Abstract
Background:
Alterations in intestinal permeability have been implicated in the pathogenesis of Crohn disease (CD). We have reported that granulocyte macrophage-colony-stimulating factor (GM-csF) is required for mucosal barrier function in mice, and elevated neutralizing GM-CsF autoantibodies (Ab) are associated with stricturing ileal disease and surgery in patients with CD. We hypothesized that children with CD with elevated GM-CSF Ab would exhibit increased intestinal permeability.
Patients and Methods:
Subjects were divided into 3 groups: 15 with CD andhigh GM-CSF Ab (≥1.6 μg/mL, GM-CSF Ab Hi), 12 with CD and low GM-CSF Ab (<1.6 μg/mL, GM-CSF Ab Lo), and 15 healthy controls. Subjects ingested a lactulose:mannitol (L:M) solution, and urinary excretion of LM was measured by high-performance liquid chromatography. Serum GM-CSF Ab, endotoxin core Ab (EndoCAb), and lipopolysaccharide-binding protein (LBP), and fecal S100A12 were determined by enzyme-linked immunosorbent assay.
Results:
The CD groups did not vary by age, sex, disease location, or activity. Neither systemic (serum LBP) nor mucosal (fecal S100A12) inflammation differed between the CD groups. Intestinal permeability as measured by the urine L:M ratio and endotoxin exposure as measured by serum EndoCAb were increased in the GM-CSF Ab Hi group compared to the GM-CSF Ab Lo group and controls.
Conclusions:
Patients with CD with elevated GM-CSF Ab exhibit an increase in bowel permeability relative to patients with CD with lower levels of GM-CSF Ab in the absence of differences in systemic or intestinal inflammation. Therapies that target the mucosal barrier may be of particular benefit in this subgroup of patients with CD.
Keywords: bowel permeability, Crohn disease, granulocyte macrophage-colony-stimulating factor antibodies, sargramostim
The incidence of the inflammatory bowel diseases Crohn disease (CD) and ulcerative colitis (UC) is increasing (1–3). The pathophysiology of CD is likely related to polygenic alterations in immunity, which interact with environmental factors such as the host flora to trigger inappropriate immune responses leading to tissue damage. it is likely that there are several phenotypic and immunogenetic forms, with CD and UC representing the broadest clinical classifications (4,5). For example, emerging data suggest that patients with CD who carry mutations in the CARD15 gene and are seroreactive to microbial products (anti-Saccharomyces cerevisiae antibodies [ASCA], anti-Escherichia coli outer membrane porin C [OmpC], Pseudomonas fluorescens [I2], and anti-flagellin antibodies [CBir1]) are more likely to have progressive small-bowel disease requiring surgery (5). Although therapeutic options have increased during the past decade, our ability to target biological therapies to specific subgroups of patients has lagged behind. This has led to an empiric step-up approach to therapy, in which more potent agents are progressively offered until an effective regimen is identified.
There have been several clinical trials of granulocyte macrophage-colony-stimulating factor (GM-CSF), or sargramostim, in the treatment of CD (6–8). One placebo-controlled trial in corticosteroid-dependent patients with CD showed a clinical response with a reduction of the Crohn Disease Activity Index by 190 points in 80% of patients, with 53% of patients achieving clinical remission (6); however, in an earlier study in patients with active CD, there were no differences in outcome between patients in the placebo and treatment arms of the study (9). The lack of efficacy in this study may be related to a high placebo-response rate or artificial selection of milder CD phenotypes (excluding subjects on corticosteroids, immunomodulators, or other biological therapies). The efficacy of GM-CSF administration in most studies suggests that alterations in GM-CSF bioactivity likely contribute to the pathogenesis of CD in at least a subset of patients (9).
There is evidence that CD may be the result of dysfunction of the innate immune system, which includes neutrophils and macrophages (10–14). This dysfunction over time may lead to increased bowel permeability, failure to clear bacterial products, and augmentation of chronic T-cell-mediated inflammation. This potential series of immune events may explain the progression of disease type in many patients with CD from primarily inflammatory early in the disease to penetrating and stricturing disease (4). GM-CSF is required for priming of monocyte/macrophage and neutrophil antimicrobial functions, and altered bioactivity of GM-CSF likely plays a role in the innate immune dysregulation in CD (15). We have previously found that endogenous neutralizing anti-GM-CSF antibodies (GM-CSF Ab) reduce GM-CSF bioactivity and monocyte/neutrophil function (16). Our recent studies have determined that GM-CSF Ab are increased in pediatric- and adult-onset small-bowel CD, elevated GM-CSF Ab increase risk for stricturing/penetrating behavior and early surgery, and GM-CSF-deficient mice exhibit defects in ileal permeability with increased bacterial translocation leading to more severe small-bowel injury (17).
The intestinal barrier likely plays a key role in the inflammatory immune response in CD. Although precise mechanisms are unknown, it is speculated that disruption of the intestinal barrier could lead to permeation of luminal antigens augmenting the immune response. There have been several studies of bowel permeability, which have shown that both patients with CD and their immediate family members have increased bowel permeability (18–21). In a single case report and in animal models of CD, it has been shown that bowel permeability precedes the development of CD (22). Whether mechanisms of increased bowel permeability in CD are primary or secondary is unknown. We speculate that the innate immune system may play a role. Whether GM-CSF Ab promote an increase in intestinal permeability in CD is not known. We hypothesized that GM-CSF Ab regulate IBD phenotype and behavior by reducing GM-CSF bioactivity and mucosal barrier function, leading to increased bacterial translocation independent of active bowel inflammation. We tested this hypothesis by measuring intestinal permeability and biomarkers of systemic and mucosal inflammation and endotoxin exposure in pediatric patients with CD and healthy controls.
PATIENTS AND METHODS
Subject Enrollment
The patient-based studies were approved by the institutional review board at Cincinnati Children’s Hospital Medical Center. CD was diagnosed using established criteria and CD phenotype was determined per the Montreal criteria (23). Subjects with CD were recruited from a previous cohort of 272 pediatric patients from whom serum GM-CSF Ab levels had already been obtained (17). Among 3 different groups, 8- to 18-year-old subjects were recruited: 15 patients with CD who had high GM-CSF Ab, 15 patients with CD who had low GM-CSF Ab, and 15 healthy controls. High GM-CSF Ab were defined by a serum level ≥1.6 μg/mL and low GM-CSF Ab were defined as a serum level below 1.6 μg/mL. The cutoff of low versus high GM-CSF Ab level is based on our previously reported data, which demonstrated that the odds of stricturing/penetrating disease increased at this threshold value (17). Patients were categorized as remission or active disease by the Physician Global Assessment. Control subjects were recruited from the Cincinnati Genomic Control Cohort excluding those with a known family history of IBD. This cohort consists of previously recruited healthy children (atopic disease allowed) from the local community; banked serum and genetic samples are available.
Lactulose:Mannitol Test
Subjects were instructed to eat dinner at 6 PM, and then fast thereafter until the first morning void. Each subject (teenagers and adults) ingested a weight-based dose solution containing 5 g lactulose, 2g mannitol, 100 g sucrose, and a commercially available flavoring dissolved in 400 mL water before bedtime (Table 1). Urine was collected overnight to include the first morning void. Collected urine was stored at −20°C until analysis. The lactulose and mannitol recovered in the urine was measured by high-performance liquid chromatography (24,25). The fractional excretion of lactulose and mannitol in the urine relative to the ingested dose was calculated as an index of intestinal permeability, and results were expressed as the lactulose:mannitol (L:M) excretion ratio.
TABLE 1.
Weight-based administration of sucrose:lactulose mannitol mixture
| Weight, kg (range) | Sucrose, g | Lactulose, g | Mannitol, g | Kool-Aid, g |
|---|---|---|---|---|
| 20 (14–24) | 40 | 2 | 0.8 | 0.6 |
| 30 (25–34) | 60 | 3 | 1.2 | 0.9 |
| 40 (35–44) | 80 | 4 | 1.6 | 1.2 |
| 50 (>45) | 100 | 5 | 2.0 | 1.5 |
Fecal S100A12 Measurement
A stool sample was collected at the time of the urine collection for evaluation of fecal S100A12 level as a biomarker of intestinal inflammation. Fecal samples were stored at −80°C until analysis. Fecal concentrations of S100A12 were determined by a double sandwich enzyme-linked immunosorbent assay (ELISA) technique described previously (26,27).
GM-CSF Ab, EndoCAb, and LBP Measurements and CARD15 Genotyping
Serum endotoxin core immunoglobulin A (IgA) antibody (EndoCAb) was measured as a marker of the immune response to endotoxin exposure, serum lipopolysaccharide-binding protein (LBP) was collected as a short-term marker of systemic inflammation, and CARD15 genotyping was performed because mutations in this gene have been associated with increased bowel permeability (20,21). Blood samples were collected under sterile conditions and serum was obtained and stored at −80°C until analysis. EndoCAb and LBP concentration were determined by ELISA, as per the manufacturer’s instructions (Hycult Biotechnology, Uden, the Netherlands). EndoCAb and LBP were evaluated only in the 2 CD disease groups because of a paucity of banked serum available in the control group. Serum concentrations of GM-CSF autoantibodies were quantified by ELISA as previously described (17). DNA was isolated from whole blood using the Puregene kit (Gentra Systems, Minneapolis, MN). Genotyping was performed for 3 common mutations in the CARD 15 gene associated with increased CD risk: NOD2R702W (SNP8), G908R (SNP12), and 1007fs (SNP13) by the Cincinnati Children’s Hospital Medical Center Genetics Core Laboratory.
Statistical Analysis
Urine samples of 15 subjects were returned in the high GMCSF Ab group, 12 in the low GM-CSF Ab group, and 15 in the control group. Stool samples were obtained and evaluated from 10 of the 15 high (Hi) GM-CSF Ab subjects, 9 of the 15 low (Lo) GM-CSF Ab subjects, and 12 of the 15 control subjects. The 3 subjects who were recruited in the low GM-CSF Ab group who returned neither urine nor stool samples were excluded from the study analyses. Statistical analyses were performed using SAS version 9.1 (SAS Institute, Inc, Cary, NC) and GraphPad PRISM version 5.02 (GraphPad. Inc, San Diego, CA). Univariate analyses were conducted to investigate any potential outliers as well as the normality of the data. We summarized normally distributed data with means and standard deviations, and skewed data with medians and interquartile ranges (IQRs). Bivariate analyses were conducted to investigate for differential distribution of demographic and other variables between the 2 CD groups. Continuous variables, if normally distributed, were analyzed using t test or 1-way analysis of variance (ANOVA) with post hoc Tukey test to compare the groups. When data were skewed, we log-transformed the data. If the data in logarithmic scale were normally distributed, then we used t test or 1-way ANOVA. Nonparametric alternatives such as the Mann-Whitney, Kruskal-Wallis, or permutation test were used on data that are not normally distributed. Discrete variables were analyzed using the Fisher exact test or the Cochran-Mantel-Haenszel test.
RESULTS
Clinical and Demographic Characteristics
Clinical and demographic characteristics of the study subjects are summarized in Table 2. The median (minimum-maximum) age in the GM-CSF Ab Lo group was 13.3 years (range 8.2–18.0), in the GM-CSF Ab Hi group 15.3 years (range 9.3–18.9), and in the control group 13.5 years (range 8.5–18.9). The control group had a higher proportion of girls (67%) than both disease groups (high 27%, low 17%); however, this difference was not significant (P = 0.57). As expected, serum GM-CSF Ab were increased in the GM-CSF Ab Hi group, compared to the GM-CSF Ab Lo group and the healthy controls. The GM-CSF Ab Lo group had a median (IQR) serum GM-CSF Ab concentration of 0.25 (0.19–0.73) μg/mL, the GM-CSF Ab Hi group had a median (IQR) serum concentration of 9.4 μg/mL (2.6–13.2) μg/mL, and the control group had a median serum concentration of 0.58 (0.31–0.63) μg/mL. There was a difference between all 3 groups (P < 0.0001), and group-to-group comparison showed a difference between the GM-CSF Ab Hi and Lo groups (P < 0.0002), the GM-CSF Ab Hi and control group (P < 0.0001), and no difference between the GM-CSF Ab Lo group and control.
TABLE 2.
Clinical and immune characteristics
| Control | GM-CSF Ab Lo | GM-CSF Ab Hi | |
|---|---|---|---|
| n | 15 | 12 | 15 |
| Age, median (range) | 13.5 (8.5–18.9) | 13.3 (8.2–18.0) | 15.3 (9.3–18.9) |
| Sex | 67% female | 17% female | 27% female |
| Serum GM-CSF Ab, μg/mL (range) | 0.57 (0.32–0.64) | 0.25 (0.2–0.73) | 9.4* (2.6–13.2) |
| Montreal classification of disease | |||
| Terminal ileum (L1) | L1: 1 (8%) | L1: 1 (7%) | |
| Colon (L2) | L2: 2 (17%) | L2: 2 (13%) | |
| Ileocolon (L3) | L3: 9 (75%) | L3: 12 (80%) | |
| Upper GI only (L4) | L4: 0 | L4: 0 | |
| Nonstricturing, nonpenetrating (B1) | B1: 12 (100%) | B1: 13 (86%) | |
| Stricturing (B2) | B2: 0 | B2: 1 (7%) | |
| Penetrating (B3) | B3: 0 | B3: 1 (7%) | |
| Age at diagnosis ≤16 y (A1) | A1: 100% | A1: 100% | |
| Medications | |||
| Corticosteroids | 1 (8%) | 2 (13%) | |
| Oral immunomodulator | 10 (83%) | 10 (67%) | |
| 5-Aminosalicylate | 7 (58%) | 3 (20%) | |
| Antibiotics | 1 (8%) | 4 (27%) | |
| Infliximab | 1 (8%) | 9 (60%)** | |
| CARD15 mutation | 1 (7%) | 4 (33%) | 3 (20%) |
| Clinical remission frequency | 10 (83%) | 9 (60%) | |
| Fecal S100A12, μg/kg (range) | 70 (59–103) | 5,500 (2050–11,000) | 5050*** (100–16,250) |
| Serum lipopolysaccharide-binding protein, μg/mL (range) | 22.2 (11.6–47.7) | 30.0 (20.8–46.8) |
GM-CSF Ab Lo = patients with CD with serum GM-CSF autoantibody <1.6 μg/mL, GM-CSF Ab Hi = patients with CD with serum GM-CSF autoantibody ≥1.6 μg/mL. Values for GM-CSF Ab, S100A12, and LBP are given as the median (IQR). GM-CSF Ab = granulocyte macrophage-colony-stimulating factor autoantibodies; GM-CSF Ab Lo = low granulocyte macrophage-colony-stimulating factor autoantibodies; GM-CSF Ab Hi = high granulocyte macrophage-colony-stimulating factor autoantibodies; LBP = lipopolysaccharide-binding protein.
P < 0.001 vs control and GM-CSF Ab Lo group;
P < 0.05 vs GM-CSF Ab Lo group;
P < 0.05 vs control.
The CD groups had no difference in the Montreal classification of disease location or behavior (23). There was no difference in exposure to any of the oral medications (5-aminosalycilates, corticosteroids, immunomodulators, or antibiotics). There was, however, a significant difference between the CD groups in the proportion receiving infliximab therapy (P = 0.04). One patient in the GM-CSF Ab Lo group was receiving infliximab therapy, whereas 9 in the GM-CSF Ab Hi group were receiving infliximab therapy at the time of urine L:M sampling. We have previously reported that serum GM-CSF Ab level does not vary with infliximab exposure in pediatric CD (17). None of the patients had received therapeutic GM-CSF administration. CARD15 mutations were identified in all 3 groups, but the frequency was not significantly different (P = 0.42). Four subjects in the GM-CSF Ab Lo group, 3 in the GM-CSF Ab Hi group, and 1 in the control group carried at least 1 of the 3 risk mutations tested.
Clinical Disease Activity and Mucosal and Systemic Inflammation
The frequency of clinical remission was 83% in the GM-CSF Ab Lo group and 60% in the GM-CSF Ab Hi group at the time of urine sampling and did not differ between the 2 groups. The surrogate markers of bowel inflammation were fecal S100A12 concentration and of systemic inflammation serum LBP concentration. Serum LBP is a positive acute-phase protein produced in the liver in response to LPS or IL-6 exposure, and thus is similar to CRP. The fecal S100A12 levels were different between the 3 groups overall (P = 0.005); in group-to-group comparison after correcting for multiple comparisons these remained different between the GMCSF Ab Hi group and controls (P = 0.015), and did not differ between the GM-CSF Ab Hi and Lo groups (P = 0.27) (Table 2). There was no significant difference in serum LBP concentration between the 2 CD groups (P = 0.38). Collectively, these data demonstrated that the 2 CD groups did not vary in terms of clinical disease activity or biomarkers of mucosal or systemic inflammation at the time of urine L:M measurements.
GM-CSF Ab and Intestinal Permeability
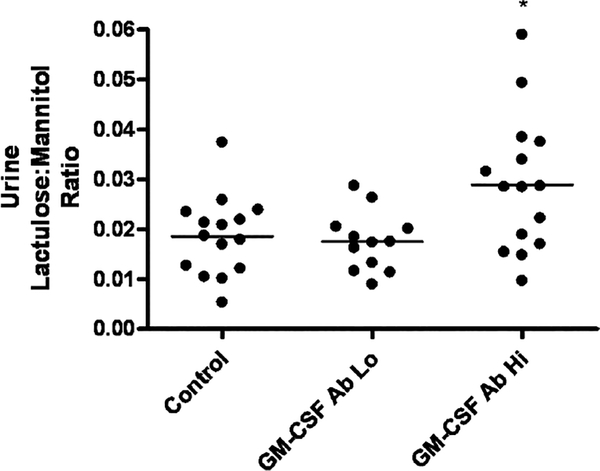
We then tested for the presence of increased intestinal permeability in the GM-CSF Ab Hi group. The marker of bowel permeability was the ratio of L:M in the urine. There was a significant difference in the urine L:M ratio between the 3 groups (P = 0.0172) (Fig. 1). Tukey post hoc group-to-group comparison (α < 0.05) showed a significant difference between the GM-CSF Ab Hi and Lo groups, and the GM-CSF Ab Hi group and control group, but no difference between the GM-CSF Ab Lo group and control. The mean (SD) urine L:M ratio in the GM-CSF Ab Hi group was equal to 0.03 (0.01), compared to 0.02 (0.01) in the GM-CSF Ab Lo group, and 0.02 (0.01) in the control group.
FIGURE 1.
Urine lactulose:mannitol ratio in CD patients stratified by GM-CSF Ab level and healthy controls. Healthy controls (n = 15) and CD patients (n = 27) ingested a mixture of sucrose, lactulose, and mannitol, and urinary concentrations of lactulose and mannitol were determined by high-pressure liquid chromatography. CD patients were stratified by serum GM-CSF Ab concentration measured by enzyme-linked immunosorbent assay. GM-CSF Ab Lo: CD patients with serum GM-CSF Ab concentration <1.6 mg/mL, GM-CSF Ab Hi: CD patients with serum GM-CSF Ab concentration ≥1.6 mg/mL. Data are shown as the urinary lactulose:mannitol excretion ratio, with the means for each group as indicated. *P = 0.007 by ANOVA with Tukey’s multiple comparison test vs controls and GM-CSF Ab Lo group. CD = Crohn disease; GM-CSF = granulocyte macrophage-colony-stimulating factor.
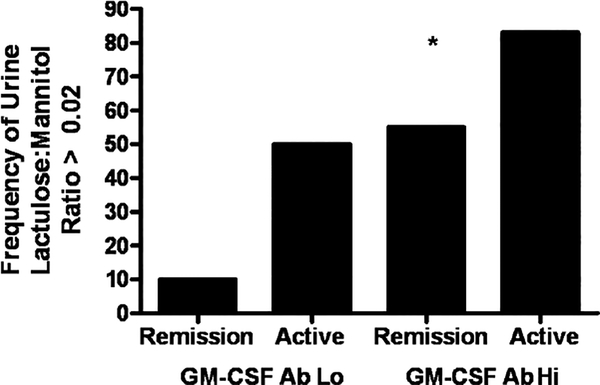
In agreement with prior studies, we found that the mean urine L:M ratio in healthy controls was equal to 0.02. We therefore asked whether the frequency of urine L:M ratio >0.02 would be increased in patients with CD with elevated GM-CSF Ab, and whether this would remain true for those in clinical remission. The frequency of urine L:M >0.020 increased from 16% in patients with CD with GM-CSF Ab Lo to 66% in those with GM-CSF Ab Hi (P = 0.01). After limiting the comparison to patients in remission at the time of urine sampling, we observed that the frequency of urine L:M >0.020 increased from 10% in patients with GM-CSF Ab Lo (n = 10) to 55% in those with GM-CSF Ab Hi (n = 9, P = 0.04, Fig. 2). These data demonstrated that elevated GM-CSF Ab were associated with increased intestinal permeability in patients with CD in clinical remission.
FIGURE 2.
Urine lactulose:mannitol ratio in CD patients stratified by GM-CSF Ab level and clinical disease activity. CD patients (n = 27) ingested a mixture of sucrose, lactulose, and mannitol, and urinary concentrations of lactulose and mannitol were determined by high-pressure liquid chromatography. CD patients were stratified by serum GM-CSF Ab concentration and clinical disease activity. GM-CSF Ab Lo: CD patients with serum GM-CSF Ab concentration <1.6 mg/mL, GM-CSF Ab Hi: CD patients with serum GM-CSF Ab concentration ≥1.6 mg/mL. The frequency of patients with urine lactulose:mannitol ratio >0.020 is shown. *P = 0.04 vs GM-CSF Ab Lo group in remission. CD = Crohn disease; GM-CSF = granulocyte macrophage-colony-stimulating factor.
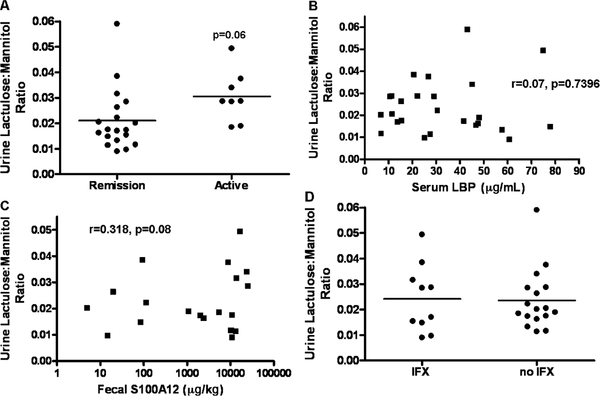
Increases in intestinal permeability in CD could be secondary, in association with increases in clinical disease activity. We observed a trend toward an increase in the urine L:M ratio in patients with CD with clinically active disease at the time of urine sampling (P = 0.06), compared to those in remission (Fig. 3A). Increases in intestinal permeability in active disease could result from increases in systemic or mucosal inflammation; however, we did not observe an association between serum LBP, as a biomarker of systemic inflammation (Fig. 3B), and the urine L:M ratio. We did observe a trend (r = 0.318, P = 0.08) toward an association between the fecal S100A12, as a biomarker of mucosal inflammation (Fig. 3C), and the urine L:M ratio. Infliximab therapy has been shown to reduce intestinal permeability; however, we did not observe a difference in the urine L:M ratio between patients who were and patients who were not exposed to infliximab at the time of urine sampling (Fig. 3D).
FIGURE 3.
Urine lactulose:mannitol ratio and clinical disease activity, systemic and mucosal inflammation, and infliximab exposure. Healthy controls (n = 15) and CD patients (n = 27) ingested a mixture of sucrose, lactulose, and mannitol, and urinary concentrations of lactulose and mannitol were determined by high-pressure liquid chromatography. A, CD patients were stratified by clinical disease activity. The relationship between (B) serum LBP and (C) fecal S100A12 and urine lactulose:mannitol ratio is shown. D, CD patients were stratified by infliximab exposure at the time of urine lactulose:mannitol measurement. Data are shown as the urinary lactulose:mannitol excretion ratio, with the means for each group as indicated in (A) and (D). CD = Crohn disease.
GM-CSF Ab and Endotoxin Exposure
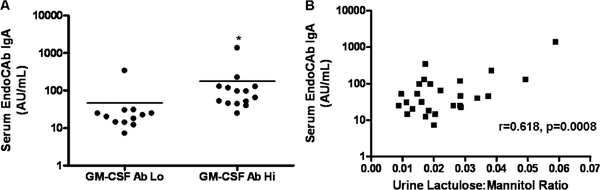
We asked whether the increase in intestinal permeability observed in the GM-CSF Ab Hi group would be associated with an increase in endotoxin exposure, as measured by circulating EndoCAb. We observed a significant difference in the serum EndoCAb concentration between the GM-CSF Ab Hi and Lo groups (P < 0.002). The median (IQR) serum EndoCAb concentration was equal to 21.20AU/mL (14.35–26.05) in the GM-CSF Ab Lo group and increased to 79.90AU/mL (46.65–125.13) in the GM-CSF Ab Hi group (Fig. 4A). Consistent with this, serum EndoCAb IgA was strongly associated with urine L:M ratio (Fig. 4B, r = 0.618, P = 0.0008). These data demonstrated that patients with CD with elevated GM-CSF Ab and increased intestinal permeability also exhibited evidence of increased endotoxin exposure.
FIGURE 4.
Serum endotoxin core antibody in CD patients stratified by GM-CSF Ab level and urine L:M ratio. Serum immunoglobulin A endotoxin core antibody (EndoCAb IgA) was determined by ELISA in CD patients (n = 27). A, CD patients were stratified by serum GM-CSF Ab concentration measured by ELISA. GM-CSF Ab Lo: CD patients with serum GM-CSF Ab concentration <1.6 μg/mL, GM-CSF Ab Hi: CD patients with serum GM-CSF Ab concentration ≥1.6 μg/mL. The means for each group were as indicated. *P< 0.0001 vs GM-CSF Ab Lo group. B, The relation between urine lactulose:mannitol ratio and serum EndoCAb IgA is shown. CD = Crohn disease; ELISA = enzyme-linked immunosorbent assay; GM-CSF = granulocyte macrophage-colony-stimulating factor.
DISCUSSION
Although it is known that patients with CD have an increased bowel permeability, the mechanisms are not well understood. We found an increase in permeability as measured by the ratio of lactulose-to-mannitol excretion in the urine in patients with CD with GM-CSF Ab Hi, compared to those with GM-CSF Ab Lo. Our findings support previous reports of elevated bowel permeability in CD, and suggest that there are phenotypic subtypes of patients with CD (those with elevated GM-CSF Ab) who are more likely to experience an increase in bowel permeability. The previous reports of family members with CD having elevated bowel permeability also suggest that CD represents a variety of genetic polymorphisms that, when combined with environmental triggers, lead to disease (21). It will be of interest in ongoing studies to determine whether elevated GM-CSF Ab and increased intestinal permeability are also present in healthy relatives of CD probands with these characteristics.
The use of L:M measurement in the urine to determine bowel permeability has been reviewed (28). These saccharide markers cross the small intestinal epithelium by the paracellular pathway. The fractional excretion of the smaller mannitol molecule is a reflection of total villus surface area, whereas the fractional excretion of lactulose has been used to infer either damage or increased permeability. Therefore, the L:M ratio is a measurement of small intestinal permeability or damage normalized to intestinal surface area. Our results are similar to those previously reported in healthy and CD populations and suggest that the assay in fact yielded reproducible and valid results supporting an increase in small intestinal permeability in the CD subgroup with elevated GM-CSF Ab.
In a previous study, individuals with increased bowel permeability exhibited higher levels of circulating B memory cells, suggesting higher antigenic exposure (29). We have now demonstrated an association of increased permeability with upregulation of GM-CSF autoantibodies, which presumably would be produced by B memory cells. Moreover, our observed elevation in the serum EndoCAb concentration in those with high GM-CSF Ab supports the findings of both increased bowel permeability and antigenic exposure. EndoCAb is a measurement of the immune reaction to the bacterial product endotoxin, and thus higher serum concentrations suggest both higher exposure through a “leaky” bowel and an augmented immune reaction to these translocated bacterial products. We have recently reported that higher levels of EndoCAb are associated with growth failure in children with CD, suggesting that permeability, antigen exposure, and the subsequent immune reaction lead to more severe disease activity (30). Ongoing studies will seek to define the population of B cells producing the GM-CSF autoantibodies.
Inflammation is known to increase bowel permeability; however, no difference was found in fecal S100A12 levels in our patients suggesting that the difference in permeability between patients with CD with serum GM-CSF Ab Hi and Lo concentration is independent of active bowel inflammation. Fecal S100 proteins are a family of calcium-binding proteins associated with phagocytes, which are expressed by the intestinal mucosa during inflammation. Fecal S100A12 is a more specific marker for granulocyte activity, which has a higher correlation with bowel mucosal inflammation, and is more specific in distinguishing children with IBD than its commonly used sister complex S100A8/S100A9 (calprotectin) (31). Also confirming the S100A12 findings was the lack of difference in serum LBP levels. LBP is a soluble acute-phase protein and can be considered an additional surrogate for systemic inflammation, similar to CRP (32). Although LBP has been described as higher in patients with CD, the absence of a difference between the high and the low GM-CSF antibody groups suggests that the difference in permeability found was independent of either local mucosal or systemic inflammation (30). We did not measure ESR or CRP, and so we cannot determine whether one of these standard clinical markers for inflammation would have been associated with serum GM-CSF Ab level or intestinal permeability.
In considering possible confounders of our study, we did note that there was a difference in the proportion of subjects in the GM-CSF Ab Hi group on infliximab therapy (60% vs 8%). Infliximab, however, is the only medication that has been shown to decrease bowel permeability (33,34). Therefore, the higher rate of exposure to infliximab may have been expected to reduce permeability in the GM-CSF Ab Hi group. In our sample, the GM-CSF Ab Hi group had a significantly elevated L:M ratio despite having a higher proportion of subjects receiving infliximab therapy at the time of sampling, supporting the finding that GM-CSF Ab influence increased bowel permeability via a primary mechanism of action.
Our finding that increased GM-CSF Ab are associated with an increase in bowel permeability suggests that an altered innate immune system affects bowel permeability, although we cannot conclude that there is a direct causal link. The exact mechanism for this interaction of GM-CSF with bowel permeability remains unknown. Defects in neutrophil function have been described in CD, and administering GM-CSF corrects impairments in neutrophil chemotaxis in patients with CD (35,36). Our previous study in mice has shown that GM-CSF is required for both paracellular permeability and bacterial clearance in the gut (17). Whether GM-CSF may also directly affect epithelial cells and thus bowel permeability via effects on tight junction protein assembly is not known. Alternately, the association between elevated GM-CSF Ab and intestinal permeability may be an epiphenomenon, in which increased permeability for other reason(s) promotes loss of tolerance to GM-CSF. Our ongoing patient-based and animal studies will test these possibilities.
Despite various strengths, there are also several limitations in our study. Although there was a difference in L:M between the 2 GM-CSF Ab groups and between the GM-CSF Ab Hi group and control, there was no difference between the GM-CSF Ab Lo group and control. This implies that either patients with CD with GM-CSF Ab Lo have normal permeability or the control group randomly had increased permeability. A possible confounder in this study was atopy. There is evidence that supports increased bowel permeability in patients with atopic disease (37,38). Atopy was not an exclusion criterion used in enrollment in the Cincinnati Genomics Control Cohort, and in reviewing the control subjects 9 of the 15 subjects had at least 1 form of atopy. In our study design, another factor that was not taken into account was the possibility of nonsteroidal antiinflammatory drug ingestion, which has also been found to increase bowel permeability (39–41).
In conclusion, we found that the loss of GM-CSF bioactivity resulting from higher levels of GM-CSF Ab is associated with increased bowel permeability. Our results suggest that a loss of innate immune function may impair barrier function in patients with CD. This lends support to the potential administration of GM-CSF in the treatment of CD; however, we suggest that targeting specific subgroups of patients with CD, who may have GM-CSF decreased bioactivity, will likely increase therapeutic yield. In addition, first-degree family members of patients with CD are known to have elevated permeability suggesting that innate immune dysfunction precedes the development of disease (22,42). Future directions based on our results may include identifying and targeting patients at risk of developing CD, restoring innate immune function, normalizing intestinal barrier function, and preemptively preventing the development of CD.
Acknowledgments:
Samples used in this research project were made available through the Cincinnati Control Cohort for Genomic and Gene Expression Studies Project, which is funded by the Children’s Hospital Research Foundation and Pediatric and Pediatric Surgical Divisional Resources within Children’s Hospital Medical Center. Kathleen Lake provided outstanding support with subject recruitment.
This work was supported by the Crohn’s and Colitis Foundation of America (L.A.D.), the Broad Medical Research Program (L.A.D.), the National Institutes of Health (NIH)-supported Cincinnati Children’s Hospital Research Foundation Digestive Health Center (1P30DK078392–01, B.C.T.), and NIH grants R01 DK058259 and R01 DK078683 (L.A.D.).
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol 2006;101:1559–68. [DOI] [PubMed] [Google Scholar]
- 2.Kugathasan S, Judd RH, Hoffmann RG, et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J Pediatr 2003;143:525–31. [DOI] [PubMed] [Google Scholar]
- 3.Vind I, Riis L, Jess T, et al. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003 −2005: a population-based study from the Danish Crohn Colitis Database. Am J Gastroenterol 2006;101:1274–82. [DOI] [PubMed] [Google Scholar]
- 4.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 2006;12(suppl 1): S3–9. [DOI] [PubMed] [Google Scholar]
- 5.Dubinsky MC, Taylor K, Targan SR, et al. Immunogenetic phenotypes in inflammatory bowel disease. World J Gastroenterol 2006;12:3645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valentine JF, Fedorak RN, Feagan B, et al. Steroid-sparing properties of sargramostim in patients with corticosteroid-dependent Crohn’ disease: a randomised, double-blind, placebo-controlled, phase 2 study. Gut 2009;58:1354–62. [DOI] [PubMed] [Google Scholar]
- 7.Takazoe M, Matsui T, Motoya S, et al. Sargramostim in patients with Crohn’s disease: results of a phase 1–2 study. J Gastroenterol 2009; 44:535–43. [DOI] [PubMed] [Google Scholar]
- 8.Dieckgraefe BK, KorzenikJR. Treatment of active Crohn’s disease with recombinant human granulocyte-macrophage colony-stimulating factor. Lancet 2002;360:1478–80. [DOI] [PubMed] [Google Scholar]
- 9.Korzenik JR, Dieckgraefe BK, Valentine JF, et al. Sargramostim for active Crohn’s disease. N Engl J Med 2005;352:2193–201. [DOI] [PubMed] [Google Scholar]
- 10.Marks DJ, Harbord MW, MacAllister R, et al. Defective acute inflammation in Crohn’s disease: a clinical investigation. Lancet 2006;367: 668–78. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 2005;307:731–4. [DOI] [PubMed] [Google Scholar]
- 12.Kramer M, Netea MG, de Jong DJ, et al. Impaired dendritic cell function in Crohn’s disease patients with NOD2 3020insC mutation. J Leukoc Biol 2006;79:860–6. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto-Furusho JK, Korzenik JR. Crohn’s disease: innate immunodeficiency? World J Gastroenterol 2006;12:6751–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comalada M, Peppelenbosch MP. Impaired innate immunity in Crohn’s disease. Trends Mol Med 2006;12:397–9. [DOI] [PubMed] [Google Scholar]
- 15.Shibata Y, Berclaz PY, Chroneos ZC, et al. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 2001;15:557–67. [DOI] [PubMed] [Google Scholar]
- 16.Uchida K, Beck DC, Yamamoto T, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. NEnglJMed 2007;356:567–79. [DOI] [PubMed] [Google Scholar]
- 17.Han X, Uchida K, Jurickova I, et al. Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn’s disease. Gastroenterology 2009;136: 1261–71.e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May GR, Sutherland LR, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn’s disease? Gastroenterology 1993;104:1627–32. [DOI] [PubMed] [Google Scholar]
- 19.Hollander D, Vadheim CM, Brettholz E, et al. Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med 1986;105:883–5. [DOI] [PubMed] [Google Scholar]
- 20.D’Inca R, Annese V, di Leo V, et al. Increased intestinal permeability and NOD2 variants in familial and sporadic Crohn’s disease. Aliment Pharmacol Ther 2006;23:1455–61. [DOI] [PubMed] [Google Scholar]
- 21.Buhner S, Buning C, Genschel J, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut 2006;55:342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn’s disease in a subject with familial risk. Gastroenterology 2000;119:1740–4. [DOI] [PubMed] [Google Scholar]
- 23.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19(suppl A):5–36. [DOI] [PubMed] [Google Scholar]
- 24.Breslin NP, Nash C, Hilsden RJ, et al. Intestinal permeability is increased in a proportion of spouses of patients with Crohn’s disease. Am J Gastroenterol 2001;96:2934–8. [DOI] [PubMed] [Google Scholar]
- 25.Meddings JB, Sutherland LR, May GR. Intestinal permeability in patients with Crohn’s disease. Gut 1994;35:1675–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foell D, Kucharzik T, Kraft M, et al. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut 2003;52:847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaiser T, Langhorst J, Wittkowski H, et al. Faecal S100A12 as a noninvasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut 2007;56:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teshima CW, Meddings JB. The measurement and clinical significance of intestinal permeability. Curr Gastroenterol Rep 2008;10:443–9. [DOI] [PubMed] [Google Scholar]
- 29.Yacyshyn BR, Pilarski LM. Expression of CD45RO on circulating CD19+ B-cells in Crohn’s disease. Gut 1993;34:1698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasternak BA, D’Mello S, Jurickova II, et al. Lipopolysaccharide exposure is linked to activation of the acute phase response and growth failure in pediatric Crohn’s disease and murine colitis. Inflamm Bowel Dis 2010;16:856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foell D, Wittkowski H, Roth J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut 2009;58:859–68. [DOI] [PubMed] [Google Scholar]
- 32.Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect 2006;8:946–52. [DOI] [PubMed] [Google Scholar]
- 33.Suenaert P, Bulteel V, Lemmens L, et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am J Gastroenterol 2002;97:2000–4. [DOI] [PubMed] [Google Scholar]
- 34.Suenaert P, Bulteel V, Vermeire S, et al. Hyperresponsiveness of the mucosal barrier in Crohn’s disease is not tumor necrosis factor-dependent. Inflamm Bowel Dis 2005;11:667–73. [DOI] [PubMed] [Google Scholar]
- 35.Harbord MW, Marks DJ, Forbes A, et al. Impaired neutrophil chemo-taxis in Crohn’s disease relates to reduced production of chemokines and can be augmented by granulocyte-colony stimulating factor. Aliment Pharmacol Ther 2006;24:651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korzenik JR, Dieckgraefe BK. Is Crohn’s disease an immunodeficiency? A hypothesis suggesting possible early events in the pathogenesis of Crohn’s disease. Dig Dis Sci 2000;45:1121–9. [DOI] [PubMed] [Google Scholar]
- 37.Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 2006;101:1288–94. [DOI] [PubMed] [Google Scholar]
- 38.Maran AG, Small M, Ferguson A, et al. Small bowel permeability in patients with nasal polyposis. Rhinology 1986;24:195–8. [PubMed] [Google Scholar]
- 39.Bjarnason I, Takeuchi K. Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. J Gastroenterol 2009;44(Suppl. 19):23–9. [DOI] [PubMed] [Google Scholar]
- 40.Kerckhoffs AP, Akkermans LM, de Smet MB, et al. Intestinal permeability in irritable bowel syndrome patients: effects of NSAIDs. Dig Dis Sci 2010;55:716–23. [DOI] [PubMed] [Google Scholar]
- 41.Hilsden RJ, Meddings JB, Sutherland LR. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn’s disease. Gastroenterology 1996;110:1395–403. [DOI] [PubMed] [Google Scholar]
- 42.Meddings J What role does intestinal permeability have in IBD pathogenesis? Inflamm Bowel Dis 2008;14(suppl 2):S138–9. [DOI] [PubMed] [Google Scholar]