Abstract
Mantle cell lymphoma (mcl) is a rare subtype of aggressive B-cell non-Hodgkin lymphoma that remains incurable with standard therapy. Patients typically require multiple lines of therapy, and those with relapsed or refractory (r/r) disease have a very poor prognosis. The Bruton tyrosine kinase (btk) inhibitor ibrutinib has proven to be an effective agent for patients with r/r mcl. Although usually well tolerated, ibrutinib can be associated with unique toxicities, requiring discontinuation in some patients. Effective and well-tolerated alternatives to ibrutinib for patients with r/r mcl are therefore needed. Novel btk inhibitors such as acalabrutinib, zanubrutinib, and tirabrutinib are designed to improve on the safety and efficacy of first-generation btk inhibitors such as ibrutinib. Data from single-arm clinical trials suggest that, compared with ibrutinib, second-generation btk inhibitors have comparable efficacy and might have a more favourable toxicity profile. Those newer btk inhibitors might therefore provide a viable treatment option for patients with r/r mcl.
Keywords: Bruton tyrosine kinase inhibitors, mantle cell lymphoma, acalabrutinib
INTRODUCTION
Mantle cell lymphoma (mcl), a rare subtype of non-Hodgkin lymphoma (nhl) with an aggressive clinical course and poor prognosis, is considered incurable in almost all affected patients1. In Canada, approximately 500 new cases of mcl are diagnosed each year, comprising 4%–10% of all nhls2–5. Mantle cell lymphoma occurs more frequently in men than in women, and it is usually diagnosed in older adults at a median age of 68 years6,7. Patients with mcl typically present with advanced-stage disease, including lymphadenopathy, bone marrow involvement, splenomegaly, circulating tumour cells, and bowel involvement; they frequently require immediate treatment6–8.
Median overall survival (os) duration from initial diagnosis of mcl has historically been poor, ranging from 29 to 51 months depending on clinical risk, as assessed by the Mantle Cell Lymphoma International Prognostic Index9. However, survival appears to have improved with the advent of newer therapies.
Standard treatment for advanced mcl is based on patient age and comorbidities. Younger, fit patients are treated with induction immunochemotherapy, followed by autologous stem-cell transplantation and rituximab maintenance. Excellent os and progression-free survival (pfs) have been obtained with aggressive regimens that incorporate cytarabine into an anthracycline-containing induction regimen followed by autologous stem-cell transplantation10. Older patients who are not appropriate for stem-cell transplantation are typically treated with chemoimmunotherapy and rituximab maintenance, with bendamustine–rituximab commonly being used as induction8,11.
Although initial treatment strategies achieve high response rates, most patients will eventually relapse and require further therapy, which generally results in shorter periods of remission with each subsequent therapy1. Therapeutic options in the relapsed setting include consideration of alternative chemoimmunotherapy and a growing list of novel therapies that have demonstrated efficacy in patients with relapsed or refractory (r/r) mcl. The latter therapies include the proteasome inhibitor bortezomib, the mechanistic target of rapamycin inhibitor temsirolimus, the immunomodulatory drug lenalidomide, and the Bruton tyrosine kinase (btk) inhibitors ibrutinib and acalabrutinib. The B-cell lymphoma 2 inhibitor venetoclax is also showing promise, but is not yet approved for mcl. Although those therapies have not been compared in randomized studies, data from phase ii trials suggest that btk inhibitors are among the most effective agents in the r/r mcl setting1,12–14.
The btk non-receptor protein kinase is an important mediator in the B-cell receptor signalling pathway, instrumental in the proliferation and survival of mcl cells1. The first-generation btk inhibitor ibrutinib has been associated with response rates of 54%–72% in patients with r/r mcl, with complete response (cr) rates in the 19%–23% range1,12,13. Although generally well tolerated, ibrutinib does have a unique toxicity profile. Some patients inevitably discontinue therapy because of associated toxicities such as cytopenias, infection, pneumonitis, diarrhea, bleeding, and atrial fibrillation15. Additionally, a large proportion of treated patients do not achieve a cr with ibrutinib, and other effective and well-tolerated options are therefore needed.
A number of second-generation btk inhibitors are in various stages of development; they are designed to improve on the safety and efficacy of first-generation btk inhibitors such as ibrutinib. The purpose of the present paper is to provide an overview of btk inhibitors for the treatment of r/r mcl, a discussion of how those agents might be used within the Canadian treatment landscape, and possible future applications in other hematologic malignancies.
CURRENT TREATMENT OPTIONS IN R/R MCL
In the United States, the U.S. Food and Drug Administration (fda) has approved a total of 4 agents for the treatment of r/r mcl, including bortezomib (approved in 2006)16, lenalidomide (approved in 2013), ibrutinib (approved in 2013), and acalabrutinib (approved in 2017)17. In addition, temsirolimus was approved in Europe in 200918. However, in Canada, only 2 agents are approved in this setting: bortezomib19 and ibrutinib20. Furthermore, public funding for ibrutinib in r/r mcl is available in only some provinces in Canada.
Despite recent approval of novel therapies, management of r/r mcl remains challenging. Overall response rates (orrs) range from 22% to 81%, depending on the type of treatment, with median pfs ranging from 3.9 months to 14.6 months12,13,21–29 Median os remains short, in the range of 1–2 years13 (Table I).
TABLE I.
Clinical efficacy of approved treatments in relapsed or refractory mantle cell lymphoma (MCL)
| Study | Patients with MCL | ORR (%) | CR (%) | Median PFS (months) |
|---|---|---|---|---|
| Acalabrutinib | ||||
| Wang et al., 201814 | 124 | 81 | 40 | NR |
|
| ||||
| Ibrutinib | ||||
| Advani et al., 201312 | 9 (of 56 total) | 54 | — | 13.6 |
| Wang et al., 201529 | 111 | 67 | 23 | 13.0 |
| Dreyling et al., 201613 | 280 | 72 | 19 | 14.6 |
|
| ||||
| Bortezomib | ||||
| Fisher et al., 200621 | 155 | 33 | 8 | 9.2 |
| Strauss et al., 200622 | 24 (of 51 total) | 29 | 4 | — |
|
| ||||
| Lenalidomide | ||||
| Wiernik et al., 200824 | 15 (of 49 total) | 53 | 7 | 5.6 |
| Zinzani et al., 201323 | 57 | 35 | 12 | 8.8 |
| Eve et al., 201325 | 26 | 31 | 7 | 3.9 |
| Goy et al., 201326 | 134 | 26 | 8 | 4.0 |
| Trneny et al., 201527 | 254 | 40 | — | 8.7 |
|
| ||||
| Temsirolimus | ||||
| Hess et al., 200928 | 162 | 22 | — | 4.8 |
| Dreyling et al., 201613 | 280 | 40 | 1 | 6.2 |
ORR = overall response rate; CR = complete response; PFS = progression-free survival.
Although no current standard of care in the r/r setting has been established, and no randomized trials comparing therapies have been published, phase i and ii trials suggest that ibrutinib might have the most promising single-agent activity of the agents currently approved in Canada, with orrs ranging from 54% to 72%, and median pfs ranging from 13.6 months to 14.6 months1,12,13. Those outcomes compare favourably with the reported median pfs for bortezomib, lenalidomide, and temsirolimus (9.2 months, 3.9–8.7 months, and 4.8–6.2 months respectively, Table I) More recently, the published phase ii ace-ly-004 study examining the efficacy of a novel btk inhibitor, acalabrutinib, in r/r mcl also demonstrated promising efficacy with an orr of 81%14. In addition, cr rates with acalabrutinib were notable at 40%. Those results appear favourable compared with results reported for ibrutinib, but the relevant studies were not comparable in terms of number and types of prior therapies, with patients in the acalabrutinib trial being less heavily pretreated (median: 2 vs. 3 prior therapies) and with differences in the types of prior therapies given (7% vs. 24% receiving lenalidomide, for example). Results of the ace-ly-004 study led, in October 2017, to the accelerated approval of acalabrutinib by the fda for the treatment of patients with mcl whose cancer has progressed after receiving at least 1 prior therapy14,30. However, acalabrutinib is not yet approved in Canada for patients with r/r mcl.
BTK INHIBITORS IN R/R MCL
The btk non-receptor protein kinase is a member of the tec protein tyrosine kinase family that is expressed in B-cells, myeloid cells, mast cells, and platelets31,32. A key component of the B-cell antigen receptor signalling cascade, btk is involved in all aspects of B-cell development, including proliferation, maturation, differentiation, apoptosis, and cell migration. As such, btk plays a critical role in the progression of B-cell lymphoproliferative disorders and is therefore an attractive target for treating B-cell malignancies.
First-Generation BTK Inhibitors
Ibrutinib
Ibrutinib is a once-daily oral btk inhibitor that binds covalently to a cysteine residue (Cys481) in the active site of the atp-binding domain of btk, inhibiting B-cell receptor signalling and thereby reducing cell growth, proliferation, survival, adhesion, and migration13. In addition to its use in r/r mcl, ibrutinib is also approved by Health Canada for the treatment of other hematologic malignancies, including chronic lymphocytic leukemia (cll)33, Waldenström macroglobulinemia34, marginal zone lymphoma35, and chronic graft-versus-host disease20.
Results of a multicentre single-arm phase ii study (pcyc-1104) led, in November 2013, to the approval of ibrutinib by the fda for the treatment of patients with mcl who have received 1 prior therapy1,29,36. Patients included in the study had a median age of 68 years, and 86% had intermediate-risk or high-risk mcl according to clinical prognostic factors, with a median of 3 prior therapies. At an initial median follow-up of 15.3 months, the orr was 68%, and the cr rate was 21% according to the International Working Group 2007 response criteria1,37. The estimated median pfs was 13.9 months, with a median duration of response (dor) of 17.5 months. At a later median follow-up of 26.7 months, the orr was 67%, and the cr rate was 23%29. The median pfs was 13 months, with a median dor of 17.5 months (Table I, supplementary Appendix 1).
The most common adverse events (aes), experienced by more than 30% of the patients, included diarrhea (54%), fatigue (50%), nausea (33%), and dyspnea (32%)1,29 (Table II, supplementary Appendix 1). Common all-grade hematologic aes were thrombocytopenia (22%), neutropenia (19%), and anemia (18%). The most frequent grade 3 or greater infections included pneumonia (8%), urinary tract infections (4%), and cellulitis (3%). All-grade bleeding events were reported in 50% of the patients. Grade 3 or greater bleeding events in more than 2% of the patients were hematuria (2%) and subdural hematoma (2%). Atrial fibrillation was reported in 11% of the patients, with grade 3 events occurring in 6% of the patients. No grade 4 or 5 events were observed. Discontinuations as a result of treatment toxicity occurred in 11% of the patients.
TABLE II.
Adverse events reported with Bruton tyrosine kinase inhibitorsa
| Variable | Key trial, by inhibitor | |||
|---|---|---|---|---|
|
| ||||
| Ibrutinib Wang et al., 201529 (n=111) | Acalabrutinib Wang et al., 201814 (n=124) | Zanubrutinib Tam et al., 201738 (n=65) | Tirabrutinib Walter et al., 201639 (n=12) | |
| Adverse events of interest (%) | ||||
| Bleeding | Observed All grades: 50 Grade 3 or greater: 6 |
Observed All grades: 31 Grade 3 or greater: 0.8 (1 case of grade 3 or greater GI hemorrhage with history of ulcer) |
Observed All grades: 25 Grade 3 or greater: 3 |
All grades: not given No increased risk |
| Atrial fibrillation | Observed Grade 3 or greater: 4.6 | Not observed | Observed All grades: 3 | Observed, but not drug-related |
|
| ||||
| Common toxicities, all grades (%) | ||||
| Diarrhea | 54 | 31 | 23 | 21 |
| Fatigue | 50 | 27 | 18 | Not given |
| Nausea | 33 | 18 | Not given | 15 |
| Headache | Not given | 38 | Not given | Not given |
|
| ||||
| Hematologic toxicities, grade 3 or greater (%) | ||||
| Neutropenia | 17 | 10 | 9 | 10 |
| Thrombocytopenia | 13 | 5 | 9 | 13 |
| Anemia | 11 | 12 | 11 | 13 |
|
| ||||
| Infections, grade 3 or greater (%) | ||||
| All reported | 28 | 13 | URT: 2 | LRT: 5 |
A number of subsequent studies examining ibrutinib in r/r mcl were conducted, and they supported the results from pcyc-110413,40 (supplementary Appendix 1). Although results from clinical trials showed that ibrutinib was well tolerated by most patients, further experience with ibrutinib, as demonstrated by real-world data, showed higher discontinuation rates than had been reported in clinical trials. A study by Mato et al.15 of patients with cll compared the type and frequency of toxicities seen with ibrutinib in clinical trials and in a real-world setting. Although that study included patients with cll and not mcl, it had interesting findings. At a median follow-up of 14.5 months, the ibrutinib discontinuation rate was estimated to be 42% (median time to ibrutinib discontinuation: 7 months). Moreover, discontinuations that occurred as a result of toxicity appeared higher in the real-world setting (52.5%) than in clinical trials (38.7%). Toxicities related to ibrutinib were the most common reason for discontinuation in all patients. The most common ibrutinib-related toxicities leading to discontinuation included atrial fibrillation (12.3%), infection (10.7%), pneumonitis (9.9%), bleeding (9%), and diarrhea (6.6%).
Two studies have examined ibrutinib combined with other molecules for the treatment of r/r mcl (supplementary Appendix 1). Those studies demonstrated orrs of 71% and 88% when ibrutinib was combined with venetoclax and rituximab respectively41,42. However, as expected, both studies reported rates of all-grade toxicities that were higher than those seen when ibrutinib was used alone.
Second-Generation BTK Inhibitors
Although the first-generation btk inhibitor ibrutinib has proven to be highly effective, it also has a number of off-target effects by inhibiting other tyrosine kinases such as epidermal growth factor receptor, interleukin 2–inducible T-cell kinase, and tec (Table III). Those off-target effects could explain some of the unique toxicities seen with ibrutinib31. Second-generation btk inhibitors were designed to have fewer off-target effects, with the goal of improving efficacy and reducing toxicity. To date, second-generation btk inhibitors examined in patients with r/r mcl include acalabrutinib14, zanubrutinib38, and tirabrutinib39. As reported in clinical trials, those newer btk inhibitors might have lower rates of the toxicities typically associated with ibrutinib such as bleeding, atrial fibrillation, and hypertension. Moreover, rates of common toxicities such as diarrhea, fatigue, and nausea might also be reduced (Table III).
TABLE III.
Molecular targets of the various Bruton tyrosine kinase (BTK) inhibitors
| Variable | Inhibitor | |||
|---|---|---|---|---|
|
| ||||
| Ibrutinib | Acalabrutinib | Zanubrutinib | Tirabrutinib | |
| Target | BTK | BTK | BTK | BTK |
|
| ||||
| Major off-targets | ITK | Minimal | ITK (weak) | TEC (weak) |
| EGFR | ||||
| TEC | ||||
| BMX | ||||
|
| ||||
| Anti-platelet activity | Yes | No | No | No |
ITK = interleukin 2–inducible T cell kinase; EGFR = epidermal growth factor receptor; TEC = Tec protein tyrosine kinase; BMX = cytoplasmic tyrosine–protein kinase BMX.
Acalabrutinib
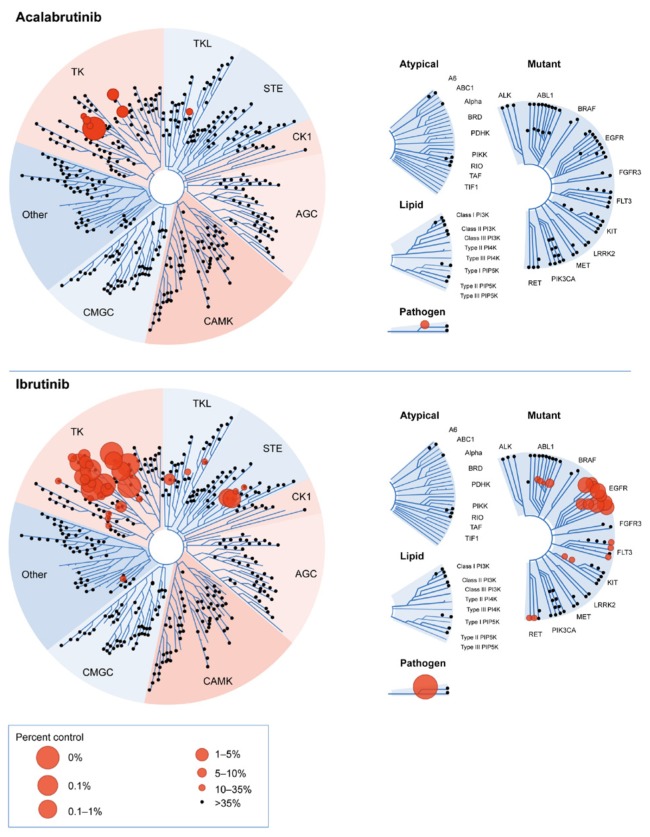
Acalabrutinib (ACP-196) is a novel, potent, orally bioavailable btk inhibitor that was specifically designed to improve on the safety and efficacy of first-generation btk inhibitors such as ibrutinib43. Acalabrutinib also binds Cys481 in the btk active site, inactivating the enzyme and resulting in the inhibition of proliferation and survival signals in malignant B cells. However, acalabrutinib is more highly selective than ibrutinib, resulting in less off-target activity. It is therefore predicted to have fewer adverse effects, with virtually no inhibition of epidermal growth factor receptor, interleukin 2–inducible T-cell kinase, and tec (Table III, Figure 1)31,32,44. Indeed, acalabrutinib does not share the off-target antiplatelet activity of ibrutinib43,45. In addition to its selectivity, the twice-daily dosing of acalabrutinib results in complete and continuous inhibition of btk signalling over time, without increased toxic effects from inhibition of other kinases14,43.
FIGURE 1.
Comparison of acalabrutinib and ibrutinib in competitive binding assays on wild-type and mutant kinases. Adapted with permission from Figure 3 in Barf et al., 201731, which was published by ASPET under the CC BY-NC Attribution 4.0 International license.
The efficacy and safety of acalabrutinib was evaluated in the single-arm phase ii ace-ly-004 study, which included 124 subjects with r/r mcl enrolled between 12 March 2015 and 5 January 201614. Patients included in the study had relapsed after, or been refractory to, 1 or more but not more than 5 prior treatment regimens, with a median of 2 prior therapies. Participants received acalabrutinib 100 mg twice daily, continuously until progression or unacceptable drug-related toxicity. The primary endpoint was investigator-assessed orr, based on the Lugano classification for nhl. Preliminary analysis of the data was performed after a median follow-up of 15.4 months. An orr of 80.6% and a cr rate of 39.5% were reported based on investigator assessment (Table I, supplementary Appendix 1). Median dor and pfs have not been reached; the estimated dor and pfs rates at 12 months were 72.1% and 63.6% respectively.
The most common aes (≥20% of patients; Table II, supplementary Appendix 1) were headache (38%), diarrhea (31%), fatigue (27%), and myalgia (21%). Headache events were mostly grade 1 (64%), and most patients (77%) experienced only 1 event. Headaches generally occurred early in treatment, at a median onset of 5 days, and were manageable, with a low rate of treatment withholding (4% of patients). The most frequently reported grades 3 and 4 aes (≥5% of patients) were neutropenia (11%) and anemia (9%). Serious aes reported in 2 or more patients included pneumonia (4.0%), anemia (3.2%), general physical health deterioration (2.4%), sepsis, tumour lysis syndrome, and vomiting (each 1.6%). No cases of atrial fibrillation occurred; 1 case of grade 3 hemorrhage was reported in a patient with a history of ulcer. Treatment discontinuations because of toxicity occurred in 5.6% of patients, with no treatment discontinuation as a result of headache. Acalabrutinib is not yet approved by Health Canada for the treatment of r/r mcl; however, based on results of the phase ii ace-ly-004 study, it was approved by the fda in October 2017 for the treatment of patients with mcl whose cancer has progressed after at least 1 prior therapy14,30.
Tirabrutinib
Tirabrutinib is a selective and irreversible btk inhibitor that induces apoptosis in the TMD-8 cell line of activated–B-cell diffuse large B-cell lymphoma32,39,46. Tirabrutinib inhibits btk signalling through akt and protein kinase D and can inhibit auto-phosphorylation of btk at the Tyr223 position through the erk, akt, and pkd signalling pathways in vitro46.
A multicentre dose-escalation study examined the efficacy and safety of tirabrutinib in patients with r/r B-cell malignancies39 (Tables I and II, supplementary Appendix 1). In 16 patients with r/r mcl whose median age was 64 years, 11 of 12 evaluable patients experienced an orr of 92% and a cr rate of 46% after a median follow-up of 309 days. The most common aes in patients with nhl (n = 62) were anemia (36%), thrombocytopenia (21%), and diarrhea (21%). In addition, 1 case of grade 3 hemorrhage and 1 case of atrial fibrillation (thought to be unrelated to the study drug) occurred. Tirabrutinib is not yet approved in Canada or in any other country for the treatment of hematologic malignancies.
Zanubrutinib
Zanubrutinib is a second-generation btk inhibitor that is also selective and irreversible38. In preclinical studies, less off-target activity was observed with zanubrutinib than with ibrutinib against a number of kinases, including interleukin 2–inducible T-cell kinase (Table III, supplementary Appendix 1)32. Both in the REC-1 mcl and TMD-8 xenograft models, zanubrutinib has induced dose-dependent antitumour effects that are superior to those seen with ibrutinib.
An open-label dose-escalation study examined the efficacy and safety of zanubrutinib in 99 patients with r/r nhl (supplementary Appendix 1)38. In patients with r/r mcl or diffuse large B-cell lymphoma (n = 65), whose median age was 70 years and who had received 2 prior therapies, the orr in the mcl subgroup was 88%, and the cr rate was 25% after a median follow-up of 9.5 months. The most common aes (Table III, supplementary Appendix 1) included petechiae (25%), diarrhea (23%), and constipation (22%). The most frequently reported grades 3 and 4 aes (≥5% of patients) were anemia (11%), thrombocytopenia (9%), neutropenia (9%), and pneumonia (6%). Severe hemorrhage and atrial fibrillation were each reported in 3% of patients. Zanubrutinib is not yet approved in Canada or in any other country for the treatment of hematologic malignancies.
FUTURE DIRECTIONS
Inhibitors of btk have been examined for the treatment of a number of hematologic malignancies in addition to mcl, including cll, Waldenström macroglobulinemia, diffuse large B-cell lymphoma, and marginal zone lymphoma (supplementary Appendix 2). Based on data from phase ii/iii studies, ibrutinib is now approved by Health Canada for patients with r/r mcl, for untreated patients with cll and 17p deletion, for patients with cll who have received at least 1 prior therapy, for patients with cll who have received at least 1 prior therapy in combination with bendamustine and rituximab, for patients with marginal zone lymphoma who have received at least 1 prior anti-CD20–based therapy, for patients with Waldenström macroglobulinemia, and for patients with steroid-dependent or refractory chronic graft-versus-host disease20.
Ongoing phase iii trials comparing acalabrutinib and other novel btk inhibitors with ibrutinib will aid in further clarifying the benefit of the second-generation btk inhibitors (supplementary Appendix 2). Regimens that combine btk inhibitors with other agents such as novel CD19 and CD20 antibodies, B-cell lymphoma 2 inhibitors, pi3k inhibitors, and alk inhibitors will likely further improve outcomes. However, it will be important to consider the combined toxicity of those agents.
In cll, a randomized trial of ibrutinib monotherapy compared with ibrutinib–rituximab did not demonstrate an improvement in pfs or os with the addition of rituximab47. However, in vitro studies suggest that acalabrutinib does not result in off-target inhibition of T-cell or natural killer cell function48. Whether the addition of an anti-CD20 monoclonal antibody to acalabrutinib results in additional benefit awaits the results of ongoing studies. Finally, it will be of interest to examine whether an alternative btk inhibitor can overcome resistance to an initial btk inhibitor, although such a result would not be expected for agents with the same binding site (for example, ibrutinib and acalabrutinib).
CANADIAN PERSPECTIVE
The availability of ibrutinib for the treatment of r/r mcl and other hematologic malignancies has clearly benefited patients by improving on the outcomes achieved with previously available agents. Despite the importance of ibrutinib for patients with r/r mcl, most patients do not achieve a cr, and some patients experience intolerable toxicities. Off-target effects such as bleeding, gastrointestinal toxicities, and atrial fibrillation are a concern for certain patients and can lead to discontinuation before progression of disease. Those toxicities can be difficult to manage and have emerged as a growing concern among hematologists as experience with ibrutinib in the management of cll increases in clinical practice. The study by Mato et al.15 confirmed that, in a real-world setting, more patients discontinued ibrutinib because of toxicity than discontinued the drug in the clinical trials. Although those findings have not yet been demonstrated in mcl, the move to incorporate btk inhibitors earlier in the treatment of mcl (with longer expected duration of response) suggests that discontinuation because of toxicity could also become a problem for patients with mcl. In addition, the greater dose of btk inhibitors given in mcl compared with cll might also result in greater toxicities for the patients with mcl. Patients who discontinue treatment (and possibly those who receive a reduced dose) are thus unable to obtain the maximal benefit of btk inhibition. In addition, many patients might not be given ibrutinib because of comorbidities, including atrial fibrillation or conditions that increase the risk of bleeding.
Access to additional therapies that are effective and well-tolerated is a key unmet need for patients with r/r mcl, for whom few effective treatment options are available. The development of novel btk inhibitors that aim to improve on the safety profile of ibrutinib by reducing off-target effects might produce therapies that are better tolerated. Early evidence suggests that second-generation btk inhibitors are highly effective and might provide viable treatment options for patients with r/r mcl. With respect to those novel agents, the greatest amount of available data relates to acalabrutinib, which might have a toxicity profile better than that for ibrutinib, although comparative trials are lacking. It is unclear, however, whether the requirement for twice-daily dosing will hinder compliance. Nevertheless, the favourable toxicity profile seen in early trials of second-generation btk inhibitors is encouraging, but must be confirmed with greater follow-up. Results of phase iii trials in cll comparing first-generation with second-generation btk inhibitors will also aid in evaluating the comparative efficacy and safety of those agents. Additionally, data from patients treated with a second-generation btk inhibitor after experiencing ibrutinib-related toxicities should clarify whether such patients could benefit from a more selective btk inhibitor. Moreover, in light of attempts to combine btk inhibitors with additional therapies in mcl patients, the use of btk inhibitors with better toxicity profiles might improve the tolerability and efficacy of such combinations. In the meantime, additional effective and well-tolerated treatment options would allow physicians to consider the safety profile of available agents, patient comorbidities, and patient preference to select what could ultimately be the best treatment for their patients.
CONCLUSIONS
Mantle cell lymphoma is an aggressive disease with especially poor outcomes in the relapsed setting. Despite improved outcomes with ibrutinib, btk inhibition is not curative in mcl, and additional treatments are needed. Real-world experience with ibrutinib demonstrates that the drug can be associated with toxicities that lead to treatment discontinuation or dose reduction for some patients. Second-generation btk inhibitors were designed to improve on the efficacy and toxicity profiles of ibrutinib. Data from phase ii trials indicate that the novel btk inhibitors are highly effective. Moreover, they have demonstrated favourable toxicity profiles and might be associated with fewer troublesome aes (including atrial fibrillation and major bleeding) than are seen with ibrutinib. Of the second-generation btk inhibitors, acalabrutinib is the furthest into development and has been approved by the fda in the relapsed setting. In the future, second-generation btk inhibitors could also form a critical component of combination regimens, whose overall toxicity profile is of key importance. Longer follow-up, real-world experience, and comparative trials should aid in establishing their role. Notwithstanding the differences between ibrutinib and second-generation btk inhibitors, the class effect seems clear in terms of efficacy in r/r mcl, and the introduction of competition in this market should be valuable for reducing the cost of those agents.
Supplementary Information
ACKNOWLEDGMENTS
The authors acknowledge financial and editorial support from AstraZeneca Canada Inc. for the development of this article. Medical writing assistance was provided by Anna Christofides of impact Medicom Inc.
Footnotes
Supplemental material available at http://www.current-oncology.com
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: CO has received honoraria from AstraZeneca, Janssen, AbbVie, Gilead, Lundbeck (Teva), Merck, and Hoffmann–La Roche Canada; NLB has received honoraria from AstraZeneca, Gilead, Hoffman La–Roche Canada, Laboratoires Servier, Seattle Genetics, Merck, Amgen, and Celgene; AC has received funding from AstraZeneca for the development of this article; LHS has received honoraria from Hoffmann–La Roche/Genentech, AbbVie, AstraZeneca, Celgene, TG Therapeutics, Amgen, Janssen, Lundbeck, and Seattle Genetics.
REFERENCES
- 1.Wang ML, Rule S, Martin P, et al. Targeting btk with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen Inc. Imbruvica (Ibrutinib) Approved with Conditions for Relapsed or Refractory Mantle Cell Lymphoma Patients [online news release] Toronto, ON: Cision Canada; 2015. [Available at: http://www.newswire.ca/news-releases/imbruvica-ibrutinib-approved-with-conditions-for-relapsed-or-refractory-mantle-cell-lymphoma-patients-520014821.html; cited 12 July 2017] [Google Scholar]
- 3.Lymphoma Canada. Health Canada approves ibrutinib for mantle cell lymphoma [Web page] Mississauga, ON: Lymphoma Canada; 2015. [Available online at: http://www.lymphoma.ca/research/health-canada-approves-ibrutinib-mantle-cell-lymphoma; cited 12 July 2017] [Google Scholar]
- 4.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by who subtype in the United States, 1992–2001. Blood. 2006;107:265–76. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008;113:791–8. doi: 10.1002/cncr.23608. [DOI] [PubMed] [Google Scholar]
- 6.Canadian Cancer Society. Mantle cell lymphoma [Web page] Toronto, ON: Canadian Cancer Society; n.d.. [Available at: http://www.cancer.ca/en/cancer-information/cancer-type/non-hodgkin-lymphoma/non-hodgkin-lymphoma/mantle-cell-lymphoma/?region=on; cited 12 July 2017] [Google Scholar]
- 7.Shah BD, Martin P, Sotomayor EM. Mantle cell lymphoma: a clinically heterogeneous disease in need of tailored approaches. Cancer Control. 2012;19:227–35. doi: 10.1177/107327481201900307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberta Health Services (ahs) Clinical Practice Guideline: Lymphoma. Edmonton, AB: ahs; 2018. [Available online at: http://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-lyhe002-lymphoma.pdf; cited 10 November 2018] [Google Scholar]
- 9.Hoster E, Dreyling M, Klapper W, et al. on behalf of the German Low Grade Lymphoma Study Group and the European Mantle Cell Lymphoma Network. A new prognostic index (mipi) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–65. doi: 10.1182/blood-2007-06-095331. [Erratum in: Blood 2008;111:5761] [DOI] [PubMed] [Google Scholar]
- 10.Hermine O, Hoster E, Walewski J, et al. on behalf of the European Mantle Cell Lymphoma Network. Addition of highdose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (mcl younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388:565–75. doi: 10.1016/S0140-6736(16)00739-X. [DOI] [PubMed] [Google Scholar]
- 11.BC Cancer. Lymphoma [Web resource] Vancouver, BC: BC Cancer; 2013. [Available at: http://www.bccancer.bc.ca/health-professionals/clinical-resources/cancer-management-guidelines/lymphoma-chronic-leukemia-myeloma/lymphoma; cited 10 November 2018] [Google Scholar]
- 12.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387:770–8. doi: 10.1016/S0140-6736(15)00667-4. [Erratum in: Lancet 2016;387:750] [DOI] [PubMed] [Google Scholar]
- 14.Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ace-ly-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659–67. doi: 10.1016/S0140-6736(17)33108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mato AR, Lamanna N, Ujjani CS, et al. Toxicities and outcomes of ibrutinib-treated patients in the United States: large retrospective analysis of 621 real world patients. Blood. 2016;128:3222. [Google Scholar]
- 16.Takada. FDA approves Velcade (bortezomib) for injection for previously untreated patients with mantle cell lymphoma [online news release] Osaka, Japan: Takeda; 2014. [Available at: https://www.takeda.com/newsroom/newsreleases/2014/fda-approves-velcade-bortezomib-for-injection-forpreviously-untreated-patients-with-mantle-cell-lymphoma; cited 10 November 2018] [Google Scholar]
- 17.Lymphoma Research Foundation. Mantle Cell Lymphoma: FDA Updates [Web resource] New York, NY: Lymphoma Research Foundation; n.d.. [Available at: https://www.lymphoma.org/aboutlymphoma/nhl/mcl/mclfdaupdates/; cited 27 June 2018] [Google Scholar]
- 18.European Medicines Agency (ema) Public Summary of Opinion on Orphan Designation: Temsirolimus for the Treatment of Mantle Cell Lymphoma. London, U.K.: ema; 2011. [Available online at: Public summary of opinion on orphan designation: Temsirolimus for the treatment of mantle cell lymphoma; cited 3 March 2019] [Google Scholar]
- 19.Actavis Inc. Act Bortezomib [product monograph] Parsippany–Troy Hills, NJ: Actavis; 2016. [Google Scholar]
- 20.Janssen Inc. Imbruvica: Ibrutinib Capsules [product monograph] Toronto, ON: Janssen; 2018. [Google Scholar]
- 21.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase ii study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–74. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 22.Strauss SJ, Maharaj L, Hoare S, et al. Bortezomib therapy in patients with relapsed or refractory lymphoma: potential correlation of in vitro sensitivity and tumor necrosis factor alpha response with clinical activity. J Clin Oncol. 2006;24:2105–12. doi: 10.1200/JCO.2005.04.6789. [DOI] [PubMed] [Google Scholar]
- 23.Zinzani PL, Vose JM, Czuczman MS, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the nhl-003 study. Ann Oncol. 2013;24:2892–7. doi: 10.1093/annonc/mdt366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:4952–7. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]
- 25.Eve HE, Carey S, Richardson SJ, et al. Single-agent lenalidomide in relapsed/refractory mantle cell lymphoma: results from a U.K. phase ii study suggest activity and possible gender differences. Br J Haematol. 2012;159:154–63. doi: 10.1111/bjh.12008. [DOI] [PubMed] [Google Scholar]
- 26.Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase ii mcl-001 (emerge) study. J Clin Oncol. 2013;31:3688–95. doi: 10.1200/JCO.2013.49.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trneny M, Lamy T, Walewski J, et al. on behalf of the sprint trial investigators and in collaboration with the European Mantle Cell Lymphoma Network. Lenalidomide versus investigator’s choice in relapsed or refractory mantle cell lymphoma (mcl-002; sprint): a phase 2, randomised, multicentre trial. Lancet Oncol. 2016;17:319–31. doi: 10.1016/S1470-2045(15)00559-8. [DOI] [PubMed] [Google Scholar]
- 28.Hess G, Herbrecht R, Romaguera J, et al. Phase iii study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–9. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 29.Wang ML, Blum KA, Martin P, et al. Long-term follow-up of mcl patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126:739–45. doi: 10.1182/blood-2015-03-635326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United States, Department of Health and Human Services, National Institutes of Health, National Cancer Institute (nci) Acalabrutinib Receives FDA Approval for Mantle Cell Lymphoma [blog post] Bethesda, MD: nci; 2017. [Available online at: https://www.cancer.gov/news-events/cancercurrents-blog/2017/acalabrutinib-fda-mantle-cell-lymphoma; cited 9 May 2018] [Google Scholar]
- 31.Barf T, Covey T, Izumi R, et al. Acalabrutinib (ACP-196): a covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363:240–52. doi: 10.1124/jpet.117.242909. [DOI] [PubMed] [Google Scholar]
- 32.Wu J, Liu C, Tsui ST, Liu D. Second-generation inhibitors of Bruton tyrosine kinase. J Hematol Oncol. 2016;9:80. doi: 10.1186/s13045-016-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrd JC, Brown JR, O’Brien S, et al. on behalf of the resonate investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372:1430–40. doi: 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]
- 35.European Society for Medical Oncology (esmo) Ibrutinib, First FDA-approved therapy for marginal zone lymphoma [online news release] Lugano, Switzerland: esmo; 2017. [Available at: http://www.esmo.org/Oncology-News/Ibrutinib-First-FDA-Approved-Therapy-for-Marginal-Zone-Lymphoma; cited 27 June 2018] [Google Scholar]
- 36.Inman S. FDA approves ibrutinib for mantle cell lymphoma [Web news article] Cranbury, NJ: Intellisphere, LLC; 2013. [Available at: http://www.onclive.com/web-exclusives/fda-approves-breakthrough-ibrutinib-for-mcl; cited 10 November 2018] [Google Scholar]
- 37.Cheson BD. The International Harmonization Project for response criteria in lymphoma clinical trials. Hematol Oncol Clin North Am. 2007;21:841–54. doi: 10.1016/j.hoc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Tam C, Grigg AP, Opat S, et al. The btk inhibitor, Bgb-3111, is safe, tolerable, and highly active in patients with relapsed/refractory B-cell malignancies: initial report of a phase 1 first-in-human trial. Blood. 2015;126:832. [Google Scholar]
- 39.Walter HS, Rule SA, Dyer MJ, et al. A phase 1 clinical trial of the selective btk inhibitor ono/gs-4059 in relapsed and refractory mature B-cell malignancies. Blood. 2016;127:411–19. doi: 10.1182/blood-2015-08-664086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M, Goy A, Martin P, et al. Efficacy and safety of single-agent ibrutinib in patients with mantle cell lymphoma who progressed after bortezomib therapy. Blood. 2014;124:4471. [Google Scholar]
- 41.Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol. 2016;17:48–56. doi: 10.1016/S1470-2045(15)00438-6. [DOI] [PubMed] [Google Scholar]
- 42.Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211–23. doi: 10.1056/NEJMoa1715519. [DOI] [PubMed] [Google Scholar]
- 43.Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323–32. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel V, Balakrishnan K, Bibikova E, et al. Comparison of acalabrutinib, a selective Bruton tyrosine kinase inhibitor, with ibrutinib in chronic lymphocytic leukemia cells. Clin Cancer Res. 2017;23:3734–43. doi: 10.1158/1078-0432.CCR-16-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bye AP, Unsworth AJ, Desborough MJ, et al. Severe platelet dysfunction in nhl patients receiving ibrutinib is absent in patients receiving acalabrutinib. Blood Adv. 2017;1:2610–23. doi: 10.1182/bloodadvances.2017011999. [Erratum in: Blood Adv 2018;2:3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J, Zhang M, Liu D. Bruton tyrosine kinase inhibitor ono/gs-4059: from bench to bedside. Oncotarget. 2017;8:7201–7. doi: 10.18632/oncotarget.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burger JA, Sivina M, Ferrajoli A, et al. Randomized trial of ibrutinib versus ibrutinib plus rituximab (Ib+R) in patients with chronic lymphocytic leukemia (cll) Blood. 2017;130(suppl 1):427. doi: 10.1182/blood-2018-10-879429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fowler NH, Coleman M, Stevens DA, et al. Acalabrutinib alone or in combination with rituximab (r) in follicular lymphoma (fl) [abstract 7549] J Clin Oncol. 2018;36 [Available online at: http://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.7549; cited 18 January 2019] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.