Abstract
Background
In the present study, we examined real-world treatment patterns for squamous cell carcinoma of the head and neck (scchn) in Canada, which are largely unknown.
Methods
Oncologists across Canada provided data for disease history, characteristics, and treatment patterns during May–July 2016 for 6–8 consecutive patients receiving first-line or second-line drug treatment for scchn (including locally advanced and recurrent or metastatic disease).
Results
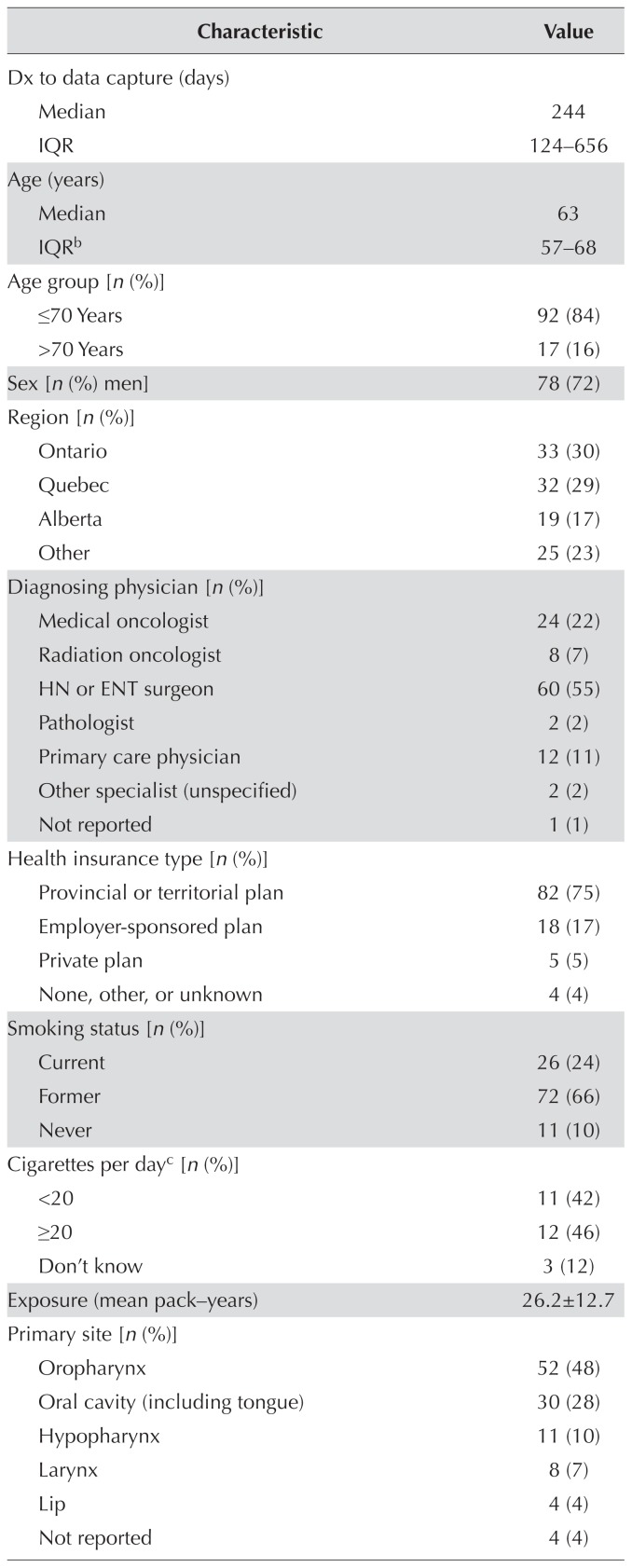
Information from 16 physicians for 109 patients receiving drug treatment for scchn was provided; 1 patient was excluded from the treatment-pattern analysis. Median age in the cohort was 63 years [interquartile range (iqr): 57–68 years], and 24% were current smokers, with a mean exposure of 26.2 ± 12.7 pack–years. The most common tumour site was the oropharynx (48%). Most patients (84%) received platinum-based regimens as first-line treatment (44% received cisplatin monotherapy). Use of cetuximab-based regimens as first-line treatment was limited (17%). Of 53 patients receiving second-line treatment, 87% received a first-line platinum-based regimen. Median time between first-line treatment with a platinum-based regimen and initiation of second-line treatment was 55 days (iqr: 20–146 days). The most common second-line regimen was cetuximab monotherapy (43%); platinum-based regimens were markedly infrequent (13%).
Conclusions
Our analysis provides real-world insight into scchn clinical practice patterns in Canada, which could inform reimbursement decision-making. High use of platinum-based regimens in first-line drug treatment was generally reflective of treatment guidelines; cetuximab use in the second-line was higher than anticipated. Additional real-world studies are needed to understand the effect of novel therapies such as immuno-oncology agents on clinical practice and outcomes, particularly for recurrent or metastatic scchn.
Keywords: Canada, cetuximab, head-and-neck cancer, platinum-based therapy, recurrent disease, metastatic disease, squamous cell carcinoma, scchn, treatment patterns
INTRODUCTION
Head-and-neck cancers encompass neoplasms of the oral cavity, pharynx, larynx, sinuses, and salivary glands1. In 2012, approximately 686,000 new cases of head-and-neck cancer and 376,000 associated deaths occurred worldwide, representing the 7th leading cancer by incidence2, with squamous cell carcinoma of the head and neck (scchn) accounting for approximately 90% of those cancers3. An estimated 4700 Canadians were diagnosed with oral cancer in 2017, resulting in 1250 deaths4. The most recent Canadian statistics for oropharyngeal cancer reported 180 new cases and 131 deaths in 20135.
Treatment for scchn depends on stage at diagnosis: Patients diagnosed early are treated with curative intent; patients diagnosed at advanced stages or with recurrent or metastatic disease (r/m scchn) are treated with the aim of prolonging remission6. Because of limited therapeutic options, r/m scchn poses a treatment challenge—particularly for the patients who present with advanced-stage disease (approximately 66%)7. Patients with locally advanced stage iii or iv disease are usually treated with combined modalities, including chemotherapy, radiotherapy, and surgery (if resectable)6,8–11. However, approximately 50% of patients with locally advanced scchn subsequently develop locoregional or distant (metastatic) recurrences7. Median overall survival (os) for patients with r/m scchn receiving treatment is 6–9 months12.
Other treatment options for patients with r/m scchn commonly include chemotherapies [platinum compounds (cisplatin, carboplatin), taxanes (docetaxel, paclitaxel), fluorouracil (5fu), or methotrexate], targeted therapies (such as the anti-epidermal growth factor receptor monoclonal antibody cetuximab), and combinations of chemotherapies with targeted agents6,13–16. Regimen choice is governed by a number of factors, including Eastern Cooperative Oncology Group performance status, symptoms related to tumour growth, comorbidities, prior treatment (including whether chemotherapy was received in the first line), patient preference, and the need for palliation13,16.
The introduction of cetuximab in combination with platinum and 5fu chemotherapy, followed by maintenance cetuximab (the extreme regimen)17, represented a significant advance in the first-line treatment of r/m scchn, supported by a phase iii randomized trial that showed a significant survival benefit compared with platinum–5fu alone18. The median os was 10.1 months for extreme compared with 7.4 months for platinum–5fu (hazard ratio for death: 0.80; 95% confidence interval: 0.64 to 0.99; p = 0.04) in patients with r/m scchn who had not received systemic chemotherapy within 6 months of study entry19. Based on published evidence, the extreme regimen has become a standard first-line treatment approach for r/m scchn in Europe9,10 and in the United States in patients considered fit enough to tolerate the regimen6,18. In Canada, the Head and Neck Cancer Disease Site Group also recommends the extreme regimen to improve os, progression-free survival, and response rate for “suitable” patients with r/m scchn20,21.
In clinical practice, other combinations such as a taxane or cisplatin plus cetuximab are sometimes used as first-line treatment for r/m scchn when patients are considered too frail for the extreme regimen18; however, no randomized controlled studies have been conducted to show benefit for those treatments. Methotrexate monotherapy—which is associated with a lower response rate, but os comparable to that with platinum–5fu22—is usually recommended for patients unable to tolerate combination chemotherapy9. Despite advances in treatment options, the prognosis for patients with scchn remains poor12,18,23. New therapies are emerging, offering a wider range of treatment options for patients with recurrent disease. Those options include the anti–PD-1 immune checkpoint inhibitors nivolumab and pembrolizumab, both of which are approved in the United States for the treatment of r/m scchn with disease progression on or after platinum-based therapy24,25.
At present, there is a dearth of published evidence describing the use of therapies in real-world clinical practice, particularly for a second or later line of therapy, and to the best of the authors’ knowledge, no studies have evaluated Canadian treatment patterns for scchn in the real-world setting. A better understanding of Canadian clinical practice patterns and clinical outcomes is needed to inform reimbursement decision-makers about treatment options in patients with scchn. The objective of the present study was therefore to identify real-world treatment patterns for patients with scchn so as to better understand the standard of care, including by line of therapy, in Canadian clinical practice.
METHODS
Study Design
This descriptive cross-sectional analysis used survey data collected from 2 May to 18 July 2016 from participating medical oncologists actively involved in the management of scchn across Canada. Details of the survey method, which has been used in more than 50 disease areas, have previously been published26.
Eligible physicians who wanted to participate in the study were identified from public listings and were required to have qualified as a medical oncologist between 1981 and 2013 and to be treating a minimum of 10 patients with scchn per month. Participating physicians completed a detailed electronic Patient Record Form reporting data for their next 6–8 consecutively treated patients who met these eligibility criteria: scchn diagnosis, 18 years of age or older, receiving active drug treatment for scchn, not enrolled in a clinical trial (at the time of consultation), and primary tumour not located on a salivary gland or the nasopharynx. Excluded from the study were patients receiving best supportive care; those undergoing either surgery or radiotherapy (or both), but no active drug treatment; and those under a “watch and wait” treatment approach. Proportional quota sampling was used to generate a quasirandom sample evenly split between patients who were receiving first-line active drug treatment and those who were receiving second-line or later active drug treatment at the point of data collection.
Data Collection
Information extracted from the electronic Patient Record Forms included demographic and clinical characteristics of the patients and complete scchn drug and non-drug treatment history (including surgery and radiotherapy). Real-world treatment patterns identified in clinical practice for the participating providers included comprehensive treatment history from diagnosis to current treatment and treatment modality sequencing (that is, surgery, radiotherapy, chemotherapy, and targeted therapy, including specific agents received).
The end of a given line of drug therapy was defined by the addition, switching, or discontinuation of a drug or by repeat of the same therapy after completion of the original course. A change in dose or a treatment holiday was not considered a new line of therapy. Line of treatment was counted relative to each patient’s first-line active drug treatment (either monotherapy or a combination regimen) with or without concurrent radiotherapy and regardless of disease progression (that is, for patients who had previously received drug therapy for non-recurrent or metastatic disease). Drug treatment for subsequent recurrent or metastatic disease was not considered “first line” for that disease stage. For the purposes of the present study, radiotherapy alone and surgery alone were not counted as lines of treatment. Best supportive care was considered a line of therapy only in the third line or later. The length of time between diagnosis and treatment initiation, the duration of first-line therapy, and the time to initiation of second-line therapy also were recorded for patients receiving drug treatment.
Statistical Analyses
Demographics, clinical characteristics, and antineoplastic treatment patterns are described using frequencies and proportions for categorical data and using means with standard deviation or medians with interquartile range (iqr) for numeric data. Time variables are reported as medians with iqr. The probability of time to progression from initiation of first-line therapy until initiation of second-line therapy was plotted using the Kaplan–Meier method and was calculated using the initiation dates of first-line and second-line therapy. The time-to-progression analysis included all patients in the sample, including those receiving first-line therapy only, who were censored at the time of data collection.
RESULTS
Demographic and Clinical Characteristics of the Patients
Data from 16 physicians for 109 patients with scchn receiving first-line or second-line drug treatment were obtained. The treatment pattern analysis (n = 108) excluded 1 patient whose treatment data were incomplete. Most patients (77%) were diagnosed either by a head-and-neck or ear/nose/throat surgeon (55%) or by a medical oncologist (22%). On average, patients saw a medical oncologist 6.7 times and a radiation oncologist 6.1 times annually. Most patients were receiving treatment as part of a provincial or territorial health insurance plan (75%). Participating patients were drawn predominantly from clinics in Ontario (30% of patients), Quebec (29%), and Alberta (17%).
Median patient age in the cohort was 63 years (iqr: 57–68 years), and 72% of the patients were men. Median time from diagnosis to the point of data capture was 244 days (iqr: 124–656 days). Of the 109 patients included in the study, 90% were current or former smokers, and the mean smoking exposure for the current smokers was 26.2 ± 12.7 pack–years. The most common primary tumour site was the oropharynx (48%). At the point of data capture, 76% of patients had locoregionally advanced disease (stages i–ivb), and 23% had recurrent or metastatic disease (stage ivc). Compared with their counterparts having stages i–ivb disease, patients with stage ivc disease at data capture were more likely to be treated with the aim of improving quality of life (60% vs. 41%) and symptom control (44% vs. 28%), and less likely to be treated with the aim of improving os (32% vs. 46%). Similarly, when physicians were asked to predict the likely next treatment step, patients with stage ivc disease were deemed more likely to progress to best supportive care (40% vs. 11%) and less likely to continue with current treatment (32% vs. 46%). At data capture, almost two thirds of the patients (64%) were clinically fit, with an Eastern Cooperative Oncology Group performance status of 0 or 1. Of the 73 patients who underwent p16 testing to determine their human papillomavirus infection status at the point of data capture, 45% tested positive (Table I).
TABLE I.
Demographics and clinical characteristics of the 109 study patientsa
| Characteristic | Value |
|---|---|
| Dx to data capture (days) | |
| Median | 244 |
| IQR | 124–656 |
|
| |
| Age (years) | |
| Median | 63 |
| IQRb | 57–68 |
|
| |
| Age group [n (%)] | |
| ≤70 Years | 92 (84) |
| >70 Years | 17 (16) |
|
| |
| Sex [n (%) men] | 78 (72) |
|
| |
| Region [n (%)] | |
| Ontario | 33 (30) |
| Quebec | 32 (29) |
| Alberta | 19 (17) |
| Other | 25 (23) |
|
| |
| Diagnosing physician [n (%)] | |
| Medical oncologist | 24 (22) |
| Radiation oncologist | 8 (7) |
| HN or ENT surgeon | 60 (55) |
| Pathologist | 2 (2) |
| Primary care physician | 12 (11) |
| Other specialist (unspecified) | 2 (2) |
| Not reported | 1 (1) |
|
| |
| Health insurance type [n (%)] | |
| Provincial or territorial plan | 82 (75) |
| Employer-sponsored plan | 18 (17) |
| Private plan | 5 (5) |
| None, other, or unknown | 4 (4) |
|
| |
| Smoking status [n (%)] | |
| Current | 26 (24) |
| Former | 72 (66) |
| Never | 11 (10) |
|
| |
| Cigarettes per dayc [n (%)] | |
| <20 | 11 (42) |
| ≥20 | 12 (46) |
| Don’t know | 3 (12) |
|
| |
| Exposure (mean pack–years) | 26.2±12.7 |
|
| |
| Primary site [n (%)] | |
| Oropharynx | 52 (48) |
| Oral cavity (including tongue) | 30 (28) |
| Hypopharynx | 11 (10) |
| Larynx | 8 (7) |
| Lip | 4 (4) |
| Not reported | 4 (4) |
|
| |
| Tumour status at diagnosis [n (%)] | |
| Resectable | 33 (30) |
| Non-resectable or not eligible for resection | 76 (70) |
|
| |
| Disease stage at Dx [n (%)] | |
| I | 11 (10) |
| II | 26 (24) |
| III | 32 (29) |
| IVAd | 18 (17) |
| IVBe | 10 (9) |
| IVCf | 9 (8) |
| Not assessed | 1 (1) |
|
| |
| Disease stage at data capture [n (%)] | |
| I | 3 (3) |
| II | 19 (17) |
| III | 14 (13) |
| IVAd | 33 (30) |
| IVBe | 14 (13) |
| IVCf | 25 (23) |
| Not assessed | 1 (1) |
|
| |
| ECOG PS [n (%)] | |
| 0 | 8 (7) |
| 1 | 62 (57) |
| ≥2 | 36 (33) |
| Not assessed | 3 (3) |
|
| |
| HPV p16 status [n (%)] | |
| Not tested | 36 (33) |
| Tested | 73 (67) |
| Positiveg | 33 (45) |
| Negativeg | 37 (51) |
| Inconclusiveg | 3 (4) |
At point of data capture unless otherwise specified.
Two patients reported to be 90 or more years of age were assigned an age of 90 years.
For current smokers.
Moderately advanced locoregional disease.
Very advanced locoregional disease.
Distant metastatic disease.
Of those tested.
Dx = diagnosis; IQR = interquartile range; HN = head-and-neck; ENT = ear, nose and throat; ECOG PS = Eastern Cooperative Oncology Group performance status; HPV = human papillomavirus.
SCCHN Treatment Overview
Median time from diagnosis to treatment initiation was 49 days (iqr: 24–131 days). At the point of data capture, 51% of the patients were receiving first-line drug treatment, 42% were receiving second-line treatment, and 6% were receiving third-line treatment (Table II).
TABLE II.
Time to treatment and treatment type in 109 patients at point of data capture
| Variable | Value |
|---|---|
| Dx to start of first-line drug therapy (days) | |
| Median | 49 |
| IQR | 24–131 |
|
| |
| End of 1st-line drug therapy to start of 2nd-line drug therapy (days) | |
| Median | 49 |
| IQR | 19–149 |
|
| |
| Start of 1st-line platinum-based drug therapy to start of 2nd-line drug therapy (days) | |
| Median | 55 |
| IQR | 20–146 |
|
| |
| Line of drug treatment at point of data capture [n (%)] | |
| First | 56 (51) |
| Second | 46 (42) |
| Third | 7 (6) |
|
| |
| Use of non-drug treatmentsa [n (%)] | |
| Surgery | 35 (32) |
| Radiotherapy | 82 (75) |
| Neither | 13 (12) |
Before initiation of drug treatment; patients might previously have received surgery or radiotherapy, or both.
Dx = diagnosis; IQR = interquartile range.
First-Line Drug Treatment Patterns
Of the 108 patients who received first-line therapy, most (84%, n = 91) received a platinum-based regimen as first-line treatment (Table III), and of those patients, 26% (n = 24) received concomitant radiotherapy. The individual regimen most frequently received was cisplatin monotherapy (44%, n = 48), and of those patients, 33% (n = 16) received concomitant radiotherapy. Use of cetuximab-based regimens as first-line treatment was limited (17%). Use of the extreme regimen (cisplatin–cetuximab or carboplatin–cetuximab plus 5fu)19 as first-line therapy was very rare (1%); however, 6% of the patients received extreme plus docetaxel. Docetaxel-containing regimens without cetuximab were received by 15 patients (14%), with 2 patients receiving docetaxel monotherapy.
TABLE III.
First-line drug treatment patterns in 108 patients
| Treatment | With radiotherapy [n (%)] | Overall | |
|---|---|---|---|
|
| |||
| No | Yes | ||
| Treatment regimens | |||
|
| |||
| Carboplatin | 2 (2) | — | 2 (2) |
|
| |||
| Carboplatin–docetaxel | — | 1 (1) | 1 (1) |
|
| |||
| Carboplatin–docetaxel–5FU | — | 1 (1) | 1 (1) |
|
| |||
| Carboplatin–5FU | 1 (1) | — | 1 (1) |
|
| |||
| Carboplatin–paclitaxel | 1 (1) | — | 1 (1) |
|
| |||
| Cetuximab | 5 (5) | 2 (2) | 7 (6) |
|
| |||
| Cisplatin | 32 (30) | 16 (15) | 48 (44) |
|
| |||
| Cisplatin–cetuximab | 3 (3) | — | 3 (3) |
|
| |||
| Cisplatin–docetaxel– | 1 (1) | — | 1 (1) |
|
| |||
| cetuximab | |||
|
| |||
| Cisplatin–docetaxel–5FU | 7 (6) | 3 (3) | 10 (9) |
|
| |||
| Cisplatin–docetaxel–5FU | 5 (5) | 1 (1) | 6 (6) |
|
| |||
| –cetuximab | |||
|
| |||
| Cisplatin–5FU | 12 (11) | 1 (1) | 13 (12) |
|
| |||
| Cisplatin–5FU–cetuximab | — | 1 (1) | 1 (1) |
|
| |||
| Cisplatin–5FU | 1 (1) | — | 1 (1) |
|
| |||
| –methotrexate | |||
|
| |||
| Cisplatin–other | 1 (1) | — | 1 (1) |
|
| |||
| Cisplatin–paclitaxel | 1 (1) | — | 1 (1) |
|
| |||
| Docetaxel | 2 (2) | — | 2 (2) |
|
| |||
| Docetaxel–5FU –methotrexate | — | 1 (1) | 1 (1) |
|
| |||
| 5FU–other | 1 (1) | — | 1 (1) |
|
| |||
| Gemcitabine | 1 (1) | 1 (1) | 2 (2) |
|
| |||
| Methotrexate | 2 (2) | 1 (1) | 3 (3) |
|
| |||
| Other | — | 1 (1) | 1 (1) |
| Regimen classa | |||
|
| |||
| Platinum-based | 67 (62) | 24 (22) | 91 (84) |
|
| |||
| Cetuximab-based | 14 (13) | 4 (4) | 18 (17) |
Not mutually exclusive; regimen classes overlap. “Other” regimen assumed not to contain platinum or cetuximab.
5FU = 5-fluorouracil.
Second-Line Drug Treatment Patterns
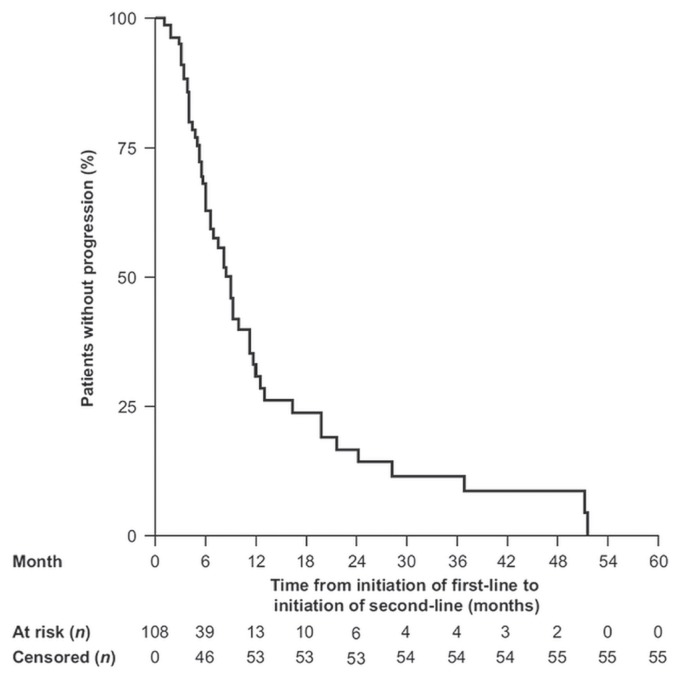
Median time to progression from initiation of first-line therapy to initiation of second-line therapy was 8.9 months (95% confidence interval: 6.4 months to 11.1 months; Figure 1). For the 53 patients who received second-line treatment, platinum-based therapies were used less frequently (13%) than they had been in the first line (Table IV). Few patients received concomitant radiotherapy during second-line therapy (n = 2). The oncologists reported a median of 49 days (iqr: 19–149) between the end of first-line and the initiation of second-line drug treatment (Table II). The second-line regimens most commonly used were cetuximab (43%), docetaxel (13%), paclitaxel (13%), and methotrexate (8%) monotherapies. Use of the extreme regimen remained low (2%). Of the patients who received second-line treatment, 87% (n = 46) had received a first-line platinum-based regimen. The median time between initiation of first-line treatment with a platinum-based regimen and initiation of second-line treatment was 55 days (iqr: 20–146 days; Table II). Nearly half the patients previously treated with platinum-based therapy (46%) received cetuximab monotherapy as second-line treatment; 13% received docetaxel monotherapy; 13%, paclitaxel monotherapy; 9%, methotrexate monotherapy; and 9%, another platinum-based regimen. In the patients who received platinum-based therapy in the first-line setting and who went on to receive second-line therapy, cetuximab monotherapy was the most frequently used regimen (45%, Table V).
FIGURE 1.
Kaplan–Meier probability of time to disease progression from initiation of first-line therapy until initiation of second-line therapy in 108 patients.
TABLE IV.
Second-line drug treatment patterns in 53 patients
| Treatment | With radiotherapy [n (%)] | Overall | |
|---|---|---|---|
|
| |||
| No | Yes | ||
| Treatment regimens | |||
|
| |||
| Capecitabine–cetuximab | 1 (2) | — | 1 (2) |
|
| |||
| Carboplatin | 1 (2) | — | 1 (2) |
|
| |||
| Carboplatin–5FU | 1 (2) | — | 1 (2) |
|
| |||
| Carboplatin–paclitaxel | — | 1 (2) | 1 (2) |
|
| |||
| Cetuximab | 22 (42) | 1 (2) | 23 (43) |
|
| |||
| Cisplatin | 1 (2) | — | 1 (2) |
|
| |||
| Cisplatin–5FU | 2 (4) | — | 2 (4) |
|
| |||
| Cisplatin–5FU–cetuximab | 1 (2) | — | 1 (2) |
|
| |||
| Docetaxel | 7 (13) | — | 7 (13) |
|
| |||
| Docetaxel–cetuximab | 1 (2) | — | 1 (2) |
|
| |||
| Erlotinib | 2 (4) | — | 2 (4) |
|
| |||
| 5FU | 1 (2) | — | 1 (2) |
|
| |||
| Methotrexate | 4 (8) | — | 4 (8) |
|
| |||
| Paclitaxel | 5 (9) | — | 7 (13) |
| Regimen classes | |||
|
| |||
| Platinum-based regimens | 6 (11) | 1 (2) | 7 (13) |
|
| |||
| Cetuximab-based regimens | 25 (47) | 1 (2) | 26 (49) |
Not mutually exclusive; regimen classes overlap.
5FU = fluorouracil.
TABLE V.
Second-line treatment in 46 patients receiving first-line platinum
| Treatment | Value [n (%)] |
|---|---|
| Monotherapy | |
|
| |
| Cetuximab | 20 (43) |
|
| |
| Cetuximab + RT | 1 (2) |
|
| |
| Docetaxel | 6 (13) |
|
| |
| Docetaxel + RT | 0 |
|
| |
| 5FU ± RT | 1 (2) |
|
| |
| Gemcitabine ± RT | 0 |
|
| |
| Methotrexate | 4 (9) |
|
| |
| Methotrexate + RT | 0 |
|
| |
| Paclitaxel | 4 (9) |
|
| |
| Paclitaxel + RT | 2 (4) |
|
| |
| Other ± RT | 2 (4) |
|
| |
| Platinum | 2 (4) |
|
| |
| Platinum + RT | 0 |
|
| |
| Combination therapy | |
|
| |
| EXTREME regimena ± RT | 1 (2) |
|
| |
| Platinum–5FU ± RT | 0 |
|
| |
| Platinum–taxane ± RT | 1 (2) |
|
| |
| Other ± RT | 2 (4) |
Platinum–5FU–cetuximab.
5FU = fluorouracil; RT = radiotherapy.
DISCUSSION
Treatment patterns observed in this study generally reflected current treatment guidelines reported for Canada8,20,27, with some notable deviations. Platinum-based chemotherapy (used concomitantly or postoperatively with radiotherapy) is considered the standard of care for the primary treatment of most patients with locally advanced (stages iii–ivb) scchn27. Most patients (84%) received platinum-based therapies in the first-line setting. According to international guidelines6,9, patients with an Eastern Cooperative Oncology Group performance status of 2 or greater should generally receive first-line monotherapy; accordingly, 6% of patients received cetuximab; 3%, methotrexate; and 2%, docetaxel. No patient received paclitaxel, carboplatin, 5fu, or capecitabine monotherapy. Docetaxel–cisplatin–5fu was received by 9% of the patients receiving first-line drug therapy. For larynx preservation, Canadian guidelines recommend either that regimen followed by radiation and surgery, or concurrent chemoradiotherapy, in preference to radiotherapy alone27. Cetuximab (as monotherapy or in combination with other agents) was received by 17% of patients in the first line; practice guidelines suggest the use of cetuximab in addition to intensified radiotherapy as an alternative to chemoradiotherapy for patients with stages iii–ivb scchn who are ineligible for concurrent platinum-based chemotherapy or those more than 70 years of age27.
Use of the extreme regimen19 was low, with only 1% of patients receiving the regimen in the first line, and only 2% receiving it in the second line, although an additional 6% of patients received extreme–docetaxel as first-line treatment. Based on observations of increased survival18, Canadian practice guidelines recommend the extreme regimen to improve survival and response rate in “suitable” untreated patients with r/m scchn20,21. However, few patients in the present study had metastatic disease at diagnosis. Additionally, extreme and other existing platinum-based treatment options are associated with severe toxicity and adverse effects on health-related quality of life28. It is unclear whether the low rates of use of extreme or other cetuximab–platinum–5fu–containing regimens observed in our study reflect such concerns.
Optimal first-line treatment selection is crucial to ensure that patients remain sufficiently healthy to receive subsequent treatments (that is, second-line and beyond) 18—particularly those with locoregional progression. In the present study, second-line drug treatment regimens varied and primarily used monotherapies—namely, cetuximab (43%), taxanes (docetaxel, 13%; paclitaxel, 13%), and methotrexate (8%). Of the patients who progressed to second-line treatment, a high proportion (87%) had received platinum-based first-line treatment; only 9% of patients who received first-line platinum-based therapy subsequently received platinum-based therapy in the second-line setting. Given the poor response rates with existing therapies for r/m scchn and disease progression during or after platinum-based chemotherapy18, the substantial health care and economic burden represented by those patients is driven largely by hospitalizations and anticancer therapy costs10,29. That observation has led to suggestions to limit the use of combination regimens for metastatic cancers and to restrict chemotherapy on the basis of performance status30.
Higher-than-expected cetuximab use was identified in the second line. A formal comparison of observed treatment patterns with guideline recommendations was not the purpose of our study, because guidelines might not reflect currently approved indications or preferred clinical practice in a given country. Additionally, physician prescribing preferences are likely to be influenced by familiarity and personal preferences. For example, chemotherapy often incorporates a combination of drugs, each of which might have already been on the market for a long time. The label might therefore not reflect the combinations used for the treatment of cancer in a particular setting, oncologists might individualize therapy, and institutional protocols might allow for flexibility. The practice patterns identified in the present study illustrate the importance of real-world evidence in describing clinical practice and informing reimbursement decision-makers.
The increasing availability of novel therapies with better adverse event profiles could potentially change the Canadian treatment algorithm for scchn. A new therapy class—immune checkpoint inhibitors—has shown promising os results as monotherapy, with tolerable toxicities31–34 and improvements in health-related quality of life32,35 in patients with r/m scchn. However, our analysis was conducted before Canadian approval of those therapies. Subsequent real-world treatment-pattern studies are needed to understand the effects of novel therapies on clinical practice and the outcomes of patients with r/m scchn and platinum-refractory disease. To the best of the authors’ knowledge, few studies have examined real-world treatment patterns for patients with scchn. A real-world study of patients with r/m scchn conducted between 2006 and 2013 in the Netherlands provided insight into local drug usage patterns10; however, its reported findings cannot be compared directly with our results because of geographic differences in treatment guidelines, clinical practice, and reimbursement policies. The scchn treatment patterns presented here provide the most reliable evidence of Canadian clinical practice to date.
Several study limitations should be noted. With respect to the study’s methods and the selection of participating physicians, a cross-sectional rather than a longitudinal approach was taken; the data therefore reflect the population with scchn at the time of data collection and cannot be used for causal inference or to project treatment patterns beyond the reporting period, because new agents have become available and clinical practice patterns could have changed. In addition, although some data were available at the time of diagnosis, most of the included data were available only at the time of capture; the study findings might therefore not have reflected the comprehensive clinical or treatment characteristics of the patients at the time of diagnosis. Furthermore, although disease stage and treatment could be identified at data capture, we could not elucidate the disease stage of the patients during prior lines of therapy. Physician inclusion was influenced by willingness to participate, resulting in a convenience sample that might not be representative of the overall population of Canadian physicians treating patients with scchn. In terms of patient selection, the patients included in the study represent a quasi-random sample because physicians were asked to select, from study initiation, the first 6–8 consecutive patients meeting the entry criteria. Providers were more likely to collect data from patients seen more frequently, who thus might have been overrepresented. Additionally, physician preferences could, in part, be influenced by provincial variations in reimbursement policy and patient access to relevant clinical trials. However, the systematic approach to recruitment was designed to reduce selection bias. No formalized diagnostic checklist was mandated in the study methods; instead, diagnosis of the target patient group was based on the judgment and diagnostic skills of the responding physicians, as being reflective of routine real-world clinical practice. With regard to treatment data, patients were selected on the basis of receiving drug therapy and only general first-line and second-line drug treatments were included. Information such as dose, frequency of therapy, or receipt of therapy by stage or tumour site was not reported. In addition, the classifications used in our analysis for line of therapy were based on receipt of drug therapy rather than on receipt of any therapy (for example, surgery or radiation) and could be open to interpretation. Consequently, it is not possible to differentiate between “line of any type of therapy” (that is, drug or non-drug treatment) and “line of drug therapy.” Finally, as in any study based on chart review, data quality relied on the accurate reporting of information by physicians.
CONCLUSIONS
The published literature contains little information about clinical practice patterns in scchn. This Canadian real-world study revealed high use of platinum-based regimens as first-line treatment. Although some variation in second-line treatment patterns was noted, cetuximab-based regimens were used most frequently. Monotherapies were used more often in the second-line than in the first-line setting, with cisplatin being the monotherapy in highest use in the first line, and cetuximab being the most common monotherapy agent in the second line. The study findings generally support the view that there is no standard second-line treatment for scchn in Canada. Management strategies are expected to evolve with the emergence of the new immuno-oncology treatment options for patients with scchn, which could lead to improved outcomes for patients in Canada.
ACKNOWLEDGMENTS
This study was sponsored by Bristol–Myers Squibb. Medical writing assistance was provided by Robyn Fowler phd, Martin Bell phd, and Karen Smoyer phd, of Evidence Scientific Solutions and was funded by Bristol–Myers Squibb.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: KB and PH were employees of Adelphi Real World at the time of the study; AATM is a full-time employee of Bristol–Myers Squibb Canada; AM is a full-time employee of Bristol–Myers Squibb Canada and received nonfinancial support from Bristol–Myers Squibb during the conduct of the study; JWS is a full-time employee and stockholder of Bristol–Myers Squibb.
REFERENCES
- 1.Wissinger E, Griebsch I, Lungershausen J, Foster T, Pashos CL. The economic burden of head and neck cancer: a systematic literature review. Pharmacoeconomics. 2014;32:865–82. doi: 10.1007/s40273-014-0169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123–41. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canadian Cancer Society. Oral cavity cancer statistics [Web page] Toronto, ON: Canadian Cancer Society; 2017. [Available at: http://www.cancer.ca/en/cancer-information/cancer-type/oral/statistics/?region=on; cited 25 September 2017] [Google Scholar]
- 5.Canadian Cancer Society Advisory Committee. Pharyngeal cancer statistics: Oropharyngeal cancer incidence and mortality [Web page] Toronto, ON: Canadian Cancer Society; 2017. [Available at: http://www.cancer.ca/en/cancer-information/cancer-type/oropharyngeal/statistics/?region=on; cited 25 September 2017] [Google Scholar]
- 6.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Head and Neck Cancers. Ver. 1.2017. Fort Washington, PA: nccn; 2017. [Current version available online at: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp (free registration required); cited 1 June 2017] [Google Scholar]
- 7.Cognetti DM, Weber RS, Lai SY. Head and neck cancer: an evolving treatment paradigm. Cancer. 2008;113(suppl):1911–32. doi: 10.1002/cncr.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert R, Devries-Aboud M, Winquist E, Waldron J, McQuestion M on behalf of the Head and Neck Cancer Disease Site Group. The Management of Head and Neck Cancer in Ontario. Toronto, ON: Cancer Care Ontario; 2009. [Available online at: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/536; cited 8 June 2017] [Google Scholar]
- 9.Gregoire V, Lefebvre JL, Licitra L, Felip E on behalf of the ehns-esmo-estro Guidelines Working Group. Squamous cell carcinoma of the head and neck: ehns-esmo-estro clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v184–6. doi: 10.1093/annonc/mdq185. [DOI] [PubMed] [Google Scholar]
- 10.van der Linden N, Buter J, Pescott CP, et al. Treatments and costs for recurrent and/or metastatic squamous cell carcinoma of the head and neck in the Netherlands. Eur Arch Otorhinolaryngol. 2016;273:455–64. doi: 10.1007/s00405-015-3495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zibelman M, Mehra R. Overview of current treatment options and investigational targeted therapies for locally advanced squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2016;39:396–406. doi: 10.1097/COC.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 12.Echarri MJ, Lopez-Martin A, Hitt R. Targeted therapy in locally advanced and recurrent/metastatic head and neck squamous cell carcinoma (la-r/m hnscc) Cancers (Basel) 2016;8 doi: 10.3390/cancers8030027. pii:E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2644–52. doi: 10.1200/JCO.2005.05.3348. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann TK. Systemic therapy strategies for head–neck carcinomas: current status. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2012;11:Doc03. doi: 10.3205/cto000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikpeazu CV. Treatment of locally recurrent and metastatic squamous cell carcinoma of head and neck. Head Neck Cancer Res. 2016;1:1–5. doi: 10.21767/2572-2107.100004. [DOI] [Google Scholar]
- 16.Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21(suppl 7):vii252–61. doi: 10.1093/annonc/mdq453. [DOI] [PubMed] [Google Scholar]
- 17.Rivera F, Garcia-Castano A, Vega N, Vega-Villegas ME, Gutierrez-Sanz L. Cetuximab in metastatic or recurrent head and neck cancer: the extreme trial. Expert Rev Anticancer Ther. 2009;9:1421–8. doi: 10.1586/era.09.113. [DOI] [PubMed] [Google Scholar]
- 18.Argiris A, Harrington KJ, Tahara M, et al. Evidence-based treatment options in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Front Oncol. 2017;7:72. doi: 10.3389/fonc.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 20.Cripps C, Winquist E, Devries MC, et al. Epidermal growth factor receptor targeted therapy in stages iii and iv head and neck cancer. Curr Oncol. 2010;17:37–48. doi: 10.3747/co.v17i3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Head and Neck Cancer Disease Site Group. Epidermal Growth Factor Receptor (EGFR) Targeted Therapy in Stage III and IV Head and Neck Cancer. Toronto, ON: Cancer Care Ontario; 2015. [Google Scholar]
- 22.Forastiere AA, Metch B, Schuller DE, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J Clin Oncol. 1992;10:1245–51. doi: 10.1200/JCO.1992.10.8.1245. [DOI] [PubMed] [Google Scholar]
- 23.Saloura V, Cohen EE, Licitra L, et al. An open-label single-arm, phase ii trial of zalutumumab, a human monoclonal anti-egfr antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol. 2014;73:1227–39. doi: 10.1007/s00280-014-2459-z. [DOI] [PubMed] [Google Scholar]
- 24.United States, Department of Health and Human Services, Food and Drug Administration (fda) Pembrolizumab (keytruda) [online news release] Silver Spring, MD: fda; 2016. [Available at: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm515627.htm; cited 25 April 2017] [Google Scholar]
- 25.United States, Department of Health and Human Services, Food and Drug Administration (fda) Nivolumab for scchn [online news release] Silver Spring, MD: fda; 2016. [Available at: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm528920.htm; cited 25 April 2017] [Google Scholar]
- 26.Anderson P, Benford M, Harris N, Karavali M, Piercy J. Realworld physician and patient behaviour across countries: disease-specific programmes—a means to understand. Curr Med Res Opin. 2008;24:3063–72. doi: 10.1185/03007990802457040. [DOI] [PubMed] [Google Scholar]
- 27.Winquist E, Agbassi C, Meyers BM, Yoo J, Chan KKW on behalf of the Head and Neck Cancer Disease Site Group. Systemic therapy in the curative treatment of head-and-neck squamous cell cancer: Cancer Care Ontario clinical practice guideline. Curr Oncol. 2017;24:e157–62. doi: 10.3747/co.24.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn MJ, D’Cruz A, Vermorken JB, et al. Clinical recommendations for defining platinum unsuitable head and neck cancer patient populations on chemoradiotherapy: a literature review. Oral Oncol. 2016;53:10–16. doi: 10.1016/j.oraloncology.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 29.DeRosa M, Cocks K, Taylor F, Bobiak S, Juarez Garcia A, Shaw JW. Analyses of healthcare resource utilization in CheckMate 141, a phase 3 study of nivolumab versus investigator’s choice in patients with recurrent or metastatic platinum-refractory squamous cell carcinoma of the head and neck [abstract PCN149]. Presented at the International Society for Pharmacoeconomics and Outcomes Research 22nd Annual International Meeting; Boston, MA, U.S.A.. 20–24 May 2017; [Available online at: https://tools.ispor.org/research_pdfs/55/pdffiles/PCN149.pdf; cited 1 March 2019] [Google Scholar]
- 30.Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med. 2011;364:2060–5. doi: 10.1056/NEJMsb1013826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow LQ, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase ib keynote-012 expansion cohort. J Clin Oncol. 2016;34:3838–45. doi: 10.1200/JCO.2016.68.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehra R, Seiwert TY, Mahipal A, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in keynote-012. Br J Cancer. 2018;119:153–9. doi: 10.1038/s41416-018-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (keynote-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–65. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 35.Harrington KJ, Ferris RL, Blumenschein G, Jr, et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017;18:1104–15. doi: 10.1016/S1470-2045(17)30421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]