Abstract
Objectives
In the present study, we explored the real-world efficacy of the immuno-oncology checkpoint inhibitor nivolumab and the tyrosine kinase inhibitor cabozantinib in the second-line setting.
Methods
Using the International Metastatic Renal Cell Carcinoma Database Consortium (imdc) dataset, a retrospective analysis of patients with metastatic renal cell carcinoma (mrcc) treated with nivolumab or cabozantinib in the second line after prior therapy targeted to the vascular endothelial growth factor receptor (vegfr) was performed. Baseline characteristics and imdc risk factors were collected. Overall survival (os) and time to treatment failure (ttf) were calculated using Kaplan–Meier curves. Overall response rates (orrs) were determined for each therapy. Multivariable Cox regression analysis was performed to determine survival differences between cabozantinib and nivolumab treatment.
Results
The analysis included 225 patients treated with nivolumab and 53 treated with cabozantinib. No significant difference in median os was observed: 22.10 months [95% confidence interval (ci): 17.18 months to not reached] with nivolumab and 23.70 months (95% ci: 15.52 months to not reached) with cabozantinib (p = 0.61). The ttf was also similar at 6.90 months (95% ci: 4.60 months to 9.20 months) with nivolumab and 7.39 months (95% ci: 5.52 months to 12.85 months) with cabozantinib (p = 0.20). The adjusted hazard ratio (hr) for nivolumab compared with cabozantinib was 1.30 (95% ci: 0.73 to 2.3), p = 0.38. When adjusted by imdc criteria and age, the hr was 1.32 (95% ci: 0.74 to 2.38), p = 0.35.
Conclusions
Real-world imdc data indicate comparable os and ttf for nivolumab and cabozantinib. Both agents are reasonable therapeutic options for patients progressing after initial first-line vegfr-targeted therapy.
Keywords: Renal cell carcinoma, metastatic; targeted therapy; checkpoint inhibitors; nivolumab; cabozantinib
INTRODUCTION
The treatment landscape for metastatic renal cell carcinoma (mrcc) has changed considerably in recent years. Inhibitors of the vascular endothelial growth factor receptor (vegfr) family and the mechanistic target of rapamycin pathways have long dominated. Positive results from the pivotal meteor and CheckMate 025 trials led, respectively, to approval of the novel agents cabozantinib and nivolumab1,2.
In the meteor trial, 658 patients with mrcc were randomized to either cabozantinib or everolimus after at least 1 previous line of treatment with vegfr tyrosine kinase inhibitor1. Cabozantinib has a unique mechanism of action whereby it targets vegfr as well as met and axl, thus targeting multiple signalling pathways at once1,3,4. The final results of the meteor trial demonstrated, for the cabozantinib and everolimus groups, an overall survival (os) of 21.4 months and 16.5 months respectively and a progression-free survival of 7.4 months and 3.8 months respectively5. Patients treated with cabozantinib experienced an independently-assessed objective response rate (orr) of 17%, compared with 3% with everolimus5. As a result, cabozantinib became the first drug in the second-line setting to show significant improvements in the endpoints of os, progression-free survival, and orr. Grade 3 or greater toxicity was observed in 68% of the patients receiving cabozantinib, and 60% of the patients required dose reductions1,5.
The CheckMate 025 trial randomized 823 patients with clear-cell mrcc to nivolumab or everolimus after 1 or 2 prior antiangiogenic therapy regimens2. Nivolumab is an inhibitory monoclonal antibody directed against PD-1, allowing for an effective T cell–mediated immune response to cancer cells2–4. In CheckMate 025, the os duration was 25 months for the nivolumab-treated group2. The progression-free survival reported for the nivolumab and everolimus groups was 4.6 months and 4.4 months respectively2. The investigator-assessed orr in the nivolumab group was 25%2. Further subgroup analyses showed that a response to nivolumab was still observed in cancers with apparently low or absent PD-L1 expression2. Grades 3 and 4 treatment-related adverse events were experienced by 19% of patients6.
Populations meeting the eligibility criteria for clinical trials might not be the same as those receiving treatment in the real-world setting7,8. Furthermore, no head-to-head comparisons of nivolumab and cabozantinib in the second-line setting have been published. Given that context, we used the International Metastatic Renal Cell Carcinoma Database Consortium (imdc) dataset to examine the real-world efficacy of both drugs in the second-line setting.
METHODS
Patient Population
Contributors of patient data to the imdc include 38 international cancer centres in Canada, the United States, Denmark, Greece, South Korea, Australia, New Zealand, Japan, Singapore, Italy, and Belgium. The data are obtained through registry, pharmacy, or consecutive clinic lists. Individual retrospective chart reviews using standardized database templates were performed to collect patient data. The data included patients accrued between 2005 and October 2017.
The patients included in the study had mrcc and had previously been treated with 1 line of vegfr-targeted therapy before starting either nivolumab or cabozantinib. Patients with non-clear-cell mrcc were included in the analysis. Institutional review board approval was obtained from each participating centre.
Statistical Analysis
Statistical analyses were performed using the SAS software application (version 9.4: SAS Institute, Cary, NC, U.S.A.). Kaplan–Meier curves were used to evaluate os and ttf. Overall survival was defined as the time from the start of either nivolumab or cabozantinib to death or last follow-up (censored). Time to treatment failure was defined as the time from the start of nivolumab or cabozantinib to treatment discontinuation because of death, progression (based on the Response Evaluation Criteria in Solid Tumors), treatment toxicity, or last follow-up (censored). Proportional hazards regression modelling was performed to adjust for baseline imbalances in imdc risk criteria as measured at initiation of cabozantinib or nivolumab therapy.
Patients were stratified into prognostic groups using the following 6 factors included in the imdc prognostic model9:
■ Score less than 80% on the Karnofsky performance scale
■ Time from diagnosis to initiation of targeted therapy less than 1 year
■ Hypercalcemia
■ Anemia
■ Neutrophilia
■ Thrombocytosis
In the analysis, all variables except for time from diagnosis to initiation of first-line targeted therapy less than 1 year were collected at the start of second-line therapy with nivolumab or cabozantinib. Patients were stratified into imdc favourable risk (0 prognostic factors), imdc intermediate risk (1–2 prognostic factors), and imdc poor risk (3–6 prognostic factors). A chi-square test was performed to examine for differences between the prognostic groups in patients receiving nivolumab or cabozantinib.
RESULTS
Baseline Characteristics of the Patients
At the time of analysis, the imdc dataset included 8798 patients, of whom 4656 (53%) went on to receive second-line therapy. In the second-line setting, 225 patients received nivolumab, and 53 received cabozantinib. The most commonly used first-line drugs in the nivolumab group (Table I) were sunitinib (53%) and pazopanib (37%). In the cabozantinib group, 56% of the patients had received sunitinib and 40% had received pazopanib in the first-line setting (Table I). Table II shows the baseline characteristics of the patients at initiation of either nivolumab or cabozantinib in the second-line setting. In the nivolumab group, 29% of the patients were 70 years of age or older; 9% in the cabozantinib group had attained that age (p = 0.0033). No other baseline parameters were significantly different between the groups. Table II also shows the imdc prognostic subgroups for each treatment group (p = 0.88).
TABLE I.
Prior therapies received in the first-line setting in the nivolum-ab and cabozantinib groups
| First-line drug | Nivolumab (n=220) | Cabozantinib (n=48) | ||
|---|---|---|---|---|
|
|
|
|||
| (n) | (%) | (n) | (%) | |
| Sunitinib | 115 | 53 | 27 | 56) |
|
| ||||
| Sorafenib | 2 | 1 | 0 | 0 |
|
| ||||
| Axitinib | 10 | 5 | 1 | 2 |
|
| ||||
| Bevacizumab | 1 | 1 | 0 | 0 |
|
| ||||
| Temsirolimus | 2 | 1 | 1 | 2 |
|
| ||||
| Pazopanib | 81 | 37 | 18 | 40 |
|
| ||||
| Everolimus | 0 | 0 | 1 | 2 |
|
| ||||
| Other | 9 | 4 | 0 | 0 |
TABLE II.
Patient characteristics at initiation of nivolumab (n = 225) or cabozantinib (n = 53)
| Characteristic | Pts (n) | Nivolumab [n (%)] | Pts (n) | Cabozantinib [n (%)] | p Value |
|---|---|---|---|---|---|
| Sex (men) | 225 | 179 (80) | 53 | 43 (81) | 0.80 |
|
| |||||
| Age >70 years | 225 | 65 (29) | 53 | 5 (9) | 0.0033 |
|
| |||||
| KPS <80 | 178 | 38 (21) | 48 | 8 (17) | 0.47 |
|
| |||||
| Dx to Tx <1 year | 222 | 107 (48) | 51 | 32 (63) | 0.061 |
|
| |||||
| Prior nephrectomy | 223 | 199 (89) | 52 | 44 (85) | 0.35 |
|
| |||||
| Hypercalcemia | 182 | 15 (8) | 44 | 1 (2) | 0.17 |
|
| |||||
| Anemia | 188 | 144 (77) | 46 | 30 (65) | 0.11 |
|
| |||||
| Neutrophilia | 193 | 13 (7) | 46 | 4 (9) | 0.64 |
|
| |||||
| Thrombocytosis | 194 | 19 (10) | 45 | 3 (7) | 0.51 |
|
| |||||
| Non–clear cell histology | 167 | 26 (16) | 42 | 8 (19) | 0.59 |
|
| |||||
| Sarcomatoid features | 164 | 27 (16) | 43 | 6 (14) | 0.69 |
|
| |||||
| Metastasis | |||||
| >1 Site | 188 | 155 (82) | 48 | 42 (88) | 0.40 |
| To brain | 162 | 10 (6) | 37 | 1 (3) | 0.40 |
| To bone | 179 | 67 (37) | 39 | 14 (36) | 0.86 |
| To liver | 165 | 36 (22) | 37 | 8 (22) | 0.98 |
|
| |||||
| IMDC risk | |||||
| Favourable | 157 | 21 (13) | 39 | 6 (15) | 0.88 |
| Intermediate | 157 | 107 (68) | 39 | 27 (69) | |
| Poor | 157 | 29 (19) | 39 | 6 (15) | |
Pts = patients with relevant data; KPS = Karnofsky performance status; Dx = diagnosis; Tx = treatment; IMDC = International Metastatic Renal Cell Carcinoma Database Consortium.
Survival Outcomes and Response Rates
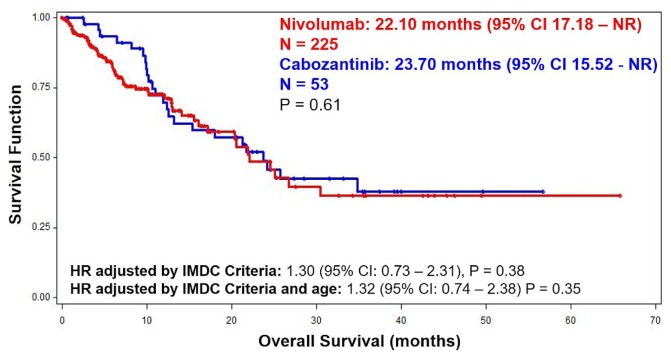
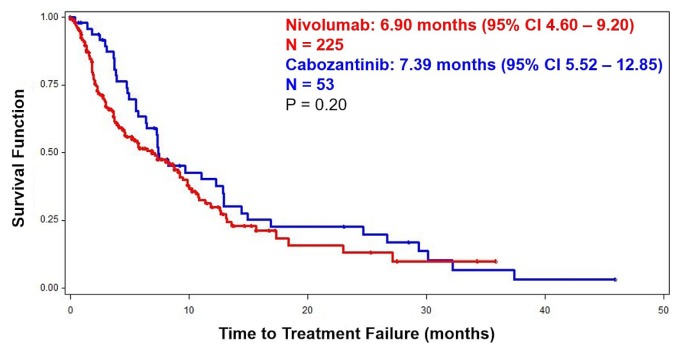
Median os duration from initiation of second-line treatment was 22.10 months for nivolumab and 23.70 months for cabozantinib (p = 0.60, Figure 1). Figure 2 shows a ttf duration of 6.90 months for nivolumab and 7.39 months for cabozantinib (p = 0.20). The orr was 21% for patients treated with nivolumab, and 20% for those treated with cabozantinib (Table III). Excluding the patients with non-clear-cell disease, os duration was 20.64 months with nivolumab [95% confidence interval (ci): 15.51 months to not reached] and 25.85 months with cabozantinib (95% ci: 12.50 months to not reached), p = 0.31; and the ttf duration was 6.47 months for the nivolumab group (95% ci: 3.71 months to 9.93 months) and 8.28 months for the cabozantinib group (95% ci: 6.41 months to 14.42 months), p = 0.24. Additionally, the orr did not change substantially when limited to patients with clear-cell disease (nivolumab 22%, cabozantinib 27%; p = 0.91).
FIGURE 1.
Kaplan–Meier curve depicting overall survival from initiation of nivolumab (n = 225) or cabozantinib (n = 53), with complete prognostic information. CI = confidence interval; NR = not reached; HR = hazard ratio; IMDC = International Metastatic Renal Cell Carcinoma Database Consortium.
FIGURE 2.
Kaplan–Meier curve depicting time to treatment failure from initiation of nivolumab (n = 225) or cabozantinib (n = 53), with complete prognostic information. CI = confidence interval.
TABLE III.
Best response at second-line therapy with cabozantinib in 40 patients and nivolumab in 140 patients
| Response | Cabozantinib [n (%)] | Nivolumab [n (%)] |
|---|---|---|
| Complete response | 1 (3) | 2 (1) |
| Partial response | 7 (18) | 28 (20) |
| Stable disease | 23 (58) | 48 (34) |
| Progressive disease | 9 (23) | 62 (44) |
| Overall response rate | 8 (20) | 30 (21) |
| p=0.85 |
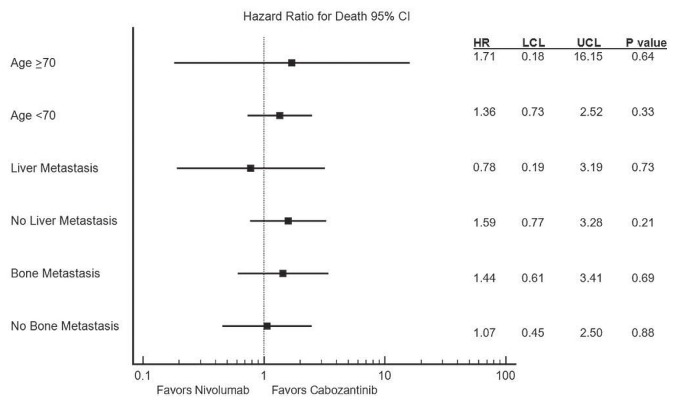
In multivariable analysis with adjustments for imdc criteria, the hazard ratio for os for nivolumab compared with cabozantinib was 1.30 (95% ci: 0.73 to 2.31), p = 0.38. Because of the differences in age in the two groups, another multivariable analysis of os adjusting for imdc criteria and for age was performed, resulting in a hazard ratio of 1.32 (95% ci: 0.74 to 2.38), p = 0.35. Figure 3 shows the hazard ratios for additional subgroups (including patients with liver and bone metastases), with no significant differences being observed for those subgroups.
FIGURE 3.
Forest plot depicting hazard ratios (HRs) for death by age group and presence or absence of liver and bone metastasis. LCL = lower confidence limit; UCL = upper confidence limit.
DISCUSSION
Populations in clinical trials often do not have a profile that matches the profile of populations seen in clinical practice10. Large retrospective cohorts such as the imdc can be more representative of the real-world population by including patients with brain metastases and non-clear-cell histology. In the imdc patient series used for the present study, only a small proportion of patients were treated in phase iii clinical trials. Our analysis did not demonstrate substantial differences between the two drugs for either os or ttf in the second-line setting. The os durations of 22.1 months for nivolumab and 23.7 months for cabozantinib were comparable to the durations reported in Check-Mate 025 (25 months) and meteor (21.4 months)2,5. The slightly increased proportion of patients with progressive disease in both treatment groups in our real-world cohort could be attributable to patients with more comorbidities, lower scores on the Karnofsky performance scale, and brain metastasis being included. Furthermore, the lack of a difference in orr for the entire cohort of patients compared with the clear-cell cohort indicates that our findings are not driven by the non-clear-cell patients that were included. Overall, the data suggest that real-world outcomes are relatively similar to those obtained in the clinical trials and that either drug is a reasonable option in the second-line treatment of mrcc. Given similar efficacy and a lack of predictive biomarkers, decisions about which drug to use in the second-line setting are currently largely pragmatic, based on toxicity profiles, patient preference, and drug availability. Some patients might prefer to receive nivolumab intravenously; others might choose cabozantinib because it can be taken orally. Patients with autoimmune conditions such as uncontrolled or active lupus erythematosus, Crohn disease, or immunodeficiency might choose cabozantinib because nivolumab’s mechanism of action requires a functional immune system. On the other hand, cabozantinib’s side effect profile might prompt patients with mrcc and refractory hypertension to choose nivolumab.
Our study is limited by its retrospective design. However, the use of consecutive patient series (for example, registries and pharmacy databases) to prevent physician recall bias and reduce selection bias helps to mitigate some of the deficiencies. In addition, given that our analysis of the data is relatively early, the number of cabozantinib patients is small. Analyzing the real-world toxicity profile of the two agents will also be important.
Recently, the CheckMate 214 trial observed a benefit of using upfront combination immunotherapy with ipilimumab–nivolumab in patients with mrcc having an intermediate or poor imdc risk11. That observation changes the treatment landscape in mrcc, because many intermediate- and poor-risk patients receiving checkpoint immunotherapy in the first-line setting might then receive cabozantinib in the second line. Many patients judged to be “favourable risk” will continue to have the choice of receiving single-agent nivolumab or cabozantinib after progression on sunitinib or pazopanib in the first line. However, some patients with an intermediate or poor imdc risk will still receive a first-line tyrosine kinase inhibitor because of comorbidities, patient preference, and inability to tolerate or lack of access to checkpoint immunotherapy at their institutions.
Lastly, further studies are required to determine whether biomarkers such as PD-L1, pbrm1, bap1, met, or other candidate markers can help to identify patients who will benefit from treatment with cabozantinib or nivolumab4,12,13.
CONCLUSIONS
Nivolumab and cabozantinib appeared to have similar efficacy in terms of both os and ttf in our real-world population. These novel agents are both reasonable therapeutic options for mrcc patients progressing after initial first-line targeted therapy with an anti-vegfr agent. Further studies are needed to identify population subgroups and predictive biomarkers that could help to better select patients likely to benefit from nivolumab or cabozantinib.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: FD declares research funding from Novartis, Pfizer, and Ipsen; TKC declares research funding from AstraZeneca, Bristol–Myers Squibb, Exelixis, Genentech, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, Roche, Tracon Pharmaceuticals, and Eisai, and consulting or advisory fees from AstraZeneca, Bayer, Bristol–Myers Squibb, Cerulean, Eisai, Foundation Medicine, Exelixis Genentech/Roche, GlaxoSmithKline, Merck, Novartis, Peloton, Pfizer, and Prometheus Labs; BB declares honoraria from Amgen, Pfizer, Janssen, and Bayer, and was also an investigator on the European Clinical Trials Database 2011-006085-40/MetaSun trial supported by Pfizer; NA declares consulting fees from Pfizer, Exelixis, Argos, and Cerulean; RK declares honoraria from Novartis, Pfizer, and Bayer; TY declares remuneration for a lecture from Pfizer Japan and Novartis Pharma Japan; DYCH declares consulting fees from Pfizer, Novartis, Bristol–Myers Squibb, Janssen Pharmaceuticals, and Astellas Pharma; IDD is supported by an Australian National Health and Medical Research Council Practitioner Fellowship (APP1102604), and has received research funding from Astellas and Exelixis. The remaining authors have no conflicts to disclose.
REFERENCES
- 1.Choueiri TK, Escudier B, Powles T, et al. on behalf of the meteor investigators. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1814–23. doi: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Escudier B, McDermott DF, et al. on behalf of the CheckMate 025 investigators. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stukalin I, Alimohamed N, Heng DY. Contemporary treatment of metastatic renal cell carcinoma. Oncol Rev. 2016;10:295. doi: 10.4081/oncol.2016.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376:354–66. doi: 10.1056/NEJMra1601333. [DOI] [PubMed] [Google Scholar]
- 5.Choueiri TK, Escudier B, Powles T, et al. on behalf of the meteor investigators. Cabozantinib versus everolimus in advanced renal cell carcinoma (meteor): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:917–27. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Sharma P, McDermott DF, et al. on behalf of the CheckMate 025 investigators. CheckMate 025 randomized phase 3 study: outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol. 2017;72:962–71. doi: 10.1016/j.eururo.2017.02.010. [Erratum in: Eur Urol 2018;73:e116–18] [DOI] [PubMed] [Google Scholar]
- 7.Gerber DE, Pruitt SL, Halm EA. Should criteria for inclusion in cancer clinical trials be expanded? J Comp Eff Res. 2015;4:289–91. doi: 10.2217/cer.15.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schilsky RL. Finding the evidence in real-world evidence: moving from data to information to knowledge. J Am Coll Surg. 2017;224:1–7. doi: 10.1016/j.jamcollsurg.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 10.Heng DYC, Choueiri TK, Rini BI, et al. Outcomes of patients with metastatic renal cell carcinoma that do not meet eligibility criteria for clinical trials. Ann Oncol. 2014;25:149–54. doi: 10.1093/annonc/mdt492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motzer RJ, Tannir NM, McDermott DF, et al. on behalf of the CheckMate 214 investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brugarolas J. pbrm1 and bap1 as novel targets for renal cell carcinoma. Cancer J. 2013;19:324–32. doi: 10.1097/PPO.0b013e3182a102d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao D, Margolis CA, Gao W, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359:801–6. doi: 10.1126/science.aan5951. [DOI] [PMC free article] [PubMed] [Google Scholar]