Abstract
Objectives
In cancer patients, chemotherapy-induced peripheral neuropathy (cipn) is a common complication, characterized by pain, loss of sensation, and numbness. Medical treatment for peripheral neuropathies has been shown to be ineffective for cipn. Acupuncture has been shown to be safe and effective in treating cancer-related symptoms and other peripheral neuropathies. For the present review, we aimed to evaluate the efficacy of acupuncture for the treatment of cipn.
Design
Comprehensive searches for relevant studies were conducted in Ovid embase, the Web of Science, Ovid medline, the Cochrane Central Register of Controlled Trials (central), cinahl (ebsco Information Services, Ipswich, MA, U.S.A.), and the ClinicalTrials.gov Web site. References from previous systematic reviews were also searched. Additional trials were found in the reference lists of relevant papers and in searches of Google Scholar and acupuncture-specific Web sites. Included studies were randomized controlled trials (rcts) of any type of acupuncture used to treat patients with cipn.
Results
Three clinical trials (203 participants) were included. Two studies found acupuncture to be effective in alleviating cipn pain and improving quality of life. One study found no benefit in improving neuropathic pain, symptoms, or quality of life. Study quality was variable and included a moderate overall risk of bias.
Conclusions
The evidence is insufficient to recommend acupuncture for the treatment or prevention of cipn. Further research is needed to evaluate the effects of acupuncture in the treatment of cipn. Given that acupuncture is considered safe and might provide relief for patients, it can be considered at the clinician’s discretion.
Keywords: Acupuncture, systematic reviews, chemotherapy-induced peripheral neuropathy, cipn, integrative oncology
INTRODUCTION
Most anticancer treatments have significant side effects. It is well understood that chemotherapy drugs are neurotoxic and can damage peripheral nerves, ultimately resulting in chemotherapy-induced peripheral neuropathy (cipn)1. Patients with cipn can experience any or all of neuropathic pain (allodynia), loss of sensation, and loss of motor function in a stocking–glove distribution.
Unfortunately, cipn is common, with 68.1% of patients developing it within the first month of chemotherapy2. Furthermore, cipn can persist for years and significantly affects quality of life for cancer patients. A recent study of 362 patients showed a highly significant (p < 0.0005) inverse correlation between cipn symptoms and quality of life3. Associations of cipn with increased depressive symptoms and reduced sleep quality have been observed4,5. More importantly, however, cipn might limit the effectiveness of anticancer treatments because a common management strategy is to reduce the dose of chemotherapy6.
Although effective symptomatic treatments for other peripheral neuropathies not induced by chemotherapy are available (for example, gabapentin, tricyclic antidepressants), the evidence for their use in cipn is inconclusive7. Duloxetine is the only symptomatic treatment for cipn backed by a randomized controlled trial (rct), and even then, the therapeutic effect is modest8. Treatments for cipn prevention backed by rcts are limited8,9. Some inconclusive data suggest that lafutidine might prevent the development of severe cipn (but without decreasing its occurrence)10. Additionally, a recent rct demonstrated that a 6-week exercise program is effective in preventing cipn11. Given the morbidity of cipn and limited conventional means for management, it is imperative to look for better alternatives.
Acupuncture therapy, associated with Traditional Chinese Medicine, has a long history in treating pain. Acupuncture is well-accepted and safe, and adverse effects are quite rare12. Moreover, acupuncture is currently practiced in oncology settings13 and has been shown to be effective in treating other cancer-related symptoms such as pain14,15; nausea and vomiting16; xerostomia induced by radiation therapy17,18; fatigue19; and anxiety, depression, and insomnia20. Acupuncture has also demonstrated benefits in treating other peripheral neuropathies such as diabetic neuropathy, Bell palsy, and carpal tunnel21.
Franconi et al.22 conducted the most recent systematic review of the use of acupuncture in cipn. They found seven records, of which three were rcts, and only two assessed patients with cipn. Both studies found acupuncture to be effective for cipn, but because the full texts of the studies were in Chinese, it is difficult to evaluate the quality of the data. Given that additional clinical trials have been conducted since that review, we felt that it was important to reassess the literature. Here, we evaluate the efficacy of acupuncture compared with controls (placebo, sham acupuncture) in treating and preventing cipn. As a secondary objective, we discuss the current understanding of acupuncture mechanisms to provide some insight into how this treatment might play a role in managing cipn. A key difference between our study and the previous systematic review is that we include only high-quality rcts. The previous review also failed to critically appraise study quality with respect to risk of bias and methodology, which we have done here, using validated tools.
METHODS
Database Search
In May 2017, we performed searches in the following databases: Ovid embase, Web of Science, Ovid medline, the Cochrane Central Register of Controlled Trials (central), cinahl (ebsco Information Services, Ipswich, MA, U.S.A.), the ClinicalTrials.gov Web site, and the AcuTrials database (Oregon College of Oriental Medicine, Portland, OR, U.S.A.). To ensure high search sensitivity, grey literature searches were performed in high-yield databases and acupuncture Web sites identified by Cogo et al.23 (Acudoc2, Index to Chiropractic Literature, PsycInfo). The systematic searches were designed with input from a medical librarian (DG) and adjusted for each database to account for indexing and keyword search functionality (supplemental Appendix 1). Additional studies from previous systematic reviews21,22,24 and Google Scholar searches were also identified.
Study Selection and Data Extraction
Using Mendeley (Elsevier, Amsterdam, Netherlands), records retrieved from our searches were aggregated, and duplicates were removed (Figure 1). Abstracts were screened for relevance, and the rationale behind screening choices were recorded in Rayyan25. Articles were considered relevant when they met the eligibility criteria for the intervention (acupuncture), population (patients with cipn), control (standard treatment, placebo, sham acupuncture, or no acupuncture), and outcomes (cipn symptoms, cipn prevention). Within the pool of relevant records, the full text was evaluated for eligibility based on an rct study design (non-rcts were excluded). The study selection process was completed by one researcher (KL), and the results were reviewed by the research supervisor (DS). Data extraction was completed using a data extraction tool adapted from the data collection form developed by the Cochrane Effective Practice and Organization of Care Group26, and the articles were critically appraised using the Cochrane Risk of Bias tool27 and the stricta checklist28. Data extraction and risk-of-bias assessments were completed by one researcher (KL).
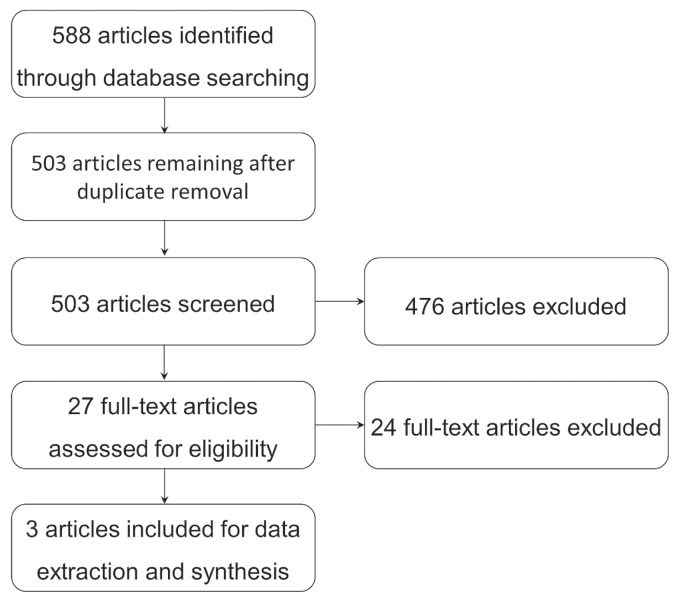
FIGURE 1.
PRISMA flow chart for the study selection process.
Inclusion Criteria
Included studies were human rcts, in which acupuncture was an independently applied intervention and cipn was one of the a priori primary or secondary outcomes evaluated. Participants in those studies must have had chemotherapy treatment and symptoms of cipn. Included studies could use any form of acupuncture treatment, including acupuncture, electro-acupuncture, or acupressure as an adjunctive or main intervention. The intervention must have been compared with an appropriate control (standard treatment, placebo, sham acupuncture, or no acupuncture). The studies must have assessed one or more of the following outcomes (adapted from Franconi et al.22):
■ Pain, as defined by any grading scale
■ Numbness, tingling, cold sensitivity, or any other signs of peripheral neuropathy
■ Subjective patient reports
■ Surrogate markers that might explain the mechanisms by which acupuncture treats cipn
■ Activities of daily living
■ Quality of life measures
■ Safety
■ Changes in chemotherapy dosing
Exclusion Criteria
Studies other than rcts (preclinical studies, observational studies, qualitative studies, case reports, and nonrandomized or non-controlled studies) were excluded. Studies assessing patients who were experiencing types of neuropathy other than cipn (diabetic neuropathy, hiv), and non-English-language studies were also excluded.
Review Protocol
Before the data from the search were extracted, the protocol for this review was registered at the prospero international prospective register of systematic reviews (registration number: CRD4201706). It can be accessed at http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017067745.
RESULTS
Search Results
The searches identified a total of 588 records (Figure 1). After duplicates were removed, the remaining 503 records were screened for relevance. During the screening process, 476 records were excluded because they were not relevant to cipn or did not use a variation of acupuncture as an intervention. The remaining twenty-seven records were assessed for eligibility based on their full text. After full-text screening, twenty-four records were excluded based on inadequate study design. Within the twenty-four excluded studies, two assessed an inappropriate outcome (prevention of cipn), one was a conference abstract of an included journal article, five did not assess cipn, one was written in Chinese, one used an inadequate placebo, seven were non-rcts, three were protocols, and four used acupuncture-like therapies not outlined in our inclusion criteria (supplemental Appendix 2). Three records were included for qualitative synthesis.
Study Characteristics
The three included studies assessed acupuncture’s efficacy in treating cipn pain and cipn-associated quality of life (Table I). The sample sizes in the studies varied from 40 to 104, with the total sample size being 203. The studies assessed patients with breast cancer and multiple myeloma experiencing cipn caused by chemotherapeutic agents including taxanes, platinum derivatives, or vinca alkaloids. Each trial used acupuncture or electro-acupuncture as the intervention, with a primary outcome associated with cipn. The controls used in the studies varied and included oral and injection methylcobalamin, delayed low-dose acupuncture treatment, hydroelectric baths, vitamin B complex capsules, and placebo (lactose capsules). The duration of the intervention varied in the range of 3–12 weeks, and the frequency of treatment varied from weekly to 3 times per week. In the studies overall, 1 adverse event (swelling, discomfort, and bruising) was reported.
TABLE I.
Summary of methods and results of included studies
| Reference | Sample size | Sex | Cancer type and stage | Chemotherapy | Acupuncture | Frequency and duration | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Intervention | Control | Intervention | Control | |||||
| Rostock et al., 201329 | 59 | Men and women | Not specified | Taxanes, platinum derivatives, or vinca alkaloids | Electroacupuncture | Three groups:
|
2–3 Times weekly for 3 weeks (total of 8 sessions) | |
| Outcomes assessed: | ||||||||
| Results: Acupuncture no more effective than control treatments: no significant difference in outcome improvement (primary or secondary) between acupuncture group and control groups. | ||||||||
|
| ||||||||
| Han et al., 201730 | 104 | Men and women | Multiple myeloma, all stages | All chemotherapy treatments | Acupuncture and methylcobalamin | Methylcobalamin | Approximately 3 times weekly for 12 weeks | Every other day or daily for 12 weeks |
| Outcomes assessed: Degree of CIPN using VAS pain scores, score on the FACT/GOG-Ntxc , and nerve conduction velocities | ||||||||
| Results: Compared with methylcobalamin, acupuncture was effective for CIPN: significant decrease in VAS pain scores (p<0.01), and FACT/GOG-Ntx scores (p<0.05); no significant difference in nerve conduction velocities between acupuncture and controls (p>0.05) | ||||||||
|
| ||||||||
| Lu et al., 201731 | 40 | Women | Breast cancer, stages I–III | Not specified | Acupuncture | Low-dose acupuncture, delayed 8 weeks after intervention | 2–3 Times weekly for 8 weeks (total of 18 sessions) | 1–2 Times weekly for 8 weeks (total of 9 sessions) |
| Outcomes assessed: Degree of CIPN using the PNQ, FACT-Ntxc, QLQ-CIPN20b | ||||||||
| Results: Acupuncture better than low-dose acupuncture for CIPN: significant improvement compared with control on the PNQ (p=0.02), the FACT-Ntx (p=0.002), and the QLQ-CIPN20 (p=0.006) | ||||||||
U.S. National Cancer Institute, Bethesda, MD.
European Organisation for Research and Treatment of Cancer, Brussels, Belgium.
FACIT.org, Elmhurst, IL, U.S.A.
CIPN = chemotherapy-induced peripheral neuropathy; QLQ-C30 = 30-question core Quality of Life Questionnaire; VAS = visual analogue scale; FACT/GOG-Ntx = Functional Assessment of Cancer Therapy/Gynecologic Oncology Group 12-item neurotoxicity scale; PNQ = Patient Neurotoxicity Questionnaire; FACT-Ntx = Functional Assessment of Cancer Therapy–Neurotoxicity; QLQ-CIPN20 = Quality of Life Questionnaire, 20-item chemotherapy-induced peripheral neuropathy scale.
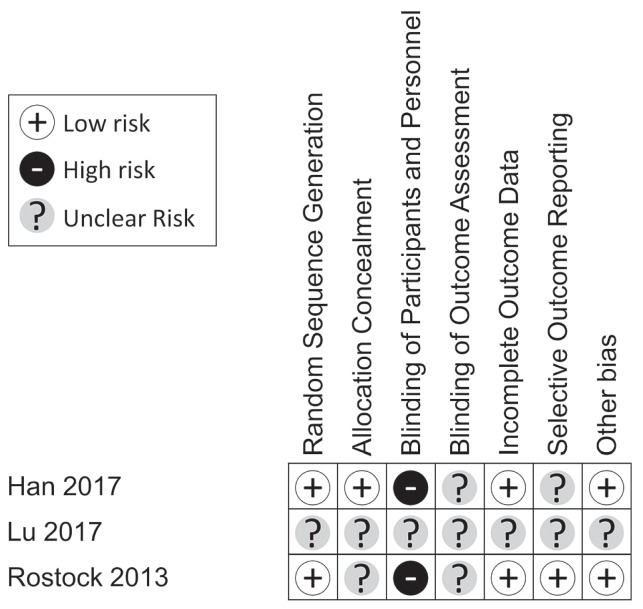
Risk of Bias
The included studies generally had a low or unclear risk of bias (Figure 2). Random sequence allocation was adequately used in two of the studies29,30, but was unclear in the study by Lu and colleagues31. Only one trial adequately concealed the participant’s assignment from the investigators30; it was unclear whether such concealment was done in the other two studies. Participants or personnel, or both, were not blinded in two studies29,30, but blinding status was not made clear in the study by Lu and colleagues31. Blinding of investigators involved in outcome assessment was not clear in any study29–31. Incomplete outcomes data were sufficiently explained and were deemed to carry a low risk of bias for all the studies except for that by Lu and colleagues. Outcomes were also not selectively reported in one study29, but selectivity of reporting was unclear in the other two30,31. Lastly, all studies (aside from the study by Lu and colleagues31) were considered to be free of other biases.
FIGURE 2.
Risk-of-bias assessment for the included studies. Studies were evaluated using the Cochrane Risk of Bias tool and were classified under each category as low, high, or unclear risk of bias.
It should be noted that, because the study by Lu and colleagues31 was a conference abstract, many of the details required for risk of bias assessment were not available. Given that our sample size was inherently limited, we felt it necessary to include that study despite the unclear risk of bias. The author was contacted to clarify relevant details, but no response was received. The study was therefore deemed to have an unclear risk of bias across all domains.
STRICTA Checklist
Table II summarizes the appraisal of study quality based on the stricta checklist. None of the studies satisfied all of the stricta criteria, but two studies met all but 4 of the checklist items29,30. Excluding the abstract by Lu and colleagues31, the style of acupuncture and the extent of treatment variance was described by all studies. No study adequately provided a rationale for the treatment provided. Two studies29,30 reported most of the details of their acupuncture technique, but one study did not report any details31. No studies reported the number of needle insertions per session. The treatment regimen (number of sessions, frequency and duration of sessions) was reported clearly in all studies. Details of other interventions administered to the acupuncture group were explained in two studies29,30, but were not mentioned in one study31. The setting and context of treatment were not adequately reported in all studies. Details and qualifications of the acupuncturists and the rationale for controls were described in two studies29,30, but not in the study by Lu and colleagues31. All studies provided a precise description of the control group29–31.
TABLE II.
STRICTA checklist assessment of reporting quality
| Item | STRICTA criterion | Reference | ||
|---|---|---|---|---|
|
|
||||
| Rostock et al., 201329 | Han et al., 201730 | Lu et al., 201731 | ||
| 1. Acupuncture rationale | a) Style of acupuncture (for example, Traditional Chinese Medicine, Japanese, Korean, Western medical, etc.) | Yes | Yes | No |
| b) Reasoning for treatment provided, literature source or consensus methods (or both), with references where appropriate | No | No | No | |
| c) Extent to which treatment was varied | Yes | Yes | No | |
|
| ||||
| 2. Details of needling | a) Number of needle insertions per subject per session (mean and range where relevant) | No | No | No |
| b) Names (or location if no standard name) of points used (unilateral or bilateral) | Yes | Yes | No | |
| c) Depth of insertion, based on a specified unit of measurement | No | Yes | No | |
| d) Response sought (for example, de qi sensation or muscle twitch response) | Yes | Yes | No | |
| e) Needle stimulation (for example, manual, electrical) | Yes | No | No | |
| f) Needle retention time | Yes | Yes | No | |
|
| ||||
| 3. Treatment regimen | a) Number of treatment sessions | Yes | Yes | Yes |
| b) Frequency and duration of treatment sessions | Yes | Yes | Yes | |
|
| ||||
| 4. Other components of treatment | a) Details of other interventions administered to the acupuncture group (for example, moxibustion, cupping, herbs, exercises) | Yes | Yes | No |
| b) Setting and context of treatment, including instructions to practitioners, and information and explanations to patients | No | No | No | |
|
| ||||
| 5. Practitioner background | Description of participating acupuncturists (qualification or professional affiliation, other relevant experience) | Yes | Yes | No |
|
| ||||
| 6. Control or comparator interventions | a) Rationale for the control or comparator in the context of the research question, with sources that justify the choice | Yes | Yes | No |
| b) Precise description of the control or comparator. If sham acupuncture or any other type of acupuncture-like control is used, complete items 1 to 3 for the comparator as well as for the intervention. | Yes | Yes | Yes | |
STRICTA = Standards for Reporting Interventions in Controlled Trials of Acupuncture.
Results of Individual Studies
Two studies showed that acupuncture was effective for cipn pain and cipn-associated quality of life30,31, and one study showed that it was ineffective for cipn pain, neuropathy score, and quality of life29.
DISCUSSION
Summary of Results
Of the three studies evaluated, two showed positive effects of acupuncture30,31. Han et al. and Lu et al. both showed that acupuncture was more effective than its control for pain management and improved functional quality of life. The study by Rostock et al.29 failed to show acupuncture’s efficacy in improving neuropathic pain, symptoms, and quality of life. Given that the studies are inconsistent, it is inconclusive whether acupuncture is efficacious for cipn. Within the three studies, only 1 mild adverse event was reported. That observation is consistent with previous studies that have shown acupuncture to be a safe therapy, with few and minor associated adverse effects12.
Of special note is a study by Greenlee et al.32 that assessed acupuncture in preventing cipn and that failed to show efficacy for acupuncture compared with placebo (sham acupuncture). We include that study in our discussion because it pertains to a similar topic and might be useful for discerning patterns.
Meta-analysis
Because of the heterogeneity of the study methods, a meta-analysis was not conducted. Studies varied in acupuncture protocol, acupuncture type, participant population, control therapy, and outcome assessment.
Concerns About the Studies
A few important points should be mentioned for the studies showing lack of benefit. The study by Rostock et al.29 was terminated prematurely because, at the interim analysis stage, electro-acupuncture failed to show efficacy compared with placebo (determined using a predefined statistical threshold). The absolute improvement in pain was also less in the electro-acupuncture group than in the control group, but the difference was not statistically significant. Moreover, the study by Greenlee et al.32 showed that acupuncture might delay recovery from cipn symptoms after chemotherapy is discontinued. Although inconclusive, those results are of concern from a caregiver’s perspective.
Patterns Within Studies
The trials using acupuncture showed a positive effect30,31, whereas the trials testing electro-acupuncture showed a lack of benefit29,32. It is possible that only standard acupuncture, rather than electro-acupuncture, is effective for cipn. Furthermore, the only study involving multiple myeloma patients30 showed benefit for acupuncture, but in the two studies involving breast cancer patients31,32, results were mixed. One study showed benefit31; the other showed lack of benefit32. Perhaps the effectiveness of acupuncture varies with the cancer population.
Because the mechanism of cipn differs depending on the chemotherapeutic agents administered1, it might be hypothesized that efficacy could vary depending on the chemotherapeutic agent used. However, the results of our study do not support that hypothesis. Of the two trials31,32 assessing patients with taxane-induced cipn, only one31 showed benefit. Another trial29 assessed cipn induced by several chemotherapeutic agents, and the study by Han30 did not specify the agent used. Thus, the literature does not suggest that the chemotherapeutic agent or agents affect the efficacy of acupuncture.
Mechanisms of Acupuncture in CIPN
It is generally accepted that chemotherapeutic agents cause cipn through oxidative stress within neurons, axonal degeneration, neuro-inflammation, and unbalanced calcium homeostasis1. Neuro-inflammation might be a mechanistic target of acupuncture. Chemotherapeutic agents cause neuro-inflammation by activating immune cells to release inflammatory cytokines1. Because acupuncture has been shown to have an anti-inflammatory effect by modulating cytokines and increasing calcitonin gene–related peptide33, it is plausible that acupuncture might improve cipn symptoms by reducing neuro-inflammation.
Other mechanisms of acupuncture have also been hypothesized. Acupuncture might reduce pain by inhibition of cyclooxogenase-234, release of endogenous opioids35,36, and modulation of nociception35,37. Acupuncture for cipn might act through a combination of those mechanisms. Given that few studies have assessed the mechanism of acupuncture in cipn, the literature does not provide clear guidance in this area.
Study Strengths and Limitations
A strength of our review is that we assessed only rcts known to have the lowest risk of bias. Those trials all compared acupuncture with a control, used adequate random sequence generation, and accounted for incomplete outcomes data. Also, one of the studies had previously published protocols that matched their report. The risk of reporting bias in that study was low30. To minimize incomplete retrieval of studies, we established our search protocol with the help of a medical librarian, and we conducted exhaustive searches in seven databases and Web sites.
The limitations of our study are that the total sample size was small (n = 203) and that the risk of bias in most categories was considered to be unclear. The small sample size was a result of too few rcts on this topic being available. There were, however, many non-controlled and nonrandomized trials that were not reviewed. In addition, our study might also have inherent reporting or selection biases, given that the entire review was conducted by one individual (KL). That bias was minimized by having the study results reviewed by the project supervisor (DS).
Challenges of Acupuncture Studies
It is fundamentally difficult to objectively assess acupuncture, because there are many variations of acupuncture, and treatment methods are practitioner-dependent. That difficulty is evident from our review: the included studies show great variation with respect to acupuncture protocol and placebo therapy.
Placebo therapy varies because there is no generally agreed-upon placebo control. Placebos vary from acupressure, to sham acupuncture, to methylcobalamin injections, to placebo pills. Even within sham acupuncture, many variations are seen (low-dose therapy, needling non-acupoint areas, or using non-penetrating Streitberger needles, for instance). Some of those placebos also prevent adequate participant blinding (that is, patients can clearly identify whether they are receiving a pill or acupuncture). Moreover, unless a machine is used to perform the acupuncture protocol, it is extremely difficult to blind the personnel administering acupuncture.
Dimitrova et al.21 recently published a standardized protocol that could be used as a guideline for future studies in peripheral neuropathy. Further studies establishing “gold standard” acupuncture protocols, placebos, and outcomes for peripheral neuropathy will allow for a more reliable, objective assessment of acupuncture. We recognize that achieving a good placebo control for acupuncture is challenging. However, it is our opinion that finding that placebo is still the approach that could establish a true sense of the specific effects of acupuncture in this setting and for other clinical conditions
Comparison to Duloxetine in Future Trials
None of the included studies compared acupuncture with duloxetine, a drug previously established to be effective for cipn. Although duloxetine is not the “gold standard” for cipn, it is currently the most effective known treatment. Thus, we would recommend that future studies use this medication as a comparator in addition to placebo or sham acupuncture controls.
CONCLUSIONS
Although two of three included studies showed efficacy, it is difficult to offer a strong recommendation for the use of acupuncture in cipn because of limited data and sample sizes. It is possible that only acupuncture, and not electro-acupuncture, is effective for cipn. Both trials using acupuncture showed a positive effect30,31, whereas the trials testing electro-acupuncture showed a lack of benefit29,32. Two studies in our review also suggested potential harms of acupuncture for cipn, although those observations were not statistically significant29,32. However, acupuncture has previously been proved to be safe, and it is already used in oncology settings12,13. Acupuncture could be chosen as an inexpensive, safe alternative treatment, but clinicians should use due diligence and vigilant monitoring when recommending acupuncture therapy. Given that the quality and quantity of the literature concerning this topic are limited, a potentially beneficial effect might exist, but future rigorous rcts with appropriate controls should be conducted.
Supplementary Information
Footnotes
Supplemental material available at http://www.current-oncology.com
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci. 2017;10:174. doi: 10.3389/fnmol.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461–70. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Gordon BS, Gbadamosi B, Jaiyesimi IA. The relationship between chemotherapy-induced neuropathy and quality of life in breast cancer survivors [abstract e22111] J Clin Oncol. 2018;36 doi: 10.1200/JCO.2018.36.15_suppl.e22111. [Available online at: http://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.e22111; cited 13 January 2019] [DOI] [Google Scholar]
- 4.Mols F, Beijers T, Vreugdenhil G, Van De Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. 2014;22:2261–9. doi: 10.1007/s00520-014-2255-7. [DOI] [PubMed] [Google Scholar]
- 5.Tofthagen C, Donovan K, Morgan MA, Shibata D, Yeh Y. Oxaliplatin-induced peripheral neuropathy’s effects on health-related quality of life of colorectal cancer survivors. Support Care Cancer. 2013;21:3307–13. doi: 10.1007/s00520-013-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–20. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 7.Pachman DR, Watson JC, Lustberg MB, et al. Management options for established chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2014;22:2281–95. doi: 10.1007/s00520-014-2289-x. [DOI] [PubMed] [Google Scholar]
- 8.Hershman DL, Lacchetti C, Dworkin RH, et al. on behalf of the American Society of Clinical Oncology. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–67. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 9.Pachman DR, Loprinzi CL, Grothey A, Ta LE. The search for treatments to reduce chemotherapy-induced peripheral neuropathy. J Clin Invest. 2014;124:72–4. doi: 10.1172/JCI73908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukaguchi M, Shibano M, Matsuura A, Mukai S. The protective effects of lafutidine for bortezomib induced peripheral neuropathy. J Blood Med. 2013;4:81–5. doi: 10.2147/JBM.S44127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleckner I, Kamen CS, Peppone LJ, et al. A urcc ncorp nationwide randomized controlled trial investigating the effect of exercise on chemotherapy-induced peripheral neuropathy in 314 cancer patients [abstract 1000] J Clin Oncol. 2016;34 doi: 10.1200/JCO.2016.34.15_suppl.10000. [Available online at: http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.10000; cited 13 January 2019] [DOI] [Google Scholar]
- 12.Lu W, Rosenthal DS. Recent advances in oncology acupuncture and safety considerations in practice. Curr Treat Options Oncol. 2010;11:141–6. doi: 10.1007/s11864-010-0126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst E, Lee MS. Acupuncture for palliative and supportive cancer care: a systematic review of systematic reviews. J Pain Symptom Manage. 2010;40:e3–5. doi: 10.1016/j.jpainsymman.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Minton O, Higginson IJ. Electroacupuncture as an adjunctive treatment to control neuropathic pain in patients with cancer. J Pain Symptom Manage. 2007;33:115–17. doi: 10.1016/j.jpainsymman.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Alimi D, Rubino C, Pichard-Léandri E, Fermand-Brulé S, Dubreuil-Lemaire ML, Hill C. Analgesic effect of auricular acupuncture for cancer pain: a randomized, blinded, controlled trial. J Clin Oncol. 2003;21:4120–6. doi: 10.1200/JCO.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Shen J, Wenger N, Glaspy J, et al. Electroacupuncture for control of myeloablative chemotherapy-induced emesis: a randomized controlled trial. JAMA. 2000;284:2755–61. doi: 10.1001/jama.284.21.2755. [DOI] [PubMed] [Google Scholar]
- 17.Johnstone PA, Peng YP, May BC, Inouye WS, Niemtzow RC. Acupuncture for pilocarpine-resistant xerostomia following radiotherapy for head and neck malignancies. Int J Radiat Oncol Biol Phys. 2001;50:353–7. doi: 10.1016/S0360-3016(00)01530-3. [DOI] [PubMed] [Google Scholar]
- 18.Johnstone PAS, Niemtzow RC, Riffenburgh RH. Acupuncture for xerostomia. Cancer. 2002;94:1151–6. doi: 10.1002/cncr.10348. [DOI] [PubMed] [Google Scholar]
- 19.Vickers AJ, Straus DJ, Fearon B, Cassileth BR. Acupuncture for postchemotherapy fatigue: a phase ii study. J Clin Oncol. 2004;22:1731–5. doi: 10.1200/JCO.2004.04.102. [DOI] [PubMed] [Google Scholar]
- 20.Dean-Clower E, Doherty-Gilman AM, Keshaviah A, et al. Acupuncture as palliative therapy for physical symptoms and quality of life for advanced cancer patients. Integr Cancer Ther. 2010;9:158–67. doi: 10.1177/1534735409360666. [DOI] [PubMed] [Google Scholar]
- 21.Dimitrova A, Murchison C, Oken B. Acupuncture for the treatment of peripheral neuropathy: a systematic review and meta-analysis. J Altern Complement Med. 2017;23:164–79. doi: 10.1089/acm.2016.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franconi G, Manni L, Schröder S, Marchetti P, Robinson N. A systematic review of experimental and clinical acupuncture in chemotherapy-induced peripheral neuropathy. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/516916. 516916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cogo E, Sampson M, Ajiferuke I, et al. Searching for controlled trials of complementary and alternative medicine: a comparison of 15 databases. Evid Based Complement Alternat Med. 2011;2011 doi: 10.1093/ecam/nep038. 858246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brami C, Bao T, Deng G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: a systematic review. Crit Rev Oncol Hematol. 2016;98:325–34. doi: 10.1016/j.critrevonc.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a Web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cochrane Collaboration, Effective Practice and Organisation of Care (epoc) EPOC Resources for Review Authors [Web page] London, U.K.: epoc; 2017. [Available at: http://epoc.cochrane.org/epoc-specific-resources-review-authors; cited 5 May 2017] [Google Scholar]
- 27.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacPherson H, Altman DG, Hammerschlag R, et al. on behalf of the stricta Revision Group. Revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (stricta): extending the consort statement. J Evid Based Med. 2010;3:140–55. doi: 10.1111/j.1756-5391.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 29.Rostock M, Jaroslawski K, Guethlin C, Ludtke R, Schroder S, Bartsch HH. Chemotherapy-induced peripheral neuropathy in cancer patients: a four-arm randomized trial on the effectiveness of electroacupuncture. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/349653. 349653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han X, Wang L, Shi H, et al. Acupuncture combined with methylcobalamin for the treatment of chemotherapy-induced peripheral neuropathy in patients with multiple myeloma. BMC Cancer. 2017;17:40. doi: 10.1186/s12885-016-3037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu W, Giobbie-Hurder A, Freedman R, et al. Acupuncture for chemotherapy-induced peripheral neuropathy in breast cancer, preliminary results of a pilot randomized controlled trial [abstract PD4-01] Cancer Res. 2017;77(suppl) doi: 10.1158/1538-7445.SABCS16-PD4-01. [Available online at: http://cancerres.aacrjournals.org/content/77/4_Supplement/PD4-01; cited 7 May 2017] [DOI] [Google Scholar]
- 32.Greenlee H, Crew KD, Capodice J, et al. Randomized sham-controlled pilot trial of weekly electro-acupuncture for the prevention of taxane-induced peripheral neuropathy in women with early stage breast cancer. Breast Cancer Res Treat. 2016;156:453–64. doi: 10.1007/s10549-016-3759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zijlstra FJ, van den Berg–de Lange I, Huygen FJ, Klein J. Anti-inflammatory actions of acupuncture. Mediators Inflamm. 2003;12:59–69. doi: 10.1080/0962935031000114943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau WK, Chan WK, Zhang JL, Yung KK, Zhang HQ. Electroacupuncture inhibits cyclooxygenase-2 up-regulation in rat spinal cord after spinal nerve ligation. Neuroscience. 2008;155:463–8. doi: 10.1016/j.neuroscience.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Lin JG, Chen WL. Acupuncture analgesia: a review of its mechanisms of actions. Am J Chin Med. 2008;36:635–45. doi: 10.1142/S0192415X08006107. [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Min BI, Na HS, Park DS. Relieving effects of electroacupuncture on mechanical allodynia in neuropathic pain model of inferior caudal trunk injury in rat: mediation by spinal opioid receptors. Brain Res. 2004;998:230–6. doi: 10.1016/j.brainres.2003.11.045. [DOI] [PubMed] [Google Scholar]
- 37.Dong ZQ, Xie H, Ma F, Li WM, Wang YQ, Wu GC. Effects of electroacupuncture on expression of somatostatin and preprosomatostatin mrna in dorsal root ganglions and spinal dorsal horn in neuropathic pain rats. Neurosci Lett. 2005;385:189–94. doi: 10.1016/j.neulet.2005.05.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.