Abstract
Background
Data showing the value of neoadjuvant chemotherapy (nact) followed by interval debulking surgery (ids) in the management of advanced-stage serous endometrial carcinoma (eca) are limited; the aim of the present study was to expand the knowledge about that treatment strategy in patients with advanced eca, including endometrioid eca.
Methods
Data were collected retrospectively from all patients with advanced-stage eca treated with nact between 2005 and 2014 at 3 oncology referral centres. Primary outcomes were the radiologic response to nact and achievement of optimal or complete ids. Secondary outcomes were recurrence rate and progression-free and overall survival.
Results
Of 102 eca cases included, a complete radiologic response was achieved in only 4 cases, with a partial response being achieved in 72% (64% of endometrioid cases, 80% of serous cases). Complete ids was achieved in 62% of the endometrioid cases and in 56% of the serous eca cases, with optimal ids achieved in 31% and 28% of those cases respectively. Survival rates were calculated for all patients with complete and optimal ids; recurrence was observed in 56% and 67% of the cases respectively, and progression-free survival was 18 months and 13 months respectively. Median survival duration was 24 months for endometrioid eca and 28 months for serous eca.
Conclusions
For patients with advanced eca who are not suitable for primary debulking, nact followed by ids can be considered regardless of histologic subtype. The treatment options for this group of patients are limited and have to be explored.
Keywords: Endometrial cancer, advanced, neoadjuvant chemotherapy, radiologic response, interval debulking surgery
INTRODUCTION
Endometrial carcinoma (eca) is the most common gynaecologic malignancy in developed countries, with a lifetime risk of 2.88% for white and 1.69% for African American women1,2. The primary treatment for these carcinomas is surgical3. However, some patients are unfit for primary surgical treatment because of either comorbidities or advanced disease. A prospective study demonstrated that neoadjuvant chemotherapy (nact) followed by interval debulking surgery (ids) is an effective alternative treatment strategy for patients with advanced serous eca4, which, like ovarian cancer, usually spreads intraperitoneally5,6. A radiologic response to nact, defined as complete or partial remission according to the Response Evaluation Criteria in Solid Tumors (recist)7, was observed in 74% of the included study participants. A few (7%) had progressive disease and were therefore not suitable for ids. At the time of ids, 13% of the patients were considered inoperable, but 80% had complete or optimal debulking4.
Another study compared surgical outcomes and survival for Fédération Internationale de Gynécologie et d’Obstétrique (figo) stage iv serous eca treated with either nact followed by ids or by complete debulking followed by adjuvant chemotherapy (ctx)8. The study showed that surgery duration and hospital stay were shorter for patients who started with nact and found no significant statistical difference in surgical outcome, progression-free survival (pfs), or overall survival (os) between the two treatment groups. A recent case–control study confirmed those results for patients with serous eca9.
Overall, the data pertaining to nact followed by ids for advanced-stage eca are based mainly on cases of serous eca, and yet advanced endometrioid eca also frequently occurs in the aging patient population with multiple comorbidities. The aim of the present retrospective multicentre study was to expand knowledge about outcomes (radiologic response, recurrence-free survival, os) after nact followed by ids in patients with advanced eca, including endometrioid eca.
METHODS
Patients
All patients with figo 2009 stage iii or iv eca treated with nact between January 2005 and January 2014 at 3 referral centres for gynecologic malignancies—BC Cancer (Vancouver, BC; n = 43), the University Hospital Leuven (Leuven, Belgium; n = 35), and the Gynaecological Oncology Centre South (Eindhoven and Tilburg, Netherlands; n = 24)—were included10. Patients were retained in the cohort if they had figo iii or iv eca treated with nact, independent of histologic subtype, and if they had received adequate follow-up during treatment. No exclusion criteria were applied. The decision for nact as the primary treatment approach was made by the treating physician or tumour board based on the extent of the disease or the patient’s performance status, or both.
Of the 35 patients from the University Hospital Leuven, 12 had previously been included in a descriptive analysis4.
Patient and tumour characteristics, laboratory and radiology results, and the number and type of ctx courses were collected from patient charts and pathology reports. The pattern of tumour spread was classified as locally extended, intra-abdominal spread, lymphatic spread, extra-abdominal spread, or some combination of those patterns. The figo stage was determined before treatment by either gynecologic investigation, imaging, diagnostic laparoscopy, or a combination. Radiologic response to nact was reported based on the recist 1.1 criteria, as used in clinical decision-making7. Information about the extent of disease, residual disease after nact, and residual disease after surgery was collected from the surgical report. The surgery was defined as complete cytoreduction (no residual disease), optimal cytoreduction (residual disease < 1 cm), or suboptimal cytoreduction (residual disease > 1 cm). Adjuvant therapy was recorded as either ctx, radiotherapy (rt), or a combination of both. After treatment, patients were monitored according to local protocols by physical examination and, when appropriate, imaging. The date of diagnosis was used as the start point for calculating survival (the exact start date of any treatment was not always available). Recurrence was defined as the first objective documentation of disease by either serology (elevated cancer antigen 125), cytology, histology, or imaging. The os was calculated based on the actual date of death; the follow-up time was either to date of death or last visit to the clinic.
The study protocol was approved by the Medical Ethics Committee of Catharina Hospital in Eindhoven, Netherlands.
Outcomes
Primary outcomes were defined as response to nact according to the recist 1.1 criteria and achievement of either complete or optimal ids after nact. Secondary outcomes were defined as recurrence rate, pfs, and os (all with reference to the outcome achieved after ids).
All outcome measurements were calculated for the cohort overall and for the histologic subtype cohorts.
Statistical Analyses
Differences in the radiologic response rate and the recurrence rate between the histopathologic subtype cohorts were calculated using the chi-square and Fisher exact tests for categorical variables and the Mann–Whitney U-test for continuous variables. Logistic regression analyses were used to estimate factors predicting complete ids: age at diagnosis, age at menopause, extent of disease before treatment, radiologic response, histology, and figo stage.
Kaplan–Meier graphs were generated for survival functions. The log-rank test was used to compare survival curves. Differences were considered statistically significant if the 2-sided p value was 0.05 or less. The IBM SPSS Statistics software application (version 20: IBM, Armonk, NY, U.S.A.) was used to perform the statistical analyses.
RESULTS
The 102 patients included 43% (n = 44) with endometrioid, 43% (n = 44) with serous, 4% (n = 4) with clear-cell, and 10% (n = 10) with other or mixed histologic subtypes of eca. Table I shows the patient and tumour characteristics. Of the patients with endometrioid eca, 29.5% (n = 13) had a grade 1 tumour at diagnosis, 36.4% (n = 16) had a grade 2 tumour, and 29.5% (n = 13) had a grade 3 tumour. Grade was not documented for the remaining 2 patients.
TABLE I.
Baseline characteristics of all included women
| Characteristic | Endometrial cancer type | ||||
|---|---|---|---|---|---|
|
|
|||||
| All | Endometrioid | Serous | Clear cell | Other | |
| Patients (n) | 102 | 44 | 44 | 4 | 10 |
|
| |||||
| Age (years) at ... | |||||
| Diagnosis | |||||
| Mean | 63 | 63 | 62 | 69 | 62 |
| Range | 43–85 | 43–85 | 44–79 | 64–72 | 52–70 |
| Menopause | |||||
| Mean | 51 | 50 | 51 | 53 | 51 |
| Range | 37–58 | 37–57 | 43–58 | 52–53 | 48–54 |
|
| |||||
| Body mass index | |||||
| Mean | 28 | 30 | 26 | 28 | 27 |
| Range | 17–52 | 17–52 | 17–45 | 24–32 | 20–44 |
|
| |||||
| Parity | |||||
| Mean | 1.8 | 1.6 | 1.8 | 3.0 | 2.0 |
| Range | 0–5 | 0–5 | 0–4 | 2–4 | 0–3 |
|
| |||||
| FIGO stage [n (%)] | |||||
| IIIA | 1 (1) | 1 (2.3) | 0 | 0 | 0 |
| IIIB | 9 (8.8) | 2 (4.5) | 5 (11.4) | 0 | 2 (20) |
| IIIC1 | 18 (17.6) | 11 (25) | 3 (6.8) | 2 (50) | 2 (20) |
| IIIC2 | 3 (2.9) | 0 | 3 (6.8) | 0 | 0 |
| IV | 69 (67.6) | 29 (65.9) | 32 (72.7) | 2 (50) | 6 (60) |
| Not specified | 2 (2) | 1 (2.3) | 1 (2.3) | 0 | 0 |
FIGO = Fédération Internationale de Gynécologie et d’Obstétrique.
Abnormal bleeding, defined as postmenopausal blood loss or metrorrhagia, was the presenting symptom in 82% of patients with endometrioid eca and in 36% of patients with serous eca; abdominal complaints were the main presenting symptom in 39% of patients with serous eca and in 11% of patients with endometrioid eca.
The endometrioid and serous carcinomas had different patterns of disease spread. In most of the endometrioid carcinoma cases, disease spread was primarily lymphatic (n = 13, 30%), followed by intra-abdominal (n = 8, 18%) and extra-abdominal (n = 7, 16%). For serous eca, spread was primarily intra-abdominal (n = 19, 43%), followed by lymphatic (n = 11, 25%) and extra-abdominal (n = 6, 14%).
In 89% of the patients (n = 91), carboplatin–paclitaxel was given as nact. Other nact strategies were carboplatin only (n = 2), carboplatin–epirubicin (n = 2), cisplatin (n = 6), and cisplatin–doxorubicin (n = 1).
Two thirds of the patients (n = 69, 68%) received 3 cycles of nact (range: 1–6 cycles) before ids. Of those 69 patients, 6 also received neoadjuvant rt, 5 of whom received concurrent cisplatin, and 1, carboplatin–paclitaxel. Of the remaining 33 patients, 8% (n = 8) received fewer than 3 cycles of nact, 9% (n = 9) received 4 cycles, 5% (n = 5) received 5 cycles, and 11% (n = 11) received 6 cycles.
Radiologic Response to NACT
Table II shows the radiologic response according to the recist criteria by histopathologic subgroup. Overall, 4% of the patients (n = 4) experienced a complete response, and 72% (n = 73) experienced a partial response. No significant difference in the radiologic response rate between the endometrioid and serous eca subgroups was evident. A complete response occurred in 7% of the patients with endometrioid eca (n = 3) and in 2% of those with serous eca (n = 1); a partial remission occurred in 64% (n = 28) and 80% (n = 35) of those subgroups respectively. Progressive disease was seen in 14% of the patients with endometrioid eca (n = 6), in 7% of those with serous eca (n = 3), in 25% of those with clear-cell histology (n = 1), and in 10% of those with other forms of eca (n = 1). While receiving nact, 2 patients died, both as a result of progressive endometrioid eca. No statistical differences were evident in the response rates of patients with figo stage iii and iv carcinomas in the endometrioid and serous eca subgroups.
TABLE II.
Radiologic response according to the Response Evaluation Criteria in Solid Tumors
| Response | Endometrial cancer type [n (%)] | ||||
|---|---|---|---|---|---|
| All (n=102) | Endometrioid (n=44) | Serous (n=44) | Clear cell (n=4) | Other (n=10) | |
| Complete | 4 (3.9) | 3 (6.8) | 1 (2.3) | ||
| Partial | 73 (71.6) | 28 (63.6) | 35 (79.5) | 3 (75) | 7 (70) |
| Stable disease | 9 (8.8) | 3 (6.8) | 5 (11.4) | 1 (10) | |
| Progressive disease | 11 (10.8) | 6 (13.6) | 3 (6.8) | 1 (25) | 1 (10) |
| Died during treatment | 2 (2) | 2 (4.5) | |||
| Imaging not available | 3 (2.9) | 2 (4.5) | 1 (10) | ||
IDS
Overall, 78% of the patients (n = 80) underwent ids, with 60% (n = 48) having a complete debulking, and 29% (n = 23) having an optimal debulking. Surgery was omitted in 22 patients, either because of extensive disease (n = 3), progressive disease (n = 9), stable disease (n = 3), no residual disease (n = 3), age (n = 1), pulmonary embolism (n = 1), or death (n = 2). Complete debulking was achieved in 62% of the endometrioid cases (n = 18) and in 56% of the serous cases (n = 22, p = 0.9). Optimal debulking was achieved in 31% (n = 9) of the endometrioid cases and in 28% (n = 11) of the serous cases. Debulking was incomplete for 2 patients in the endometrioid group and for 6 patients in the serous group. Table III shows the surgical outcomes.
TABLE III.
Outcome of interval debulking surgery after neoadjuvant chemotherapy
| Outcome | Endometrial cancer type [n (%)] | ||||
|---|---|---|---|---|---|
|
|
|||||
| All (n=102) | Endometrioid (n=44) | Serous (n=44) | Clear cell (n=4) | Other (n=10) | |
| No surgery | 22 (21.6) | 14 (31.8) | 5 (11.4) | 1 (25) | 1 (10) |
|
| |||||
| Surgery | 80 (78.4) | 30 (68,2) | 39 (88.6) | 3 (75) | 9 (90) |
| Complete | 48 (60) | 18 (62.1) | 22 (56.4) | 2 (66.7) | 6 (66.7) |
| Optimal | 23 (28.9) | 9 (31) | 11 (28.2) | 3 (33.3) | |
| Incomplete | 9 (11.3) | 3 (10.3) | 6 (15.4) | 1 (33.3) | |
When surgical outcome was compared for these patients with figo stages iii and iv disease, no difference in outcome between the histopathologic subtypes was found. Overall, no significant difference was evident with respect to the surgical outcome between the endometrioid and serous eca cases. The logistic regression analysis showed no significant contributors to the achievement of complete ids.
Pathology results for residual tumour after surgery showed no residual tumour or necrosis in 9 patients (11.25%), endometrial intraepithelial carcinoma in 4 patients (5%), and vital tumour in 67 patients (83.75%).
Adjuvant Therapy After Surgery
Adjuvant treatment consisted of ctx, rt, or hormonal therapy. Adjuvant ctx was given in 69% of the patients who had a complete or optimal debulking surgery (n = 49). Only 7% (n = 5) received a combination of adjuvant ctx and rt after complete or optimal debulking.
Adjuvant rt consisted of at least external-beam radiation and was in most cases combined with vaginal brachytherapy. Patients with endometrioid eca more often received adjuvant rt [46% (n = 20) vs. 23% (n = 10) for patients with serous eca].
Recurrence and Survival
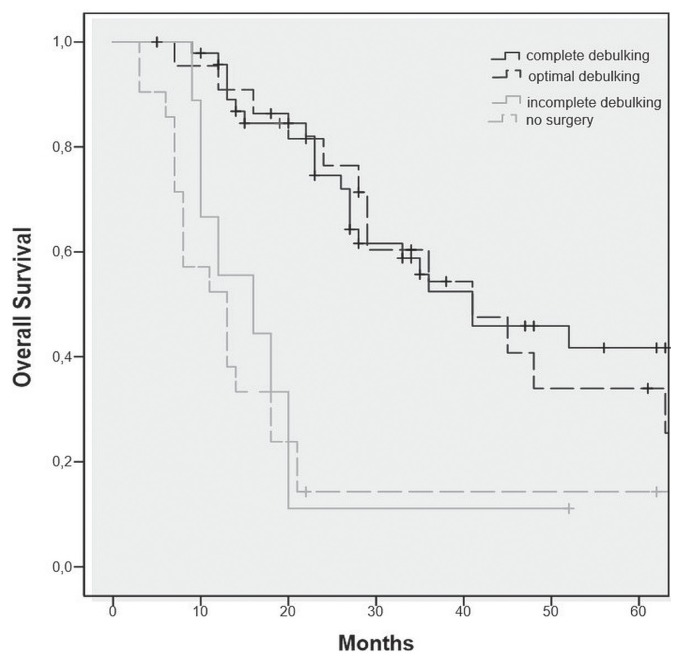
The os rate was calculated for complete, optimal, and incomplete debulking, and for no surgery (Figure 1). Median os was 41 months after complete and optimal debulking, 16 months after incomplete debulking, and 13 months for patients who did not undergo surgery. Based on those results (no difference in os between complete and optimal debulking), the recurrence and os rates were calculated for patients who underwent complete and optimal ids after nact (n = 71).
FIGURE 1.
Overall survival curve for all included women.
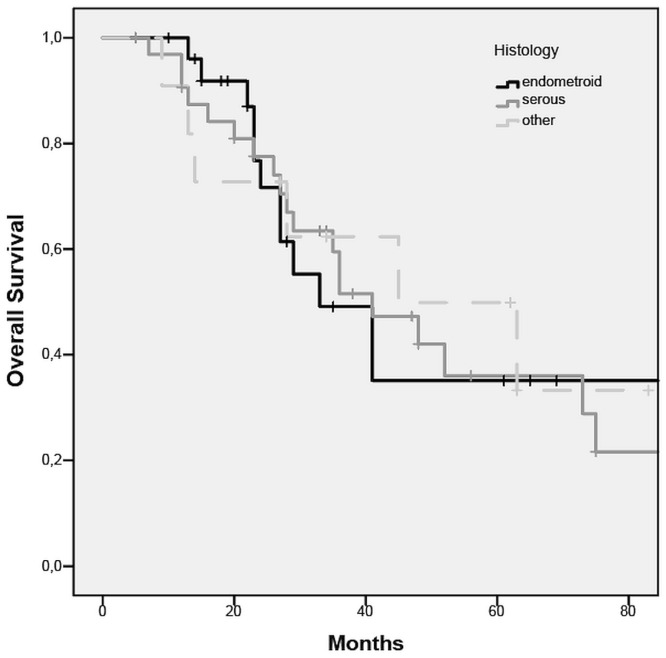
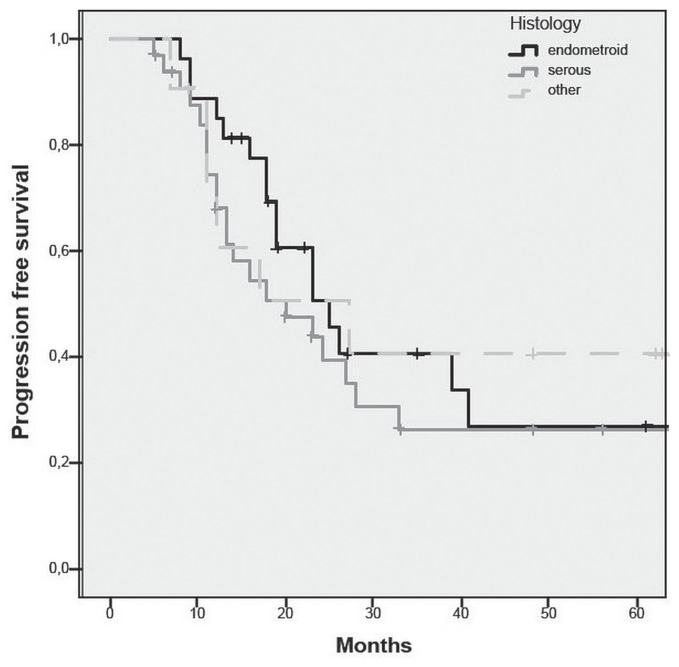
The overall recurrence rate for patients after nact and complete or optimal ids was 60.6% (n = 43). For patients with endometrioid eca, the recurrence rate was 56% (n = 15), with a median time to recurrence of 18 months; for the patients with serous eca, the recurrence rate was 67% (n = 22), with a median time to recurrence of 13 months. The recurrence rate was not significantly different in the endometrioid and serous subgroups (p = 0.6, Figure 2). Median os was 27 months overall; it was 24 months for the endometrioid subgroup and 28 months for the serous subgroup (p = 0.5, Figure 3).
FIGURE 2.
Progression-free survival after complete or optimal debulking for serous and endometrioid endometrial cancer.
FIGURE 3.
Overall survival after complete or optimal debulking for serous and endometrioid endometrial cancer.
DISCUSSION
The present study demonstrates that nact followed by complete or optimal ids is an effective treatment option for patients with advanced eca who are considered not suitable for primary debulking surgery, regardless of histologic type. A survival benefit of 25–28 months might accrue for patients who have complete or optimal debulking compared with those having incomplete debulking or no surgery; however, the latter results have to be interpreted with care, because the sample size is small, and the patients who did not have surgery constitute a heterogeneous group with poor response to nact.
The results of the present study expand the possibilities for the clinical management of advanced eca, a disease notorious for its poor outcome. A recent narrative review of nact for advanced-stage eca similarly concluded that, based on limited current data, nact followed by ids could be considered for patients with poor performance status or patients who the surgeon believes would have suboptimal debulking if surgery were to be attempted11.
The treatment strategy of nact followed by ids is commonly used in patients with advanced ovarian cancer. Both the European Organisation for Research and Treatment of Cancer 55971 trial12 and the recently published chorus trial13 demonstrated that, in patients with advanced-stage ovarian cancer, nact followed by ids, compared with primary debulking surgery, has a similar outcome and fewer complications. Interestingly, the results of the foregoing studies with respect to surgical outcome (complete and optimal debulking) are comparable to our findings for patients with advanced serous eca12. A small retrospective study comparing nact with primary debulking surgery for stage iv serous eca (n = 44) did not find a significant difference in surgical outcome or pfs and os between the two strategies. And yet patients receiving nact experienced significantly reduced surgery durations and shorter hospital stays8.
The results of our analyses, including all histologic subtypes of eca, are in line with previously published studies showing a high rate of response to nact and a high rate of complete ids after nact specifically for advanced serous eca4,8,9,14–16. A retrospective study by Bristow et al.17 about the role of primary cytoreductive surgery in figo stage iv serous eca showed a survival benefit for patients who had complete debulking compared with those who had optimal or incomplete debulking.
The present study also substantiates the importance of debulking surgery in patients with eca, even with the benefit of nact, as discussed in the European Society for Medical Oncology–European Society of Gynaecological Oncology–European Society for Radiotherapy and Oncology Consensus Conference on Endometrial Cancer18.
We observed wide variation with respect to adjuvant treatment, which was often patient-tailored. That observation is in line with our expectations, because no guidelines for this treatment strategy have been established, although a recent publication contains recommendations18. Most patients in the present study received at least 3 cycles of ctx, with rt applied more often in cases of endometrioid eca than in cases of serous eca.
The survival benefit observed with complete and optimal ids after nact probably depends equally on patient factors and tumour biology19,20. The patients who did not have surgery were a heterogeneous group comprising those who had a complete radiologic response and those with progressive disease or high morbidity who might not have been suitable for ids, regardless of the response to nact. And yet the numbers are small, and hence the results should be interpreted with caution. In future, it might be possible to use molecular markers such as stathmin21 or topoisomerase 2-alpha22 to predict the preoperative response to nact. Whether those or other biomarkers could be used in clinical practice should be further explored.
Another interesting development is the introduction of the ctx response score as a prognostic factor for survival in patients with high-stage ovarian cancer treated with nact followed by ids. The ctx response score has low inter-observer variation and high reproducibility, and shows a significant correlation with pfs23,24. Its prognostic value for os when correcting for completeness of cytoreduction varies with the study in which it is used24–26. Given that no central histopathology review of the specimens in our study was conducted, we cannot draw any conclusions about that effect in our patients with eca.
We observed an overall recurrence rate of 61% after nact and complete or optimal debulking. In the recently published data from portec-3, a recurrence rate of 31% was reported for patients with figo stage iii eca receiving adjuvant chemoradiotherapy and 42% for patients receiving adjuvant rt, based on 5-year failure-free survival. Unfortunately, because of the limited number of patients and the fact that that most patients received adjuvant ctx with or without radiation, we could not compare the various adjuvant treatment approaches in our study cohort undergoing ids. As expected, compared with outcomes in portec-327, outcomes in the present study were substantially worse, given the highly selected groups with good performance status in portec-3 and the patients not suitable for a primary surgical approach in our study.
The strength of the present study is that it constitutes the largest series so far of patients with advanced eca of all histology types who were treated with nact followed by ids. We did not limit the study to serous and endometrioid eca because the histologic diagnosis was based on preoperative histology, and as recently demonstrated in a cross-sectional study and a meta-analysis, agreement on tumour grade between the preoperative endometrial sampling and the final diagnosis is only moderate28,29. Moreover, data were collected in 3 well-organized referral centres in different countries.
A major limitation of the study is its retrospective nature. Information about the decision to offer nact followed by ids was therefore individual and not well documented in all cases, making for a heterogeneous group. The decision for the particular treatment strategy might have been influenced by performance status and the presence of comorbidities, which might lead to selection bias. We have no additional information about those data. Another fact to consider when interpreting the data is that the figo stage was not determined by surgery, and hence patients could potentially have been “understaged.” Moreover, only patients who were treated at the referral centres were included; however, that factor is unlikely to have caused a selection bias, because all patients with gynecologic cancer who do not receive standard treatment are discussed at the tumour board of the referral centres. It was not possible to retrieve information about how many women with advanced eca were treated with primary debulking surgery. Finally, no central pathology review of tumour histology before treatment was conducted. It is well known that the differential diagnosis between primary uterine serous and primary ovarian serous cancer can be challenging in small samples of tumour tissue, even with the aid of additional markers30. However, we do not think that uncertainty in the differential would have altered the outcomes in this study, given that a large cohort analysis (n = 12,336) showed no difference in outcome between serous cancers of ovarian and uterine origin31.
As demonstrated in the present multicentre study, the incidence of patients with advanced eca not suitable for primary surgical treatment is low; improving knowledge about treatment strategies for this specific group of patients is therefore difficult. Central international registration of these rare uterine cancers as initiated by the European Network of Individual Treatment in Endometrial Cancer and sharing the outcome of treatment modalities could contribute to improved decision-making for individual patients with advanced age and comorbidities32.
CONCLUSIONS
The present observational study indicates that nact followed by ids is a suitable noninferior treatment strategy for patients with advanced eca who are not considered suitable for primary surgery, regardless of histopathologic subtype. Compared with incomplete or no surgery, complete or optimal debulking after nact could lead to a survival benefit of 25–28 months; however, those data must be interpreted with care, given the retrospective nature of the study. No significant difference was evident in radiologic response rate, percentage of complete ids, recurrence rate, pfs, or os between the endometrioid and serous eca subtypes. The treatment options for this group of patients are limited and have to be explored.
ACKNOWLEDGMENTS
An abstract of this work was presented as an e-poster at the European Society of Gynaecological Oncology (esgo) 2015 European Gynaecological Oncology Congress; Nice, France; 24–27 October 2015; and some of the data were presented by FFA at the 2017 European Gynaecological Oncology Congress; Vienna, Austria; 4–7 November 2017.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none. FFA is a senior researcher for the Research Fund Flanders. The present research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Cancer Research UK. Uterine cancer statistics [Web resource] London, UK: Cancer Research UK; n.d.. [Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer; cited 15 January 2018] [Google Scholar]
- 2.Oliver KE, Enewold LR, Zhu K, et al. Racial disparities in histopathologic characteristics of uterine cancer are present in older, not younger blacks in an equal-access environment. Gynecol Oncol. 2011;123:76–81. doi: 10.1016/j.ygyno.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379:1352–60. doi: 10.1016/S0140-6736(12)60442-5. [DOI] [PubMed] [Google Scholar]
- 4.Vandenput I, Van Calster B, Capoen A, et al. Neoadjuvant chemotherapy followed by interval debulking surgery in patients with serous endometrial cancer with transperitoneal spread (stage iv): a new preferred treatment? Br J Cancer. 2009;101:244–9. doi: 10.1038/sj.bjc.6605157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 6.Cirisano FD, Jr, Robboy SJ, Dodge RK, et al. Epidemiologic and surgicopathologic findings of papillary serous and clear cell endometrial cancers when compared to endometrioid carcinoma. Gynecol Oncol. 1999;74:385–94. doi: 10.1006/gyno.1999.5505. [DOI] [PubMed] [Google Scholar]
- 7.van Persijn van Meerten EL, Gelderblom H, Bloem JL. recist revised: implications for the radiologist. A review article on the modified recist guideline. Eur Radiol. 2010;20:1456–67. doi: 10.1007/s00330-009-1685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson-Ryan I, Frolova AI, Liu J, et al. Neoadjuvant chemotherapy versus primary cytoreductive surgery for stage iv uterine serous carcinoma. Int J Gynecol Cancer. 2015;25:63–8. doi: 10.1097/IGC.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogani G, Ditto A, Leone Roberti Maggiore U, et al. Neoadjuvant chemotherapy followed by interval debulking surgery for unresectable stage ivb serous endometrial cancer. Tumori. 2018 doi: 10.1177/0300891618784785. 300891618784785. [DOI] [PubMed] [Google Scholar]
- 10.Creasman W. Revised figo staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Rabinovich A. Neo-adjuvant chemotherapy for advanced stage endometrial carcinoma: a glimmer of hope in select patients. Arch Gynecol Obstet. 2016;293:47–53. doi: 10.1007/s00404-015-3841-8. [DOI] [PubMed] [Google Scholar]
- 12.Vergote I, Trope CG, Amant F, et al. on behalf of the European Organisation for Research and Treatment of Cancer–Gynaecological Cancer Group and the ncic Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage iiic or iv ovarian cancer. N Engl J Med. 2010;363:943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 13.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (chorus): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–57. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 14.Resnik E, Taxy JB. Neoadjuvant chemotherapy in uterine papillary serous carcinoma. Gynecol Oncol. 1996;62:123–7. doi: 10.1006/gyno.1996.0201. [DOI] [PubMed] [Google Scholar]
- 15.Price FV, Amin RM, Sumkin J. Complete clinical responses to neoadjuvant chemotherapy for uterine serous carcinoma. Gynecol Oncol. 1999;73:140–4. doi: 10.1006/gyno.1998.5303. [DOI] [PubMed] [Google Scholar]
- 16.Le TD, Yamada SD, Rutgers JL, DiSaia PJ. Complete response of a stage iv uterine papillary serous carcinoma to neoadjuvant chemotherapy with Taxol and carboplatin. Gynecol Oncol. 1999;73:461–3. doi: 10.1006/gyno.1999.5361. [DOI] [PubMed] [Google Scholar]
- 17.Bristow RE, Duska LR, Montz FJ. The role of cytoreductive surgery in the management of stage iv uterine papillary serous carcinoma. Gynecol Oncol. 2001;81:92–9. doi: 10.1006/gyno.2000.6110. [DOI] [PubMed] [Google Scholar]
- 18.Colombo N, Creutzberg C, Amant F, et al. on behalf of the esmo-esgo-estro Endometrial Consensus Conference Working Group. esmo-esgo-estro Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26:2–30. doi: 10.1097/IGC.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvesen HB, Haldorsen IS, Trovik J. Markers for individualised therapy in endometrial carcinoma. Lancet Oncol. 2012;13:e353–61. doi: 10.1016/S1470-2045(12)70213-9. [DOI] [PubMed] [Google Scholar]
- 20.Werner HM, Salvesen HB. Current status of molecular biomarkers in endometrial cancer. Curr Oncol Rep. 2014;16:403. doi: 10.1007/s11912-014-0403-3. [DOI] [PubMed] [Google Scholar]
- 21.Werner HM, Trovik J, Halle MK, et al. Stathmin protein level, a potential predictive marker for taxane treatment response in endometrial cancer. PLoS One. 2014;9:e90141. doi: 10.1371/journal.pone.0090141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito F, Furukawa N, Nakai T. Evaluation of top2a as a predictive marker for endometrial cancer with taxane-containing adjuvant chemotherapy. Int J Gynecol Cancer. 2016;26:325–30. doi: 10.1097/IGC.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 23.Bohm S, Faruqi A, Said I, et al. Chemotherapy response score: development and validation of a system to quantify histopathologic response to neoadjuvant chemotherapy in tubo-ovarian high-grade serous carcinoma. J Clin Oncol. 2015;33:2457–63. doi: 10.1200/JCO.2014.60.5212. [DOI] [PubMed] [Google Scholar]
- 24.Singh P, Kaushal V, Rai B, et al. The chemotherapy response score is a useful histological predictor of prognosis in highgrade serous carcinoma. Histopathology. 2018;72:619–25. doi: 10.1111/his.13399. [DOI] [PubMed] [Google Scholar]
- 25.Coghlan E, Meniawy TM, Munro A, et al. Prognostic role of histological tumor regression in patients receiving neoadjuvant chemotherapy for high-grade serous tubo-ovarian carcinoma. Int J Gynecol Cancer. 2017;27:708–13. doi: 10.1097/IGC.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 26.Rajkumar S, Polson A, Nath R, et al. Prognostic implications of histological tumor regression (Bohm’s score) in patients receiving neoadjuvant chemotherapy for high grade serous tubal and ovarian carcinoma. Gynecol Oncol. 2018;151:264–8. doi: 10.1016/j.ygyno.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 27.de Boer SM, Powell ME, Mileshkin L, et al. on behalf of the portec Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (portec-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batista TP, Cavalcanti CL, Tejo AA, Bezerra AL. Accuracy of preoperative endometrial sampling diagnosis for predicting the final pathology grading in uterine endometrioid carcinoma. Eur J Surg Oncol. 2016;42:1367–71. doi: 10.1016/j.ejso.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Visser NCM, Reijnen C, Massuger LFAG, Nagtegaal ID, Bulten J, Pijnenborg JMA. Accuracy of endometrial sampling in endometrial carcinoma: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:803–13. doi: 10.1097/AOG.0000000000002261. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Garcia-Buitrago MT, Koru-Sengul T, Schuman S, Ganjei-Azar P. An immunohistochemical panel to distinguish ovarian from uterine serous papillary carcinomas. Int J Gynecol Pathol. 2013;32:476–81. doi: 10.1097/PGP.0b013e31826ddc4e. [DOI] [PubMed] [Google Scholar]
- 31.Usach I, Blansit K, Chen LM, et al. Survival differences in women with serous tubal, ovarian, peritoneal, and uterine carcinomas. Am J Obstet Gynecol. 2015;212:188.e1–6. doi: 10.1016/j.ajog.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 32.European Society of Gynaecological Oncology (esgo) European Network of Individual Treatment in Endometrial Cancer [Web page] Geneva, Switzerland: esgo; n.d.. [Available at: https://www.esgo.org/network/enitec; cited 15 January 2018] [Google Scholar]