Abstract
Introduction
Diffuse large B-cell lymphoma (dlbcl) accounts for 30%–40% of all non-Hodgkin lymphomas. Approximately 60% of patients are cured with standard treatment. Targeted treatments are being investigated and might improve disease outcomes; however, their effect on cancer drug budgets will be significant. For the present study, we conducted an analysis of real-world costs for dlbcl patients treated in British Columbia, useful for health care system planning.
Methods
Patient records from a retrospective cohort of patients diagnosed with dlbcl in British Columbia during 2004–2013 were anonymously linked across multiple administrative data sources: systemic therapy, radiotherapy, hospitalizations, oncologist services, outpatient medications, and fee-for-service physician services. Using generalized linear modelling regression, time-dependent costs (in 2015 Canadian dollars) were estimated in 6-month intervals over a 5-year period. The inverse probability weighting method was applied to account for censored observations. Nonparametric bootstrapping was used to estimate standard errors for the mean cost at each time interval.
Results
The cohort consisted of 678 patients (5-year overall survival: 67%). Mean age at diagnosis was 64 ± 14 years; median follow-up was 3.2 years. Mean total cost of care was highest in the first 6 months after diagnosis ($29,120; 95% confidence interval: $28,986 to $29,170) and after disease progression ($18,480; 95% confidence interval: $15,187 to $24,772). Systemic therapy and hospitalization costs were the largest cost drivers. At each time interval, costs were observed to be positively skewed.
Conclusions
Our results depict real-world costs for the treatment of dlbcl patients with standard chop-r therapy. Cost-model parameters are also provided for economic modelling of dlbcl interventions.
Keywords: Diffuse large B-cell lymphoma; costs, real-world; censored costing; inverse probability weighting
INTRODUCTION
Diffuse large B-cell lymphoma (dlbcl) is the most common form of non-Hodgkin lymphoma, representing approximately 30%–40% of all non-Hodgkin lymphoma cases1. Treatment is usually administered shortly after diagnosis2,3. Standard treatment for dlbcl has remained a combination of chemotherapy drugs including cyclophosphamide, doxorubicin, vincristine, and prednisone (chop). Rituximab was added to chop in the early 2000s, and chop-r has remained the standard treatment regime for nearly two decades4,5. Approximately 60% of patients are cured with chop-r, and differences in response to chop-r might be explained in part by the discovery of at least two distinct and clinically meaningful subtypes of dlbcl4,6,7. The efficacy and toxicity of subtype-specific treatments are currently being investigated in clinical trials4,8.
When introduced, the budgetary impact of chop-r compared with chop was signif icant, est imated at approximately $11,000 (2015 Canadian dollars) over a 15-year time horizon. Although associated with a significant survival benefit5, the use of chop-r placed upward pressure on cancer drug budgets. As subtype-specific treatments become more common in oncology, the sustainability of cancer drug budgets is at the forefront of policy discussions9,10. Having an understanding of the real-world costs of dlbcl treatment can be helpful for health care system planning and budget impact assessments2,11. Here, we present a real-world analysis of the costs incurred for the treatment of dlbcl over a 5-year period in British Columbia. Coefficients from our cost model are included for potential use in future economic models and analyses. Ethics approval for the study was obtained from the University of British Columbia–BC Cancer Research Ethics Board.
METHODS
Data Sources
We conducted a secondary analysis of administrative health data to obtain an understanding of direct medical costs in 6 categories (Table I): systemic therapy, including chemotherapy, nursing, pharmacy, and clerk services; radiotherapy; hospitalizations; outpatient prescription drugs; outpatient fee-for-service physician services; and oncologist services. A cohort of patients diagnosed with dlbcl was established using records from the database maintained by the Centre for Lymphoid Cancer. That database includes clinical outcomes information for patients with lymphoid cancer treated at the provincial cancer agency as determined by standard clinical practice guidelines. Inclusion criteria were established to produce a cohort of patients diagnosed and treated in British Columbia between 1 January 2004 and 30 June 2013 who were 18 years of age or older and hiv-negative. Characteristics retrieved included age, sex, patient status at follow-up, and dates of disease diagnosis, progression, death, and last available follow-up. Anonymized unique patient identifiers were used to link patient records from the Centre for Lymphoid Cancer database to all other individual-level data.
TABLE I.
Sources of cost data and unit costs
| Cost category | Source for … | |
|---|---|---|
|
| ||
| Data | Unit cost | |
| Systemic therapy | BC Cancer, Systemic Therapy Program (BC Cancer Registrya) | Drug reimbursement cost |
|
| ||
| Radiotherapy | CAIS scheduling and appointment records (BC Cancer Registrya) | Literature-derived cost per course of treatment12 |
| BC Cancer, Radiotherapy warehouse (BC Cancer Registrya) | ||
|
| ||
| Hospitalizations | CIHI, Discharge Abstract Databaseb | Cost of a standard hospital stay for British Columbia13 |
|
| ||
| Outpatient services provided by fee-for-service practitioners | British Columbia MSPc | MSP fee item paid |
|
| ||
| Outpatient prescription medications | British Columbia PharmaNetd | Drug reimbursement, professional dispensing fees |
|
| ||
| Oncologist services | CAIS scheduling and appointment records (BC Cancer Registrya) | New patient visit: MSP item 33510 |
| British Columbia MSP 2014 fee schedule | Follow-up visit: MSP item 33507 | |
BC Cancer. BC Cancer Registry [https://www.popdata.bc.ca/data/health/bccancer]. Ver. 2, data extract. Vancouver, BC: Population Data BC: 2015. [Approved by: BC Cancer, 2014]
Canadian Institute for Health Information. Discharge Abstract Database (hospital separations) [https://www.popdata.bc.ca/data/health/dad]. Ver. 2, data extract. Vancouver, BC: Population Data BC; 2015. [Approved by: Ministry of Health, 2014]
British Columbia, Ministry of Health. Medical Services Plan, Payment Information File [https://www.popdata.bc.ca/data/health/msp]. Ver. 2, data extract. Vancouver, BC: Population Data BC; 2015. [Approved by: Ministry of Health, 2014]
British Columbia, Ministry of Health. PharmaNet [https://www.popdata.bc.ca/data/health/PharmaNet]. Ver. 2, data extract. Vancouver, BC: Ministry of Health, 2014. [Approved by: Data Stewardship Committee; 2014]
CAIS = Cancer Agency Information System; CIHI = Canadian Institute for Health Information; MSP = Medical Services Plan.
Analytic Techniques
Total observation time was divided into 1 or 2 treatment phases depending on the occurrence of relapse. First-line treatment was defined as the time from date of diagnosis to either date of relapse or date of last follow-up. Second-line treatment was defined as the time from date of disease relapse (after primary treatment) to date of last follow-up. Total observation time for each subject was divided into 6-month intervals to align with a typical course of chop-r treatment14. Censoring variables were used to indicate patient status as either alive at last follow-up, relapsed, or died. All direct medical costs were included in the analysis and were converted to 2015 Canadian dollars15.
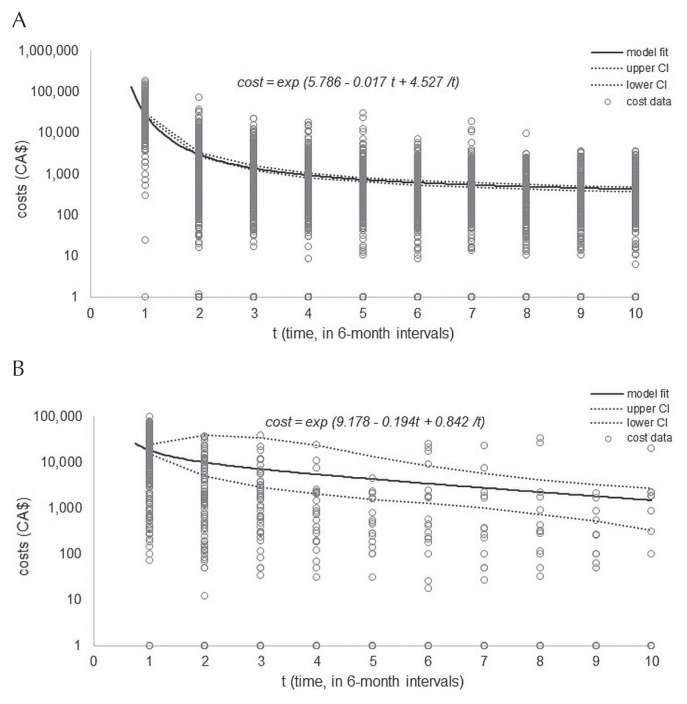
Treatment-related costs during the observation period were calculated for each patient by multiplying units of resource consumption by the unit cost for each expense category (Table I). Once all category-specific costs were estimated, the values were summed to calculate the total cost for each 6-month interval. To account for data censoring, the nonparametric inverse probability weighting method was applied to predict the conditional survival probability for each time interval, given the multi-record nature of the data11,16. When plotted, costs were observed to be inversely proportional to time (Figure 1). We thus fit a generalized linear regression model to the data, assuming a log-link (State/SE) function, gamma distribution with time (6-month interval), and inverse time (1/time) as response variables. Nonparametric bootstrapping (1000 bootstrapped samples) was used to estimate standard errors of the means. The 95% confidence intervals of the means were estimated using the corresponding percentiles from the bootstrap distribution. Table II presents mean cost estimates, confidence intervals, and standard errors for first-line and second-line treatment periods over a period of 5 years. All data analyses were conducted in the SAS (version 9.4: SAS Institute, Cary, NC, U.S.A.) or Stata/SE (version 15.1: StataCorp LP, College Station, TX, U.S.A.) software application.
FIGURE 1.
Fitted cost model showing censoring-adjusted costs for the cohort, plotted against time (t) in 6-month intervals. (A) Costs for first-line treatment. (B) Costs for second-line treatment. The solid black line indicates the fitted cost model; the dotted lines indicate the 95% confidence limits.
TABLE II.
Cost by phase of treatment, including proportions of the total cost by cost category
| Phase of treatment and time period | Cost (2015 CA$) | Proportion of cost attributed to each cost category (%)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Interval | Months | Mean | SE | 95% CI | Systemic therapy | Oncologist servicesb | Hospital services | Outpatient services | Outpatient prescription medications | RT services |
| First-line treatment, period since diagnosis | ||||||||||
| 1 | 0–6 | 29,120 | 1,477 | 28,986 to 29,170 | 56 | 10 | 20 | 5 | 8 | 1 |
| 2 | 7–12 | 4,393 | 239 | 4,383 to 4,425 | 7 | 30 | 15 | 11 | 20 | 16 |
| 3 | 13–18 | 2,389 | 113 | 2,384 to 2,414 | 4 | 30 | 11 | 14 | 30 | 12 |
| 4 | 19–24 | 1,790 | 186 | 1,785 to 1,809 | 3 | 26 | 10 | 12 | 36 | 15 |
| 5 | 25–30 | 1,525 | 154 | 1,519 to 1,538 | 2 | 21 | 13 | 11 | 43 | 10 |
| 6 | 31–36 | 1,385 | 133 | 1,378 to 1,394 | 1 | 26 | — | 8 | 62 | 3 |
| 7 | 37–42 | 1,305 | 119 | 1,296 to 1,311 | — | 19 | 8 | 7 | 66 | — |
| 8 | 43–48 | 1,258 | 110 | 1,248 to 1,262 | — | 20 | 3 | 4 | 73 | — |
| 9 | 49–54 | 1,232 | 107 | 1,220 to 1,233 | — | 18 | — | 1 | 80 | — |
| 10 | 55–60 | 1,219 | 110 | 1,205 to 1,219 | — | 15 | — | 2 | 84 | — |
|
| ||||||||||
| Second-line treatment, period since relapse | ||||||||||
| 1 | 0–6 | 18,480 | 2,451 | 15,187 to 24,772 | 17 | 3 | 62 | 4 | 8 | 6 |
| 2 | 7–12 | 9,970 | 10,078 | 5,099 to 39,244 | 6 | 7 | 53 | 5 | 13 | 16 |
| 3 | 13–18 | 7,122 | 9,937 | 2,861 to 34,544 | 18 | 7 | 42 | 2 | 16 | 14 |
| 4 | 19–24 | 5,458 | 6,563 | 2,010 to 23,529 | 41 | 22 | 17 | 3 | 18 | — |
| 5 | 25–30 | 4,302 | 3,737 | 1,554 to 13,653 | 28 | 46 | — | 2 | 24 | — |
| 6 | 31–36 | 3,438 | 2,050 | 1,262 to 8,213 | 25 | 10 | 60 | — | 5 | — |
| 7 | 37–42 | 2,770 | 1,213 | 1,009 to 5,658 | 36 | 5 | 54 | 2 | 3 | — |
| 8 | 43–48 | 2,243 | 895 | 731 to 4,131 | 1 | 6 | 89 | — | 4 | — |
| 9 | 49–54 | 1,822 | 713 | 528 to 3,328 | — | 63 | — | — | 37 | — |
| 10 | 55–60 | 1,484 | 633 | 334 to 2,713 | 2 | 25 | 60 | — | 13 | |
Boldface type indicates proportions representing more than one quarter (25%) of the total cost.
Includes new-patient and follow-up visits.
SE = standard error; CI = confidence interval; RT = radiotherapy.
RESULTS
Table III summarizes patient characteristics. Most participants (58%) were men, had been diagnosed with advanced-stage disease (53%), and had received a mean of 5 cycles of chop-r. The BC Cancer Systemic Therapy Program chemotherapy protocols suggest 3 cycles to 6–8 cycles of chop-r for patients with limited- and advanced-stage disease14. Mean follow-up was 3.2 years. As depicted in Figure 1, costs are incurred mostly in the first 6 months after either the date of diagnosis or the date of disease relapse. That observation reflects the fact that most patients (60%) were in remission after first-line treatment—a finding comparable to results reported in the literature4.
TABLE III.
Cohort characteristics
| Characteristic | Valuea |
|---|---|
| Patients (n) | 678 |
|
| |
| Mean chemotherapy cyclesb | 5±1.6 |
|
| |
| Mean age (years) | 64±14 |
|
| |
| Age group [n (%)] | |
| 0–19 Years | <5 (<1) |
| 20–59 Years | 229 (34) |
| 60–69 Years | 182 (27) |
| 70–79 Years | 179 (26) |
| 80+ Years | 85 (13) |
|
| |
| Sex [n (%) male] | 391 (58) |
|
| |
| ECOG PS at Dx [n (%)] | |
| 0 or 1 | 422 (62) |
| 2 or 3 | 233 (34) |
| 4 | 23 (3) |
|
| |
| Stage of disease at Dxc [n (%)] | |
| I/II | 317 (47) |
| III/IV | 361 (53) |
|
| |
| Patient status at end of follow-up period [n (%)] | |
| In remission | 406 (60) |
| Relapsed | 259 (38) |
| Lost to follow-up | 13 (2) |
Some percentages might not add to 100 because of rounding.
First-line treatment with CHOP-R (cyclophosphamide–doxorubicin –vincristine–prednisone, plus rituximab).
Using the Ann Arbor staging system.
ECOG PS = Eastern Cooperative Oncology Group performance status; Dx = diagnosis.
Costs were modelled over time using the equation
where t is time (in 6-month intervals), a is the coefficient of the constant term (first-line: 5.786; second-line: 9.178), b is the coefficient for time (first-line: −0.017; second-line: −0.194), and c is the coefficient for inverse time (first-line: 4.527; second-line: 0.842). Coefficient values were estimated from the generalized linear regression model.
Our model estimated the mean cost of care in the first 6 months after diagnosis to be $29,120 (95% confidence interval: $28,986 to $29,170); it was $18,480 (95% confidence interval: $15,187 to $24,772) in the first 6 months after relapse (Table II). Inpatient hospitalization for second-line treatment was the highest driver of cost (62%), most of which can be attributed to patients (3%) who underwent autologous stem-cell transplantation (asct) as second-line treatment. Systemic therapy represented the largest driver of costs in the first 6 months after diagnosis (56%). In both the first-line and second-line treatment periods, radiotherapy services accounted for a similar proportion of the total cost (1%–16%).
DISCUSSION AND CONCLUSIONS
Here, we present an analysis of the real-world costs incurred by a cohort of patients diagnosed with dlbcl in the province of British Columbia. Costs are presented 6-month time intervals, supplemented with information about model fitting so that costs can be modelled over time.
Our estimated costs are lower than those in other dlbcl costing studies. In Ontario, the censoring-adjusted total cost of treatment for dlbcl was estimated to be approximately $70,200 (2015 Canadian dollars) for all ages for the first year after diagnosis, including home and community care services11. A study using micro-costing techniques conducted in Alberta estimated a mean cost of $40,191 for treatment during first-line therapy, $5,294 for disease assessment, and $3,905 for follow-up costs over a mean duration of 450 days (all costs in 2015 Canadian dollars)2. Data about staging tests and other diagnostics, such as flow cytometry and magnetic resonance imaging, were not included in our analysis. Patient characteristics might also differ between studies. For example, studies with a higher proportion of patients undergoing costly procedures such as asct might report higher hospitalization costs. In addition, differences in costing methods, analysis timeframes (pre-diagnostic, for instance), analytical approaches, and unit costs (hospital cost per case, for instance) also make it challenging to ensure similarity for cost comparisons13. However, the positive skewness of our cost data at each time interval is similar to reports from other authors, suggesting that costs are incurred mostly by a small proportion of patients. As in other studies, hospitalizations accounted for the largest proportion of the costs incurred during second-line treatment11. The shift away from hospitalization costs toward outpatient prescription medications in our study could place a greater financial burden on patients.
As with any secondary analysis of administrative data, our study has limitations. Missing records and varying completeness of data has likely affected our results. For example, home and community care services were excluded from our analysis because of incompleteness of records. The average estimated cost for patients who underwent asct in our analysis is lower than estimates reported elsewhere. The total inpatient and outpatient costs of an asct procedure were reported to be approximately $58,793 (2015 Canadian dollars)2 in Alberta and $47,076 (2015 Canadian dollars) in the Netherlands17. Mean inpatient costs for an asct procedure in our analysis was approximately $30,000, although harvesting costs and other laboratory activities might not be fully captured in our datasets. We also observed that most patients who progressed and died (58%) did so within 6 months of their date of progression, thus limiting the period for which costs could be captured.
Despite its limitations, this work has provided some valuable insights. Providing mean costs over time for dlbcl care is useful for health care system planning, especially considering the potential effects that targeted treatments can have on how dlbcl is managed8,18. Our work has also made use of costing methods that account for censoring and made explicit how costs were modelled over time. Those approaches can be useful for future research involving modelling of dlbcl costs and contribute to the broader costing literature.
ACKNOWLEDGMENTS
The Ontario Institute for Cancer Research (OICR) is funded by the Government of Ontario through the Ministry of Economic Development, Job Creation and Trade. The Canadian Centre for Applied Research in Cancer Control (ARCC) receives core funding from the Canadian Cancer Society (grant no. 2015-703549). Both OICR and ARCC are proud to support the publication of this costing series.
All inferences, opinions, and conclusions drawn in this publication are those of the authors and do not reflect the opinions or policies of the Data Stewards. This research was supported by Genome BC/Genome Canada (141LYM and 271LYM), the Canadian Institutes of Health Research, the BC Cancer Foundation, and ARCC. ARCC is funded by the Canadian Cancer Society (2015-703549).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Cancer Society. Diffuse large B-cell lymphoma [Web page] Toronto, ON: Canadian Cancer Society; n.d. [Available at: http://www.cancer.ca/en/cancer-information/cancer-type/non-hodgkin-lymphoma/non-hodgkin-lymphoma/diffuse-large-b-cell-lymphoma/?region=bc; cited 20 June 2016] [Google Scholar]
- 2.Lee RC, Zou D, Demetrick DJ, Difrancesco LM, Fassbender K, Stewart D. Costs associated with diffuse large B-cell lymphoma patient treatment in a Canadian integrated cancer care center. Value Health. 2008;11:221–30. doi: 10.1111/j.1524-4733.2007.00227.x. [DOI] [PubMed] [Google Scholar]
- 3.Maurer MJ, Ghesquieres H, Link BK, et al. Diagnosis-to-treatment interval is an important clinical factor in newly diagnosed diffuse large B-cell lymphoma and has implication for bias in clinical trials. J Clin Oncol. 2018;36:1603–10. doi: 10.1200/JCO.2017.76.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125:22–32. doi: 10.1182/blood-2014-05-577189. [DOI] [PubMed] [Google Scholar]
- 5.Johnston KM, Marra CA, Connors JM, Najafzadeh M, Sehn L, Peacock SJ. Cost-effectiveness of the addition of rituximab to chop chemotherapy in first-line treatment for diffuse large B-cell lymphoma in a population-based observational cohort in British Columbia, Canada. Value Health. 2010;13:703–11. doi: 10.1111/j.1524-4733.2010.00737.x. [DOI] [PubMed] [Google Scholar]
- 6.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined chop plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–33. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 7.Scott DW, Mottok A, Ennishi D, et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J Clin Oncol. 2015;33:2848–56. doi: 10.1200/JCO.2014.60.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Staton AD, Ayer T, Goldstein DA, Koff JL, Flowers CR. Exploring the potential cost-effectiveness of precision medicine treatment strategies for diffuse large B-cell lymphoma. Leuk Lymphoma. 2018;59:1700–9. doi: 10.1080/10428194.2017.1390230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cressman S, Browman GP, Hoch J, Kovacic L, Peacock S. A time-trend economic analysis of cancer drug trials. Oncologist. 2015;20:729–36. doi: 10.1634/theoncologist.2014-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khor S, Beca J, Krahn M, et al. Real world costs and cost-effectiveness of rituximab for diffuse large B-cell lymphoma patients: a population-based analysis. BMC Cancer. 2014;14:586. doi: 10.1186/1471-2407-14-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yong JH, McGowan T, Redmond-Misner R, et al. Estimating the costs of intensity-modulated and 3-dimensional conformal radiotherapy in Ontario. Curr Oncol. 2016;23:e228–38. doi: 10.3747/co.23.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canadian Institute for Health Information (cihi) Your Health System: Cost of a Standard Hospital Stay [Web page] Ottawa, ON: cihi; n.d. [Available online at: https://yourhealthsystem.cihi.ca/hsp/inbrief?lang=en#!/indicators/015/cost-of-a-standard-hospital-stay/;mapC1;mapLevel2;/; cited 15 September 2018] [Google Scholar]
- 14.Cancer BC. BC Cancer Protocol Summary for Treatment of Lymphoma with Doxorubicin, Cyclophosphamide, Vincristine, Prednisone and Rituximab (CHOP-R) Vancouver, BC: BC Cancer; 2001. [revised 2019]. [Available online at: http://www.bccancer.bc.ca/chemotherapy-protocols-site/Documents/Lymphoma-Myeloma/LYCHOPR_Protocol.pdf; cited 20 July 2016] [Google Scholar]
- 15.Statistics Canada. Consumer Price Index, annual average, not seasonally adjusted [Web page] Ottawa, ON: Statistics Canada; 2019. [Available at: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000501; cited 5 September 2015] [Google Scholar]
- 16.Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika. 2000;87:329–43. doi: 10.1093/biomet/87.2.329. [DOI] [Google Scholar]
- 17.Blommestein HM, Verelst SG, Huijgens PC, Blijlevens NM, Cornelissen JJ, Uyl-de Groot CA. Real-world costs of autologous and allogeneic stem cell transplantations for haematological diseases: a multicentre study. Ann Hematol. 2012;91:1945–52. doi: 10.1007/s00277-012-1530-2. [DOI] [PubMed] [Google Scholar]
- 18.Di Rocco A, De Angelis F, Ansuinelli M, Foa R, Martelli M. Is now the time for molecular driven therapy for diffuse large B-cell lymphoma? Expert Rev Hematol. 2017;10:761–74. doi: 10.1080/17474086.2017.1356714. [DOI] [PubMed] [Google Scholar]