Abstract
Background
In Canada, requests for public reimbursement of cancer drugs are predominately initiated by pharmaceutical manufacturers. Clinician-led submissions provide a mechanism to initiate the drug funding process when industry does not submit a request for funding consideration. Although such requests are resource-intensive to produce, Cancer Care Ontario (cco) has the capacity to facilitate clinician-led submissions. In 2014, cco began developing a cancer drug prioritization framework that allocates resources to systematically address a growing number of clinician-identified funding gaps with clinician-led submissions.
Methods
Cancer site–specific drug advisory committees established by cco consist of health care practitioners whose roles include identifying and prioritizing funding gaps. The committees submit their identified gaps to a cross-cancer-site prioritization exercise in which the requests are ranked based on a set of guiding principles derived from health technology assessment. The requests are then sequentially allocated the resources needed to meet submission requirements. Whether the funding gap is of provincial or pan-Canadian relevance determines where the submission is filed for assessment.
Results
Since its inception, the cco framework has identified 17 funding gaps in 9 cancer sites. In 4 prioritizations, the framework supported 6 submissions. As of June 2018, the framework had contributed to the eventual funding of more than 9 new drug–indication pairs, with more awaiting funding consideration.
Conclusions
The cco prioritization framework has enabled clinicians to effectively and systematically identify, prioritize, and fill funding gaps not addressed by industry. Ultimately, the framework helps to ensure that patients can access evidence-informed and cost-effective therapies. The framework will continue to evolve as it encounters new challenges, including funding requests for rare indications.
Keywords: Drug funding, health technology assessment, prioritization, clinician-led funding submissions
INTRODUCTION
After regulatory approval by Health Canada, all drugs undergo health technology assessment (hta) to evaluate the supporting clinical and economic evidence before public reimbursement is considered. Most requests for public reimbursement (funding) of oncology drugs are submitted to the pan-Canadian Oncology Drug Review (pcodr) at the Canadian Agency for Drugs and Technologies in Health. An hta program, pcodr makes funding recommendations to most of Canada’s provinces and territories. In Quebec, drug funding requests are evaluated by the Institut national d’excellence en santé et en services sociaux. In Ontario, province-specific funding requests that are not of pan-Canadian relevance are instead evaluated by the Ontario Steering Committee for Cancer Drugs (osccd). The osccd’s objective is to provide evidence-based clinical, health research, and health economic guidance to the executive officer of Ontario Public Drug Programs (https://www.cancercareontario.ca/en/cancer-treatments/chemotherapy/funding-reimbursement/ontario-steering-committee-cancer-drugs). Importantly, submissions assessed by pcodr are not subsequently assessed by osccd. To ensure appropriate and efficient use of public resources, drugs available through public drug programs are commonly funded according to specific clinical criteria, relying on hta recommendations to guide the funding decisions. The use of hta has been shown to be crucial for maintaining a sustainable health care system, and it recognizes the need for high-quality evidence-informed care when faced with finite resources and the increasing costs of anticancer treatment1,2.
In Canada, the consideration process for oncology drug funding is predominantly driven by the pharmaceutical industry. Since pcodr was established in 2011, more than 95% of the funding requests submitted have come from drug manufacturers (https://www.cadth.ca/pcodr/find-areview). However, drugs with generic equivalents, with low potential for profit, or without a Notice of Compliance from Health Canada for a given indication are rarely submitted by industry for funding consideration, creating a number of potential unmet patient needs and drug funding gaps.
Clinician-led funding submissions provide a mechanism to address drug funding gaps arising from lack of industry submissions. The manufacturer plays little to no role in those types of submissions. Instead, clinicians and other non-industry professionals are responsible for fulfilling all the necessary submission requirements. Submission requirements for Ontario or pan-Canadian review are similar, including a comprehensive clinical summary, a pharmacoeconomic analysis, and a budget impact assessment. In particular, the need for a comprehensive pharmacoeconomic analysis has historically limited the number of clinician-driven drug funding submissions. Ontario’s provincial cancer agency, cco, has built capacity to support the development of clinician-led submissions. The agency has clinicians, a submission pharmacist, health economists, and various supporting staff who understand submission and funding requirements.
Before 2015, cco had no explicit framework for supporting clinician-led submissions. Submissions were completed through a “first in, first out” mechanism, wherein funding gaps were addressed in the order that they were identified, regardless of clinical need or priority. As more funding gaps were identified, the need to prioritize and systematically address those gaps became increasingly apparent. In 2014, cco began developing a prioritization framework (pf) to address the growing number of clinician-identified funding gaps. The pf was designed to evaluate and prioritize funding gaps for all cancer sites, guided by a set of principles relevant to hta.
Since its establishment in 2015, the pf has guided the prioritization of several oncology funding submissions. The objective of the present paper was to describe the standardized framework for prioritizing oncology-drug funding submissions for all cancer sites at the provincial level.
METHODS
Identifying Funding Gaps Not Addressed by Manufacturer Drug Submissions
Cancer Care Ontario administers several cancer-drug funding programs. To support and advise those programs, cco established 9 cancer site-specific drug advisory committees (dacs). A key role for the dacs is to identify oncology-drug funding gaps not addressed by industry and to facilitate clinician-led submissions. The dacs represent skin, lung, breast, gastrointestinal, hematologic, gynecologic, genitourinary, central nervous system, and head, neck, and thyroid malignancies. Each dac is composed of volunteers who are active health care practitioners, including academic and practicing oncologists and oncology pharmacists. The dac members are based in various regions throughout Ontario to ensure geographic representation across the province.
Each dac is led by an Ontario cancer lead (ocl)—a paid clinician consultant for cco who works collaboratively to oversee a variety of initiatives concerning their cancer sites. The ocls are responsible for championing or electing a clinical champion from the dac to guide and oversee the dac-initiated funding submission. The ocls also represent their cancer site’s funding gaps at all prioritization exercises.
Members of the dacs meet regularly with cco to identify, discuss, and prioritize funding gaps affecting their cancer sites. The dacs also apply their unique clinical perspective to evaluate a drug’s use, benefits, value, and safety with the aim of creating recommendations for drug implementations, funding algorithms, manufacturer submissions, and other oncology funding issues.
Development of the PF
Collaboratively, cco and the ocls developed the pf to guide the ranking of dac-identified funding gaps and to ensure that funding submissions are completed in order of priority, emphasizing patient and provincial needs. The guiding principles that constitute the pf were developed in consultation with provincial cancer experts and after a review of existing hta frameworks. One of the main frameworks examined was the pcodr Expert Review Committee Deliberative Framework. Adapted from Johnson et al.3, pcodr’s framework uses 4 principles, described in Table I, to make funding recommendations and guide drug-funding decisions3.
TABLE I.
Principles governing the pan-Canadian Oncology Drug Review’s evaluative framework4
| Criterion | Definition |
|---|---|
| Overall clinical benefit | |
| A measure of the net health benefit of using the drug to diagnose or manage a cancer-related condition (for example, lung cancer) or cancer care–related issue (for example, skeletal-related events in metastatic disease) | |
|
| |
| Alignment with patient values | |
| An assessment made after considering information about patient values | |
|
| |
| Cost effectiveness | |
| A measure of the net efficiency of the drug and companion technology compared with other drug and nondrug alternatives (no cut-off threshold) | |
|
| |
| Feasibility of adoption into the health system | |
| An assessment of the ease with which the drug can be adopted into the overall health care and cancer care systems | |
Although the pcodr criteria included principles that cco valued for its own framework (namely that the target audience include policymakers and decision-makers and not be limited to clinicians and patients), those criteria were not adopted outright for a few reasons. First, unlike many hta frameworks, the principles for cco’s pf were not intended to equate to prioritization for funding; rather, they are used only to prioritize funding gaps with high clinical need for which funding submissions will be developed. Principles such as cost-effectiveness were therefore not directly applicable to that goal and were not included in the pf. Instead, the principle “potential for cost-savings” was incorporated to assess the economic impact at an early stage in the submission process. A full cost-effectiveness analysis is conducted during the submission workup for requests with a high priority. Second, pcodr’s framework is intended for pan-Canadian recommendations; it lacks criteria that explicitly address the needs of Ontario patients in the oncology context. Principles such as “likelihood of success”—which recognizes submissions that have the highest chance of success, are associated with strong evidence, and address a relevant gap—were also added to the cco pf.
The PF
Currently, cco’s pf consists of a set of 7 hta-derived guiding principles (Table II) that are used to prioritize drug-funding gaps identified by the dacs. No explicit weights are assigned to the principles, and so each ocl decides the merit of each request on a case-by-case basis by applying the principles to the evidence and context of the request. That process recognizes that every funding request is unique and that certain principles might be valued differently in diverse scenarios5,6. The sub-criteria represent various considerations that constitute each guiding principle, but that are not completely exhaustive.
TABLE II.
Guiding principles governing Cancer Care Ontario’s prioritization framework
| Principle | Definition | Sub-criteria |
|---|---|---|
| Strength of clinical evidence | Assesses the literature or studies used to demonstrate the clinical benefit in patients receiving the therapy |
|
| Magnitude of clinical benefit | Examines the effect the therapy would have on patient outcomes and the health care system |
|
| Effect for patients in Ontario | Examines the province-specific effect of the therapy on patient outcomes and the health care system |
|
| Patient need (unmet need) | Examines other therapies currently available to patients and the standard of care for the disease |
|
| Likelihood of success | Considers the strength of the overall submission and the likelihood of receiving a positive funding recommendation from Ontario Steering Committee for Cancer Drugs or the pan-Canadian Oncology Drug Review |
|
| Opportunity for cost savings | Considers economic factors that might support system sustainability, such as the effects for budgets or resources and the potential for system savings |
|
| Curative compared with palliative | Considers whether the treatment is of curative or palliative intent |
|
RESULTS
The Prioritization Process
The dac funding requests are prioritized twice annually at a cross-cancer-site prioritization exercise. The exercise has 4 main steps:
-
▪ Internal prioritization
First, the dacs individually identify and prioritize funding gaps for their own cancer site. During dac meetings, members discuss emerging evidence and the current manufacturer submission pipeline. They also identify unmet needs from their clinical practice. Although there is no set framework for internal prioritization, funding gaps are generally ranked based on their significance, urgency, and feasibility of submission. The decision to bring forward a funding request to the cross-cancer-site prioritization exercise is strongly influenced by the perceived clinician urgency of the funding gap. At that point, the dac must ensure that the manufacturer has no intention to submit for the indication at hand; that the funding gap has not previously received a negative funding recommendation at the national level (because such requests are not allowed to be resubmitted unless the body of evidence or circumstances have changed substantially); and the dac is prepared to dedicate a significant amount of time over the coming months to support the submission. To ensure transparency and the integrity of any potential submissions, dac members must also declare any conflicts of interest. Members with significant conflicts of interest must recuse themselves from this aspect of the prioritization process.
-
▪ Potential funding mechanism determination
Ontario has several funding mechanisms through which cancer drugs are publicly reimbursed (supplemental Appendix 1). The funding mechanism for each drug–indication pair is determined by several factors—primarily, where the drug is administered (in hospital, at an outpatient systemic treatment clinic, dispensed at community pharmacy), but also cost, level of evidence, and volume or unique circumstances7,8. The dacs work with cco to assess an appropriate potential funding mechanism for their gap. The final funding decision is ultimately made by the Ministry of Health and Long-Term Care, and therefore cco and hta review committees can merely advise on the funding stream.
-
▪ Cross-cancer-site prioritization
The ocls work with cco to complete a pre-submission document for eligible funding gaps. The template is designed to briefly summarize relevant information about the funding gap, including the potential funding request, funding stream, clinical condition, evidence, and data sources. Requests are usually based on one or more published pivotal trials, but to facilitate timeliness of funding efforts, can also be based on data presented in high-impact abstracts. The document also includes an overview of the clinical, economic, and epidemiologic data, including potential cost, patient numbers, and currently available alternatives. The conflicts of interest documented by the dac member are also disclosed on the form.
A Canada-wide jurisdiction scan to determine the funding status for each request in other provinces across the country is then conducted by cco staff. The jurisdiction scan also assesses pan-Canadian interest in the request, in which case, any potential submission will be submitted to pcodr in the interest of funding alignment. Each pre-submission document is circulated to all ocls 2 weeks before the cross-cancer-site prioritization in preparation for the exercise. The dacs are not required to bring forward a request to each prioritization exercise, nor are they limited in the number of requests they may bring forward.
The cross-cancer-site prioritization exercise itself is a 2-hour meeting chaired by the clinical lead of the Provincial Drug Reimbursement Programs and cco, and attended by the ocls. At the exercise, each ocl delivers a summary presentation to the other attendees that outlines their cancer site’s funding gap and highlights the need for funding. After all the presentations, the multidisciplinary group is given time to ask questions and discuss the funding requests. Each ocl then ranks all the funding requests, including their own, anonymously by ballot.
-
▪ Submission workup
Ballots are tabulated, and the funding request with the highest mean rank is given priority access to the resources available at cco to support a clinician-led submission. The subsequent mean ranking of the other submissions determines their place in the queue.
Results of the Framework
Since 2015, cco’s dacs have identified more than 50 funding gaps, 17 of which were deemed appropriate for the pf and a clinician-led submission. The other funding gaps were not brought forward by the ocls or dacs for a variety of reasons, including a manufacturer intention to submit, insufficient data to support a submission at the particular time, or inappropriateness for any of the eligible funding streams described in supplementary Table 1.
As of May 2018, 4 rounds of prioritization had been completed, through which cco supported a total of 6 high-priority submissions, with a 7th in progress (Table III). All submissions thus far supported by the pf have been submitted to the osccd and have since been evaluated through the provincial hta processes. The pf has led to funding in Ontario of 4 drugs for more than 9 indications arising from 4 positive funding recommendations. The discrepancy between the number of recommendations and the funded indications reflects the fact that some submissions contain more than 1 drug–indication pair (Table III). The remaining 2 submissions are undergoing evaluation, and funding recommendations are expected within the calendar year. At the time of writing, cco was working on a submission with their lung dac that was expected to go to the national (pcodr) review process by the end of 2018.
TABLE III.
Clinician-led submissions to Cancer Care Ontario’s Drug Advisory Committee since 2015
| Drug Advisory Committee | Submitted to | Year assessed | Status | |
|---|---|---|---|---|
| Site | Funding request | |||
| Gastrointestinal | Capecitabine–oxaliplatin (XELOX) as adjuvant therapy for stage III colorectal cancer | OSCCD | 2015 | Funded under NDFP and ODB |
| Gynecology | Liposomal doxorubicin with carboplatin in ovarian cancer with platinum-sensitive occurrence | OSCCD | 2017 | Funded under NDFP |
| Hematology | Bortezomib retreatment for relapsed or refractory myeloma | OSCCD | 2017 | Funded under NDFP |
| Multiplea | Capecitabine for multiple evidence-informed regimens | OSCCD | 2017 | Funded under ODB as general benefit |
| Breast | Pending | OSCCD | 2018 | Pending |
| Hematology | Pending | OSCCD | 2018 | Pending |
| Lung | In progress | In progress | In progress | In progress |
Including gastrointestinal and breast.
OSCCD = Ontario Steering Committee for Cancer Drugs; NDFP = New Drug Funding Program; ODB = Ontario Drug Benefit.
If work has not started on a funding request by the time of the next prioritization exercise, that request must be reprioritized if the dac still wishes to address the gap. Because the average submission takes 3–6 months to develop, the twice-annual frequency of prioritization means that some requests must be re-evaluated for priority against a new collection of funding gaps. The process thus ensures that the most recent evidence and funding requests are continuously assessed and compared with the existing submission queue. Funding requests that have not started workup for a submission by the next prioritization exercise are usually brought back for re-prioritization unless withdrawn. Funding requests could be withdrawn from the submission queue as a result of changes in dac priorities or in the event that the funding gap is addressed through an alternative review or funding mechanism.
Evaluation
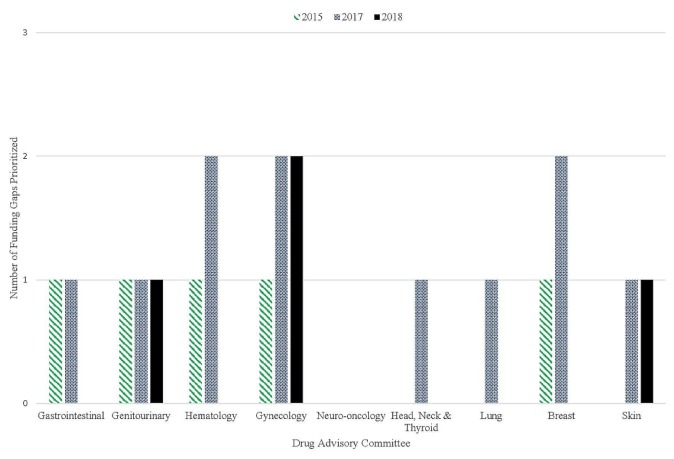
To evaluate the impact of the framework, we considered the total number of funding requests brought forward, the number of funding requests addressed, the number of new funding requests at each round of prioritization, the number of funding requests based on newly reported evidence, and the number of prior submissions funded by the next prioritization exercise. Our most recent assessment of dac funding gaps indicated that several cancer-site groups have had their historical funding gap numbers reduced since implementation of the pf (Table IV). All 5 original funding gaps prioritized in 2015 have now been addressed, although only 3 were addressed through the pf (Table IV). Furthermore, only 4 requests were brought forward to the most recent prioritization in March 2018 (Figure 1). The new requests appear to be based on recent trial data as opposed to historical funding gaps.
TABLE IV.
Funding requests submitted to each round of cross-cancer-site prioritization
| Prioritization round | Funding requests | Submissions via the prioritization framework | Submissions awaiting funding consideration | |||
|---|---|---|---|---|---|---|
| Overall | New | Based on evidence from preceding 2 years | Complete | Funded | ||
| 1 (Feb 2015) | 5 | 5 | 1 | 3 | 3 | 0 |
| 2 (Mar 2017) | 8 | 8 | 3 | 2 | 1 | 1 |
| 3 (Sep 2017) | 6 | 3 | 5 | 1 | 0 | 1 |
| 4 (Mar 2018) | 4 | 1 | 4 | 0 | 0 | 0 |
FIGURE 1.
Number of funding gaps brought forward each year of prioritization, stratified by Drug Advisory Committee (cancer site).
DISCUSSION
While it is important that manufacturers seeking reimbursement for their drug products contribute to assessments that use rigorous evaluative processes, there is also a need to ensure that treatment options are not solely driven by manufacturer interests. Although compiling a high-quality, comprehensive submission for reimbursement consideration is highly resource-intensive, cco has invested in the resources necessary to support the development of submissions that address clinician-identified funding gaps. Submissions supported by cco have increased the overall number of clinician-led submissions to both national and local hta committees. (In addition to addressing Ontario-based funding gaps, cco has, to date, supported 3 of the total 4 non-industry submissions to pcodr). In recognition of the demand for and importance of such an undertaking, cco began developing a cancer-drug pf to optimize resources and to address, in a systematic manner, a growing number of clinician-identified funding gaps. The identification of funding gaps not addressed by industry is facilitated by cco’s cancer-site experts. The identified gaps are prioritized twice annually using a set of 7 guiding criteria to rank eligible funding requests. The criteria are derived from hta principles and were established in a review of current hta frameworks and after consultation with provincial cancer experts. The funding request with the highest ranking is then given priority access to the resources available at cco to support a clinician-led submission.
An increasing number of health care systems are adopting hta as one of the mechanisms to inform decisions about the allocation of health care resources2,9. Having become a common tool to assist evidence-based healthcare decisions, hta assesses competing technologies2 and guides reimbursement decisions and recommendations for heath technology adoption. The need for such assessments is largely a result of the resource constraints that challenge the implementation of new therapies, especially as expenditures in cancer treatment increase1. Making sure that the highest priority requests are addressed in a timely manner and that funders receive the best value for money spent on treatments that maximize patient benefit is also an urgent need. Together, those needs emphasize the requirement for a mechanism to prioritize funding requests. To address these needs, the pf uses criteria that are derived from hta principles and that are consistent with other evaluative frameworks including 6-steppps, the Canadian Agency for Drugs and Technologies in Health, and the European Society for Medical Oncology10–12.
Because the results of decisions about how to allocate submission resources will ultimately affect the health and well-being of patients, fairness was an important consideration in the development of the pf13,14. To establish a fair process, cco ensured that its framework was based on clear and consistent principles, developed with the members involved and grounded in a multi-aspect and evidence-based approach14–16. The involvement of all cancer-site representatives helps to ensure equity in cancer-site access to submission resources, regardless of tumour type. Equity is promoted by engaging clinicians in provincial funding initiatives and recognizing that value is complex and multidimensional, and should be assessed from multiple perspectives5,6,11,14. As a result, cco could look to expand the number of cancer sites represented by dacs in the future. The framework also recognizes the importance of clinician involvement in resource allocation decision-making, especially because of the expertise required to assess clinical aspects of proposed therapies in the oncology context17. Furthermore, for health resource allocation decisions to be best incorporated into clinical practice, they have to be informed by local clinical leaders18.
One of the main strengths of cco’s pf is its ongoing revision process. Through 4 rounds of prioritization, several revisions have been made to the framework. After each round of prioritization, the ocls and cco meet to discuss and revise the pf through consensus. That work can include revisions or additions to the guiding principles, change in the frequency of prioritization, or modifications to procedure and design. Since the inception of the pf, cco in 2017 increased the frequency of prioritization from ad hoc to twice annually so as to reprioritize funding gaps more frequently; changed the voting system to a ranking system; and removed the guiding principle “dac readiness to submit,” given that it was made a pre-submission requirement that dacs be prepared to support a submission if they bring a request forward.
Limitations of the process include the lack of a formal appeal process. As suggested in Norman Daniels’ “Accountability for Reasonableness,” a procedurally fair process requires opportunities to revise and challenge decisions that stakeholders make11,14,15. For the moment, cco has not established a mechanism through which ocls, dacs, or cco staff can challenge the outcome of the prioritization exercise. Such a mechanism could become increasingly important in managing stakeholder (for example, the dacs) expectations as requests that are increasing complex, have varying evidence, or deal with supportive care drugs are brought forward. (Such requests are unlikely to be prioritized high, however.) Further, cco has not made the prioritization results or deliberations public, which limits the transparency of the process. Other limitations include managing dac or ocl conflicts of interest and managing requests for rare diseases or mutations. The latter type of request often has weaker evidence because of the low prevalence of some genomic mutations19, and that low prevalence makes conducting randomized trials and developing comparative data challenging, despite a clinical need often perceived as pressing19.
Potential opportunities to further evaluate or improve the pf could include a broad survey of all involved stakeholders. Such a survey would allow the dacs and cco staff to convey their perspectives about the fairness and efficiency of the process. As well, incorporating patient values in the prioritization process to inform the principles encompassing patient benefit (similar to pcodr’s deliberative framework) could enhance this process3,4. Patient values were not incorporated into the current framework; however, certain patient value components (for example, unmet need) have been included3,20. Use of real-world evidence from other jurisdictions to supplement clinical trial data might also provide benefit when evaluating funding requests.
Currently, we are unaware of other Canadian provinces or territories that have established and use a pf for the purpose described in this paper. We recognize that many jurisdictions will have their own mechanism for evaluating drugs, but it is not clear if or how drugs are prioritized in the oncology space or, further, for clinician-led submissions.
CONCLUSIONS
The cco pf has enabled Ontario clinicians to address—in a systematic manner and in order of priority—funding gaps not addressed by industry. The process promotes a sustainable drug funding system and ultimately provides patients with access to important evidence-informed and cost-effective therapy. The framework values fairness and promotes evidence-based decision-making through its guiding hta principles. This report of the cco pf is meant to facilitate the transparency of the process and in the hopes that it might provide guidance to other jurisdictions in establishing a similar framework.
Supplementary Information
ACKNOWLEDGMENTS
We thank all cco staff involved in the creation and implementation of the prioritization exercise, as well as those involved in developing the submissions. We also thank the dac members for all their contributions to the process.
Footnotes
Supplemental material available at http://www.current-oncology.com
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.de Oliveira C, Weir S, Rangrej J, et al. The economic burden of cancer care in Canada: a population-based cost study. CMAJ Open. 2018;6:E1–10. doi: 10.9778/cmajo.20170144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephens JM, Handke B, Doshi J. International survey of methods used in health technology assessment (hta): does practice meet the principles proposed for good research? Comp Eff Res. 2012;2:29–44. [Google Scholar]
- 3.Johnson AP, Sikich NJ, Evans G, et al. Health technology assessment: a comprehensive framework for evidence-based recommendations in Ontario. Int J Technol Assess Health Care. 2009;25:141–50. doi: 10.1017/S0266462309090199. [DOI] [PubMed] [Google Scholar]
- 4.Pan-Canadian Oncology Drug Review (pcodr) pCODR Expert Review Committee: Deliberative Framework. Toronto, ON: pcodr; 2016. [Available online at: https://www.cadth.ca/sites/default/files/pcodr/The%20pCODR%20Expert%20Review%20Committee%20%28pERC%29/pcodr_perc_deliberative_frame.pdf; cited 16 May 2018] [Google Scholar]
- 5.Guindo LA, Wagner M, Baltussen R, et al. From efficacy to equity: literature review of decision criteria for resource allocation and healthcare decision making. Cost Eff Resour Alloc. 2012;10:9. doi: 10.1186/1478-7547-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans WK, Cheung MC, Chan KK. Measuring value and benefit —a matter of perspective. Lancet Oncol. 2017;18:839–40. doi: 10.1016/S1470-2045(17)30423-0. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Care Ontario. Drug Formulary—Funding and Reimbursement [Web page] Toronto, ON: Cancer Care Ontario; n.d.. [Available at: https://www.cancercareontario.ca/en/cancer-treatments/chemotherapy/funding-reimbursement; cited 16 May 2018] [Google Scholar]
- 8.Government of Ontario. Get coverage for prescription drugs [Web page] Toronto, ON: Government of Ontario; 2018. [Available at: https://www.ontario.ca/page/get-coverage-prescription-drugs; cited 16 May 2018] [Google Scholar]
- 9.Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7:518–28. doi: 10.1111/j.1524-4733.2004.75003.x. [DOI] [PubMed] [Google Scholar]
- 10.Cheng S, McDonald EJ, Cheung MC, et al. Do the American Society of Clinical Oncology Value Framework and the European Society of Medical Oncology Magnitude of Clinical Benefit Scale measure the same construct of clinical benefit? J Clin Oncol. 2017;35:2764–71. doi: 10.1200/JCO.2016.71.6894. [DOI] [PubMed] [Google Scholar]
- 11.Browman GP, Manns B, Hagen N, et al. 6-steppps: a modular tool to facilitate clinician participation in fair decisions for funding new cancer drugs. J Oncol Pract. 2008;4:2–7. doi: 10.1200/JOP.0812001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canadian Agency for Drugs and Technologies in Health (cadth) Health Technology Assessment and Optimal Use: Medical Devices; Diagnostic Tests; Medical, Surgical, and Dental Procedures. Ottawa, ON: cadth; 2018. [Google Scholar]
- 13.Gruskin S, Daniels N. Process is the point: justice and human rights: priority setting and fair deliberative process. Am J Public Health. 2008;98:1573–7. doi: 10.2105/AJPH.2007.123182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniels N. Accountability for reasonableness. BMJ. 2000;321:1300. doi: 10.1136/bmj.321.7272.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin DK, Giacomini M, Singer PA. Fairness, accountability for reasonableness, and the views of priority setting decision-makers. Health Policy. 2002;61:279–90. doi: 10.1016/S0168-8510(01)00237-8. [DOI] [PubMed] [Google Scholar]
- 16.Bentley C, Abelson J, Burgess MM, et al. Making Fair and Sustainable Decisions About Funding for Cancer Drugs in Canada: Final Report. Vancouver, BC: Canadian Centre for Applied Research in Cancer Control; 2017. [Google Scholar]
- 17.de Groot F, Capri S, Castanier JC, et al. Ethical hurdles in the prioritization of oncology care. Appl Health Econ Health Policy. 2017;15:119–26. doi: 10.1007/s40258-016-0288-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown BB, Young J, Smith DP, et al. Clinician-led improvement in cancer care (clicc)—testing a multifaceted implementation strategy to increase evidence-based prostate cancer care: phased randomised controlled trial—study protocol. Implement Sci. 2014;9:64. doi: 10.1186/1748-5908-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andre F. Developing anticancer drugs in orphan molecular entities—a paradigm under construction. N Engl J Med. 2018;378:763–5. doi: 10.1056/NEJMe1716821. [DOI] [PubMed] [Google Scholar]
- 20.Skedgel C. The prioritization preferences of pan-Canadian Oncology Drug Review members and the Canadian public : a stated-preferences comparison. Curr Oncol. 2016;23:322–8. doi: 10.3747/co.23.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.