Abstract
Background
Improved treatments resulting in a rising number of survivors of breast cancer (bca) calls for optimization of current specialist-based follow-up care. In the present study, we evaluated well survivors of bca with respect to their supportive care needs and attitudes toward follow-up with various care providers, in varying settings, or mediated by technology (for example, videoconference or e-mail).
Methods
A cross-sectional paper survey of well survivors of early-stage pT1–2N0 bca undergoing posttreatment follow-up was completed. Descriptive and univariable logistic regression analyses were performed to examine associations between survivor characteristics, supportive care needs, and perceived satisfaction with follow-up options. Qualitative responses were analyzed using conventional content analysis.
Results
The 190 well survivors of bca who participated (79% response rate) had an average age of 63 ± 10 years. Median time since first follow-up was 21 months. Most had high perceived satisfaction with in-person specialist care (96%, 177 of 185). The second most accepted model was shared care involving specialist and primary care provider follow-up (54%, 102 of 190). Other models received less than 50% perceived satisfaction. Factors associated with higher perceived satisfaction with non-specialist care or virtual follow-up by a specialist included less formal education (p < 0.01) and more met supportive care needs (p < 0.05). Concerns with virtual follow-up included the perceived impersonal nature of virtual care, potential for inadequate care, and confidentiality.
Conclusions
Well survivors of bca want specialists involved in their follow-up care. Compared with virtual follow-up, in-person follow-up is perceived as more reassuring. Certain survivor characteristics (for example, met supportive care needs) might signal survivor readiness for virtual or non-specialist follow-up. Future work should examine multi-stakeholder perspectives about barriers to and facilitators of shared multimodal follow-up care.
Keywords: Breast cancer, follow-up, survivorship, supportive care needs, preferences, virtual care
INTRODUCTION
Breast cancer (bca) is the most common cancer in women worldwide1. In the United States and Canada, there are currently more than 3 million survivors of bca, and more than 250,000 patients are newly diagnosed annually2,3. After treatment, survivors of bca require follow-up care to monitor for recurrences, to manage acute and late treatment-related side-effects, and to address supportive care needs4. Traditionally, follow-up care has been delivered by oncology specialists at a cancer centre. However, with a predicted shortage of oncologists by 20205 and improved treatments resulting in a rising number of survivors6, a specialist-only model is not sustainable and might not optimally serve the needs and preferences of well survivors of bca.
Several alternatives to conventional specialist-based in-person follow-up care for the well survivor population have emerged, involving various care providers [specialist nurses and primary care providers (pcps), among others], clinical settings (for example, cancer centres, community-based hospitals, pcp clinics), and virtual visits (telephone, Web, e-mail)7,8. In the absence of clear evidence advocating for the superiority of any one model, practices for bca follow-up are heterogeneous and show variability in care provider, setting, and modality.
In-person clinic-based primary care follow-up for well survivors of bca has, by far, been the most commonly evaluated alternative to the in-person cancer centre–based specialist model, with results demonstrating equivalent survival outcomes and health-related quality of life regardless of the type of care provider9–11. Although the evidence supports involvement of pcps in well survivor care, adoption of that model has not been widespread. The current literature suggests that some survivors prefer the expertise of specialists and find that transitioning to a pcp does not provide the continuity of care and support that they desire12.
Other follow-up models receiving increasing attention include shared care and virtual follow-up visits. Common shared-care models are specialist–pcp or specialist–bca nurse teams. The bca nurse models have been reported to improve support for the psychosocial and informational needs of survivors13. Virtual follow-up visits include a range of information and communication technology–based delivery models that replace or complement in-person follow-up appointments8. Such virtual care services are more convenient for many survivors, particularly those located in rural regions8. In one study, cancer survivors reported high satisfaction for virtual visits with their oncologist, with 95% indicating that it was equivalent to an in-person visit14. Additionally, e-health applications have been cited as a convenient and timely way to address supportive care needs, such as monitoring quality of life, providing personalized educational information, and navigating the health system15.
Care transitions between providers, settings, and modalities are opportunities to tailor care to the individual needs and preferences of survivors. Previous studies have assessed the importance of using survivor profiles (for example, demographics, health and psychosocial needs, perceptions of care providers) to personalize cancer care16. For example, O’Malley et al.16 identified the relationship of greater psychosocial needs and medical comorbidities with increased demand for health education during follow-up care. However, using the supportive care needs and preferences of survivors to personalize follow-up care models requires further investigation.
The purpose of the present study was to examine the relationship between survivor characteristics, supportive care needs, and perceived satisfaction with various models of posttreatment follow-up care. We examined 6 alternatives to traditional specialist-based follow-up that have been receiving increased attention in the literature, that might be more convenient for survivors, and that could optimize specialist involvement in follow-up care. Our cross-sectional survey asked well survivors of bca about their follow-up care use, characteristics (demographics, disease, use of technology), supportive care needs, and perceived satisfaction with various types of follow-up care. “Perceived satisfaction” (that is, feelings or attitudes about a situation, object, or action) is a measure correlating with acceptability17,18. Additionally, we collected qualitative open-ended survey responses so as to better understand survivor preferences, barriers, and facilitators for implementing various models of follow-up. Overall, the study provides guidance about optimal care transitions from the perspective of survivor supportive care needs and follow-up care preferences.
METHODS
Participants
Study participants were recruited from the radiation oncology clinics of a large academic cancer centre in Toronto, Ontario. Eligibility criteria included survivors who were female, who had previously been diagnosed with a pT1–2N0 estrogen receptor–positive or progesterone receptor–positive (or both) and her2-negative bca, who had completed radiation treatment, who were undergoing follow-up care, and who were English-speaking. Higher-risk survivor populations (for example, hormone receptor–negative and her2-positive) were excluded for this well follow-up study. Follow-up accorded with guidelines from Health Canada’s Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer4. Standard follow-up care for well survivors of bca included history and physical exam (including clinical breast exam) at least every 6 months for 5 years, with an annual mammogram. Survivors were ineligible if they did not complete primary treatment, were less than 18 years of age, could not read or speak English, or had already been transitioned or discharged from the cancer centre. We chose to focus on the perspectives of survivors receiving post–radiation treatment follow*up care to inform the interest on the part of radiation oncology specialists for transitioning to different models of follow-up care delivery. Given that most patients with early-stage bca undergo trimodality therapy, those receiving surgery or chemotherapy were not excluded.
Study Design
The study protocol received institutional research ethics board approval. Our cross-sectional paper survey used a convenience sample with a corresponding medical chart review. Eligible survivors were identified from oncology clinic lists and from review of patient medical records. To obtain diversity in patient–provider relationships and follow-up practices, participants were recruited from 8 different radiation oncology follow-up clinics. All eligible survivors were approached to participate during routine follow-up appointments from August 2012 to May 2013. The research assistant (with permission from the treating physician) approached each eligible patient after they had registered with the clinic receptionist for their visit. Participants provided informed consent and were given the option to complete a self-administered paper questionnaire in the clinic or to take it home and return it by mail. Non-responders received up to 3 reminder telephone calls.
Measures
The questionnaire (Table I) collected information about participant demographics (6 items), health care service use (4 items), Internet use (7 items), supportive care needs (35 items, 1 open-ended question), perceived satisfaction with follow-up care options (7 items), and views about follow-up care options (1 open-ended item). The options in the participant demographics, health care service, Internet use, and perceived satisfaction with follow-up care sections were based on previous research with survivors of thyroid17, bowel19, breast20, and testicular cancer21 and input from a multidisciplinary breast oncology clinical team. The supportive care needs of the participants were assessed using the validated casun (Cancer Survivors’ Unmet Needs) measure22. Personal health information extracted from medical records included age, date of diagnosis, treatments (that is, surgery, radiation therapy, chemotherapy), date of first follow-up appointment, and postal code.
TABLE I.
Surveyed information
| Category | Description |
|---|---|
| Participant characteristics | From the survey: Age, relationship status, country of birth, first spoken language, education, income, and employment status |
| From the medical record: Age, date of diagnosis, treatments (for example, surgery, radiation therapy, chemotherapy), date of first follow-up appointment, and postal code | |
|
| |
| Health care service use | Number of visits to family doctor, surgeon, oncologists, and other health professionals for cancer-related issues in the preceding year |
|
| |
| Internet use | Use of the Internet to search for information about breast cancer (yes or no); frequency of Internet use (daily, weekly, monthly, less than once per month); use of Facebook, Twitter, online discussion forums, Wikipedia (yes, no, unsure); interest in communicating with the health care team through a secure online support network (yes, no, unsure) |
|
| |
| Supportive care needs |
Cancer Survivors’ Unmet Needs (CaSUN)22 measure: Includes 35 unmet need items and 5 domains (existential survivorship, comprehensive cancer care, information, quality of life, and relationships); response options were simplified into three categories:
|
|
| |
| Perceived satisfaction with follow-up care models | Assessed by asking patient survivors how much they agreed or disagreed (on a 5-point Likert scale: 1, strongly disagree; 2, disagree; 3, neutral; 4, agree; 5, strongly agree) with each follow-up option, using the wording: “I would be satisfied for my follow-up appointments to be with ” Options included a breast cancer specialist at our institution; a breast cancer specialist in the local community; a primary care provider (PCP) in the local community; a PCP–nurse team specializing in cancer follow-up at a hospital that is partnered with our institution; shared with a breast cancer specialist and the PCP; a breast cancer specialist from our institution through secure videoconferencing; and a breast cancer specialist from our institution through secure e-mail or Web site. Perceptions were also assessed by asking survivors to “Take a moment to tell us your thoughts about these options” in one open-ended question. |
Statistical Analysis
The statistical analysis was performed using the SAS software application (version 9.4: SAS Institute, Cary, NC, U.S.A.). Sample means and standard deviations were calculated for continuous or discrete variables (or medians with interquartile range and ranges if variables were not normally distributed), and proportions were calculated for categorical variables. The relationships of key variables with the outcomes of interest were examined in univariable analysis for exploratory investigation. Specifically, the relationships of age, education (elementary or high-school vs. college or university), employment status (student, unemployed, part-time, full-time, retired), time since first follow-up appointment, health care service use (doctor visits for bca-related issues), travel distance (one way), Internet use (everyday vs. other), and total met and unmet supportive care needs with perceived satisfaction with primary care, shared care, specialist by videoconference, and specialist by e-mail were explored. Given that the outcomes of interest were ordinal (from “strongly disagree” to “strongly agree”), the use of ordinal logistic regression as the main analysis method was selected; p values less than 0.05 were considered significant for the exploratory analysis.
Analysis of open-ended survey responses followed the procedures for conventional qualitative content analysis23. Two team members (JLB, JYYK) independently read and coded the entire dataset. Data were read to derive codes that flowed from the data by highlighting exact words in the text that appeared to capture key thoughts or concepts. Independent coding results were compared, resulting in minor modifications to the coding scheme. Codes were sorted into categories, and categories were used to organize codes into meaningful themes. The qualitative findings were used to explain participant responses to the follow-up options presented and to identify additional variables that might explain the variance in perceived satisfaction with follow-up options on the part of the participants.
An average travel distance in kilometres from a survivor’s home to our institution was calculated based on postal code data, using the Google Maps Web mapping software (Google, Mountain View, CA, U.S.A.) and Google Maps travel calculator.
RESULTS
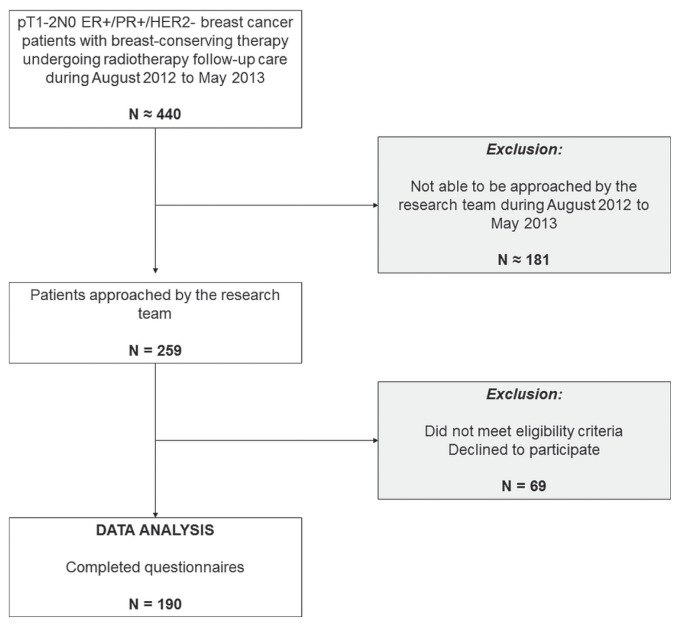
Of 259 individuals who were approached to participate in the study, 18 did not meet the eligibility criteria, and 51 declined to participate (Figure 1). The 190 participants who completed the questionnaire represent a 79% (190 of 241) response rate. For context, approximately 440 new pT1–2N0 estrogen receptor–positive or progesterone receptor–positive (or both) bca patients are seen by the radiation oncology department annually.
FIGURE 1.
Patient recruitment. ER+ = estrogen receptor–positive; PR+ = progesterone receptor–positive; HER2 = human epidermal growth factor receptor 2.
Participant Characteristics
Average age of the participants was 63 years, with 100 of the 190 (53%) having been born in Canada, 88 (46%) having been born in another country, and 2 (1%) not stating their birthplace. Most participants were married, retired, had greater than secondary school education, and had a household income greater than $80,000. All participants underwent at least surgery and radiotherapy treatments for their bca management. Median time since first follow-up appointment had been 21 months (Table II).
TABLE II.
Characteristics of 190 participants
| Characteristic | Value |
|---|---|
| Mean age (years) | 63±10 |
|
| |
| Nationality [n (%)] | |
| Born in Canada | 100 (53) |
| Born in another country | 88 (46) |
| Unknown | 2 (1) |
|
| |
| Relationship status [n (%)] | |
| Single, divorced, widowed | 73 (38) |
| Married or in a relationship | 112 (59) |
| Unknown | 5 (3) |
|
| |
| Education [n (%)] | |
| Secondary school or less | 39 (21) |
| College, technical school, university | 145 (76) |
| Unknown | 6 (3) |
|
| |
| Employment [n (%)] | |
| Unemployed | 18 (9) |
| Part-time | 16 (8) |
| Full-time | 65 (34) |
| Retired | 87 (46) |
| Unknown | 4 (2) |
|
| |
| Total household income [n (%)] | |
| ≤$40,000 | 34 (18) |
| $40,001–$80,000 | 54 (28) |
| ≥$80,001 | 76 (40) |
| Unknown | 26 (14) |
|
| |
| Mean one-way travel distance to treatment centre (km) | 22±28 |
|
| |
| Treatment received [n (%)] | |
| Surgery | 190 (100) |
| Chemotherapy | 40 (21) |
| Radiation | 190 (100) |
|
| |
| Time since first follow-up (months) | |
| Median | 21 |
| IQR | 29 |
144 Responses.
IQR = interquartile range.
Quantitative Responses
Existing Follow-Up Care and Related Health Care Services Use
Survivors attended a median of 4 health care professional visits per year for follow-up of their cancer or other general cancer-related issues. Most (76%, 145 of 190) identified their bca specialist as the person in charge of their cancer follow-up care. However, 98% (187 of 190) had access to a pcp.
Health-Related Internet Use and Preferences
Of the 190 participants, 131 (69%, 131 of 190) had used the Internet to search for information about bca. Familiarity with the Internet was characterized by extent of Internet use and engagement in various Internet activities. Of those survivors, 56% (107 of 190) reported using the Internet daily. Most reported using Wikipedia (53%, 100 of 190), and fewer than half reported using interactive Web sites such as Facebook (33%, 62 of 190), Twitter (9%, 17 of 190), online discussion forums (27%, 52 of 190), or blogs (5%, 9 of 190). Nevertheless, most of the survivors indicated that they would be interested in using online resources created by the cancer centre, including private messaging tools designed for communication with the health care team (52%, 98 of 190).
Supportive Care Needs
The highest domains of met and unmet supportive needs were existential survivorship needs (for example, managing concerns about cancer recurrence, anxiety over body image) and comprehensive cancer care (for example, hospital parking, care coordination). Of the participants, 47% [87 of 187 (3 participants did not respond)] reported at least 1 unmet need item. Top unmet needs were access to an ongoing case manager, availability of hospital parking, and concerns about cancer recurrence. Those items were reported by fewer than 20% of the participants (Table III).
TABLE III.
Unmet need items most frequently reported by breast cancer participants (needs are not mutually exclusive)
| Rank | Unmet need item | Selected [n (%)] |
|---|---|---|
| 1 | An ongoing case manager to whom I can go to find out about services whenever they are needed | 32 (17) |
|
| ||
| 2 | More accessible hospital parking | 30 (16) |
|
| ||
| 3 | Help to manage my concerns about the cancer coming back | 24 (13) |
|
| ||
| 4 | Help to reduce stress in my life | 23 (12) |
| Help to cope with others not acknowledging the impact that cancer has had on my life | 23 (12) | |
| Help to deal with my own and others’ expectations of me as a cancer survivor | 23 (12) | |
|
| ||
| 5 | To know that all my doctors talk to each other to coordinate my care | 22 (12) |
|
| ||
| 6 | Access to complementary and alternative therapy | 19 (10) |
|
| ||
| 7 | Help to adjust to changes in the way I feel about my body | 19 (10) |
|
| ||
| 8 | Help to manage ongoing symptoms and side effects | 18 (9) |
| Help to make decisions about my life in the context of uncertainty | 18 (9) | |
|
| ||
| 9 | Help to adjust to changes in my quality of life as a result of the cancer | 17 (9) |
| Help to cope with changes to my belief that nothing bad will ever happen in my life | 17 (9) | |
|
| ||
| 10 | Emotional support | 16 (8) |
Perceived Satisfaction with Follow-Up Care Options
Of these survivors, 93% (177 of 190) agreed or strongly agreed that they were satisfied with in-person specialist follow-up. The second most accepted model was shared care involving specialist and pcp follow-up (54%, 102 of 190). The other follow-up care models received less than 50% perceived satisfaction (Table IV).
TABLE IV.
Perceived satisfaction with breast cancer follow-up care models
| Follow-up format | Responses (n) | Mean score | Assigned score [n (%)] | ||||
|---|---|---|---|---|---|---|---|
| 5 Strongly agree |
4 Agree |
3 Neutral |
2 Disagree |
1 Strongly Disagree |
|||
| Tertiary care specialist | 185 | 4.8 | 161 (87) | 16 (9) | 4 (2) | 2 (1) | 2 (1) |
| Specialist and PCP | 180 | 3.6 | 71 (39) | 31 (17) | 36 (20) | 20 (11) | 22 (12) |
| PCP–nurse team with training in oncology | 180 | 3.1 | 32 (18) | 45 (25) | 42 (23) | 29 (16) | 32 (18) |
| Community specialist | 178 | 2.9 | 47 (26) | 22 (12) | 29 (16) | 26 (15) | 54 (30) |
| Community PCP | 183 | 2.2 | 18 (10) | 17 (9) | 33 (18) | 34 (19) | 81 (44) |
| Specialist by videoconferencing | 184 | 2.0 | 11 (6) | 18 (10) | 22 (12) | 40 (22) | 93 (51) |
| Specialist by e-mail | 184 | 1.9 | 12 (7) | 16 (9) | 22 (12) | 27 (15) | 107 (58) |
PCP = primary care provider.
Factors Associated with Perceived Satisfaction with Follow-up Care Options
Less formal education was associated with greater perceived satisfaction for follow-up care by a pcp [odds ratio (or): 3.0; 95% confidence interval (ci): 1.5 to 5.8; p = 0.001] and by community specialists (or: 2.4; 95% ci: 1.3 to 4.8; p = 0.006). Having more met supportive care needs was associated with greater perceived satisfaction for care delivered in virtual visits such as videoconferencing (or: 1.1; 95% ci: 1.0 to 1.1; p = 0.012) and e-mail (or: 1.1; 95% ci: 1.0 to 1.1; p = 0.016). Conversely, greater perceived satisfaction with videoconferencing (or: 3.1; 95% ci: 1.6 to 6.0; p = 0.001) and e-mail (or: 2.4; 95% ci: 1.2 to 4.7; p = 0.015) were inversely correlated with health-care related Internet experience. None of the other factors, including those commonly associated with Internet use (for example, age and education) or those related to convenience or clinical need (for example, travel distance from the cancer centre, health services use, or longer time in follow-up care) were associated with perceived satisfaction with virtual follow-up care options. Table V outlines key associated factors.
TABLE V.
Factors associated with perceived satisfaction by 190 patients of alternative breast cancer follow-up care models
| Follow-up format | Associated factor | OR | 95% CI | p Value |
|---|---|---|---|---|
| Specialist and PCP | Lives closer to specialist centre | 1.0 | 1.0 to 1.02 | 0.046 |
|
| ||||
| PCP–nurse team | Fewer visits to oncology health care professionals | 1.1 | 1.0 to 1.1 | 0.026 |
|
| ||||
| Community specialist | Fewer years of formal education | 2.4 | 1.3 to 4.8 | 0.006 |
|
| ||||
| Community PCP | Fewer years of formal education | 3.0 | 1.5 to 5.8 | 0.001 |
| Less frequent Internet use | 2.3 | 1.3 to 4.0 | 0.003 | |
|
| ||||
| Specialist by videoconferencing | More met needs | 1.1 | 1.0 to 1.1 | 0.012 |
| Not using the Internet to search for information about breast cancer | 3.1 | 1.6 to 6.0 | 0.001 | |
|
| ||||
| Specialist by e-mail | More met needs | 1.1 | 1.0 to 1.1 | 0.016 |
| Not using the Internet to search for information about breast cancer | 2.4 | 1.2 to 4.7 | 0.015 | |
OR = odds ratio; CI = confidence interval; PCP = primary care provider.
Qualitative Responses
Of our 190 participants, 110 (58%) provided views about the follow-up care options presented in the survey. Comments emphasized the advantages of specialist follow-up, including perceived expertise, consistency, and reassurance. Participants explained that they preferred in-person follow-up care with their specialist at the cancer centre because they believed that their specialist was the most qualified, knew their case the best, and provided the best care. Some survivors identified advantages of virtual follow-up (for example, for bloodwork, minor questions, convenience, and reduced travel and parking costs) and explained that virtual follow-up would be an efficient and reasonable option when they had fewer needs and less risk of cancer recurrence. Those findings support the quantitative findings, which revealed that participants with more met supportive care needs were more likely to be satisfied with virtual follow-up. However, many participants commented on perceived disadvantages of virtual follow-up. Those disadvantages included the perceived impersonal nature of virtual care, potential for inadequate care (for example, loss of the physical exam and limited opportunity for questions and discussion), and apprehension about confidentiality. In particular, the perceived impersonal nature of communication using technology was raised by 25% of participants (28 of 110) as a concern. As one participant explained, “Breast cancer was a profoundly personal experience for me. Videoconference or e-mail would be too impersonal for me.” Table VI shows themes, with representative quotes from the participants.
TABLE VI.
Perspectives of 110 patients about breast cancer follow-up care models
| Category | Concept | Representative quote |
|---|---|---|
| Specialist follow-up | ||
| Advantages | Expertise | I would prefer to come to [this specialist centre] where they are dealing with this all the time. |
| Consistency | I would like to see a person that is part of a team that knows me. | |
| Reassurance | Cancer patients feel vulnerable, and we need reassurance that we get the best follow-up available. | |
|
| ||
| Virtual follow-up | ||
| Advantages | Accessibility | if I lived in a remote rural community, videoconference might be okay. |
| Convenience | E-mail as an option would be preferred ... especially if there are no problems or issues. | |
| Economics | Too expensive to park! | |
| Disadvantages | Impersonal nature | I still feel that the personal touch is what helps reassure me. |
| Inadequate care | I still require physical exam! | |
| Lack of access to technology | I don’t own a computer! | |
| Concern about confidentiality | I would not want health-related e-mails—how secure could they really be? What if your iPhone is stolen? | |
DISCUSSION
For this study, we surveyed well survivors of early-stage bca currently receiving posttreatment follow-up by cancer specialists at a large tertiary care centre. Compared with the general population, participants had above-average education, average socioeconomic status, and above-average access to a pcp24–26. Despite being clinically amenable to alternative follow-up options, our participants had not yet been transitioned out of tertiary care follow-up because of survivorship needs or a preference for continuity of care, or both27. The results provide guidance for optimal care transitions from the perspective of survivor supportive care needs and preferences.
In examining current health care use in the study population, the frequency of oncology visits by survivors was on the higher end of the recommended guidelines—that is, a median of 4 visits annually compared with the 2–4 visits recommended10,28,29. That observation is consistent with findings published by Grunfeld et al.29, which demonstrated both overuse and underuse of oncology visits throughout our region. The greater number of oncology visits might in part explain the low number of unmet supportive care needs reported by the cohort.
In general, this well bca survivor population had a low prevalence of unmet needs: fewer than half the participants reported 1 or more unmet needs. That prevalence is lower than the prevalence of unmet needs reported by survivors of head-and-neck cancers (61%)30 and lung cancers (78%)31. It is also lower than the prevalence reported in other bca studies (61%)32. However, of reported unmet supportive care needs, the items requiring the most attention were those in the existential survivorship needs domain. Needs in that challenging domain have consistently been reported by other groups as well32–34. Existential survivorship services refer to psychosocial oncology supports that address the social, emotional, and spiritual needs of a survivor. All cancer care providers, including the medical team and allied health workers, are responsible for ensuring the psychosocial health of survivors and their families35. It would appear that traditional in-person specialist follow-up with access to an on-site multidisciplinary cancer care team (for example, with palliative care and psychosocial oncology services) might have been helpful in partly addressing that unmet need in our cohort. Further improvement in follow-up care for existential survivorship needs could be achieved through supplementation of current practice with nurse-led follow-up, cancer rehabilitation programs, or virtual care, all of which have demonstrated efficacy in this particular supportive care domain13,36,37.
For survivors with a high number of met needs, our study shows that transition to virtual follow-up care might be acceptable. As one participant explained, “E-mail as an option would be preferred ... especially if there are no problems or issues.” Interestingly, even survivors with minimal health-related Internet experience were amenable to virtual-only follow-up visits. In fact, less health-related Internet use was correlated with higher perceived satisfaction with virtual follow-up visits. That counterintuitive finding suggests that familiarity with technology does not equate with positive perceptions of virtual care. However, it is important to emphasize that only 15% of survivors of bca in our cohort reported that they would be satisfied with virtual-only follow-up care. Similar findings were reported in a study of Canadian thyroid cancer survivors17 and also in a study of U.S. survivors of bca38. In particular, Mayer et al. reported that virtual visits were ranked least likely to improve survival and to decrease cancer-related worry among survivors of bca38.
In their qualitative responses, study participants expressed several key concerns with virtual follow-up care that can help reach a better understanding their apprehension about the virtual option. Their concerns revolved around a fear of inadequate care. Previous studies similarly support the idea that survivors can be wary of the quality of Internet-based health care services39. The most common concern about virtual care in our cohort was loss of personalized care and a need for reassurance. In a previous study, thyroid cancer survivors explained that they would feel more secure with in-person follow-up care, which they believed would be more personal and reassuring than virtual care would be17. Well survivors of bca in the present study similarly explained that the personal touch received from in-person visits is reassuring, and that that reassurance is a critical component of follow-up care.
Another major barrier to virtual-only follow-up care reported by survivors in our cohort and in a prior study with thyroid cancer survivors17 was the loss of the clinical exam. Many survivors placed large emphasis on the utility of the clinical breast exam, which they felt is a valuable missing item in virtual-only follow-up care models. However, based on the evidence, the sensitivity of physical examination is 27.6% compared with 77.6% for mammography40, which would be included in a virtual visit model. Furthermore, performance of the clinical breast exam shows variability between providers41; in fact, using the U.S. National Cancer Database, it was recently demonstrated that physical examination had only modest value in detecting bca recurrences42.
Both of the foregoing issues—the need for reassurance and the loss of the clinical exam—could be solved with survivor education and reassurance, and physician training. They also suggest that performance expectancy about the virtual visit by survivors is an important determinant of their acceptance and likely adoption of virtual visits. That finding aligns with the Unified Theory of Acceptance of Information Technology (version 2), which explains that performance expectancy (defined as the degree to which using a technology will provide benefits to consumers in performing certain activities) is the strongest predictor of intention to use a technology43. Physician help in clarifying the quality and benefits of the virtual care being introduced could address survivor anxiety about the performance expectancy of the technology. With respect to the issue of the missing clinical exam by an oncologist in non-cancer-centre–based care, physicians would need to inform and reassure survivors that the physical exam (including the clinical breast exam) can be adequately performed by community pcps and will be supplemented by mammography (which has a higher sensitivity for cancer detection of 78% compared with 28% for the clinical exam)40–42.
With respect to other non-specialist-only follow-up models, the second most highly rated option was a shared-care model that integrates specialists with primary care (endorsed by 54% of our cohort). In addition, survivors living close to the specialist cancer care centre reported higher perceived satisfaction with cancer centre specialist involvement through a shared-care model. That observation suggests that there might be a cost (travel distance) and benefit (convenient access to supportive care services) trade-off to consider when assigning survivors to cancer centre follow-up compared with community care follow-up. Follow-up by primary care alone was the least preferred in-person care option, endorsed by 19% of our cohort. Survivors with less formal education were found to be more accepting of pcp care. That observation might be related to increased comfort on the part of those survivors in communicating in-person with their established pcp rather than in navigating any or all of potentially unfamiliar technologies, medical jargon, and relatively new relationships with multiple specialists in a fast-paced environment. Increased satisfaction with follow-up has previously been associated with opportunities for survivors to ask questions about their cancer and to receive easy-to-understand explanations44. For some survivors, such opportunities might be better facilitated through pcps. Also, survivors requiring fewer oncology-related health care visits per year indicated higher perceived satisfaction with follow-up from pcp–nurse teams with training in oncology. The omission of specialist care at a tertiary care cancer centre might be acceptable for those survivors because of their lower need for supportive care services provided at the centre.
Strengths of the present study include the high response rate, implementation of a validated supportive care needs assessment tool, and use of both quantitative and qualitative methods to examine survivor perceptions of follow-up models. Limitations of the study include recruitment through radiation oncology clinics at a single academic cancer centre offering specialist follow-up, convenience sampling, and exclusion of survivors lacking English proficiency.
CONCLUSIONS
Overall, the present study suggests that its sample of well survivors of bca want specialist involvement in their follow-up care and that they prefer in-person follow-up, which, compared with virtual follow-up, is perceived to be more reassuring. Our study also identified survivor characteristics that could signal survivor readiness for, and acceptance of, alternative models of follow-up care. For example, survivors who have had many of their supportive care needs met could be ready for transition to virtual care. Guiding transitions to alternative follow-up options based on survivor needs and preferences might yield greater survivor satisfaction and compliance. Future research should investigate the optimal model of multimodal shared care, involving specialist and pcp follow-up and using a combination of in-person and virtual interactions, and should identify multi-stakeholder perspectives about the barriers and facilitators to implementation of such care. Additionally, cost-effectiveness analyses of current follow-up models compared with virtual follow-up models is required. Lastly, our study has highlighted a need to enhance communication between physicians and survivors to clarify the quality and efficacy of follow-up cancer care and to build trust when transitioning to new care models.
ACKNOWLEDGMENTS
This research was supported by the Pat Nichols and Bob Tundermann Cancer Care Fund and the ellicsr Cancer Rehabilitation and Survivorship Program at Princess Margaret Cancer Centre. The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. We thank the patients who volunteered to participate in the study.
This research was previously presented in part at estro 36; Vienna, Austria; 5–9 May 2017; and the Princess Margaret Cancer Centre Personalizing Cancer Medicine Conference; Toronto, ON; 6–7 February 2017.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: TP has received honoraria from Celgene Canada Inc. The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. Cancer Incidence and Mortality Worldwide: GLOBOCAN 2012. Ver. 1.0. Lyon, France: International Agency for Research on Cancer; 2013. [Google Scholar]
- 2.United States, Department of Health and Human Services, National Institutes of Health, National Cancer Institute (nci) SEER Cancer Statistics Review, 1975–2013. Bethesda, MD: nci; 2016. [Google Scholar]
- 3.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2015. Toronto, ON: Canadian Cancer Society; 2015. [Google Scholar]
- 4.Grunfeld E, Dhesy-Thind S, Levine M on behalf of the Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer: follow-up after treatment for breast cancer (summary of the 2005 update) CMAJ. 2005;172:1319–20. doi: 10.1503/cmaj.045062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists: challenges to assuring access to oncology services. J Oncol Pract. 2007;3:79–86. doi: 10.1200/JOP.0723601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke M, Collins R, Darby S, et al. on behalf of Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 7.Vanhuyse M, Bedard PL, Sheiner J, Fitzgerald B, Clemons M. Transfer of follow-up care to family physicians for early-stage breast cancer. Clin Oncol (R Coll Radiol) 2007;19:172–6. doi: 10.1016/j.clon.2007.01.115. [DOI] [PubMed] [Google Scholar]
- 8.Dickinson R, Hall S, Sinclair J, Bond C, Murchie P. Using technology to deliver cancer follow-up: a systematic review. BMC Cancer. 2014;14:311. doi: 10.1186/1471-2407-14-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khatcheressian J, Hurley P, Bantug E, et al. on behalf of the American Society of Clinical Oncology. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:961–5. doi: 10.1200/JCO.2012.45.9859. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Ver. 1.2016. Fort Washington, PA: nccn; 2014. [Current version available online at: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (free registration required); cited 5 December 2016] [Google Scholar]
- 11.Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v8–30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 12.Lewis RA, Neal RD, Hendry M, et al. Patients’ and healthcare professionals’ views of cancer follow-up: systematic review. Br J Gen Pract. 2009;59:e248–59. doi: 10.3399/bjgp09X453576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pennery E, Mallet J. A preliminary study of patients’ perceptions of routine follow-up after treatment for breast cancer. Eur J Oncol Nurs. 2000;4:138–45. doi: 10.1054/ejon.2000.0092. [DOI] [PubMed] [Google Scholar]
- 14.Hede K. Teleoncology gaining acceptance with physicians, patients. J Natl Cancer Inst. 2010;102:1531–3. doi: 10.1093/jnci/djq426. [DOI] [PubMed] [Google Scholar]
- 15.Lubberding S, van Uden-Kraan CF, Te Velde EA, Cuijpers P, Leemans CR, Verdonck-de Leeuw IM. Improving access to supportive cancer care through an eHealth application: a qualitative needs assessment among cancer survivors. J Clin Nurs. 2015;24:1367–79. doi: 10.1111/jocn.12753. [DOI] [PubMed] [Google Scholar]
- 16.O’Malley DM, Hudson SV, Ohman-Strickland PA, et al. Follow-up care education and information: identifying cancer survivors in need of more guidance. J Cancer Educ. 2016;31:63–9. doi: 10.1007/s13187-014-0775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bender JL, Wiljer D, Sawka AM, Tsang R, Alkazaz N, Brierley JD. Thyroid cancer survivors’ perceptions of survivorship care follow-up options: a cross-sectional, mixed-methods survey. Support Care Cancer. 2016;24:2007–15. doi: 10.1007/s00520-015-2981-5. [DOI] [PubMed] [Google Scholar]
- 18.Liaw SS, Huang HM. Perceived satisfaction, perceived usefulness and interactive learning environments as predictors to self-regulation in e-learning environments. Comput Educ. 2013;60:14–24. doi: 10.1016/j.compedu.2012.07.015. [DOI] [Google Scholar]
- 19.Baravelli C, Krishnasamy M, Pezaro C, et al. The views of bowel cancer survivors and health care professionals regarding survivorship care plans and post treatment follow up. J Cancer Surviv. 2009;3:99–108. doi: 10.1007/s11764-009-0086-1. [DOI] [PubMed] [Google Scholar]
- 20.Bender JL. The Web of Support: A Multi-method Study Examining the Role of Online Communities As a Source or Peer-to-Peer Supportive Care for Breast Cancer Survivors [phd thesis] Toronto, ON: University of Toronto Libraries; 2012. [Google Scholar]
- 21.Bender JL, Wiljer D, To MJ, et al. Testicular cancer survivors’ supportive care needs and use of online support: a cross-sectional survey. Support Care Cancer. 2012;20:2737–46. doi: 10.1007/s00520-012-1395-x. [DOI] [PubMed] [Google Scholar]
- 22.Hodgkinson K, Butow P, Hunt GE, et al. The development and evaluation of a measure to assess cancer survivors’ unmet supportive care needs: the casun (Cancer Survivors’ Unmet Needs measure) Psychooncology. 2007;16:796–804. doi: 10.1002/pon.1137. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh H, Shannon S. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–88. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 24.Statistics Canada. Education in Canada: Attainment, Field of Study and Location of Study [Web resource] Ottawa, ON: Statistics Canada; 2016. [Available at: https://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-012-x/99-012-x2011001-eng.cfm; cited 7 March 2017] [Google Scholar]
- 25.Statistics Canada. Distribution of total income by census family type and age of older partner, parent or individual [Web page] Ottawa, ON: Statistics Canada; 2016. [Available at: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/famil108a-eng.htm; cited 7 March 2017] [Google Scholar]
- 26.Canadian Institute for Health Information (cihi) Commonwealth Fund Survey 2016. Ottawa, ON: cihi; 2016. [Google Scholar]
- 27.Kantsiper M, McDonald EL, Geller G, Shockney L, Snyder C, Wolff AC. Transitioning to breast cancer survivorship: perspectives of patients, cancer specialists, and primary care providers. J Gen Intern Med. 2009;24(suppl 2):S459–66. doi: 10.1007/s11606-009-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Care Ontario. Breast Cancer Well Follow-Up Pathway Map. Toronto, ON: Cancer Care Ontario; 2015. [Available online at: https://cancercare.on.ca/common/pages/UserFile.aspx?fileId=349028; cited 7 January 2017] [Google Scholar]
- 29.Grunfeld E, Hodgson DC, Del Giudice ME, Moineddin R. Population-based longitudinal study of follow-up care for breast cancer survivors. J Oncol Pract. 2010;6:174–81. doi: 10.1200/JOP.200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giuliani M, McQuestion M, Jones J, et al. Prevalence and nature of survivorship needs in patients with head and neck cancer. Head Neck. 2016;38:1097–103. doi: 10.1002/hed.24411. [DOI] [PubMed] [Google Scholar]
- 31.Giuliani ME, Milne RA, Puts M, et al. The prevalence and nature of supportive care needs in lung cancer patients. Curr Oncol. 2016;23:258–65. doi: 10.3747/co.23.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodgkinson K, Butow P, Hunt GE, Pendlebury S, Hobbs KM, Wain G. Breast cancer survivors’ supportive care needs 2–10 years after diagnosis. Support Care Cancer. 2007;15:515–23. doi: 10.1007/s00520-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 33.Edib Z, Kumarasamy V, Binti Abdullah N, Rizal AM, Al-Dubai SA. Most prevalent unmet supportive care needs and quality of life of breast cancer patients in a tertiary hospital in Malaysia. Health Qual Life Outcomes. 2016;14:26. doi: 10.1186/s12955-016-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiszer C, Dolbeault S, Sultan S, Brédart A. Prevalence, intensity, and predictors of the supportive care needs of women diagnosed with breast cancer: a systematic review. Psychooncology. 2014;23:361–74. doi: 10.1002/pon.3432. [DOI] [PubMed] [Google Scholar]
- 35.Turnbull Macdonald GC, Baldassarre F, Brown P, et al. Psychosocial care for cancer: a framework to guide practice, and actionable recommendations for Ontario. Curr Oncol. 2012;19:209–16. doi: 10.3747/co.19.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaver K, Williamson S, Chalmers K. Telephone follow-up after treatment for breast cancer: views and experiences of patients and specialist breast care nurses. J Clin Nurs. 2010;19:2916–24. doi: 10.1111/j.1365-2702.2010.03197.x. [DOI] [PubMed] [Google Scholar]
- 37.Scott DA, Mills M, Black A, et al. Multidimensional rehabilitation programmes for adult cancer survivors. Cochrane Database Syst Rev. 2013:CD007730. doi: 10.1002/14651858.CD007730.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer EL, Gropper AB, Neville BA, et al. Breast cancer survivors’ perceptions of survivorship care options. J Clin Oncol. 2012;30:158–63. doi: 10.1200/JCO.2011.36.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldman-Stewart D, Tong C, Brundage M, Bender J, Robinson J. Prostate cancer patients’ experience and preferences for acquiring information early in their care. Can Urol Assoc J. 2018;12:E219–25. doi: 10.5489/cuaj.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast us and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–75. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 41.McDonald S, Saslow D, Alciati MH. Performance and reporting of clinical breast examination: a review of the literature. CA Cancer J Clin. 2004;54:345–61. doi: 10.3322/canjclin.54.6.345. [DOI] [PubMed] [Google Scholar]
- 42.Neuman HB, Schumacher JR, Francescatti AB, et al. Utility of clinical breast exams in detecting local-regional breast events after breast-conservation in women with a personal history of high-risk breast cancer. Ann Surg Oncol. 2016;23:3385–91. doi: 10.1245/s10434-016-5483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkatesh V, Thong JYL, Xu X. Consumer acceptance and use of information technology: extending the unified theory of acceptance and use of technology. MIS Quarterly. 2012;36:157–78. doi: 10.2307/41410412. [DOI] [Google Scholar]
- 44.Thind A, Liu Y, Maly RC. Patient satisfaction with breast cancer follow-up care provided by family physicians. J Am Board Fam Med. 2011;24:710–16. doi: 10.3122/jabfm.2011.06.100288. [DOI] [PMC free article] [PubMed] [Google Scholar]