ameboid, ‘perturbed’ phenotype suited to phagocytosing apoptotic cells and debris (Hanisch and Kettenmann, 2007; Ransohoff and Perry, 2009; Zanier et al., 2015). Because microglia can express a range of these features at any given time, one must be cautious to avoid oversimplifying their activation states using obsolete, dichotomous terminology (e.g., M1 versus M2) (Ginhoux et al., 2016; Martinez and Gordon, 2014; Ransohoff, 2016).
Here, we refer to microglia based on function. Although a ramified surveying phenotype is important for BBB maintenance, ameboid microglia are prevalent during early embryonic development, reflecting an activated and rapidly proliferating state. Ameboid microglia documented in humans as early as gestational week 4.5 transition to a ramified surveillance phenotype as they migrate distally from the ventricular zone (Monier et al., 2007). It is tempting to speculate that these microglia may be in a proangiogenic and/or immunosuppressive activation state. Confirmation of activated microglia requires staining for secreted cytokines: immunosuppressive microglia secrete IL4, IL10 and TGFβ, which suppress the effects of proinflammatory cytokines on CXCL8; while proinflammatory microglia secrete IL1b and TNFa, which in turn promote secretion of CXCL8 (IL8) (Ehrlich et al., 1998).
A growing body of evidence supports the notion that microglia are regulators of the dynamic BBB at the site of the NVU throughout development and into adulthood (Bolton et al., 2017; Schafer and Stevens, 2010; Squarzoni et al., 2014; Stolp and Dziegielewska, 2009). Their role in mediating neuroinflammation contributes to diseases and pathological conditions in the brain (da Fonseca et al., 2014). Based on the integral role of microglia in the function of the NVU, chemical disruption of macrophage/microglia sensing is predicted to disrupt brain angiogenesis and BBB development (Bolton et al., 2017). For example, microglia activation can modulate barrier induction through IL1B, which suppresses Shh signaling in astrocytes in vitro resulting in loss of the barrier markers, claudin 5, occludin, and ZO-1 (Wang et al., 2014).
2.3.3. Radial glia
2.3.3.a. Radial glia provide a scaffold for sprouting angiogenesis
Radial glia are NPCs that give rise to neurons, oligodendrocytes, and astrocytes (Hartfuss et al., 2001) (Figure 3). These cells have bilateral processes that span the rudimentary brain from the ventricle to pial surfaces (Figure 1B) (Kriegstein and Alvarez-Buylla, 2009; Rakic, 1972), and which guide tip cells along an orthogonal path toward the ventricle during NRP1 mediated sprouting angiogenesis (Gerhardt et al., 2004). Several studies suggest that this subset of radial glia are astrocyte precursors (Engelhardt and Liebner, 2014; Gerhardt et al., 2003; Gerhardt et al., 2004; Hartfuss et al., 2001; Molofsky et al., 2012). For example, mature astrocytes are known to provide a scaffold for migrating tip cells in the retina model (Engelhardt and Liebner, 2014; Gerhardt et al., 2003; Gerhardt et al., 2004). Radial glia that differentiate into astrocytes express BLBP, GLAST, and RC2 (Molofsky et al., 2012). Radial glia subsets that express a similar phenotype (RC2/BLBP) also serve as scaffolds for migrating neurons during embryonic neurogenesis (Hartfuss et al., 2001). These markers are consistent with markers that characterize astrocytes at later stages (i.e., GLAST, BLBP, and GFAP). More recently, ALDH1L1 was identified as an early (E9.5) radial glia marker in cells that become astrocytes (Cahoy et al., 2008; Molofsky et al., 2012). Taken together, the radial glia that guide growing capillaries are likely precursors to the astrocytes that first appear in the newborn NVU (Figure 1B).
2.3.3.b. Radial glia expansion precedes neurogenesis
Neuroepithelial cell expansion prior to the onset of cortical neurogenesis (mouse E10.5 (Haubensak et al., 2004); human gestational week 6 (Howard et al., 2006)) leads to a robust base population of radial glia in the ventricular zone of the neural tube, subjacent to the developing brain ventricles (Dwyer et al., 2016; Paridaen and Huttner, 2014). While some radial glia cells proliferate via symmetric division, others undergo asymmetric division to give rise to the differentiated neurons, basal/intermediate progenitor cells, and glia in the brain (Morest and Silver, 2003; Paridaen and Huttner, 2014). Mitotic spindle orientation determines whether these cells will generate identical or differentiated daughter cells based on the inheritance of apical plasma membrane proteins, which modulates Notch signaling (Gotz and Huttner, 2005; Paridaen and Huttner, 2014).
2.3.3.c. Neurogenesis establishes four cortical layers
Radial glia proliferation and differentiation drives cortical layer development. Differentiated daughter cells (Chenn and McConnell, 1995) migrate soon after they are born along the parent cell’s process in an orthogonal path between the ventricle and outer cortex (Boulanger-Weill et al., 2017; Noctor et al., 2001). Intermediate/basal progenitor cells that express TBR2 (Bulfone et al., 1999) migrate to the subventricular zone, which is formed by the expansion of intermediate neurons and glia between rat E15 and E17 (Noctor et al., 2008) (mouse E13.5 – 15.5 (Hill, 2016)) or human gestational week 11.5 (Hansen et al., 2010). Meanwhile, radial glia on the apical side of the ventricular zone give rise to neurons that migrate to the outermost layer of the developing brain in a pattern that builds the cortical layers from the “inside out” with the ventricular zone forming first, followed by the subventricular zone, intermediate zone, and cortical plate (Paridaen and Huttner, 2014). The four layers are established by the completion of cortical neurogenesis at rat E20 (Noctor et al., 2008) (mouse E18.5 (Hill, 2016). In humans, this timepoint corresponds well with the peak density of progenitor cells in the subventricular zone at 16 – 19 gestational weeks (Malik et al., 2013). In a process unique to primates, this initial growth phase is followed by a substantial increase in brain surface size during mid-gestation (human weeks 13 to 17) when non-epithelial radial glia-like cells proliferate in the outer subventricular zone (Hansen et al., 2010). Altogether, radial glia, intermediate basal neurons, and cortical neurons interact with invading endothelial cells to form and maintain the developing BBB.
2.3.4. Pericytes
Pericytes are mural cells that promote the formation of barrier properties, which are evident in mouse endothelial cells as early as E10 (Armulik et al., 2010; Bauer et al., 1993; Gaengel et al., 2009; Gerhardt and Betsholtz, 2003; Winkler et al., 2011). Pericytes are derived from the neuroectoderm in the forebrain or mesoderm in the mid- and hindbrain (reviewed in (Winkler et al., 2011); Figure 3). Although forebrain pericytes have unique origins in the neural crest, mesodermal derived pericytes express VEGFR2 (Gerhardt and Betsholtz, 2003; Korn et al., 2002). Pericyte precursors in the rudimentary forebrain express PDGFRB and NG2 by E11.5 in mice, and are first detected associated with endothelial cells in the mouse brain at E9.0–9.5 (Bauer et al., 1993; Yamanishi et al., 2012). In comparison, the pericyte marker, PDGFRB, appears in human fetal brains as early as 8–10 weeks gestation (Maxwell et al., 1998) and pericytes associate closely with endothelial cells by 11 weeks (Allsopp and Gamble, 1979; Papageorghiou et al., 2014). The endothelial cell-pericyte association is evidenced by a common basement membrane between the two cell types interrupted only by direct intercellular contacts in the form of peg-socket junctions (Armulik et al., 2010)Allsopp, 1979 #506}. The basement membrane is composed mainly of agrin, fibronectin, and laminin, and as developmental markers are less detectable in the mature NVU once astrocytic endfeet ensheath the common basement membrane surface (Barber and Lieth, 1997; Krum et al., 1991).
2.3.4.a. Pericytes orchestrate barrier induction
Pericytes are distinguishable at the earliest stages of BBB development at least a week before astrocytes appear in mice and have key roles in both angiogenesis and BBB differentiation (Daneman et al., 2010). These roles include scaffolding, spatially specific angiogenesis suppression to modulate vesicular outgrowth, tight junction induction, and suppression of fenestration formation (Daneman et al., 2010; Ioannidou et al., 2006; Virgintino et al., 2007). Daneman et al. (2010) demonstrated that pericytes form a seal over endothelial cells that is required for tight junction formation (measured by increased transendothelial electrical resistance (TEER) and limited entry of 3 kDa dextran into the brain), but not for expression of the tight junction markers occludin and claudin 5 (Daneman et al., 2010). However, mutants lacking pericytes exhibited increased expression of Icam1, Alcam, and Lgals3 in blood vessels. These leukocyte adhesion molecules facilitate interaction of immune cells and the microvasculature; thus, pericytes likely have a role in coordinating immune surveillance by microglia during barriergenesis (Daneman et al., 2010).
Angiopoietins are additional proteins expressed by pericytes that link microglia to barriergenesis as ligands of the TIE2 receptor that is expressed on macrophages and other NVU cells (Armulik et al., 2010; Dumont et al., 1993). Angiopoietins are key pericyte markers associated with barrier induction (Daneman et al., 2010). Increased expression of Angpt2 (Ang2) and reduced expression of Angpt1 (Ang1) in pericyte mutants supports the hypothesis that pericytes reduce endothelial cell permeability. As noted above, ANG1 and ANG2 are TIE2 receptor ligands with complementary functions (Figure 4). While ANG1 decreases permeability by inducing tight junction formation via occludin and ZO-2 (TJP2) expression (Hori et al., 2004; Lee et al., 2009), ANG2 increases permeability following injury, possibly by inducing apoptosis (Nag et al., 2005). Notably, TIE2 is expressed on endothelial cells (Dumont et al., 1993), macrophages, and pericytes (reviewed in (Armulik et al., 2010)), positioning it as a key protein for orchestrating coordinated action by these cells during BBB development (Figure 4).
Pericytes also have a role in suppressing fenestration formation in endothelial cells of the BBB. Mutants with reduced pericyte function have increased levels of Plvap, which is needed for transcytosis and increases during BBB breakdown (Daneman et al., 2010). PLVAP1 (PV-1) is also required for proper fenestra formation and is the only known marker of fenestrations (Ioannidou et al., 2006). Collectively, the work by Daneman and colleagues suggests that pericytes contribute to the fenestration-poor phenotype in endothelial cells of the BBB via suppression of PLVAP (Daneman et al., 2010; Ioannidou et al., 2006). Thus, pericytes are essential to induce the BBB phenotype by initiating tight junction formation and decreasing fenestrations, thereby decreasing transcytosis in endothelial cells (Armulik et al., 2010). Ultimately, endothelial cells initiate pericyte-mediated barriergenesis by recruiting pericytes to growing microvessels.
2.3.4.b. Recruitment and differentiation follows cues from endothelial cells
Pericytes or their precursors are recruited by EC-tip cells that express PDGFBB homodimer during the earliest stages of angiogenesis (Ozerdem and Stallcup, 2003; Winkler et al., 2011). Although the PDGF signal induces pericytes to proliferate in vivo and attach to endothelial cells (Lindblom et al., 2003; Winkler et al., 2011), the presence of PDGFRB-positive cells (i.e., pericytes) in PDGF knockouts suggests that PDGF is not the primary signaling pathway that contributes to initial pericyte differentiation (Lindahl et al., 1997). Rather, evidence suggests that TGFB1 signaling is the primary pathway that orchestrates pericyte differentiation and behavior, placing it at the overlapping interface of angiogenesis and BBB differentiation (Figure 4).
TGFB1 is implicated in pericyte differentiation by studies showing induction of smooth muscle cells/pericytes (α-SMA-positive cells) from the neural crest (Chen and Lechleider, 2004; Gaengel et al., 2009). Both endothelial cells and pericytes express TGFB1 and its receptor, TGFBR2 (Winkler et al., 2011). Endothelial cells induce pericytes by secreting TGFB1, which activates TGFBR2 in pericytes, leading to the establishment of adherens junctions (N-cadherin) between the two cell types in a NOTCH-mediated process (Figure 4) (Li et al., 2011; Winkler et al., 2011). Pericyte attachment to endothelial cells suppresses angiogenesis followed by SMAD-mediated BBB differentiation and maturation (e.g., establishment of tight junctions) via the TGFBR2-ALK5-SMAD2/3 pathway (Winkler et al., 2011).
TGFB1 signaling in pericytes is also closely regulated by GPR124 (ADGRA2), which is induced by TGFB1 and also regulates expression of TGFB1-dependent genes, supporting a complex regulatory role between GPR124 and TGFB1 signaling. GPR124 is expressed in both endothelial cells and pericytes and is required for brain angiogenesis at mouse E10.5 (Anderson et al., 2011). In sum, TGFB1 induces pericyte-mediated BBB differentiation leading to angiogenesis suppression and likely directs pericyte migration, whereas GPR124 regulates these processes (Anderson et al., 2011). In addition to its modulatory role on TGFB1 signaling, GPR124 also promotes angiogenesis by inducing Wnt (7a and 7b)/β-catenin signaling (Zhou and Nathans, 2014).
2.3.4.c. Pericytes influence vascular remodeling and stabilization
Wnt signals are highly expressed in the neuroepithelium during early angiogenesis and Wnt/β-catenin signaling is essential for vasculature formation in the developing brain (Stenman et al., 2008), but not the rest of the body (Daneman et al., 2009). Wnt/β-catenin signaling impacts the activity of multiple receptors through β-catenin complexes (see review by (Dejana, 2010)). For example, in the presence of Wnt ligand, β-catenin upregulates Notch signaling in endothelial cells in vivo in favor of the stalk cell phenotype (Corada et al., 2010). This process is regulated in part by NRARP, a Wnt cofactor and negative feedback transcription regulator, which also increases β-catenin signaling via LEF1 (Phng et al., 2009). Thus, as angiogenesis proceeds along a Wnt gradient, Notch signaling can be limited while Wnt signaling is promoted in stalk cells, thereby facilitating vessel stabilization and coordinating the sprouting of new vessel junctions (Phng et al., 2009). GPR124 promotes Wnt signaling through its role as a Wnt ligand-specific coactivator of the receptor/coreceptor pair, FZD4/LRP5 (Figure 3) (Zhou and Nathans, 2014).
GPR124 activates the Wnt/FZD4/β-catenin pathway in vitro in a manner that mimics TSPAN12 activation of the Norrin (NDP)/FZD4/β-catenin pathway (Zhou and Nathans, 2014). TSPAN12 is a Norrin ligand-specific coactivator of FZD4/LRP5 that is also expressed in the brain and important for BBB function (Chen et al., 2015; Junge et al., 2009). Zhou et al. (2014) proposed that this functional redundancy during early CNS development (i.e., before mid-gestation (Ye et al., 2011)) increases the signal to noise ratio, thus ensuring sufficient Wnt signaling to induce BBB characteristics in endothelial cells, including GLUT1 (Wnt/β-catenin) and claudin 3 (β-catenin) (Daneman et al., 2009; Kuhnert et al., 2010; Liebner et al., 2008; Zhou and Nathans, 2014). Norrin also induces Wnt signaling via FZD4 in vivo, leading to formation or reinforcement of barrier characteristics; namely, more GLUT1, more claudin 5, and less PLVAP by postnatal day 60 in mice (Wang et al., 2012a), although this has not been directly demonstrated in embryonic brains and may therefore be more prominent during the later stage of BBB maturation or maintenance. Zhou et al. (2014) also suggested that coactivators such as GPR124 allow endothelial cells to discriminate from among multiple Wnt sources, thereby allowing some specificity in reaction to Wnt signaling. This may have a role in how Wnt induces angiogenesis and barrier characteristics in endothelial cells. Taken together, GPR124 is a key molecule that modulates BBB development via TGFB1 and Wnt/β-catenin signaling (Figure 4).
2.3.5. Astrocytes
2.3.4.a. Astrocyte precursors characterize the embryonic NVU
Although mature astrocytes are part of the adult NVU, they are not detected in the human fetal cortex until approximately 30 weeks of gestation (Roessmann and Gambetti, 1986). Induction of the brain microvascular endothelial cell phenotype occurs in the absence of mature astrocytes, underscoring the likelihood that the earliest BBB differentiation signals derive from pericytes or radial glia subsets that will mature into astrocytes shortly before or after birth (Lippmann et al., 2012). An example of an early differentiation signal is the expression of RALDH1 (ALDH1A1; the enzyme needed to synthesize retinoic acid) by radial glia, which may impact barriergenesis by modulating Wnt signaling (Mizee et al., 2013). Notably, endothelial cells express retinoic acid receptor beta (RARB) as early as 10 weeks gestation in human brains (Bonney et al., 2016; Mizee et al., 2013).
Astrocyte precursors may also induce barrier maturation through Shh signaling (Alvarez et al., 2011). Mouse primary astrocytes reduce BBB permeability and upregulate tight junction protein (ZO-1, occludin, and claudin 5) expression in co-cultured mouse primary microcapillary brain endothelial cells via Shh signaling (Wang et al., 2014). Furthermore, Shh knockout significantly reduces tight junction marker (ZO-1, occludin, and claudin 5) expression in E13.5 mice, implicating Shh in barrier formation in vivo (Alvarez et al., 2011). The same study reported reduced astrocyte association with endothelial cells and reduced tight junction markers at postnatal day 19 in Shh−/− mice (Alvarez et al., 2011). Moreover, Shh is expressed in astrocyte precursors (GFAP+ cells) from human fetuses as early as age 16–22 weeks, and Shh from astrocyte conditioned medium enhances tight junction protein expression in addition to increasing TEER in co-cultured microvascular brain endothelial cells (Alvarez et al., 2011).
2.3.5.b. Mature astrocytes appear in the cortex during late gestation
Mature astrocytes (Lippmann et al., 2012) are first detectable by immunohistochemical staining for GFAP in the human fetal brain stem at 15 weeks gestation, followed by detection in the cortex by 30 weeks (Roessmann and Gambetti, 1986). In comparison, GFAP mRNA is first detected in the mouse hindbrain at E16 and is fully distributed in the brain by postnatal day 5 (Landry et al., 1990). TGFBR2-expressing radial glia differentiate into astrocytes in response to TGFB1 during barrier maturation (Stipursky et al., 2014), although this doesn’t take place in humans until after birth (Daneman, 2012). Notably, TGFB1 signaling is also responsible for pericyte-endothelial cell communication during BBB maturation (Li et al., 2011).
Astrocytes are key components of the mature NVU that comprise the majority of cells in the brain, supporting a role in NVU maturation via endfoot-localized secretion of key growth factors, extracellular matrix, and cytokines at sites of contact with the endothelial cell-pericyte basement membrane (Wiese et al., 2012). Astrocyte endfeet reinforce the BBB through many of the same signaling pathways previously described for barrier induction, including Shh, VEGF, ANG1, ANG2, FGF, GDNF, and RA, among other BBB-reinforcing signals (Alvarez et al., 2013). For example, they secrete GDNF, a ligand for GDNF receptors (GFRA1) expressed by endothelial cells, which induces/reinforces tight junctions (Igarashi et al., 1999). Astrocytes also secrete the matrix glycoprotein, tenascin C (TNC), which may influence BBB maturation based on its prominent role in maintaining proper balance among neurons, astrocytes, and oligodendrocytes in the cortex (Irintchev et al., 2005) while modulating astrocyte proliferation and axon elongation (Nishio et al., 2003; Wiese et al., 2012). Astrocytes are also the primary source of triiodothyronine (T3; thyroid hormone) in the brain, which requires T3 for proper development (Zoeller and Crofton, 2000). Astrocytes use type 2 deiodinase (D2; DIO2) to convert circulating thyroxine (T4) to T3, which is delivered to neurons via endfeet contacts (Morte and Bernal, 2014). By postnatal day 15 in mice, 50% of the T3 is delivered to the brain via astrocyte endfeet (Morte and Bernal, 2014). Taken together, presence of endfeet connections, peripheral contact between neurons and the endothelial cell-basement membrane sheath, and microglia of the intact NVU are all likely indicators of close communication between the NVU and the rest of the brain that is essential for BBB maturation (Figure 1A).
2.4. Maturation reinforces barrier characteristics
It is important to keep in mind that BBB ‘maturity’ is not synonymous with BBB function (Ek et al., 2012; Saunders et al., 2009; Saunders et al., 2012). If the physical and physiological characteristics of the adult NVU are used to define maturity, then the human brain is not technically mature until a person is at least 20 years old (Semple et al., 2013). For example, while some BBB barrier functions are present shortly after vessel formation (Bauer et al., 1993), other aspects of BBB maturation such as mature astrocytes do not appear until birth or shortly thereafter in the mouse (Roessmann and Gambetti, 1986) when they differentiate from radial glia precursors (Stipursky et al., 2014). Furthermore, the human NVU is less mature at birth when astrocyte endfoot connections cover only 61% of the brain microvasculature (El-Khoury et al., 2006) compared to 89% coverage observed in a single adult specimen (Virgintino et al., 1997). For comparison, coverage in the mouse newborn is 64% versus 98% in adults (Saunders et al., 2016). Despite the differences between the neonatal and adult NVU, the BBB is already functional as early as human embryonic week 14 (Andrews et al., 2017; Virgintino et al., 2004) based on the presence and corresponding performance of tight junctions in mammalian models (Daneman et al., 2010). Overall, maturation occurs throughout life as the early, functional NVU adapts to life-stage specific needs of the embryo, fetus, neonate, juvenile, and adult (Ek et al., 2012; Saunders et al., 2009; Saunders et al., 2012).
BBB maturation is the process by which nascent BBB characteristics are reinforced through enhanced intercellular communication among the cells of the NVU, some of which differentiate into mature phenotypes (e.g., radial glia subsets become astrocytes) during this period. Accordingly, many of the same signaling cues (e.g., ANG1/TIE2) that induce differentiation are also important during maturation, which commences shortly after angiogenesis is complete (Winkler et al., 2011). The most distinguishing physical feature of the BBB is tight junctions. After initial formation, tight junction properties are reinforced by expressing additional marker proteins. Thus, in addition to early tight junction makers (i.e., claudins, occludin, and ZO-1), other tight junction markers such as ZO-2, ZO-3 (TJP3), and cingulin appear as the maturation process progresses (Wolburg and Lippoldt, 2002).
2.4.1. Fluid-flow shear forces enhance barriergenesis
In addition to molecular signaling, physical factors such as shear stress have been identified as critical to barriergenesis and maturation of the BBB. Shear stress results from the force applied to endothelial cells by the flow of blood through the microvasculature. Here, ‘shear’ refers to fluid sliding along the luminal edge of the cells, more so than perpendicular pressure on the vessel wall (Cucullo et al., 2011). Evidence exists that shear stress contributes to barrier integrity by inducing integrins and adhesion molecules that are important in tight junction formation (Tzima et al., 2005). Notably, VE-cadherin (CDH5), but not N-cadherin, mediates tight junction formation in response to shear stress (Coon et al., 2015). Shear stress enhances endothelial cell function through induction of transporter and enzyme expression (Cucullo et al., 2011; Desai et al., 2002). Evidence indicates that endothelial cells cultured in the presence of shear stress develop tighter barriers indicated by higher TEER levels (Wolfe and Ahsan, 2013).
2.4.2. BBB molecular transport is both active and passive
The BBB is designed to block unwanted substances from entering the brain while allowing access to nutrients or hormones (e.g., glucose, amino acids, thyroid hormone) (Laterra J, 1999). These functions are achieved through physical barriers, and passive, facilitative, or active transporters. Generally, higher water-octanol partition coefficients are associated with more chemical entry into the brain (Laterra J, 1999). Highly lipophilic compounds like nicotine and ethanol easily diffuse across the plasma membrane of barrier endothelial cells, and gases readily diffuse through the barrier (Laterra J, 1999). Whereas water flows freely along osmotic gradients through aquaporin channels, large water-soluble (polar) substances generally cannot cross without a transporter (Laterra J, 1999). Also, some smaller water-soluble substances that would otherwise gain access bind with plasma proteins, resulting in a complex that is too large to diffuse through the barrier (Laterra J, 1999). Membrane-bound transporters mediate passage of targeted nutrients and other substances with low lipid solubility to the brain (Laterra J, 1999). Transporters known as carrier proteins may either passively allow transport along a concentration gradient (i.e., facilitated diffusion) or use energy in the form of ATP to transport molecules against a gradient. Active transport allows for an appropriate level of nutrients within the brain (influx), and either concentration of, or removal of, toxicants. Below we briefly summarize key transporters and barrier properties representing minimum requirements that we believe are necessary for BBB models. For a more detailed discussion on solute movement across the BBB, the reader is referred to the following detailed reviews: (Golden and Pollack, 2003; Pardridge, 2012; Sanchez-Covarrubias et al., 2014; Scherrmann, 2002). BBB transporters and metabolizing enzymes in the BBB have also been reviewed (Ek et al., 2012; Morris et al., 2017). The transport of chemicals across the BBB serves as a primary component of characterizing the pharmacokinetics of potential neurotoxicants, specifically, local metabolism at the site of the barrier and distribution of xenobiotic to the neural compartment.
2.4.3. Influx transporters provide materials required for growth
Transporters allow entry of a variety of ligands including nutrients (e.g., glucose), hormones (e.g, insulin, leptin, thyroid hormone (T4)), enzymes, growth factors, and blood plasma proteins (e.g., transferrin) (Ek et al., 2012). In addition, these transporters can facilitate the distribution of xenobiotics to the brain for both pharmaceuticals (Ouzzine et al., 2014; Pardridge, 2012) and environmental toxicants (Ek et al., 2012; Jiang et al., 2010; Liu et al., 2006). An understanding of these transporters will allow for a more complete characterization of chemical and nutrient pharmacokinetics within the brain.
While facilitated diffusion allows for the energy-independent entry of nutrients such as amino acids into the brain, active transport requires energy to move molecular species against their concentration gradient in order to reach the brain. For example, glucose serves as the primary energy source for the brain and requires specialized active influx transporters to move glucose from an area of low concentration (the blood) to an area of much higher concentration (the brain) (Ek et al., 2012). Glucose is transported into the brain via GLUT1 (SLC2A1) (Dick et al., 1984). Neonates use glucose, lactate, and ketone bodies as energy sources, while adults on a typical diet only use glucose (Vannucci and Simpson, 2003). However, children with GLUT1 deficiency respond well to a ketogenic diet, which shifts the primary energy source back to ketone bodies (Klepper et al., 2005).
GLUT1 is essential for embryonic survival and development (Moley, 2001; Wang, D. et al., 2006), consistent with 64% conserved amino acid sequence similarity in the lamprey, the earliest appearing vertebrate with an endothelial BBB, compared to the human protein (Figure 2). GLUT1 levels in rat and rabbit brains increase after birth until they reach adult levels (Cornford and Cornford, 1986; Vannucci, 1994). This peak in GLUT1 expression coincides with an increased rate of glucose transport into the brain as animals age (Cornford and Cornford, 1986; Cremer et al., 1979). Despite similarities in transport rates, there appear to be species differences in GLUT1 affinity for glucose. In rabbits, GLUT1 affinity for glucose is higher at birth than in adulthood (Cornford and Cornford, 1986). In contrast, GLUT1 affinity for glucose is similar in adult humans and preterm infants. However, maximal velocity (Vmax) was reduced in the preterm infants, reflecting a decreased expression of luminal transporters (Powers et al., 1998). This decreased rate of glucose transport could result in a population susceptible to environmental toxicants that specifically target GLUT1, further inhibiting the rate of glucose uptake into the brain.
Although species differences exist with respect to glucose transport and affinity, neonatal rabbit and rat studies consistently demonstrate higher transport rates for arginine, choline, and adenine compared to adults. This increased amino acid uptake reflects a greater need for these nutrients during development (Banos et al., 1978; Braun et al., 1980; Cornford and Cornford, 1986). The higher transport rates in neonates support the tenet that the BBB is functional at birth and uniquely responsive to the nutritional needs of the brain at this stage in development (Braun et al., 1980; Ek et al., 2012; Saunders et al., 2012). The importance of nutrition in the developing brain is further evidenced by the link between SLC7A5 (LAT1) mutation and autism in humans (Tarlungeanu et al., 2016). Although this example has a genetic basis, the function of SLC7A5 could also conceivably be altered through chemical exposure. In addition to altering transporter function, chemicals can also utilize functioning transporters to enter the brain. For example, equilibrated nucleoside transporter 1 (ENT1) encoded by the SLC29A1 gene, represents a similar nutrient transporter expressed in the BBB and mediates the transport of nucleosides into the brain (Ek et al., 2012). Using results from the 2016 ToxCast data release, 53 ToxCast chemicals were identified as active at this molecular target (Richard et al., 2016) indicating the need to investigate chemical induced perturbations to transporters necessary for essential nutrient uptake.
In addition to chemical induced transport inhibition, transporters including GLUT1, amino acid transporters, DMT1, and OATP2, can also move neurotoxicants into the brain (e.g., arsenic, lead, and valproic acid) (Ek et al., 2012). For example, human or rat GLUT1 injected into frog oocytes transports arsenic trioxide (Liu et al., 2006) and methylated arsenic (Jiang et al., 2010), suggesting that this toxicant crosses the BBB via GLUT1. Additionally, although there is evidence that lead in the form of PbOH+ can passively diffuse across the BBB (Bradbury and Deane, 1993), it may also attach to amino acids thereby gaining access through amino acid transporters (Ek et al., 2012). Furthermore, in vitro studies using HUVEC cells demonstrate that the iron transporter, DMT1, also transports lead across the BBB, particularly when iron levels are low (Wang et al., 2011). A third example comes from recent research suggesting that the thyroid hormone transporter, OATP2, may transport valproic acid into rat brain microvascular endothelial cells (Guo and Jiang, 2016). All three examples represent cases where influx transporters, both active and passive, represent key areas of research for xenobiotic distribution to the brain.
2.4.4. Efflux transporters protect the brain from xenobiotics
Efflux transporters aim to remove molecular species from the brain back into circulation. In the case of environmental toxicants, rates of transport out of the brain serve as a key pharmacokinetic variable for understanding distribution to the brain. For example, in addition to using influx transporters, lead can also enter the brain through calcium ion channels by mimicking calcium ions (Kerper and Hinkle, 1997). However, because of this similarity to calcium ions, lead can also be actively exported via the plasma membrane calcium ATPase (Bradbury and Deane, 1993). Additionally, a group of efflux transporters including ATP-binding cassette (ABC) proteins functions to remove both metabolic wastes and xenobiotics representing a wide range of structures from the brain (Hartz and Bauer, 2010; Higgins, 1992; Montanari and Ecker, 2015).
A key member of the ABC transporters, P-glycoprotein (P-gly, ABCB1, MDR1), is a selective efflux transporter that is abundant in the BBB and serves a prominent role in removing xenobiotics (Hartz and Bauer, 2011). P-gly is not only located at the plasma membrane, but also other sites including the nuclear envelope, nucleus, and mitochondria, underscoring its importance in cellular toxicity protection (Babakhanian et al., 2007). Notably, P-gly expression and activity is induced by TNFA, further underscoring the importance of this growth factor in BBB differentiation and maturation (Hartz and Bauer, 2010). Mrd1a (P-gly transcript) is expressed by E10.5 in mouse brain endothelial cells (Qin and Sato, 1995). While P-gly is one of the most prominent and arguably most important efflux transporters regarding toxicity testing, multidrug resistance proteins, BCRP and other efflux transporters are also active at the BBB (Golden and Pollack, 2003). The expression of efflux transporters is responsive to a variety of stimuli, including growth factors secreted by NPCs and astrocytes, in addition to oxidative stress, inflammation, and the presence of xenobiotics (Strazielle and Ghersi-Egea, 2015).
Transporters are present and presumably functional at the earliest stages of BBB development because this is the time that nutrients including glucose, amino acids and their building blocks (choline, purines, nucleosides) are in high demand (Abbott et al., 2010; Saunders et al., 2012). Interestingly, although P-gly is present in the developing brain, its expression is lower in newborns compared to adults (Gazzin et al., 2008). Selective transport at different life stages may be the predominant underlying cause of age-based differences in BBB permeability. Changes in barrier transporter selectivity during maturation have led some to interpret increased prevalence of certain substances on the inside of the barrier as an indication of an ‘immature’ BBB; however, evidence supports the assertion that the BBB selectively and deliberately transports some of these substances to meet developmental needs specific to the fetus, neonate, child, and adult (Ek et al., 2012; Saunders et al., 2012).
2.4.5. Metabolizing enzymes transform chemicals at the site of the BBB
In addition to selective transporters, metabolizing enzymes within NVU cells provide a source of fuel (e.g., through glycolysis), metabolize chemicals to an inactive or easily excreted form (Pardridge, 1983), or in some cases bioactivate a chemical into a more toxic form (e.g., metabolism of 7,12-dimethylbenz(a)anthracene by endothelial cells in the choroid plexus (Granberg et al., 2003) or glucuronidation of morphine to the active metabolite, morphine-6-β-D glucuronide (Ouzzine et al., 2014)). Some of these enzymes serve as extra protection by inactivating xenobiotics that escape exclusion via efflux transporters (Engelhardt and Liebner, 2014). The predominant phase I xenobiotic metabolizing enzymes expressed within brain microvascular endothelial cells include CYP1B1 and CYP2U1 (Dauchy et al., 2008; Shawahna et al., 2011), while at least one study reported that the predominant phase II enzyme is GSTP1 (Shawahna et al., 2011).
To assess the metabolic capacity of the BBB, recent studies have shifted attention to investigating phase I and phase II metabolism at the site of the barrier. To assess cytochrome P450 metabolism, Hellman et al. (2017) utilized an ex vivo locust model to investigate local phase I metabolic rates of selected pharmaceuticals at the site of the BBB (Hellman et al., 2016). While this invertebrate model does not have an endothelial cell based physical barrier, the authors demonstrated CYP activity through xenobiotic metabolism. Even though these CYP enzymes are not identical to human CYPs expressed in barrier endothelial cells, we may expect a similar outcome in the vertebrate BBB based on amino acid sequence similarities between other insect and human metabolizing enzymes (e.g., ALDH1A1) of the BBB (Figure 2). In addition, phase II metabolism such as glucuronidation by UDP-glucuronosyltransferases (UGT) has been demonstrated in rat brain (Ghersi-Egea et al., 1988). A complete review of UGT metabolism by Ouzzine and coworkers demonstrated that numerous xenobiotics are metabolized through UGT enzymes, contributing to the local detoxification and altered pharmacokinetics at the target tissue of interest (Ouzzine et al., 2014). This understanding of the metabolic component of xenobiotic pharmacokinetics will play an important role in understanding the amount of chemical that reaches the brain following environmental exposure. While CYP1B1 has endogenous substrates such as estradiol and retinol (Nebert and Dalton, 2006), its expression is also increased as a result of AHR activation by xenobiotics such as TCDD or diesel exhaust particles (Jacob et al., 2011). This increased expression is presumably so that CYP1B1 can inactivate the xenobiotic, and has implications for BBB metabolism based on the high expression of CYP1B1 in brain microvessels (Dauchy et al., 2008). Notably, CYP1B1 also has a direct role in BBB formation beyond that of xenobiotic metabolism. It is highly expressed in both pericytes and endothelial cells and promotes angiogenesis (Tang et al., 2009) and pericyte-mediated vessel stabilization in mouse retinas (Palenski et al., 2013).
2.5. Section 1 summary
The first section of this review covered the molecular events that contribute to BBB development. While it is evident that the signaling events within or between all five cell types of the NVU are important for this process, key proteins with prominent roles in endothelial cells, microglia, pericytes, and radial glia (precursors to astrocytes and neurons) are candidate targets of toxicants that may perturb early BBB formation or function. With this in mind, we highlighted a shortlist of candidate targets and the key cells in which they have prominent roles during early BBB development. The manual selection of these candidate targets was based on the literature evidence that they are required for early BBB formation, yet they may not be critical for angiogenesis. The list includes TIE2 (TEK) (endothelial cells, microglia, and pericytes); NRP1 (endothelial cells, microglia, and radial glia); CSF1-R (microglia); and GPR124 (ADGRA2) (pericytes). These receptors (yellow highlights) and other key signaling molecules implicated in BBB development are summarized in Figure 4. Although this is not an exhaustive list, they may be molecular initiating events useful in building adverse outcome pathways for developmental BBB disruption, identify biomarkers for in vitro HCI, and build in silico models of NVU development and BBB formation.
3. Adverse outcome pathways for developmental BBB disruption
One way to begin utilizing this large amount of information surrounding the developing BBB is through adverse outcome pathway (AOP) development. Here, a BBB-specific AOP would allow for an increased ability to screen chemicals from a potential BBB molecular initiating event (MIE) to determine how the resulting tissue-level perturbations resolve as neurological adverse outcomes. This focus on BBB formation from the perspective of DNT entails identifying impacts on brain development and function (i.e., adverse outcomes) following gestational exposure to compounds that target cell types responsible for BBB development and maturity (i.e., the developing NVU). These adverse outcomes (AOs) may be a result of tissue-level direct effects (e.g., reduced vessel branching) or indirect effects such as localized inflammation. AOs in the form of teratogenic effects (e.g., microcephaly) are most likely to be associated with toxicant exposure during early gestation when brain patterning is established. Furthermore, altered intercellular signaling for cell types of the NVU may result in AOs that are neurobehavioral in nature, possibly with accompanying lesions that are only detectable at the molecular or cellular level (Stolp and Dziegielewska, 2009; Theoharides and Zhang, 2011). Accordingly, AOPs for BBB disruption should include MIEs at all stages of BBB formation (i.e., angiogenesis, differentiation, and maturation), KEs including cellular responses that occur during gestation or postnatally, and AOs such as neurobehavioral impairments that manifest during early childhood. Taken together, a complete prediction of BBB disruption will comprise a network of integrated AOPs including many events for which there is currently no data.
The BBB is formed by multiple developmental processes, including angiogenesis. Thus an AOP for developmental BBB disruption will necessarily share many features with the angiogenesis AOP previously developed (Knudsen and Kleinstreuer, 2011). The challenge, however, will be to distinguish MIEs and KEs underlying BBB formation, differentiation, and function, that are distinct from basic angiogenesis. One approach to meeting this challenge is to focus on the cells of the NVU that have unique roles during BBB formation (e.g., pericytes and microglia) and identify receptors specific to BBB development (e.g., GPR124 (ADGRA2)), but not absolutely essential for angiogenesis (e.g., VEGFA). Accordingly, the molecular signals specific to BBB differentiation and function may have effects on cells of the NVU that are likely subtler than angiogenesis disruption and do not necessarily lead to obvious birth defects. A preliminary framework for BBB developmental disruption is shown in Figure 5. What follows is a review of current methodologies available for use in developing robust AOPs specific to BBB development for chemical safety and screening purposes.
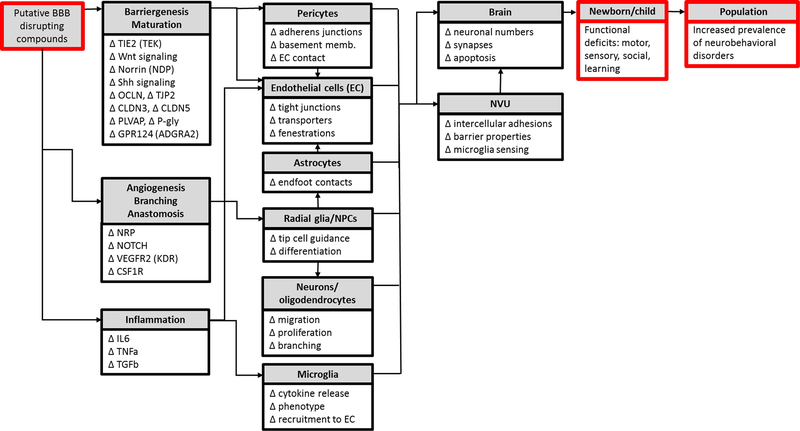
Figure 5: Preliminary AOP framework for BBB development and function.
An adverse outcome pathway (AOP) for BBB development should include the proposed molecular initiating events (MIEs), key events (KEs), and adverse outcomes (AOs) resulting from embryonic or fetal exposure to a putative BBB disrupting compound. The list of potential MIEs/KEs included here encompasses molecules important for BBB development (summarized in Figure 4). Perturbation of these molecules is indicated by ∆ to indicate that any change (either an increase or decrease in expression) could potentially lead to BBB disruption. These molecular events underlie the function of the cells of the NVU. Altered cellular function is expected to impact NVU and/or brain development and function, leading to adverse outcomes such as neurodevelopmental toxicity and neurobehavioral disorders.
3.1. Classification models help predict putative BBB disruptors
Classification models serve to link putative chemical disruptors to their adverse outcome through MIEs and connected key events (KEs) (Villeneuve et al., 2014a, b). Upon establishment of an AOP of developmental BBB disruption, the classification of potential MIE disrupting chemicals constitutes the next step in predicting BBB-specific chemical disruptors. To create the necessary linkage between chemical and in vivo effect, our group has previously used ToxRefDB as a source of phenotypes of interest (e.g., reproductive toxicity) on which to anchor classification models (Kleinstreuer et al., 2011; Leung et al., 2016b; Sipes et al., 2011). In the case of BBB development, there is insufficient information in ToxRefDB to link disrupted BBB formation, a phenotype not represented in the database), to chemical exposures (Knudsen et al., 2009).
While we may hypothesize that neurological changes may follow BBB disruption, there are too many assumptions surrounding this research gap to start with neurobehavioral outcomes as the AO for our classification model. Therefore, although neurobehavioral changes are ultimately the AO of interest in light of children’s environmental health, there is a huge ‘black box’ in place of a link between early key events and many adverse neurodevelopmental outcomes (Bal-Price et al., 2015; Shafer et al., 2005). This is true for the relationship between disrupted BBB development and adverse neurobehavioral outcomes. Here we propose a framework of how an AOP of BBB disruption may lead to neurobehavioral changes; however, this is speculative until more data is available linking the various key events represented in the framework (Figure 5).
A manageable first step is to focus on the first part of the overall AOP by identifying MIEs that lead to BBB disruption; however, this is still a new area of research with not enough data on the effects of chemical exposures on BBB development. Although DNT compounds are known (Aschner et al., 2017; Corada et al., 2010; Kadereit et al., 2012; Mundy et al., 2015), there is little direct evidence in the literature that neurotoxicity follows BBB disruption. In this case, the paucity of evidence does not mean there is no association between DNT and BBB disruption, but rather the studies to determine this have not yet been conducted. This is a rather new field that will offer key insights as more sophisticated organotypic culture models of the NVU are built (Saili et al., 2017). Moreover, in vivo models and translational human studies promise to advance the field. For example, a recent study demonstrated that reduced BBB expression of the amino acid transporter, SLC7A5, leads to autism-like behaviors in rats and mutation in this gene was more common in children with autism (Tarlungeanu et al., 2016). SLC7A5 disruption through exposure to diesel exhaust particles (Le Vee et al., 2016), serves as an example of a primary effect of this amino acid transporter and is a promising MIE for an AOP of BBB disruption. In addition, a chemical exposure representing a secondary effect on the BBB is methylglyoxal, which leads to formation of advanced glycation endproducts (AGEs) that disrupt the BBB (Hussain et al., 2016). Peripheral methylglyoxal exposure could potentially elicit these effects without crossing the BBB by inducing circulating macrophages to release high mobility group box 1 protein (HMGB1) into the serum (Wang et al., 1999; Yang et al., 2015). In this case, HMGB1 acts as a cytokine and binds AGE receptors (RAGEs) on circulating macrophages or endothelial cells of the BBB (Kokkola et al., 2005). While endothelial cell binding may have direct effects on BBB permeability, the inflammatory signal may also be transduced across the BBB leading to abluminal effects on the NVU such as perturbed microglia that enhance a localized neuroinflammatory response and neural damage.
Until a broader base of empirical data is established, classifying developmental neurovascular toxicity associated with BBB disruption will require a nuanced and manually curated AOP-based approach that relies upon an integration of the literature (summarized in the biowiring diagram in Figure 4) and associated AOPs (outlined in Figure 5); and a non a priori, phenotype-based approach previously demonstrated by our group for identifying signatures of developmental toxicity (Kleinstreuer et al., 2011; Leung et al., 2016b; Sipes et al., 2011). In this case, the phenotypes of interest representing disrupted BBB development can be observed using in vitro NVU models (e.g., organotypic culture models (Saili et al., 2017; van der Helm et al., 2016)), or a combination of neurogenesis and angiogenesis assays used to screen a representative suite of ToxCast chemicals (Belair et al., 2016; Nguyen et al., accepted) . Once a classification model of developmental neurovascular toxicity is built, one can use it to predict putative BBB toxicants.
AOPs built upon MIEs and signaling pathways that underlie proper BBB development can guide in vitro testing for links between molecular events and cellular activity (KEs) (Villeneuve et al., 2014a, b). This approach will require a leap from typical R&D testing, which has employed BBB models primarily to test whether chemical exposure impacts permeability (Banerjee et al., 2016; Kaisar et al., 2017). Tight junction-dependent permeability is most often quantified by TEER measurements and whether representative hydrophilic compounds (e.g., dextrose or dyes) cross the barrier (Appelt-Menzel et al., 2017). These measurements are compared to the qualitative or quantitative expression of tight junction proteins (e.g., ZO-1), which are determined through histology or gene expression assays, respectively (Appelt-Menzel et al., 2017). Other features of the BBB that are often studied include the abundance or function of selective transporters (e.g. P-gly or BCRA), which are measured by rates of ligand transport, immunohistochemical markers, or transporter-specific gene expression (Abbott, 2013; Appelt-Menzel et al., 2017; Shawahna et al., 2011). Although these parameters are molecular in nature, it is difficult to derive information other than permeability effects using currently available models (Appelt-Menzel et al., 2017).
3.2. In vitro models allow for rapid screening using human cells
Ideally, BBB-based AOPs will prove useful for identifying DNT-specific cellular and tissue level KEs that may be associated with developmental BBB disruption. In vitro BBB models for DNT testing should therefore provide a platform for obtaining more than just the classical measurements of BBB function. An optimal in vitro BBB model should allow us to map perturbations of molecular endpoints to higher level features of NVU development such as inter-cellular contact and communication. To this end, 3D organotypic culture models are likely to provide the data needed to ascertain these subtle changes to BBB formation following chemical insult (Saili et al., 2017; van der Helm et al., 2016). For example, an ability to track dynamic cell-cell interactions in real-time could allow us to answer whether chemical exposure disrupts pericyte-endothelial cell association, impairs the formation of contacts between astrocytic endfeet or neuron synapses and endothelial cells/pericytes, or promotes association of microglia with endothelial cells. Additionally, the use of multicellular NVU models will facilitate studies of the role of microglia in orchestrating adaptive responses to toxicant exposures (Achyuta et al., 2013). Therefore, a useful OCM will allow us to explore not just whether, but also how these processes occur.
The ability to answer these types of questions using NVU models will begin to shed light on the molecular targets of BBB toxicants. As these targets are identified, we can begin to link KEs by shared MIEs, which one day may contribute to understanding the mechanisms underlying childhood neurobehavioral diseases putatively linked to misregulated BBB development and function or neuroinflammation (Bal-Price et al., 2015; Bolton et al., 2017; Hanamsagar and Bilbo, 2016). Thus, mechanistic data obtained from a 3D, organotypic culture model of the NVU would both complement and validate molecular endpoint data from in vitro screening assays (i.e., ToxCast (Richard et al., 2016)) that comprise molecular signatures of toxicity for putative developmental BBB toxicants. Several state-of-the-art NVU models that may be adapted to accomplish these goals have been described within the last decade (e.g., (Adriani et al., 2016; Brown et al., 2015; Herland et al., 2016; Kim et al., 2015; Sellgren et al., 2015; Shao et al., 2016)). For reviews, see (Helms et al., 2016) and (Saili et al., 2017).
Based on the shortlist of key proteins from the biowiring diagram in this review (Figure 4); TIE2 (TEK), NRP1, CSF1-R, and GPR124 (ADGRA2) are candidate biomarkers (measurable via HCI) whose activation may serve as MIEs following chemical exposures. For example, to test the hypothesis that a given chemical reduces BBB integrity via a microglia-mediated neuroinflammatory response, one could expose a 3D, organotypic culture model of the NVU to the chemical and measure these receptors in endothelial cells, pericytes, and/or microglia; in addition to assessing putative key events including expression changes in tight junction markers, inflammatory cytokines, and the extent to which pericytes and microglia associate with the endothelial cells.
3.3. In vivo models provide valuable links to adverse outcomes
Once a link between chemical exposure, MIEs, and KEs is established, one can test the hypothesis that BBB disruption leads to DNT by exposing model organisms to putative BBB disrupting compounds, assessing for signs of BBB disruption in vivo, and testing for associated neurobehavioral impairments. Many of the tests needed to establish connections between KEs can be conducted in mammalian models typically used for neurobehavioral testing (e.g., mice and rats) (Cory-Slechta et al., 2001; Fang et al., 2010). Although mouse models offer the advantage of shared homology with most human proteins implicated in BBB development (Figure 2); other vertebrate models such as embryonic zebrafish (Bugel et al., 2014) are a potentially useful tool for studying the BBB in vivo.
Zebrafish offer several advantages for AOP development (Perkins et al., 2013). Zebrafish share protein sequence homology for many of the key receptors implicated in BBB development (Figure 2), and use of this model facilitates reduction of the number of animals used in toxicity research (Perkins et al., 2013). Moreover, a recently developed transgenic zebrafish that expresses fluorescent GLUT1 as a marker of the BBB (Umans et al., 2017) could be used to screen for putative BBB disrupting chemicals similar to the approach that was used by Tal et al. (2017) to identify angiogenesis inhibitors from the ToxCast library (Tal et al., 2017). Furthermore, this model also offers in vivo visualization (e.g. pericyte-endothelial cell interaction (Ando et al., 2016)) and could be used to measure tight junction formation as a further indicator of BBB integrity (Jeong et al., 2008), in addition to locomotor (Irons et al., 2013), learning (Saili et al., 2012), and social behavior (Qin et al., 2014) tests to potentially link KEs to neurobehavioral AOs.
3.4. In silico models simulate the in vivo NVU
In addition to investigating molecular and cellular effects of toxicant exposure, in vitro platforms that capture embryonic BBB development or represent all cells of the NVU are valuable for informing ‘virtual tissue models’ (VTMs) of BBB development. VTMs are computational representations of tissue development (e.g., angiogenesis (Kleinstreuer et al., 2013), genital tubercle fusion (Leung et al., 2016a), or palate fusion (Hutson et al., 2017)), which can be interrogated by simulated toxicant exposure, as long as the molecular targets of the toxicant have been identified (e.g., through ToxCast testing).
VTMs represent novel possibilities for the future of developmental toxicity testing, which in an effort to replace and reduce the use of animals (NRC, 2007), may one day rely heavily on simulations to identify priority chemicals for subsequent testing in living systems. Although the ultimate goal is to build models representing in vivo systems, sophisticated in vitro models will serve as simplified templates on which to base in silico models. Therefore, similar to in vitro models, good in silico NVU models should not only allow for real-time observations of intercellular actions leading to NVU/BBB development, but also characterization and quantification of the molecular signaling events underlying cellular behavior. Here we can incorporate the shortlist of hypothesized key receptors in BBB development from Figure 4 (i.e., TIE2 (TEK), NRP1, CSF1-R, and GPR124 (ADGRA2)) into these models. A successful virtual NVU model will predict developmental misregulation that recapitulates in vivo observations when key parameters are interrogated with chemical toxicity profiles derived from the AOP-based classification model. Moreover, in silico models offer the opportunity to test whether ‘cybermorphs’ missing any of our shortlist of key proteins exhibit aberrant BBB development.
3.5. Limitations and future directions
In developing adverse outcome pathways for BBB development, numerous limitations exist to allow for a complete understanding of the links between disrupted BBB development and neurobehavioral changes (i.e. the ‘black box’). The foremost consideration is that neurobehavioral symptoms of disorders such as ASD are difficult to connect to an underlying pathophysiological change. For example, an autism diagnosis is currently based on observable behaviors, not on anatomical or molecular level pathophysiology. However, as this relatively new field of research grows, candidate gene expression-level biomarkers (e.g., SLC7A5 mutation (Tarlungeanu et al., 2016)) are being identified. Interestingly, at least two of the recently identified autism candidate genes, NRP1 and MMP2 (Diaz-Beltran et al., 2017), are integral to BBB development (Figure 4).
An additional limitation is how the BBB itself is characterized following chemical insult. As described above, BBB permeability of circulating chemicals into the brain serves as the primary indicator of disruption. However, several pharmacokinetic parameters have been shown to be affected by xenobiotic exposure such as local rates of metabolism and influx/efflux active transport in addition to simple permeability. In addition, local xenobiotic metabolism may play a role in altering the pharmacokinetics of certain xenobiotics traversing the BBB. While studies have begun to characterize these phase I and phase II metabolizing enzymes, additional mammalian and human cell line studies will provide a more complete metabolic characterization of the BBB to determine the overall distribution of chemicals to the brain.
Taken as a whole, these pharmacokinetic properties of the BBB will allow for a more accurate representation of the local chemical flux across the barrier and the pharmacodynamic consequences from these local chemical concentrations. Another limitation to keep in mind is that there are many differences in the timing of BBB formation events between rodents (a primary source of data on BBB development) and humans (see Figure 3). Awareness of this discrepancy should help researchers focus on the strengths of existing animal model data (describing molecular interactions and sequences of events during early embryonic development), while protecting against overpredicting human outcomes.
While the majority of this AOP section has focused on establishing potential MIEs of BBB development, future work utilizing in vitro, in vivo, and in silico methods will allow for a complete description of how each MIE is translated as various cell-, tissue-, and organ-specific key events to arrive at the adverse outcome.
4. Closing remarks
This review aims to set the stage for in vitro and in silico modeling of the BBB from a children’s environmental health perspective. While BBB patterning is established within the first trimester and the BBB is arguably functional by human embryonic week 14 (Andrews et al., 2017; Virgintino et al., 2004), BBB maturation continues beyond birth and the brain, including the NVU, is not mature until a person reaches their early 20s. This highlights cells of the NVU as potential targets whose disruption could lead to altered BBB differentiation at earlier stages of development or misregulated intercellular communication at later stages, all of which could underlie the etiology of persistent AOs such as neurobehavioral effects. Approaching BBB research from a children’s environmental health context will require technological advances that facilitate studying the BBB as a target organ of toxicity, while moving beyond traditional BBB research methods that either overlook or mischaracterize the unique features of the developing BBB. As others have demonstrated, the developing BBB is not ‘leaky,’ but rather uniquely suited to respond appropriately to the microenvironment in a developmentally appropriate manner (Ek et al., 2012; Saunders et al., 2016; Saunders et al., 2009). Combining this perspective with the vision of the NVU as a target organ of interest supported by a clear outline of the signaling events and timing underlying BBB formation, differentiation, and function promises to advance the field to identify putative BBB toxicants that may contribute to the etiology of childhood neurobehavioral disorders.
Table 1:
Abbreviations. Bold terms indicate NCBI human gene symbols (common aliases in parentheses) for key proteins or signaling molecules that are included in the blood-brain barrier development biowiring diagram shown in Figure 4. Plain text abbreviations that do not occur in Figure 4 reflect usage by the original author of the cited text, with standard human gene symbols (NCBI) provided in parentheses.
| Column 1 |
Column 2 |
Column 3 |
|||
|---|---|---|---|---|---|
| Abbreviation | Name | Abbreviation | Name | Abbreviation | Name |
| ABC | ATP-binding cassette transporters | FGF2 (BFGF) | fibroblast growth factor 2 | PECAM1 | platelet and endothelial cell adhesion molecule 1 |
| ABCBl (MDRl, P-gly) | ATP binding cassette subfamily B member 1 (P-glycoprotein) | FLT1 (VEGFR1) | fms related tyrosine kinase 1 (VEGF receptor 1) | PLVAP1 (PV-1) | plasmalemma vesicle associated protein |
| ADGRA2 (GPR124) | adhesion G protein-coupled receptor A2 | FLT4 (VEGFR3) | fms related tyrosine kinase 4 (VEGF receptor 3) | PNVP | perineural vascular plexus |
| AGE | advanced glycation endproducts | FOXC2 | forkhead box C2 | PTCH1 | patch |
| AHR | aryl hydrocarbon receptor | FZD4 | frizzled4 | R&D | research and development |
| ALCAM | activated leukocyte cell adhesion molecule | GD | GD followed by a number indicates human gestational day | RA | retinoic acid |
| ALDH1A1 (RALDH1) | aldehyde dehydrogenase 1 family member A1 | GDNF | glial cell line-derived neurotropic factor | RAGE | AGE receptor |
| ALDH1L1 | aldehyde dehydrogenase 1 family member L1 | GFAP | glial fibrillary acidic protein | RARB | retinoic acid receptor beta |
| ALK5 | TGFB receptor 1 | GFRA1 | GDNF family receptor alpha 1 | RBI | rostral blood island |
| ANG1 (Angpt1) | angiopoietin 1 | GLAST (SLC1A3) | solute carrier family 1 member 3 | RC2 (Nes) | nestin (mouse) |
| ANG2 (Angpt2) | angiopoietin 2 | GSTP1 | glutathione S-transferase pi 1 | RECK | reversion inducing cysteine rich protein with kazal motifs |
| AO | adverse outcome | HCI | high content imaging | RELN | reelin |
| AOP | adverse outcome pathway | HES1 | hes family bHLH transcription factor 1 | SELE | selectin |
| ASCL1 | achaete-scute family bHLH transcription factor 1 | HMGB1 | high mobility group box 1 | SEMA3A | semaphorin 3A |
| ASD | autism spectrum disorder | HTS | high throughput screening | Shh | sonic hedgehog |
| BBB | blood-brain barrier | HUVEC | human umbilical vein endothelial cell | SLC29A1 (ENT1) | solute carrier family 29 member 1 |
| BCRP (ABCG2) | ATP binding cassette subfamily G member 2 | ICAM1 | intercellular adhesion molecule 1 | SLC2A1 (GLUT1) | solute carrier family 2 member 1 |
| BLBP (FABP7) | fatty acid binding protein 7 | IL10 | interleukin 10 | SLC3A2 | solute carrier family 3 member 2 |
| BMP | bone morphogenetic protein | IL1B | interleukin 1 beta | SLC7A5 (LAT1) | solute carrier family 7 member 5 |
| CCL2 | C-C motif chemokine ligand 2 | IL34 | interleukin 34 | SLIT2 | slit guidance ligand 2 |
| CCL20 | C-C motif chemokine ligand 20 | IL4 | interleukin 4 | α-SMA | smooth muscle alpha actin (antibody) |
| CD11b (ITGAM) | integrin subunit alpha M | IL6 | interleukin 6 | SMAD2/3 | SMAD family member 2/3 |
| CD11c (ITGAX) | integrin subunit alpha X | KDR (VEGFR2) | kinase insert domain receptor (VEGF receptor 2) | SMAD2/3 | SMAD family member 2/3 |
| CD45 (PTPRC) | protein tyrosine phosphatase, receptor type C | KE | key event | SMO | smoothened |
| CDH1 | cadherin 1 | LEF1 | lymphoid enhancer binding factor 1 | T3 | triiodothyronine |
| CDH2 | cadherin | LGALS3 | galectin 3 | T4 | thyroxine |
| CDH5 (VE-cadherin) | cadherin 5 | LRP5 | Lipoprotein receptor-related protein | TCDD | 2,3,7,8-tetrachlorodibenzodioxin |
| CLDN3 | claudin 3 | MIE | molecular initiating event | TEER | transendothelial electrical resistance |
| CLDN5 | claudin 5 | MMP2/9 | matrix metallopeptidase 2/9 | TEK (TIE2) | TEK receptor tyrosine kinase |
| CNS | central nervous system | NDP | norrin | TGFB1 | transforming growth factor beta 1 |
| CNTF | ciliary neurotrophic factor | NEUROG1 | neurogenin 1 | TGFBR2 | TGFB receptor 2 |
| CSF1 | colony stimulating factor 1 | NEUROG2 | neurogenin 2 | THR | thyroid hormone receptor |
| CSF1-R | colony stimulating factor 1 receptor | NG2 (CSPG4) | chondroitin sulfate proteoglycan 4 | TJP1 (ZO-1) | tight junction protein 1 |
| CS | Carnegie stage | NICD | notch intracellular domain | TJP2 (ZO-2) | tight junction protein 2 |
| CX3CR1 | C-X3-C motif chemokine receptor 1 | NO | nitric oxide | TNC | tenascin C |
| CXCL2 | C-X-C motif chemokine ligand 2 | NOS | nitric oxide synthase | TNF (TNFA) | tumor necrosis factor |
| CXCL8 (IL8) | C-X-C motif chemokine ligand 8 (interleukin 8) | NOTCH | notch | Tox21 | Toxicology Testing in the 21st Century |
| CYP1B1 | cytochrome P450 family 1 subfamily B member 1 | NPC | neuroprogenitor cell | ToxCast | Toxicity ForeCaster |
| CYP2U1 | cytochrome P450 family 2 subfamily U member 1 | NRARP | notch-regulated ankyrin repeat protein | TS | Thieler stage |
| DIO2 | iodothyronine deiodinase 2 | NRP1 | neuropilin 1 | ToxRefDB | Toxicity Reference Database |
| DLL4 | delta like canonical Notch ligand 4 | NVU | neurovascular unit | TSPAN12 | tetra-spanin protein 12 |
| DNT | developmental neurotoxicity | OATP (SLCO1A2) | solute carrier organic anion transporter family member 1A2 | UGT | UDP-glucuronosyltransferase |
| DMT1 (SLC11A2) | divalent metal transporter | OATP2 | organic anion transporting polypeptide 2 | VCAM1 | vascular cell adhesion molecule 1 |
| E | E followed by a number indicates mouse embryonic day | OCLN | occludin | VEGFA | vascular endothelial growth factor A |
| EGF | epidermal growth factor | PCAP | pial capillary anastomotic plexus | VEGFC | vascular endothelial growth factor C |
| EC | endothelial cell | PDGFB | platelet derived growth factor subunit B | Vmax | maximum velocity |
| EOMES (TBR2) | eomesodermin | PDGFBB | PDGFB homodimer | VTM | virtual tissue model |
| FGF | fibroblast growth factor | PDGFRB | platelet derived growth factor subunit B receptor | Wnt | Wnt signaling pathway |
5. Acknowledgements
Kevin Crofton (EPA/ORD/NCCT), John Cowden (EPA/ORD/NCCT), and William Boyes (EPA/ORD/NHEERL) provided valuable edits and feedback. The beta version of the ToxPi software used to produce Figure 2 was provided by David Reif (dmreif@ncsu.edu) as part of U.S. EPA STAR #R835802. Molly Windsor (CSRA) provided the organism sketches used in Figure 2. Bellina Veronisi (EPA/ORD/NHEERL, retired) contributed to an earlier version of this manuscript.
Funding: This work was jointly funded by the Chemical Safety for Sustainability (CSS) Research Program (U.S. EPA/ORD), U.S. EPA/ORD/NCCT contract EP-G16D-00235 with Leidos, and the Singapore Agency for Science, Technology and Research (A*STAR / SIgN).
Footnotes
6. Conflict of interest statement
The authors declare no conflicts.
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
7. Literature cited
- Abbott NJ, 2013. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36(3), 437–449. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Lane NJ, Bundgaard M, 1992. A fibre matrix model for the restricting junction of the blood-brain barrier in a cephalopod mollusc: implications for capillary and epithelial permeability. J Neurocytol 21(4), 304–311. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ, 2010. Structure and function of the blood-brain barrier. Neurobiol Dis 37(1), 13–25. [DOI] [PubMed] [Google Scholar]
- Achyuta AK, Conway AJ, Crouse RB, Bannister EC, Lee RN, Katnik CP, Behensky AA, Cuevas J, Sundaram SS, 2013. A modular approach to create a neurovascular unit-on-a-chip. Lab Chip 13(4), 542–553. [DOI] [PubMed] [Google Scholar]
- Adriani G, Ma D, Pavesi A, Kamm RD, Goh EL, 2016. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip 17(3), 448–459. [DOI] [PubMed] [Google Scholar]
- Aguilera KY, Brekken RA, 2014. Recruitment and retention: factors that affect pericyte migration. Cell Mol Life Sci 71(2), 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp G, Gamble HJ, 1979. An electron microscopic study of the pericytes of the developing capillaries in human fetal brain and muscle. J Anat 128(Pt 1), 155–168. [PMC free article] [PubMed] [Google Scholar]
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A, 2011. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334(6063), 1727–1731. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Katayama T, Prat A, 2013. Glial influence on the blood brain barrier. Glia 61(12), 1939–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KD, Pan L, Yang XM, Hughes VC, Walls JR, Dominguez MG, Simmons MV, Burfeind P, Xue Y, Wei Y, Macdonald LE, Thurston G, Daly C, Lin HC, Economides AN, Valenzuela DM, Murphy AJ, Yancopoulos GD, Gale NW, 2011. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc Natl Acad Sci U S A 108(7), 2807–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Fukuhara S, Izumi N, Nakajima H, Fukui H, Kelsh RN, Mochizuki N, 2016. Clarification of mural cell coverage of vascular endothelial cells by live imaging of zebrafish. Development 143(8), 1328–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews AM, Lutton EM, Cannella LA, Reichenbach N, Razmpour R, Seasock MJ, Kaspin SJ, Merkel SF, Langford D, Persidsky Y, Ramirez SH, 2017. Characterization of human fetal brain endothelial cells reveals barrier properties suitable for in vitro modeling of the BBB with syngenic co-cultures. J Cereb Blood Flow Metab, 271678X17708690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelt-Menzel A, Cubukova A, Gunther K, Edenhofer F, Piontek J, Krause G, Stuber T, Walles H, Neuhaus W, Metzger M, 2017. Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri- and Multipotent Stem Cells. Stem Cell Reports 8(4), 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C, 2010. Pericytes regulate the blood-brain barrier. Nature 468(7323), 557–561. [DOI] [PubMed] [Google Scholar]
- Aschner M, Ceccatelli S, Daneshian M, Fritsche E, Hasiwa N, Hartung T, Hogberg HT, Leist M, Li A, Mundi WR, Padilla S, Piersma AH, Bal-Price A, Seiler A, Westerink RH, Zimmer B, Lein PJ, 2017. Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: example lists and criteria for their selection and use. ALTEX 34(1), 49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K, 2009. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10(3), 165–177. [DOI] [PubMed] [Google Scholar]
- Babakhanian K, Bendayan M, Bendayan R, 2007. Localization of P-glycoprotein at the nuclear envelope of rat brain cells. Biochem Biophys Res Commun 361(2), 301–306. [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Crofton KM, Sachana M, Shafer TJ, Behl M, Forsby A, Hargreaves A, Landesmann B, Lein PJ, Louisse J, Monnet-Tschudi F, Paini A, Rolaki A, Schrattenholz A, Sunol C, van Thriel C, Whelan M, Fritsche E, 2015. Putative adverse outcome pathways relevant to neurotoxicity. Crit Rev Toxicol 45(1), 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee J, Shi Y, Azevedo HS, 2016. In vitro blood-brain barrier models for drug research: state-of-the-art and new perspectives on reconstituting these models on artificial basement membrane platforms. Drug Discov Today 21(9), 1367–1386. [DOI] [PubMed] [Google Scholar]
- Banos G, Daniel PM, Pratt OE, 1978. The effect of age upon the entry of some amino acids into the brain, and their incorporation into cerebral protein. Dev Med Child Neurol 20(3), 335–346. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Lieth E, 1997. Agrin accumulates in the brain microvascular basal lamina during development of the blood-brain barrier. Dev Dyn 208(1), 62–74. [DOI] [PubMed] [Google Scholar]
- Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D, 1996. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87(8), 3336–3343. [PubMed] [Google Scholar]
- Baron MH, Isern J, Fraser ST, 2012. The embryonic origins of erythropoiesis in mammals. Blood 119(21), 4828–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer HC, Bauer H, Lametschwandtner A, Amberger A, Ruiz P, Steiner M, 1993. Neovascularization and the appearance of morphological characteristics of the blood-brain barrier in the embryonic mouse central nervous system. Brain Res Dev Brain Res 75(2), 269–278. [DOI] [PubMed] [Google Scholar]
- Belair DG, Schwartz MP, Knudsen T, Murphy WL, 2016. Human iPSC-derived endothelial cell sprouting assay in synthetic hydrogel arrays. Acta Biomater 39, 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Marinero S, Hassanzadeh T, Natesan D, Le D, Belliveau C, Mason SN, Auten RL, Bilbo SD, 2017. Gestational Exposure to Air Pollution Alters Cortical Volume, Microglial Morphology, and Microglia-Neuron Interactions in a Sex-Specific Manner. Front Synaptic Neurosci 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney S, Harrison-Uy S, Mishra S, MacPherson AM, Choe Y, Li D, Jaminet SC, Fruttiger M, Pleasure SJ, Siegenthaler JA, 2016. Diverse Functions of Retinoic Acid in Brain Vascular Development. J Neurosci 36(29), 7786–7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger-Weill J, Candat V, Jouary A, Romano SA, Perez-Schuster V, Sumbre G, 2017. Functional Interactions between Newborn and Mature Neurons Leading to Integration into Established Neuronal Circuits. Curr Biol 27(12), 1707–1720 e1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MW, Deane R, 1993. Permeability of the blood-brain barrier to lead. Neurotoxicology 14(2–3), 131–136. [PubMed] [Google Scholar]
- Braun LD, Cornford EM, Oldendorf WH, 1980. Newborn rabbit blood-brain barrier is selectively permeable and differs substantially from the adult. J Neurochem 34(1), 147–152. [DOI] [PubMed] [Google Scholar]
- Brown JA, Pensabene V, Markov DA, Allwardt V, Neely MD, Shi M, Britt CM, Hoilett OS, Yang Q, Brewer BM, Samson PC, McCawley LJ, May JM, Webb DJ, Li D, Bowman AB, Reiserer RS, Wikswo JP, 2015. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics 9(5), 054124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugel SM, Tanguay RL, Planchart A, 2014. Zebrafish: A marvel of high-throughput biology for 21st century toxicology. Curr Environ Health Rep 1(4), 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfone A, Martinez S, Marigo V, Campanella M, Basile A, Quaderi N, Gattuso C, Rubenstein JL, Ballabio A, 1999. Expression pattern of the Tbr2 (Eomesodermin) gene during mouse and chick brain development. Mech Dev 84(1–2), 133–138. [DOI] [PubMed] [Google Scholar]
- Bundgaard M, 1982. Brain barrier systems in the lamprey. I. Ultrastructure and permeability of cerebral blood vessels. Brain Res 240(1), 55–64. [DOI] [PubMed] [Google Scholar]
- Bundgaard M, Abbott NJ, 2008. All vertebrates started out with a glial blood-brain barrier 4–500 million years ago. Glia 56(7), 699–708. [DOI] [PubMed] [Google Scholar]
- Bundgaard M, van Deurs B, 1982. Brain barrier systems in the lamprey. II. Ultrastructure and permeability of the choroid plexus. Brain Res 240(1), 65–75. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA, 2008. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28(1), 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Lechleider RJ, 2004. Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res 94(9), 1195–1202. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang Y, Tang J, Liu F, Hu Q, Luo C, Tang J, Feng H, Zhang JH, 2015. Norrin protected blood-brain barrier via frizzled-4/beta-catenin pathway after subarachnoid hemorrhage in rats. Stroke 46(2), 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, McConnell SK, 1995. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell 82(4), 631–641. [DOI] [PubMed] [Google Scholar]
- Congress, t., 2016. Frank R. Lautenberg Chemical Safety for the 21st Century Act, Public Law 114–182. [Google Scholar]
- Coon BG, Baeyens N, Han J, Budatha M, Ross TD, Fang JS, Yun S, Thomas JL, Schwartz MA, 2015. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol 208(7), 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, Iruela-Arispe ML, Adams RH, Dejana E, 2010. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell 18(6), 938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornford EM, Cornford ME, 1986. Nutrient transport and the blood-brain barrier in developing animals. Fed Proc 45(7), 2065–2072. [PubMed] [Google Scholar]
- Cory-Slechta DA, Crofton KM, Foran JA, Ross JF, Sheets LP, Weiss B, Mileson B, 2001. Methods to identify and characterize developmental neurotoxicity for human health risk assessment. I: behavioral effects. Environ Health Perspect 109 Suppl 1, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Chawengsaksophak K, Rossant J, 2005. Endothelial cells and VEGF in vascular development. Nature 438(7070), 937–945. [DOI] [PubMed] [Google Scholar]
- Cremer JE, Cunningham VJ, Pardridge WM, Braun LD, Oldendorf WH, 1979. Kinetics of blood-brain barrier transport of pyruvate, lactate and glucose in suckling, weanling and adult rats. J Neurochem 33(2), 439–445. [DOI] [PubMed] [Google Scholar]
- Cucullo L, Marchi N, Hossain M, Janigro D, 2011. A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J Cereb Blood Flow Metab 31(2), 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, Lima FR, 2014. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci 8, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, 2012. The blood-brain barrier in health and disease. Ann Neurol 72(5), 648–672. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA, 2009. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A 106(2), 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA, 2010. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468(7323), 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchy S, Dutheil F, Weaver RJ, Chassoux F, Daumas-Duport C, Couraud PO, Scherrmann JM, De Waziers I, Decleves X, 2008. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem 107(6), 1518–1528. [DOI] [PubMed] [Google Scholar]
- Dejana E, 2010. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res 107(8), 943–952. [DOI] [PubMed] [Google Scholar]
- Desai SY, Marroni M, Cucullo L, Krizanac-Bengez L, Mayberg MR, Hossain MT, Grant GG, Janigro D, 2002. Mechanisms of endothelial survival under shear stress. Endothelium 9(2), 89–102. [DOI] [PubMed] [Google Scholar]
- Diaz-Beltran L, Esteban FJ, Varma M, Ortuzk A, David M, Wall DP, 2017. Cross-disorder comparative analysis of comorbid conditions reveals novel autism candidate genes. BMC Genomics 18(1), 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AP, Harik SI, Klip A, Walker DM, 1984. Identification and characterization of the glucose transporter of the blood-brain barrier by cytochalasin B binding and immunological reactivity. Proc Natl Acad Sci U S A 81(22), 7233–7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan LJ, Nagy A, Fong GH, 2003. Gastrulation and angiogenesis, not endothelial specification, is sensitive to partial deficiency in vascular endothelial growth factor-a in mice. Biol Reprod 69(6), 1852–1858. [DOI] [PubMed] [Google Scholar]
- Dudvarski Stankovic N, Teodorczyk M, Ploen R, Zipp F, Schmidt MH, 2016. Microglia-blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathol 131(3), 347–363. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl GJ, Fong GH, Auerbach R, Breitman ML, 1993. The endothelial-specific receptor tyrosine kinase, tek, is a member of a new subfamily of receptors. Oncogene 8(5), 1293–1301. [PubMed] [Google Scholar]
- Dwyer ND, Chen B, Chou SJ, Hippenmeyer S, Nguyen L, Ghashghaei HT, 2016. Neural Stem Cells to Cerebral Cortex: Emerging Mechanisms Regulating Progenitor Behavior and Productivity. J Neurosci 36(45), 11394–11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle KL, Mitrofanis J, 1998. Development of glia and blood vessels in the internal capsule of rats. J Neurocytol 27(2), 127–139. [DOI] [PubMed] [Google Scholar]
- Ehrlich LC, Hu S, Sheng WS, Sutton RL, Rockswold GL, Peterson PK, Chao CC, 1998. Cytokine regulation of human microglial cell IL-8 production. J Immunol 160(4), 1944–1948. [PubMed] [Google Scholar]
- Ek CJ, Dziegielewska KM, Habgood MD, Saunders NR, 2012. Barriers in the developing brain and Neurotoxicology. Neurotoxicology 33(3), 586–604. [DOI] [PubMed] [Google Scholar]
- El-Khoury N, Braun A, Hu F, Pandey M, Nedergaard M, Lagamma EF, Ballabh P, 2006. Astrocyte end-feet in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res 59(5), 673–679. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcin C, Montiel-Nava C, Patel V, Paula CS, Wang C, Yasamy MT, Fombonne E, 2012. Global prevalence of autism and other pervasive developmental disorders. Autism Res 5(3), 160–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Liebner S, 2014. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res 355(3), 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, Walker DG, Stern DM, Yan S, Schmidt AM, Chen JX, Yan SS, 2010. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J 24(4), 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C, 2010. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116(5), 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Plein A, Maden CH, Ruhrberg C, 2013. The embryonic mouse hindbrain as a qualitative and quantitative model for studying the molecular and cellular mechanisms of angiogenesis. Nat Protoc 8(2), 418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A, Diekmann H, Goldsmith P, 2013. Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS One 8(10), e77548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaengel K, Genove G, Armulik A, Betsholtz C, 2009. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29(5), 630–638. [DOI] [PubMed] [Google Scholar]
- Gazzin S, Strazielle N, Schmitt C, Fevre-Montange M, Ostrow JD, Tiribelli C, Ghersi-Egea JF, 2008. Differential expression of the multidrug resistance-related proteins ABCb1 and ABCc1 between blood-brain interfaces. J Comp Neurol 510(5), 497–507. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C, 2003. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 314(1), 15–23. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C, 2003. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161(6), 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C, 2004. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn 231(3), 503–509. [DOI] [PubMed] [Google Scholar]
- Ghersi-Egea JF, Minn A, Siest G, 1988. A new aspect of the protective functions of the blood-brain barrier: activities of four drug-metabolizing enzymes in isolated rat brain microvessels. Life Sci 42(24), 2515–2523. [DOI] [PubMed] [Google Scholar]
- Gilbert SE, 2013. Developmental Biology, 10th ed. Sinauer Associates, Inc., Sunderland, MA. [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M, 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330(6005), 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Lim S, Hoeffel G, Low D, Huber T, 2013. Origin and differentiation of microglia. Front Cell Neurosci 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK, 2016. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol 17(1), 34–40. [DOI] [PubMed] [Google Scholar]
- Golden PL, Pollack GM, 2003. Blood-brain barrier efflux transport. J Pharm Sci 92(9), 1739–1753. [DOI] [PubMed] [Google Scholar]
- Goldie LC, Nix MK, Hirschi KK, 2008. Embryonic vasculogenesis and hematopoietic specification. Organogenesis 4(4), 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Huttner WB, 2005. The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6(10), 777–788. [DOI] [PubMed] [Google Scholar]
- Granberg L, Ostergren A, Brandt I, Brittebo EB, 2003. CYP1A1 and CYP1B1 in blood-brain interfaces: CYP1A1-dependent bioactivation of 7,12-dimethylbenz(a)anthracene in endothelial cells. Drug Metab Dispos 31(3), 259–265. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ, 2014. Neurobehavioural effects of developmental toxicity. Lancet Neurol 13(3), 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, Frei K, Ginhoux F, Merad M, Becher B, 2012. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37(6), 1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Jiang L, 2016. Organic anion transporting polypeptide 2 transports valproic acid in rat brain microvascular endothelial cells. Neurol Res 38(7), 634–639. [DOI] [PubMed] [Google Scholar]
- Hanamsagar R, Bilbo SD, 2016. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol 160, 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H, 2007. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11), 1387–1394. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PR, Kriegstein AR, 2010. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464(7288), 554–561. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M, 2001. Characterization of CNS precursor subtypes and radial glia. Dev Biol 229(1), 15–30. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, 2010. Regulation of ABC transporters at the blood-brain barrier: new targets for CNS therapy. Mol Interv 10(5), 293–304. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B, 2011. ABC transporters in the CNS - an inventory. Curr Pharm Biotechnol 12(4), 656–673. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB, 2004. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A 101(9), 3196–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward P, Kalmar T, Arias AM, 2008. Wnt/Notch signalling and information processing during development. Development 135(3), 411–424. [DOI] [PubMed] [Google Scholar]
- Hellman K, Aadal Nielsen P, Ek F, Olsson R, 2016. An ex Vivo Model for Evaluating Blood-Brain Barrier Permeability, Efflux, and Drug Metabolism. ACS Chem Neurosci 7(5), 668–680. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Gerhardt H, 2007. VEGF and Notch signaling: the yin and yang of angiogenic sprouting. Cell Adh Migr 1(3), 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms HC, Abbott NJ, Burek M, Cecchelli R, Couraud PO, Deli MA, Forster C, Galla HJ, Romero IA, Shusta EV, Stebbins MJ, Vandenhaute E, Weksler B, Brodin B, 2016. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab 36(5), 862–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C, 1999. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 126(17), 3735–3745. [DOI] [PubMed] [Google Scholar]
- Herland A, van der Meer AD, FitzGerald EA, Park TE, Sleeboom JJ, Ingber DE, 2016. Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip. PLoS One 11(3), e0150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer DB, Meredith RM, 2017. Environmental toxicology: Sensitive periods of development and neurodevelopmental disorders. Neurotoxicology 58, 23–41. [DOI] [PubMed] [Google Scholar]
- Higgins CF, 1992. ABC transporters: from microorganisms to man. Annu Rev Cell Biol 8, 67–113. [DOI] [PubMed] [Google Scholar]
- Hill MA, 2016. Embryology Carnegie Stage Comparison. https://embryology.med.unsw.edu.au/embryology/index.php/Carnegie_Stage_Comparison. (Accessed August 10th 2016).
- Hindle SJ, Bainton RJ, 2014. Barrier mechanisms in the Drosophila blood-brain barrier. Front Neurosci 8, 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffel G, Ginhoux F, 2015. Ontogeny of Tissue-Resident Macrophages. Front Immunol 6, 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan KA, Ambler CA, Chapman DL, Bautch VL, 2004. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development 131(7), 1503–1513. [DOI] [PubMed] [Google Scholar]
- Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T, 2004. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem 89(2), 503–513. [DOI] [PubMed] [Google Scholar]
- Howard B, Chen Y, Zecevic N, 2006. Cortical progenitor cells in the developing human telencephalon. Glia 53(1), 57–66. [DOI] [PubMed] [Google Scholar]
- Hussain M, Bork K, Gnanapragassam VS, Bennmann D, Jacobs K, Navarette-Santos A, Hofmann B, Simm A, Danker K, Horstkorte R, 2016. Novel insights in the dysfunction of human blood-brain barrier after glycation. Mech Ageing Dev 155, 48–54. [DOI] [PubMed] [Google Scholar]
- Hutson MS, Leung MC, Baker NC, Spencer RM, Knudsen TB, 2017. Computational Model of Secondary Palate Fusion and Disruption. Chem Res Toxicol 30(4), 965–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi Y, Utsumi H, Chiba H, Yamada-Sasamori Y, Tobioka H, Kamimura Y, Furuuchi K, Kokai Y, Nakagawa T, Mori M, Sawada N, 1999. Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood-brain barrier. Biochem Biophys Res Commun 261(1), 108–112. [DOI] [PubMed] [Google Scholar]
- Ioannidou S, Deinhardt K, Miotla J, Bradley J, Cheung E, Samuelsson S, Ng YS, Shima DT, 2006. An in vitro assay reveals a role for the diaphragm protein PV-1 in endothelial fenestra morphogenesis. Proc Natl Acad Sci U S A 103(45), 16770–16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irintchev A, Rollenhagen A, Troncoso E, Kiss JZ, Schachner M, 2005. Structural and functional aberrations in the cerebral cortex of tenascin-C deficient mice. Cereb Cortex 15(7), 950–962. [DOI] [PubMed] [Google Scholar]
- Irons TD, Kelly PE, Hunter DL, Macphail RC, Padilla S, 2013. Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol Biochem Behav 103(4), 792–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A, Hartz AM, Potin S, Coumoul X, Yousif S, Scherrmann JM, Bauer B, Decleves X, 2011. Aryl hydrocarbon receptor-dependent upregulation of Cyp1b1 by TCDD and diesel exhaust particles in rat brain microvessels. Fluids Barriers CNS 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JM, Gewolb C, Bautch VL, 2009. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development 136(5), 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch M, Leppanen VM, Saharinen P, Alitalo K, 2013. Receptor tyrosine kinase-mediated angiogenesis. Cold Spring Harb Perspect Biol 5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JY, Kwon HB, Ahn JC, Kang D, Kwon SH, Park JA, Kim KW, 2008. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res Bull 75(5), 619–628. [DOI] [PubMed] [Google Scholar]
- Jiang X, McDermott JR, Ajees AA, Rosen BP, Liu Z, 2010. Trivalent arsenicals and glucose use different translocation pathways in mammalian GLUT1. Metallomics 2(3), 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, Costa M, Rice DS, Ye W, 2009. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell 139(2), 299–311. [DOI] [PubMed] [Google Scholar]
- Kadereit S, Zimmer B, van Thriel C, Hengstler JG, Leist M, 2012. Compound selection for in vitro modeling of developmental neurotoxicity. Front Biosci (Landmark Ed) 17, 2442–2460. [DOI] [PubMed] [Google Scholar]
- Kaisar MA, Sajja RK, Prasad S, Abhyankar VV, Liles T, Cucullo L, 2017. New experimental models of the blood-brain barrier for CNS drug discovery. Expert Opin Drug Discov 12(1), 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H, 1999. A requirement for neuropilin-1 in embryonic vessel formation. Development 126(21), 4895–4902. [DOI] [PubMed] [Google Scholar]
- Kerper LE, Hinkle PM, 1997. Lead uptake in brain capillary endothelial cells: activation by calcium store depletion. Toxicol Appl Pharmacol 146(1), 127–133. [DOI] [PubMed] [Google Scholar]
- Kim JA, Kim HN, Im SK, Chung S, Kang JY, Choi N, 2015. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics 9(2), 024115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstreuer N, Dix D, Rountree M, Baker N, Sipes N, Reif D, Spencer R, Knudsen T, 2013. A computational model predicting disruption of blood vessel development. PLoS Comput Biol 9(4), e1002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstreuer NC, Judson RS, Reif DM, Sipes NS, Singh AV, Chandler KJ, Dewoskin R, Dix DJ, Kavlock RJ, Knudsen TB, 2011. Environmental impact on vascular development predicted by high-throughput screening. Environ Health Perspect 119(11), 1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper J, Scheffer H, Leiendecker B, Gertsen E, Binder S, Leferink M, Hertzberg C, Nake A, Voit T, Willemsen MA, 2005. Seizure control and acceptance of the ketogenic diet in GLUT1 deficiency syndrome: a 2- to 5-year follow-up of 15 children enrolled prospectively. Neuropediatrics 36(5), 302–308. [DOI] [PubMed] [Google Scholar]
- Knudsen TB, Kleinstreuer NC, 2011. Disruption of embryonic vascular development in predictive toxicology. Birth Defects Res C Embryo Today 93(4), 312–323. [DOI] [PubMed] [Google Scholar]
- Knudsen TB, Martin MT, Kavlock RJ, Judson RS, Dix DJ, Singh AV, 2009. Profiling the activity of environmental chemicals in prenatal developmental toxicity studies using the U.S. EPA’s ToxRefDB. Reprod Toxicol 28(2), 209–219. [DOI] [PubMed] [Google Scholar]
- Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE, 2005. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol 61(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Korn J, Christ B, Kurz H, 2002. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J Comp Neurol 442(1), 78–88. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A, 2009. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32, 149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krum JM, More NS, Rosenstein JM, 1991. Brain angiogenesis: variations in vascular basement membrane glycoprotein immunoreactivity. Exp Neurol 111(2), 152–165. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, Kuo CJ, 2010. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science 330(6006), 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLone CA, Villeneuve DL, Lyons D, Helgen HW, Robinson SL, Swintek JA, Saari TW, Ankley GT, 2016. Editor’s Highlight: Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS): A Web-Based Tool for Addressing the Challenges of Cross-Species Extrapolation of Chemical Toxicity. Toxicol Sci 153(2), 228–245. [DOI] [PubMed] [Google Scholar]
- Landry CF, Ivy GO, Brown IR, 1990. Developmental expression of glial fibrillary acidic protein mRNA in the rat brain analyzed by in situ hybridization. J Neurosci Res 25(2), 194–203. [DOI] [PubMed] [Google Scholar]
- Laterra J KR, Betz LA, Goldstein GW, 1999. Blood—Brain Barrier, 6th edition ed. Lippincott-Raven, Philadelphia. [Google Scholar]
- Le Vee M, Jouan E, Lecureur V, Fardel O, 2016. Aryl hydrocarbon receptor-dependent up-regulation of the heterodimeric amino acid transporter LAT1 (SLC7A5)/CD98hc (SLC3A2) by diesel exhaust particle extract in human bronchial epithelial cells. Toxicol Appl Pharmacol 290, 74–85. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim WJ, Jun HO, Choi YK, Kim KW, 2009. Angiopoietin-1 reduces vascular endothelial growth factor-induced brain endothelial permeability via upregulation of ZO-2. Int J Mol Med 23(2), 279–284. [PubMed] [Google Scholar]
- Leung MC, Hutson MS, Seifert AW, Spencer RM, Knudsen TB, 2016a. Computational modeling and simulation of genital tubercle development. Reprod Toxicol 64, 151–161. [DOI] [PubMed] [Google Scholar]
- Leung MC, Phuong J, Baker NC, Sipes NS, Klinefelter GR, Martin MT, McLaurin KW, Setzer RW, Darney SP, Judson RS, Knudsen TB, 2016b. Systems Toxicology of Male Reproductive Development: Profiling 774 Chemicals for Molecular Targets and Adverse Outcomes. Environ Health Perspect 124(7), 1050–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lan Y, Wang Y, Wang J, Yang G, Meng F, Han H, Meng A, Wang Y, Yang X, 2011. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell 20(3), 291–302. [DOI] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E, 2008. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol 183(3), 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C, 1997. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277(5323), 242–245. [DOI] [PubMed] [Google Scholar]
- Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C, 2003. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev 17(15), 1835–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, Palecek SP, Shusta EV, 2012. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol 30(8), 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Sanchez MA, Jiang X, Boles E, Landfear SM, Rosen BP, 2006. Mammalian glucose permease GLUT1 facilitates transport of arsenic trioxide and methylarsonous acid. Biochem Biophys Res Commun 351(2), 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Vinukonda G, Vose LR, Diamond D, Bhimavarapu BB, Hu F, Zia MT, Hevner R, Zecevic N, Ballabh P, 2013. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci 33(2), 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M, 2012. The human brain intracerebral microvascular system: development and structure. Front Neuroanat 6, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, 2014. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell M, Galanopoulos T, Neville-Golden J, Hedley-Whyte ET, Antoniades HN, 1998. Cellular localization of PDGF mRNAs in developing human forebrain. Neuropathol Appl Neurobiol 24(5), 337–345. [DOI] [PubMed] [Google Scholar]
- Mito T, Konomi H, Houdou S, Takashima S, 1991. Immunohistochemical study of the vasculature in the developing brain. Pediatr Neurol 7(1), 18–22. [DOI] [PubMed] [Google Scholar]
- Mizee MR, Wooldrik D, Lakeman KA, van het Hof B, Drexhage JA, Geerts D, Bugiani M, Aronica E, Mebius RE, Prat A, de Vries HE, Reijerkerk A, 2013. Retinoic acid induces blood-brain barrier development. J Neurosci 33(4), 1660–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moley KH, 2001. Hyperglycemia and apoptosis: mechanisms for congenital malformations and pregnancy loss in diabetic women. Trends Endocrinol Metab 12(2), 78–82. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH, 2012. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 26(9), 891–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier A, Adle-Biassette H, Delezoide AL, Evrard P, Gressens P, Verney C, 2007. Entry and distribution of microglial cells in human embryonic and fetal cerebral cortex. J Neuropathol Exp Neurol 66(5), 372–382. [DOI] [PubMed] [Google Scholar]
- Montanari F, Ecker GF, 2015. Prediction of drug-ABC-transporter interaction--Recent advances and future challenges. Adv Drug Deliv Rev 86, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morest DK, Silver J, 2003. Precursors of neurons, neuroglia, and ependymal cells in the CNS: what are they? Where are they from? How do they get where they are going? Glia 43(1), 6–18. [DOI] [PubMed] [Google Scholar]
- Morris ME, Rodriguez-Cruz V, Felmlee MA, 2017. SLC and ABC Transporters: Expression, Localization, and Species Differences at the Blood-Brain and the Blood-Cerebrospinal Fluid Barriers. AAPS J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morte B, Bernal J, 2014. Thyroid hormone action: astrocyte-neuron communication. Front Endocrinol (Lausanne) 5, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy WR, Padilla S, Breier JM, Crofton KM, Gilbert ME, Herr DW, Jensen KF, Radio NM, Raffaele KC, Schumacher K, Shafer TJ, Cowden J, 2015. Expanding the test set: Chemicals with potential to disrupt mammalian brain development. Neurotoxicol Teratol 52(Pt A), 25–35. [DOI] [PubMed] [Google Scholar]
- Nag S, Papneja T, Venugopalan R, Stewart DJ, 2005. Increased angiopoietin2 expression is associated with endothelial apoptosis and blood-brain barrier breakdown. Lab Invest 85(10), 1189–1198. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, 2006. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer 6(12), 947–960. [DOI] [PubMed] [Google Scholar]
- Nguyen EH, Daly WT, Le NNT, Farnoodian M, Belari DG, Schwartz MP, Lebakken CS, Ananiev GE, Saghiri MA, Knudsen TB, Sheibani N, Murphy WL, accepted. Versatile Synthetic Alternatives to Matrigel for Vascular Toxicity Screening and Stem Cell Expansion. Nature BMC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio T, Kawaguchi S, Iseda T, Kawasaki T, Hase T, 2003. Secretion of tenascin-C by cultured astrocytes: regulation of cell proliferation and process elongation. Brain Res 990(1–2), 129–140. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR, 2001. Neurons derived from radial glial cells establish radial units in neocortex. Nature 409(6821), 714–720. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Kriegstein AR, 2008. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol 508(1), 28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC, 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC. [Google Scholar]
- Obermeier B, Daneman R, Ransohoff RM, 2013. Development, maintenance and disruption of the blood-brain barrier. Nat Med 19(12), 1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzzine M, Gulberti S, Ramalanjaona N, Magdalou J, Fournel-Gigleux S, 2014. The UDP-glucuronosyltransferases of the blood-brain barrier: their role in drug metabolism and detoxication. Front Cell Neurosci 8, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U, Stallcup WB, 2003. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis 6(3), 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palenski TL, Sorenson CM, Jefcoate CR, Sheibani N, 2013. Lack of Cyp1b1 promotes the proliferative and migratory phenotype of perivascular supporting cells. Lab Invest 93(6), 646–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J, McGrath KE, Kingsley PD, 1995. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood 86(1), 156–163. [PubMed] [Google Scholar]
- Papageorghiou AT, Kennedy SH, Salomon LJ, Ohuma EO, Cheikh Ismail L, Barros FC, Lambert A, Carvalho M, Jaffer YA, Bertino E, Gravett MG, Altman DG, Purwar M, Noble JA, Pang R, Victora CG, Bhutta ZA, Villar J, International F, Newborn Growth Consortium for the 21st, C., 2014. International standards for early fetal size and pregnancy dating based on ultrasound measurement of crown-rump length in the first trimester of pregnancy. Ultrasound Obstet Gynecol 44(6), 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanaud L, Yassine F, Dieterlen-Lievre F, 1989. Relationship between vasculogenesis, angiogenesis and haemopoiesis during avian ontogeny. Development 105(3), 473–485. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, 1983. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev 63(4), 1481–1535. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, 2012. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 32(11), 1959–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paridaen JT, Huttner WB, 2014. Neurogenesis during development of the vertebrate central nervous system. EMBO Rep 15(4), 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins EJ, Ankley GT, Crofton KM, Garcia-Reyero N, LaLone CA, Johnson MS, Tietge JE, Villeneuve DL, 2013. Current perspectives on the use of alternative species in human health and ecological hazard assessments. Environ Health Perspect 121(9), 1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, Ondr JK, Rao S, Lang RA, Thurston G, Gerhardt H, 2009. Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev Cell 16(1), 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WJ, Rosenbaum JL, Dence CS, Markham J, Videen TO, 1998. Cerebral glucose transport and metabolism in preterm human infants. J Cereb Blood Flow Metab 18(6), 632–638. [DOI] [PubMed] [Google Scholar]
- Qin M, Wong A, Seguin D, Gerlai R, 2014. Induction of social behavior in zebrafish: live versus computer animated fish as stimuli. Zebrafish 11(3), 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Sato TN, 1995. Mouse multidrug resistance 1a/3 gene is the earliest known endothelial cell differentiation marker during blood-brain barrier development. Dev Dyn 202(2), 172–180. [DOI] [PubMed] [Google Scholar]
- Raab S, Beck H, Gaumann A, Yuce A, Gerber HP, Plate K, Hammes HP, Ferrara N, Breier G, 2004. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost 91(3), 595–605. [DOI] [PubMed] [Google Scholar]
- Rakic P, 1972. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol 145(1), 61–83. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, 2016. How neuroinflammation contributes to neurodegeneration. Science 353(6301), 777–783. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH, 2009. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 27, 119–145. [DOI] [PubMed] [Google Scholar]
- Reif DM, Martin MT, Tan SW, Houck KA, Judson RS, Richard AM, Knudsen TB, Dix DJ, Kavlock RJ, 2010. Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ Health Perspect 118(12), 1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard AM, Judson RS, Houck KA, Grulke CM, Volarath P, Thillainadarajah I, Yang C, Rathman J, Martin MT, Wambaugh JF, Knudsen TB, Kancherla J, Mansouri K, Patlewicz G, Williams AJ, Little SB, Crofton KM, Thomas RS, 2016. ToxCast Chemical Landscape: Paving the Road to 21st Century Toxicology. Chem Res Toxicol 29(8), 1225–1251. [DOI] [PubMed] [Google Scholar]
- Robertson PL, Du Bois M, Bowman PD, Goldstein GW, 1985. Angiogenesis in developing rat brain: an in vivo and in vitro study. Brain Res 355(2), 219–223. [DOI] [PubMed] [Google Scholar]
- Roessmann U, Gambetti P, 1986. Astrocytes in the developing human brain. An immunohistochemical study. Acta Neuropathol 70(3–4), 308–313. [DOI] [PubMed] [Google Scholar]
- Rymo SF, Gerhardt H, Wolfhagen Sand F, Lang R, Uv A, Betsholtz C, 2011. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS One 6(1), e15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saili K, Zurlinden T, Knudsen T, 2017. Modeling the Neurovascular Unit in vitro and in silico, in: William Slikker J (Ed.) Handbook of Developmental Neurotoxicology; in press. [Google Scholar]
- Saili KS, Corvi MM, Weber DN, Patel AU, Das SR, Przybyla J, Anderson KA, Tanguay RL, 2012. Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology 291(1–3), 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Covarrubias L, Slosky LM, Thompson BJ, Davis TP, Ronaldson PT, 2014. Transporters at CNS barrier sites: obstacles or opportunities for drug delivery? Curr Pharm Des 20(10), 1422–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NR, Dziegielewska KM, Unsicker K, Ek CJ, 2016. Delayed astrocytic contact with cerebral blood vessels in FGF-2 deficient mice does not compromise permeability properties at the developing blood-brain barrier. Dev Neurobiol 76(11), 1201–1212. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Joakim Ek C, Dziegielewska KM, 2009. The neonatal blood-brain barrier is functionally effective, and immaturity does not explain differential targeting of AAV9. Nat Biotechnol 27(9), 804–805; author reply 805. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Liddelow SA, Dziegielewska KM, 2012. Barrier mechanisms in the developing brain. Front Pharmacol 3, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Stevens B, 2010. Synapse elimination during development and disease: immune molecules take centre stage. Biochem Soc Trans 38(2), 476–481. [DOI] [PubMed] [Google Scholar]
- Scherrmann JM, 2002. Drug delivery to brain via the blood-brain barrier. Vascul Pharmacol 38(6), 349–354. [DOI] [PubMed] [Google Scholar]
- Sellgren KL, Hawkins BT, Grego S, 2015. An optically transparent membrane supports shear stress studies in a three-dimensional microfluidic neurovascular unit model. Biomicrofluidics 9(6), 061102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ, 2013. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol 106-107, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer TJ, Meyer DA, Crofton KM, 2005. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect 113(2), 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Gao D, Chen Y, Jin F, Hu G, Jiang Y, Liu H, 2016. Development of a blood-brain barrier model in a membrane-based microchip for characterization of drug permeability and cytotoxicity for drug screening. Anal Chim Acta 934, 186–193. [DOI] [PubMed] [Google Scholar]
- Shawahna R, Uchida Y, Decleves X, Ohtsuki S, Yousif S, Dauchy S, Jacob A, Chassoux F, Daumas-Duport C, Couraud PO, Terasaki T, Scherrmann JM, 2011. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm 8(4), 1332–1341. [DOI] [PubMed] [Google Scholar]
- Sipes NS, Martin MT, Reif DM, Kleinstreuer NC, Judson RS, Singh AV, Chandler KJ, Dix DJ, Kavlock RJ, Knudsen TB, 2011. Predictive models of prenatal developmental toxicity from ToxCast high-throughput screening data. Toxicol Sci 124(1), 109–127. [DOI] [PubMed] [Google Scholar]
- Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, Garel S, 2014. Microglia modulate wiring of the embryonic forebrain. Cell Rep 8(5), 1271–1279. [DOI] [PubMed] [Google Scholar]
- Stefater JA 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Williams BO, Wills-Karp M, Pollard JW, Yamaguchi T, Ferrara N, Gerhardt H, Lang RA, 2011. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature 474(7352), 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP, 2008. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322(5905), 1247–1250. [DOI] [PubMed] [Google Scholar]
- Stipursky J, Francis D, Dezonne RS, Bergamo de Araujo AP, Souza L, Moraes CA, Alcantara Gomes FC, 2014. TGF-beta1 promotes cerebral cortex radial glia-astrocyte differentiation in vivo. Front Cell Neurosci 8, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp HB, Dziegielewska KM, 2009. Review: Role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathol Appl Neurobiol 35(2), 132–146. [DOI] [PubMed] [Google Scholar]
- Strazielle N, Ghersi-Egea JF, 2015. Efflux transporters in blood-brain interfaces of the developing brain. Front Neurosci 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal T, Kilty C, Smith A, LaLone C, Kennedy B, Tennant A, McCollum CW, Bondesson M, Knudsen T, Padilla S, Kleinstreuer N, 2017. Screening for angiogenic inhibitors in zebrafish to evaluate a predictive model for developmental vascular toxicity. Reprod Toxicol 70, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomaki A, Aranda E, Miura N, Yla-Herttuala S, Fruttiger M, Makinen T, Eichmann A, Pollard JW, Gerhardt H, Alitalo K, 2011. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol 13(10), 1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N, 2009. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood 113(3), 744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlungeanu DC, Deliu E, Dotter CP, Kara M, Janiesch PC, Scalise M, Galluccio M, Tesulov M, Morelli E, Sonmez FM, Bilguvar K, Ohgaki R, Kanai Y, Johansen A, Esharif S, Ben-Omran T, Topcu M, Schlessinger A, Indiveri C, Duncan KE, Caglayan AO, Gunel M, Gleeson JG, Novarino G, 2016. Impaired Amino Acid Transport at the Blood Brain Barrier Is a Cause of Autism Spectrum Disorder. Cell 167(6), 1481–1494 e1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata M, Wall I, Joyce A, Vieira JM, Kessaris N, Ruhrberg C, 2016. Regulation of embryonic neurogenesis by germinal zone vasculature. Proc Natl Acad Sci U S A 113(47), 13414–13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Zhang B, 2011. Neuro-inflammation, blood-brain barrier, seizures and autism. J Neuroinflammation 8, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji R, Crofton KM, 2012. Developmental neurotoxicity guideline study: issues with methodology, evaluation and regulation. Congenit Anom (Kyoto) 52(3), 122–128. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA, 2005. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437(7057), 426–431. [DOI] [PubMed] [Google Scholar]
- Umans RA, Henson HE, Mu F, Parupalli C, Ju B, Peters JL, Lanham KA, Plavicki JS, Taylor MR, 2017. CNS angiogenesis and barriergenesis occur simultaneously. Dev Biol 425(2), 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm MW, van der Meer AD, Eijkel JC, van den Berg A, Segerink LI, 2016. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 4(1), e1142493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, 1994. Developmental expression of GLUT1 and GLUT3 glucose transporters in rat brain. J Neurochem 62(1), 240–246. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Simpson IA, 2003. Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab 285(5), E1127– 1134. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M, 2014a. Adverse outcome pathway (AOP) development I: strategies and principles. Toxicol Sci 142(2), 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M, 2014b. Adverse outcome pathway development II: best practices. Toxicol Sci 142(2), 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgintino D, Errede M, Robertson D, Capobianco C, Girolamo F, Vimercati A, Bertossi M, Roncali L, 2004. Immunolocalization of tight junction proteins in the adult and developing human brain. Histochem Cell Biol 122(1), 51–59. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup WB, Perris R, Roncali L, 2007. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis 10(1), 35–45. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Monaghan P, Robertson D, Errede M, Bertossi M, Ambrosi G, Roncali L, 1997. An immunohistochemical and morphometric study on astrocytes and microvasculature in the human cerebral cortex. Histochem J 29(9), 655–660. [DOI] [PubMed] [Google Scholar]
- Wang D, Pascual JM, Yang H, Engelstad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC, 2006. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet 15(7), 1169–1179. [DOI] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ, 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285(5425), 248–251. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M, 2006. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci 26(22), 5996–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Luo W, Zhang W, Liu M, Song H, Chen J, 2011. Involvement of DMT1 +IRE in the transport of lead in an in vitro BBB model. Toxicol In Vitro 25(4), 991–998. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin S, Sonobe Y, Cheng Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T, Takeuchi H, Suzumura A, 2014. Interleukin-1beta induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS One 9(10), e110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J, 2012a. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151(6), 1332–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M, 2012b. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13(8), 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Nandi S, Chitu V, Yeung YG, Yu W, Huang M, Williams LT, Lin H, Stanley ER, 2010. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol 88(3), 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese S, Karus M, Faissner A, 2012. Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharmacol 3, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV, 2011. Central nervous system pericytes in health and disease. Nat Neurosci 14(11), 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A, 2002. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol 38(6), 323–337. [DOI] [PubMed] [Google Scholar]
- Wolfe RP, Ahsan T, 2013. Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnol Bioeng 110(4), 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RO, Christiani D, 2010. Gene-environment interaction and children’s health and development. Curr Opin Pediatr 22(2), 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW, 2013. Macrophage biology in development, homeostasis and disease. Nature 496(7446), 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang T, Wu Y, Jin W, Wen Z, 2016. Microglia Colonization of Developing Zebrafish Midbrain Is Promoted by Apoptotic Neuron and Lysophosphatidylcholine. Dev Cell 38(2), 214–222. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu L, He S, Wu Y, Jin W, Yu T, Qu JY, Wen Z, 2015. Temporal-Spatial Resolution Fate Mapping Reveals Distinct Origins for Embryonic and Adult Microglia in Zebrafish. Dev Cell 34(6), 632–641. [DOI] [PubMed] [Google Scholar]
- Xue L, Cai JY, Ma J, Huang Z, Guo MX, Fu LZ, Shi YB, Li WX, 2013. Global expression profiling reveals genetic programs underlying the developmental divergence between mouse and human embryogenesis. BMC Genomics 14, 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi E, Takahashi M, Saga Y, Osumi N, 2012. Penetration and differentiation of cephalic neural crest-derived cells in the developing mouse telencephalon. Dev Growth Differ 54(9), 785–800. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Chavan SS, Andersson U, 2015. High Mobility Group Box Protein 1 (HMGB1): The Prototypical Endogenous Danger Molecule. Mol Med 21 Suppl 1, S6–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Smallwood P, Nathans J, 2011. Expression of the Norrie disease gene (Ndp) in developing and adult mouse eye, ear, and brain. Gene Expr Patterns 11(1–2), 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanier ER, Fumagalli S, Perego C, Pischiutta F, De Simoni MG, 2015. Shape descriptors of the “never resting” microglia in three different acute brain injury models in mice. Intensive Care Med Exp 3(1), 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Nathans J, 2014. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev Cell 31(2), 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Yan Z, Feng M, Peng D, Guo Y, Hu X, Ren L, Sun Y, 2014. Identification of sturgeon IgD bridges the evolutionary gap between elasmobranchs and teleosts. Dev Comp Immunol 42(2), 138–147. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Crofton KM, 2000. Thyroid hormone action in fetal brain development and potential for disruption by environmental chemicals. Neurotoxicology 21(6), 935–945. [PubMed] [Google Scholar]