Abstract
Background
The role of splenectomy in proximal gastric cancer is still debated. The objective of the present meta-analysis was to provide more-robust evidence about the effect of spleen-preserving total gastrectomy on postoperative infectious complications, overall morbidity, and 5-year overall survival (os).
Methods
PubMed, embase, and the Web of Science were consulted. Pooled effect measures were calculated using an inverse-variance weighted or Mantel–Haenszel in random effects meta-analysis. Heterogeneity was evaluated using I2 index and Cochran Q-test.
Results
Three randomized controlled trials published between 2000 and 2018 were included. Overall, 451 patients (50.1%) underwent open total gastrectomy with spleen preservation and 448 (49.9%) underwent open total gastrectomy with splenectomy. The patients ranged in age from 24 to 78 years. No differences were found in the number of harvested lymph nodes (p = 0.317), the reoperation rate (p = 0.871), or hospital length of stay (p = 0.347). The estimated pooled risk ratios for infectious complications, overall morbidity, and mortality were 1.53 [95% confidence interval (ci): 1.09 to 2.14; p = 0.016], 1.51 (95% ci: 1.11 to 2.05; p = 0.008), and 1.23 (95% ci: 0.40 to 3.71; p = 0.719) respectively. The estimated pooled hazard ratio for 5-year os was 1.06 (95% ci: 0.78 to 1.45; p = 0.707).
Conclusions
Spleen-preserving total gastrectomy should be considered in patients with curable gastric cancer because it is significantly associated with decreased postoperative infectious complications and overall morbidity, with no difference in the 5-year os. Those observations appear worthwhile for establishing better evidence-based treatment for gastric cancer.
Keywords: Gastric cancer, splenectomy, spleen preservation, infectious complications, 5-year overall survival
INTRODUCTION
Gastric cancer is one of the most common cancers worldwide1. It has been estimated that almost 1 million new cases of stomach cancer occurred in 2012, making that disease the 5th most common malignancy2,3. Despite randomized trials that have failed to demonstrate a survival benefit of D2 nodal dissection4–6, modified D2 lymphadenectomy with spleen preservation is generally accepted as the standard of care in selected subgroups of patients7.
Lymph node metastases at the splenic hilum are found in up to 10% of patients with gastric and gastroesophageal junction tumours8,9. Some authors have recommended splenectomy to completely dissect the lymph nodes around the splenic artery and hilum10. However, the effect of splenectomy on long-term prognosis appears to be marginal11– 16. Furthermore, the importance of the spleen as a part of the immune system and its role in macrophage storage and protection against gram-negative infections are well established17,18. Postoperative complications are significantly higher after gastrectomy combined with splenectomy than after gastrectomy alone19, but the effect of splenectomy on postoperative infectious complications and overall survival (os) is still unclear.
The aim of the present meta-analysis was to assess the incidence of postoperative infectious complications and the 5-year os in patients undergoing total gastrectomy with or without splenectomy so as to better define the risk–benefit ratio of those procedures and to guide clinical decision-making.
METHODS
Search Strategy and Study Selection
The study was conducted according to the prisma (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement20. An extensive literature search spanning 2000–2018 was conducted independently by two authors (AA, SS) to identify published series written in English about spleen-preservation gastrectomy (g) compared with gastrectomy with splenectomy (gs) for proximal gastric cancer. PubMed, embase, and the Web of Science databases were consulted using the terms “stomach cancer” and “splenectomy” or “spleen resection” or “splenic preservation.”
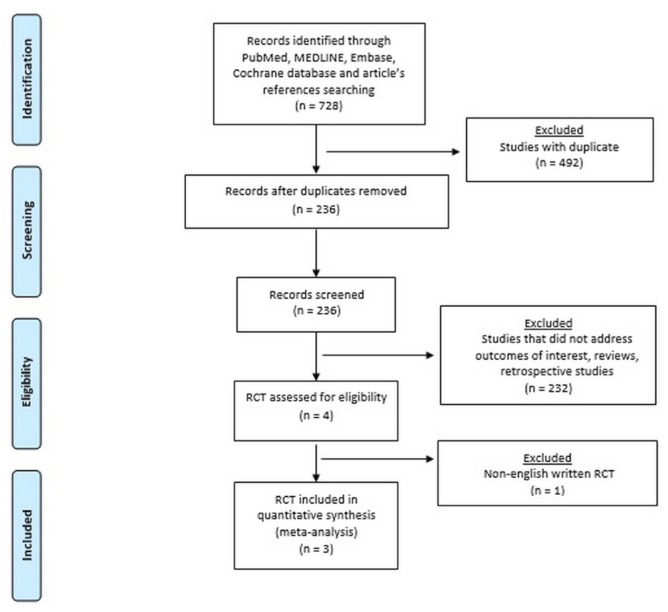
Randomized controlled trials (rcts) that compared the effectiveness or safety of gs and g were included. Abstracts, case reports, case series, retrospective observational studies, and articles not in English were excluded (Figure 1). Two authors (AA, SS) independently extracted data from eligible studies. The extracted data included study characteristics (first author name, year, country, and journal of publication), number of patients, time frame, clinical and demographic characteristics of the patient population, surgical approach, postoperative outcomes, and 5-year os. Disagreements about study exclusion and data extraction were resolved by consensus; if no agreement could be reached, a third senior author (LB) made the decision.
FIGURE 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses, 2009) diagram of study selection.
Inclusion and Exclusion Criteria and Surgical Technique
In all included studies, patients with resectable gastric cancer eligible for curative surgery were evaluated by preoperative endoscopy, biopsy, and computed tomography imaging. Patients with early gastric cancer were included for randomization. Patients were randomly allocated to a given treatment after staging laparotomy. Intraoperative pancreatic or splenic tumour infiltration, liver or peritoneal metastasis, station 10 macroscopic lymph node metastasis, Borrmann type 4 (linitis plastica), and positive peritoneal lavage cytology were exclusion criteria. All patients had a D2 lymphadenectomy. In the g group, dissection of station 11 was performed by removing lymph nodes en bloc with fatty tissue along the axis of the splenic artery. In the gs group, the spleen was removed en bloc with station 10, and the splenic artery was ligated and cut distal to the origin of the great pancreatic artery. The small number of patients with iatrogenic splenic injury were included in the analysis. Gastric tumours other than adenocarcinoma (that is, lymphoma and adenosquamous carcinoma) were excluded.
Outcomes
The primary outcome was the rate of postoperative infectious complications. The secondary outcomes were overall morbidity, postoperative mortality, 5-year os, operative time, number of lymph nodes harvested, reoperation rate, and length of hospital stay. If an outcome was unclear, we sought further information from the authors of the relevant study. Each infectious complication was defined, identified, and diagnosed in accordance with the U.S. Centers for Disease Control and Prevention guidelines21.
Study Quality Appraisal
Two authors (AA, SS) independently assessed the methodologic quality of the selected trials. These criteria were used for the assessment:
■ Method of randomization
■ Allocation concealment
■ Baseline comparability of study groups
■ Blinding
■ Completeness of follow-up
Trials were graded as follows: A, adequate; B, unclear; and C, inadequate on each criterion. Thus, each rct was graded as having low, moderate, or high risk of bias. Disagreements were resolved by discussion.
Statistical Analysis
The results of the systematic review were summarized qualitatively into a frequentist random effects meta-analysis of pooled risk ratio and raw mean difference. The inversevariance method and DerSimonian–Laird estimator for variance of true effect size (τ2) were applied22. Heterogeneity between the studies was evaluated by I2 index and Cochran Q-test23. Statistical heterogeneity was considered significant when the p value was less than 0.10 or the I2 index was greater than 50%24. Wald-type 95% confidence intervals were computed for pooled measures; otherwise, 95% confidence intervals for the I2 index were calculated according Higgins and Thompson25. The prediction interval for the treatment effect of a new study was calculated according to Borenstein23. Hazard ratios and relative standard errors for time-to-event outcomes by the Kaplan–Meier method were approximated using the formula described by Parmar26. Kaplan–Meyer curves were digitalized using the GetData Graph Digitizer software (http://getdata-graph-digitizer.com/). Variance for continuous outcomes was estimated from ranges according to Hozo et al.27. Because sample sizes were not the same in all studies, we performed sensitivity analyses by rerunning the analysis, excluding one study each time, to verify the robustness of the overall results. A Z-score test was performed. Two-sided p values were considered statistically significant when less than 0.05. All analyses were carried out using the R software application (version 3.2.2: The R Foundation, Vienna, Austria).
RESULTS
Systematic Review
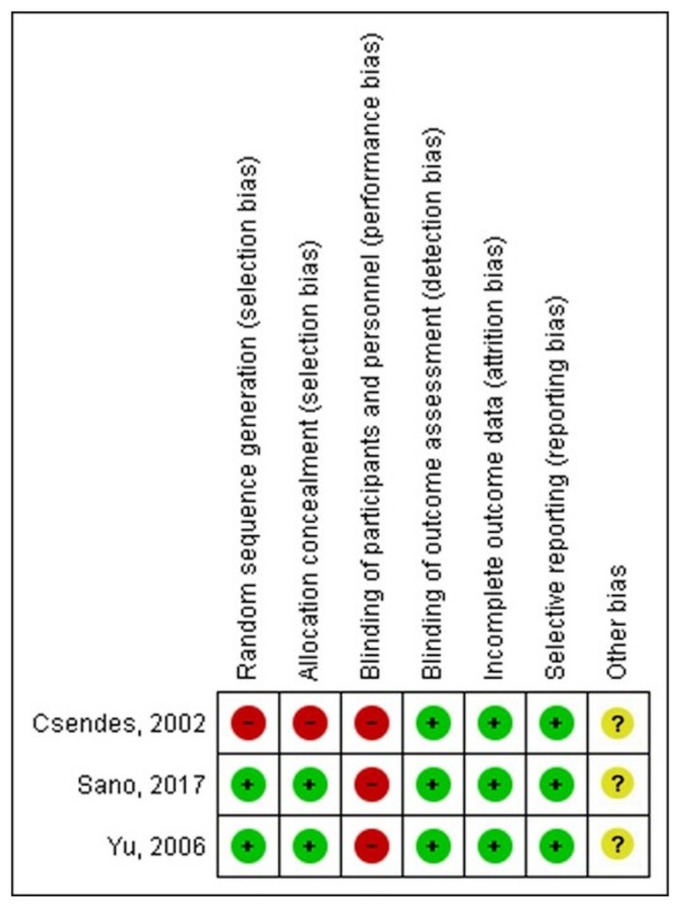
Three rcts published between 2000 and 2018 met the inclusion criteria. There was one publication each from Chile, South Korea, and Japan. Figure 2 shows the quality assessment of the trials.
FIGURE 2.
Risk of bias was assessed using the Cochrane Risk of Bias tool.
Table I shows demographic, clinical, and surgical variables for the patient sample. Of the 899 included patients, 451 (50.2%) underwent g, and 448 (49.8%) underwent gs. Patients ranged in age from 24 to 78 years, and most were men (90%). Body mass index was reported for the patients in one study. Comorbidities and American Society of Anesthesiologists score were not reported. All patients underwent an open surgical operation, and reconstruction methods were at the surgeon’s discretion. Bursectomy was not mandatory. Perioperative care, anesthesia management, and technical details of the operations were not specified.
TABLE I.
Demographics and clinical data for the 899 study patients
| Variable | Splenectomy with total gastrectomy | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Csendes et al., 200215 Chile |
Yu et al., 200616 South Korea |
Sano et al., 201719 Japan |
||||
|
|
|
|
||||
| Yes | No | Yes | No | Yes | No | |
| Patients (n) | 90 | 97 | 104 | 103 | 254 | 251 |
|
| ||||||
| Sex (n) | ||||||
| Men | 60 | 65 | 72 | 72 | 196 | 204 |
| Women | 30 | 32 | 32 | 31 | 58 | 47 |
|
| ||||||
| Age (years) | ||||||
| Mean | 62.7 | 62.7 | 57 | 57 | 65 | 65 |
| Range | 24–78 | 31–78 | 27–75 | 30–75 | ||
|
| ||||||
| Stage (n) | ||||||
| I | Not reported | Not reported | 35 | 28 | 94 | 108 |
| II | 21 | 24 | 74 | 70 | ||
| III | 27 | 27 | 70 | 53 | ||
| IV | 21 | 24 | 16 | 20 | ||
|
| ||||||
| Histology (n) | ||||||
| Differentiated | Not reported | Not reported | 33 | 33 | 118 | 136 |
| Undifferentiated | 71 | 70 | 136 | 115 | ||
|
| ||||||
| Operative time (minutes) | ||||||
| Mean | 218 | 208 | Not | Not | 231 | 224 |
| Range | 120–440 | 90–450 | reported | reported | 112–440 | 108–485 |
|
| ||||||
| Retrieved nodes (n) | ||||||
| Median | 30 | 40 | 40 | 64 | 59 | |
| Range | 22–38 | 5–93 | 4–94 | 19–156 | 16–158 | |
|
| ||||||
| Reoperation (n) | 10 | 9 | Not reported | Not reported | 3 | 4 |
|
| ||||||
| Infectious complications (n) | 35 | 24 | 8 | 7 | 23 | 14 |
|
| ||||||
| Overall morbidity (n) | 45 | 40 | 16 | 9 | 77 | 42 |
|
| ||||||
| Mortality (n) | 4 | 3 | 2 | 1 | 1 | 2 |
|
| ||||||
| 5-Year overall survival (%) | 41.9 | 36.2 | 54.8 | 48.5 | 73.6 | 74.5 |
The operative time ranged from 90 to 485 minutes in the g group and from 112 to 440 minutes in the gs group. Median intraoperative blood loss and perioperative blood transfusions were reported in only one study. The pathologic tumour stage and tumour histology were reported in two studies (Table I). The number of retrieved lymph nodes ranged from 4 to 158 in the g group and from 5 to 156 in the gs group.
The reoperation rate ranged from 1.6% to 9.3% in the g group and from 1.2% to 11.1% in the gs group. Postoperative overall morbidity ranged from 8.7% to 41.2% in the g group and from 15.4% to 50% in the gs group. Only one study specifically reported the rate of anastomotic and pancreatic fistulae. The hospital length of stay ranged from 9 to 60 days in the g group and from 9 to 71 days in the gs group. In-hospital mortality ranged from 0.8% to 3.1% in the g group and from 0.4% to 4.4% in the gs group.
Median follow-up duration was reported in two studies and ranged from 64.8 months to 71.8 months. In two studies, aggregated os was reported; in one study, os was stratified according to tumour stage. One study reported the 5-year relapse-free survival. The long-term consequences of splenectomy were analyzed in one study, which found no differences in terms of pneumonia and other infections.
Meta-analysis
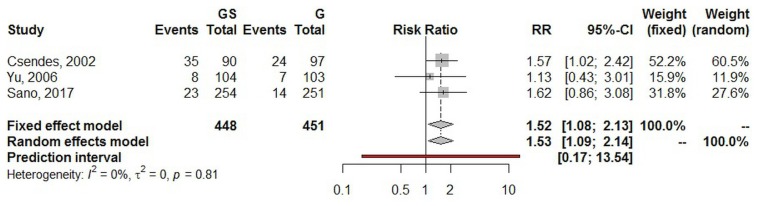
In addition to the systematic review, we performed a frequentist meta-analysis. Using a random effects model, the estimated pooled risk ratio for postoperative infectious complications (three studies, 899 patients in total) was 1.53 [95% confidence interval (ci): 1.09 to 2.14; p = 0.016]. The lower and upper limits of prediction were 0.17 and 13.54 respectively. Heterogeneity was zero (I2 = 0%; 95% ci: 0.0% to 49.7%; p = 0.813), and the τ2 was 0.0. The sensitivity analysis yielded a risk ratio estimate of 1.52 (95% ci: 1.08 to 2.13; Figure 3).
FIGURE 3.
Forest plot of postoperative infectious complications. GS = total gastrectomy with splenectomy; G = total gastrectomy; RR = risk ratio; CI = confidence interval.
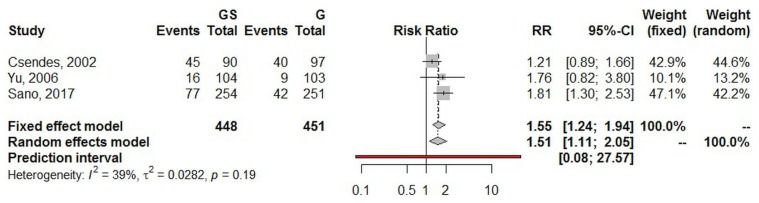
Using a random effects model, the estimated pooled risk ratio for postoperative overall morbidity (three studies, 899 patients in total) was 1.51 (95% ci: 1.11 to 2.05; p = 0.008). The lower and upper limits of prediction were 0.08 and 27.57 respectively. Heterogeneity was nonsignificant (I2 = 39.1%; 95% ci: 0.0% to 81.1%; p = 0.194), and the τ2 was 0.02. The sensitivity analysis yielded a risk ratio estimate of 1.55 (95% ci: 1.2 to 1.94; Figure 4).
FIGURE 4.
Forest plot of overall morbidity. GS = total gastrectomy with splenectomy; G = total gastrectomy; RR = risk ratio; CI = confidence interval.
Using a random effects model, the estimated pooled mean difference for hospital length of stay (two studies, 394 patients in total) was 1.50 (95% ci: −1.63 to 4.63; p = 0.347). Heterogeneity was moderate (I2 = 54.2%; 95% ci: 0.0% to 88.7%; p = 0.139), and the τ2 was 2.7.
Using a random effects model, the estimated pooled mean difference for harvested lymph nodes (two studies, 712 patients in total) was 2.50 (95% ci: −2.40 to 7.40; p = 0.317). Heterogeneity was moderate (I2 = 65.7%; 95% ci: 0.0% to 92.2%; p = 0.087), and the τ2 was 8.2.
Using a random effects model, the estimated pooled risk ratio for reoperation (two studies, 692 patients in total) was 1.06 (95% ci: 0.51 to 2.23; p = 0.871). Heterogeneity was zero (I2 = 0%, p = 0.583), and the τ2 was 0.0.
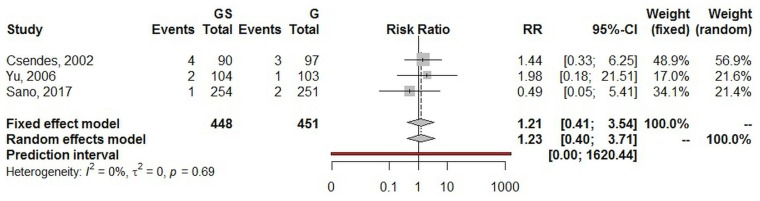
Using a random effects model, the estimated pooled risk ratio for mortality (three studies, 899 patients in total) was 1.23 (95% ci: 0.40 to 3.71; p = 0.719). The lower and upper limits of prediction were 0.01 and 1620.4 respectively. Heterogeneity was zero (I2 = 0%; 95% ci: 0.0% to 72.4%; p = 0.685), and the τ2 was 0.0. The sensitivity analysis yielded a risk ratio estimate of 1.21 (95% ci: 0.42 to 3.55; Figure 5).
FIGURE 5.
Forest plot of mortality. GS = total gastrectomy with splenectomy; G = total gastrectomy; RR = risk ratio; CI = confidence interval.
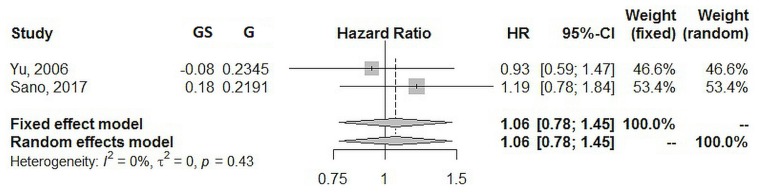
Using a random effects model, the estimated pooled hazard ratio for 5-year os (two studies, 713 patients in total) was 1.06 (95% ci: 0.78 to 1.45; p = 0.707). Heterogeneity was zero (I2 = 0%, p = 0.430), and the τ2 was 0.0 (Figure 6).
FIGURE 6.
Forest plot of 5-year overall survival. GS = total gastrectomy with splenectomy; G = total gastrectomy; HR = hazard ratio; CI = confidence interval.
DISCUSSION
In the meta-analysis, we show that the rates of postoperative infectious complications and of overall morbidity were significantly lower in patients undergoing g for carcinoma of the upper third of the stomach. In contrast, no differences were found in the number of harvested lymph nodes, the reoperation rate, hospital length of stay, overall mortality, or 5-year os.
The spleen is part of the reticuloendothelial system, and its contribution to systemic immunologic surveillance through the synthesis of opsonins and antibodies is crucial. Postoperative infectious complications after splenectomy have previously been postulated to potentially be attributable to a partial loss of immunologic function28,29.
Postoperative surgical site infection (ssi) is one of the most common complications after gastrectomy. It can lead to prolonged hospital stay and increased health care costs, and can also adversely affect os and disease-free survival30,31. The incidence of postoperative ssi after elective gastrectomy has been reported to be up to 20%, combining superficial and deep incisional ssi32. A recent study from Japan involving 685 patients undergoing open elective total or distal gastrectomy showed a 6.1% overall incidence of superficial incisional ssi33. The incidence of organ or space ssi was significant and was reported to be higher after total gastrectomy than after distal gastrectomy (10.4% vs. 5.8%). In a recent national clinical database study in Japan that included 39,253 patients who underwent total gastrectomy, the overall ssi rate was 8.1%, and splenectomy was a risk factor for postoperative ssi, anastomotic leak, and pancreatic fistula34. In our systematic review, the overall aggregated incidence of postoperative infectious complications was 12.3%, and the estimated pooled risk ratio in the gs group compared with the g group was 1.53 (95% ci: 1.09 to 2.14; p = 0.016), thus reflecting the importance of splenic preservation in maintaining immunomodulation activity to prevent postoperative infectious complications. Notably, heterogeneity in the analysis was zero, adding significance to the result.
Our estimated pooled risk ratio for postoperative overall morbidity in the gs group compared with the g group was 1.51 (p = 0.008), an observation that is in line with previous rcts15,16,19 and easily understandable, given the fact that surgical complications are influenced by the extent of the surgical procedure itself. As seen in previous studies, no statistically significant differences were found in terms of harvested lymph nodes, reoperation rate, and hospital length of stay35.
For gastric cancer, R0 resection with D2 lymphadenectomy is recommended as the standard curative surgical treatment36,37. In Asian countries, extended lymph node dissection is regarded as essential in the treatment of gastric cancer, and splenectomy has been suggested to achieve complete clearance of nodal station 10 in patients with curable T2–4 cancers invading the greater gastric curvature36. However, in Western countries, trials from the Netherlands, the U.K. Medical Research Council, and Italy38–40 failed to demonstrate any initial survival advantage with D2 resection. It has been postulated that, after splenectomy, long-term T-cell suppression, with consequent immunosuppression, could negatively affect immune surveillance, leading to worse os41.
Despite those findings, consensus opinion is that medically fit patients should undergo D2 dissection at a high-volume centre38. Previous retrospective and single-centre studies set out to examine the effect of splenectomy on 5-year os, but results were contrasting, incomplete, and biased42–45. To overcome those limitations, three rcts were conducted contemporaneously. Csendes et al.15 found a trend toward a better 5-year os rate in patients who underwent gs than in patients who underwent spleen-preserving g (42% vs. 36%). Similarly, Yu and colleagues16 described a slightly better 5-year os rate in patients who underwent gs (54.8% vs. 48.8%). In both studies, the results were not statistically significant. Recently, in a large multicentre rct, Sano et al.19 found statistically significant noninferiority for spleen-preserving g compared with gs (76.4% vs. 75.1%, p = 0.025). Our study produced similar results, with an estimated pooled 5-year os hazard ratio of 1.06 (p = 0.707), and no differences between the two groups. Notably, heterogeneity was zero, thus conferring additional credibility to that outcome.
The results of our study might not be generalizable given that the patients came mainly from Asia and South America and the surgery was performed at high-volume centres with appropriate surgical expertise. Because the data were aggregated, the confounding effects of tumour stage and perioperative chemotherapy on os could not be determined. In addition, no specific data about comorbidities and American Society of Anesthesiologists score were reported, and so no inferences could be drawn concerning an association with survival. However, despite those limitations, our systematic review and meta-analysis includes only contemporary rcts and represents the first meta-analysis focusing on postoperative infectious complications and the hazard ratio for 5-year os. Moreover, heterogeneity relating to the primary study outcome was zero.
CONCLUSIONS
Spleen-preserving open total gastrectomy has the potential to significantly lower rates of postoperative infectious complications and overall morbidity, with no difference in 5-year os. Although the interaction between postoperative infectious complications and long-term prognosis after open gastrectomy with or without splenectomy remains unclear, splenectomies are expected to decline in number into the future. Because minimally invasive surgery is becoming a recommended option for gastric cancer and appears to be comparable to open gastrectomy in short- and long-term results, further studies are required to evaluate the potential value of the laparoscopic approach in further reducing the risk of infectious complications and improving long-term survival.
ACKNOWLEDGMENTS
This work was supported by the Associazione Italiana Ricerca Esofago.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [Erratum in: CA Cancer J Clin 2011;61:134] [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Bonenkamp JJ, Hermans J, Sasako M, et al. on behalf of the Dutch Gastric Cancer Group. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–14. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 5.Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the mrc randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347:995–9. doi: 10.1016/S0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]
- 6.Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch Gastric Cancer Group trial. J Clin Oncol. 2004;22:2069–77. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Ott K, Lordick F, Blank S, Büchler M. Gastric cancer: surgery in 2011. Langenbecks Arch Surg. 2011;396:743–58. doi: 10.1007/s00423-010-0738-7. [DOI] [PubMed] [Google Scholar]
- 8.Mönig SP, Collet PH, Baldus SE, et al. Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol. 2001;76:89–92. doi: 10.1002/1096-9098(200102)76:2<89::AID-JSO1016>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.Sasada S, Ninomiya M, Nishizaki M, et al. Frequency of lymph node metastasis to the splenic hilus and effect of splenectomy in proximal gastric cancer. Anticancer Res. 2009;29:3347–51. [PubMed] [Google Scholar]
- 10.Sano T, Sasako M, Yamamoto S, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy—Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767–73. doi: 10.1200/JCO.2004.10.184. [DOI] [PubMed] [Google Scholar]
- 11.Erturk S, Ersan Y, Cicek Y, Dogusoy G, Senocak M. Effect of simultaneous splenectomy on the survival of patients undergoing curative gastrectomy for proximal gastric carcinoma. Surg Today. 2003;33:254–8. doi: 10.1007/s005950300057. [DOI] [PubMed] [Google Scholar]
- 12.Fatouros M, Roukos DH, Lorenz M, et al. Impact of spleen preservation in patients with gastric cancer. Anticancer Res. 2005;25:3023–30. [PubMed] [Google Scholar]
- 13.Lee KY, Noh SH, Hyung WJ, et al. Impact of splenectomy for lymph node dissection on long-term surgical outcome in gastric cancer. Ann Surg Oncol. 2001;8:402–6. doi: 10.1007/s10434-001-0402-0. [DOI] [PubMed] [Google Scholar]
- 14.Weitz J, Jaques DP, Brennan M, Karpeh M. Association of splenectomy with postoperative complications in patients with proximal gastric and gastroesophageal junction cancer. Ann Surg Oncol. 2004;11:682–9. doi: 10.1245/ASO.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 15.Csendes A, Burdiles P, Rojas J, Braghetto I, Diaz JC, Maluenda F. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery. 2002;131:401–7. doi: 10.1067/msy.2002.121891. [DOI] [PubMed] [Google Scholar]
- 16.Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg. 2006;93:559–63. doi: 10.1002/bjs.5353. [DOI] [PubMed] [Google Scholar]
- 17.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–16. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 18.Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect. 2001;43:182–6. doi: 10.1053/jinf.2001.0904. [DOI] [PubMed] [Google Scholar]
- 19.Sano T, Sasako M, Mizusawa J, et al. on behalf of the Stomach Cancer Study Group of the Japan Clinical Oncology Group. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. 2017;265:277–83. doi: 10.1097/SLA.0000000000001814. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG on behalf of the prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (cdc) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132. doi: 10.1016/S0196-6553(99)70088-X. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, UK: John Wiley and Sons; 2009. [DOI] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adachi Y, Kamakura T, Mori M, Maehara Y, Sugimachi K. Role of lymph node dissection and splenectomy in node-positive gastric carcinoma. Surgery. 1994;116:837–41. [PubMed] [Google Scholar]
- 29.Zhang CH, Zhan WH, He YL, Chen CQ, Huang MJ, Cai SR. Spleen preservation in radical surgery for gastric cardia cancer. Ann Surg Oncol. 2007;14:1312–19. doi: 10.1245/s10434-006-9190-x. [DOI] [PubMed] [Google Scholar]
- 30.Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intraabdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575–83. doi: 10.1245/s10434-012-2720-9. [DOI] [PubMed] [Google Scholar]
- 31.Sierzega M, Kolodziejczyk P, Kulig J on behalf of the Polish Gastric Cancer Study Group. Impact of anastomotic leakage on long-term survival after total gastrectomy for carcinoma of the stomach. Br J Surg. 2010;97:1035–42. doi: 10.1002/bjs.7038. [DOI] [PubMed] [Google Scholar]
- 32.Özmen T, Javadov M, Yeğen CS. Factors affecting surgical site infection rate after elective gastric cancer surgery. Ulus Cerrahi Derg. 2015;32:178–84. doi: 10.5152/UCD.2015.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Endo S, Tsujinaka T, Fujitani K, et al. Risk factors for superficial incisional surgical site infection after gastrectomy: analysis of patients enrolled in a prospective randomized trial comparing skin closure methods. Gastric Cancer. 2016;19:639–44. doi: 10.1007/s10120-015-0494-z. [DOI] [PubMed] [Google Scholar]
- 34.Kikuchi H, Miyata H, Konno H, et al. Development and external validation of preoperative risk models for operative morbidities after total gastrectomy using a Japanese Web-based nationwide registry. Gastric Cancer. 2017;20:987–97. doi: 10.1007/s10120-017-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang K, Chen XZ, Hu JK, Zhang B, Chen ZX, Chen JP. Effectiveness and safety of splenectomy for gastric carcinoma: a meta-analysis. World J Gastroenterol. 2009;15:5352–9. doi: 10.3748/wjg.15.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D on behalf of the esmo Guidelines Committee. Gastric cancer: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38–49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 38.Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069–77. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 39.Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the mrc randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–30. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Degiuli M, Sasako M, Ponti A, et al. on behalf of the Italian Gastric Cancer Study Group. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg. 2014;101:23–31. doi: 10.1002/bjs.9345. [DOI] [PubMed] [Google Scholar]
- 41.Okuno K, Tanaka A, Shigeoka H, et al. Suppression of T-cell function in gastric cancer patients after total gastrectomy with splenectomy: implications of splenic autotransplantation. Gastric Cancer. 1999;2:20–5. doi: 10.1007/s101200050016. [DOI] [PubMed] [Google Scholar]
- 42.Wanebo HJ, Kennedy BJ, Winchester DP, Stewart AK, Fremgen AM. Role of splenectomy in gastric cancer surgery: adverse effect of elective splenectomy on longterm survival. J Am Coll Surg. 1997;185:177–84. doi: 10.1016/S1072-7515(01)00901-2. [DOI] [PubMed] [Google Scholar]
- 43.Kanayama H, Hamazoe R, Osaki Y, Shimizu N, Maeta M, Koga S. Immunosuppressive factor from the spleen in gastric cancer patients. Cancer. 1985;56:1963–6. doi: 10.1002/1097-0142(19851015)56:8<1963::AID-CNCR2820560812>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 44.Nashimoto A, Yabusaki H, Matsuki A. The significance of splenectomy for advanced proximal gastric cancer. Int J Surg Oncol. 2012;2012 doi: 10.1155/2012/301530. 301530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otsuji E, Yamaguchi T, Sawai K, Ohara M, Takahashi T. End results of simultaneous splenectomy in patients undergoing total gastrectomy for gastric carcinoma. Surgery. 1996;120:40–4. doi: 10.1016/S0039-6060(96)80239-X. [DOI] [PubMed] [Google Scholar]