Abstract
Pulmonary sarcomatoid carcinoma (psc) is a rare subtype of non-small-cell lung carcinoma with a poor prognosis and poor response to chemotherapy and radiotherapy. A previous study reported that psc expresses high levels of PD-L1, suggesting the potential efficacy of immune checkpoint inhibitors in these tumours. We report 2 cases of patients with a lung sarcomatoid carcinoma. Both patients initially underwent curative lung resection, but developed early recurrent disease. Because PD-L1 was highly expressed in the tumour cells, we initiated therapy with nivolumab, which showed good efficacy, almost complete radiologic tumour remission, and a remarkable improvement in the condition of those patients. Immune checkpoint inhibitors targeting PD-1 might be a valuable therapy option for pscs.
Keywords: Sarcomatoid carcinoma, lung cancer, immunotherapy, PD-L1 expression
INTRODUCTION
Primary pulmonary sarcomatoid carcinoma (psc) is a rare subtype of non-small-cell lung cancer (nsclc). It has a complex differential diagnosis and accounts for 0.1%–0.4% of all lung malignancies1. The disease is more prevalent in older adults (mean age: 60–70 years) and occurs mainly in men and smokers. The prognosis for affected patients is poor, the 5-year survival rate being approximately 20%2. Even in the earlier tumour stages, the prognosis is worse for psc than for other nsclcs3.
Treatment for pscs is usually similar to that for other nsclcs; however, because of the low prevalence of psc, little knowledge is available about the biology of the tumour and the best treatment strategies. Therapeutic management and the effect of conventional chemotherapy for psc therefore remain controversial. Vieira et al.4 and Yendamuri et al.5 reported that pscs are generally resistant to conventional chemotherapy. Other authors have described advanced disease to be nonresponsive to palliative chemotherapy6. However, Huang et al.7 showed a positive effect of postoperative chemotherapy on psc. Genetic alterations are rare in psc, and patients are often ineligible for molecularly targeted therapy8,9.
Antibodies that target the PD-1 cell membrane antigen have emerged as a new therapy for advanced nsclc. Several different trials showed substantial clinical activity for anti–PD-1 antibodies for advanced cancers, leading to the approval of those agents, including nivolumab for melanoma and squamous cell lung cancer10. A recently published case series showed therapeutic success with nivolumab for the treatment of pleomorphic carcinomas of the lung11. Here, we describe 2 cases of metastasized sarcomatoid carcinoma of the lung treated with nivolumab, focusing on the efficacy of nivolumab and the clinical course of the disease.
CASE DESCRIPTIONS
Case 1
A 57-year-old man was referred to our department for evaluation of a pulmonary mass in the left upper lobe, which was identified on computed tomography (ct) imaging of the thorax. He underwent rigid bronchoscopy with endobronchial ultrasonography, and transbronchial needle aspiration was used to obtain multiple samples from the mediastinal lymph nodes. Cytopathology analysis classified the tumour as a nsclc, with no mediastinal lymph node metastasis. Because of local tumour expansion (cT4, tumour > 9 cm), a neoadjuvant therapeutic approach was discussed at the institutional tumour board.
The patient subsequently received induction chemotherapy with 4 cycles of cisplatin–gemcitabine, which was tolerated without any problems. Restaging showed partial remission of the tumour. Complete resection of the tumour was performed with intrapericardially extended left upper lobectomy with pericardial resection. Neither intraoperative nor postoperative complications were observed, and the patient was discharged 10 days after surgery.
Pathology examination of the specimen revealed a sarcomatoid carcinoma of the lung with an R0 resection. Four weeks after discharge from hospital, the patient presented with progressive dyspnea accompanied by hypoxemic respiratory insufficiency. Imaging by ct showed diffuse pleural thickening, and a thoracoscopy revealed diffuse and extensive pleural carcinomatosis, which was verified by multiple pleural biopsies. Bronchoscopy showed almost complete tumour obstruction of the left lower lobe bronchus and an unsuspicious left upper lobe bronchial stump. Biopsies of the tumour were tested for PD-L1 expression and showed 80%–90% positivity. The patient showed rapid tumour progression with lymphangiosis carcinomatosa of the lung, left adrenal gland metastasis, and contralateral reactive pleural effusion [Figure 1(A,B)]. Consistent with the radiologic progression, the general condition of the patient worsened daily, with increasing immobility, advancing chest pain, and increasing hypoxemic insufficiency.
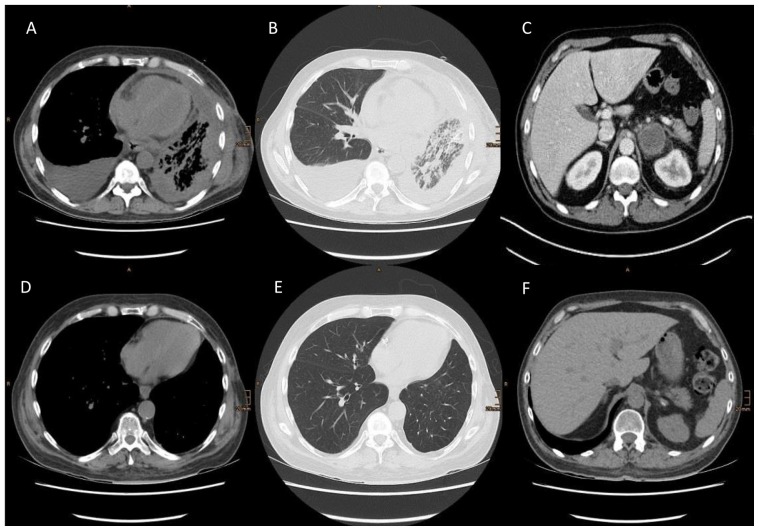
FIGURE 1.
(A–C) Computed tomography images 6 weeks after R0 resection of the left upper lobe, showing a diffuse left-sided pleural carcinomatosis, lymphangitis of the lung, a right-sided reactive pleural effusion (cytology showed no malignant cells), and metastasis in the left adrenal gland. (D–F) After 6 months of therapy with nivolumab, computed tomography shows nearly complete thoracic tumour regression and impressive regression of the adrenal gland metastasis.
After presenting this case to the tumour board of our institution, we initiated therapy with 3 mg/m2 nivolumab. That therapy was tolerated without any adverse effects. Fortunately, the clinical tumour symptoms decreased quickly. Follow-up ct imaging of the thorax and abdomen after 3 months of treatment showed a morphologic response of the lung and pleura. The patient experienced great improvement in quality of life and no more hypoxemic insufficiency.
Six months after the initiation of nivolumab, the patient remains in an improved stable clinical condition, and the most recent ct imaging of thorax showed almost complete thoracic tumour remission [Figure 1(C–F)].
Case 2
A 60-year-old man presented with a 10 cm tumour of the left lung, with chest wall infiltration. After transthoracic needle biopsy, the tumour was diagnosed as a sarcomatoid carcinoma. No distant metastases were found during staging procedures. The patient then underwent an extended left upper lobectomy with en bloc chest wall resection and complete resection of the tumour. The postoperative course was uneventful, and the patient underwent adjuvant chemotherapy consisting of cisplatin (80 mg/m2, day 1) and vinorelbine (25 mg/m2, days 1 and 8).
Two months after completion of chemotherapy, ct imaging showed a local tumour recurrence of the chest wall, and the tumour was again resected (R0 resection). Histopathology examination again found a sarcomatoid carcinoma with 100% PD-L1 expression. The patient was discharged without any complications.
After 2 months, the patient complained about progressive pain in the left chest wall. Imaging by ct revealed another tumour relapse of the chest wall and, in addition, of the left axillary region. A second line of therapy with nivolumab (3 mg/m2) was administered. After 3 cycles of nivolumab, we observed significant tumour shrinkage and impressive pain relief. After 8 months of therapy, ct imaging showed clear tumour remission (Figure 2).
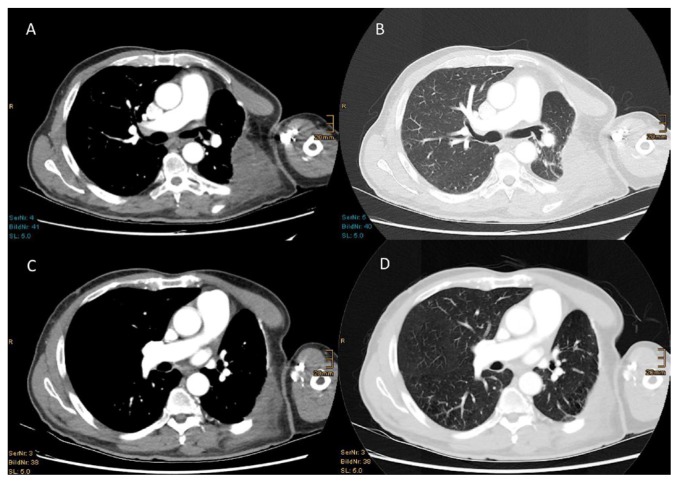
FIGURE 2.
(A,B) Computed tomography images 10 weeks after R0 resection show an extended tumour relapse at the left chest wall. (C,D) After 8 months of therapy with nivolumab, computed tomography shows significant regression of the thoracic tumour.
The patient is still in tumour care, but is asymptomatic and in excellent condition.
DISCUSSION
Sarcomatoid carcinomas of the lung are a rare form of nsclc, generally associated with poor prognosis. In this case series, we describe 2 patients with metastasized sarcomatoid lung carcinomas. Because both carcinomas showed high positivity for PD-L1, immunotherapy with nivolumab was initiated. That therapy resulted in fast and extensive tumour regression and relief of tumour symptoms, accompanied by an improvement in the general condition of both patients.
Our cases demonstrate high sensitivity of sarcomatoid lung carcinoma to nivolumab and, to some extent, indicate a therapeutic effect. In addition to the known effects of nivolumab in squamous cell cancers and adenocarcinomas of the lung, it might therefore also be a promising therapy for the rare tumours reported here.
The sarcomatoid histologic subtype of nsclc is usually considered to be chemoresistant; tumour control using a PD-1 inhibitor in these cases is therefore extremely valuable4. To the best of our knowledge, very few data about immunotherapy for sarcomatoid carcinomas are available.
High PD-L1 expression in sarcomatoid lung carcinomas has been described previously12,13. Velcheti et al.12 found that 9 of 13 patients with sarcomatoid carcinomas of the lung (69.2%) were positive for PD-L1 and that the observed levels were higher than those seen in conventional nsclcs. Considering several features of pscs such as PD-L1 expression and exuberant immune cell infiltration in tumours, immunotherapy targeted to the PD-1/PD-L1 pathway could be a therapeutic avenue for this disease. In our opinion, PD-L1 testing should be performed in these situations.
The use of antibodies against PD-1, which block inhibitory T-cell checkpoints, is a promising therapy for advanced cancers14. Nivolumab represents a new treatment option for patients requiring second-line treatment for metastatic nsclc, for which that drug has been well established15,16.
The correlation between PD-L1 staining and therapy —and particularly the efficiency of PD-L1 inhibitors—is currently a controversial topic. Although PD-L1 testing has no predictive value for determining the efficiency of second-line therapy with nivolumab for squamous cell carcinomas, many studies have shown a correlation between PD-L1 expression on tumour cells and tumour response rate, progression-free survival, and overall survival17,18. If PD-L1 expression exceeds 50%, therapy with checkpoint inhibitors shows superior efficiency compared with conventional chemotherapy. Furthermore, a study showed that therapy with pembrolizumab was superior to platin-based combination chemotherapy for the first-line therapy of tumours with PD-L1 expression greater than 50%19.
SUMMARY
As demonstrated in our cases, nivolumab should be considered as a therapeutic approach for sarcomatoid carcinomas, in which the drug might provide additional therapeutic benefits. However, patients and treating physicians must be aware of the spectrum of side effects associated with immunotherapies, and they have to carefully weigh the potential complications and risks of these new treatment options. Although it would be difficult to achieve, given the limited number of patients with psc, larger prospective randomized studies are needed to evaluate the potential benefits of immunotherapy for psc, possibly even as a first-line therapy.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: KK has received honoraria from Bristol–Myers Squibb, Merck Sharp and Dohme, and Roche. The other authors have no conflicts of interest to declare.
REFERENCES
- 1.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon, France: International Agency for Research on Cancer; 2015. pp. 88–94. [DOI] [PubMed] [Google Scholar]
- 2.Koss MN, Hochholzer L, Frommelt RA. Carcinosarcomas of the lung: a clinico-pathologic study of 66 patients. Am J Surg Pathol. 1999;23:1514–26. doi: 10.1097/00000478-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Roesel C, Terjung S, Weinreich G, et al. Sarcomatoid carcinoma of the lung: a rare histological subtype of non–small cell lung cancer with a poor prognosis even at earlier tumour stages. Interact Cardiovasc Thorac Surg. 2017;24:407–13. doi: 10.1093/icvts/ivw392. [DOI] [PubMed] [Google Scholar]
- 4.Vieira T, Girard N, Ung M, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol. 2013;8:1574–7. doi: 10.1097/01.JTO.0000437008.00554.90. [DOI] [PubMed] [Google Scholar]
- 5.Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery. 2012;152:397–402. doi: 10.1016/j.surg.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Bae HM, Min HS, Lee SH, et al. Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer. 2007;58:112–15. doi: 10.1016/j.lungcan.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. World J Surg Oncol. 2013;11:252. doi: 10.1186/1477-7819-11-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Italiano A, Cortot AB, Ilie M, et al. EGFR and KRAS status of primary sarcomatoid carcinomas of the lung: implications for anti-egfr treatment of a rare lung malignancy. Int J Cancer. 2009;125:2479–82. doi: 10.1002/ijc.24610. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Liu Y, Chen C, et al. The value of biomarkers in patients with sarcomatoid carcinomas of the lung: molecular analysis of 33 cases. Clin Lung Cancer. 2012;13:288–96. doi: 10.1016/j.cllc.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti–PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–65. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanazu M, Uenami T, Yano Y, et al. Case series of pleomorphic carcinomas of the lung treated with nivolumab. Thorac Cancer. 2017;8:724–8. doi: 10.1111/1759-7714.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) J Thorac Oncol. 2013;8:803–5. doi: 10.1097/JTO.0b013e318292be18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Kim MY, Koh J, et al. Programmed death-1 ligand 1 and 2 are highly expressed in pleomorphic carcinomas of the lung: comparison of sarcomatous and carcinomatous areas. Eur J Cancer. 2015;51:2698–707. doi: 10.1016/j.ejca.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garon EB, Rizvi NA, Hui R, et al. on behalf of the keynote-001 investigators. Pembrolizumab for the treatment of nonsmall-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 18.Fehrenbacher L, Spira A, Ballinger M, et al. on behalf of the poplar Study Group. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (poplar): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–46. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 19.Reck M, Rodríguez-Abreu D, Robinson AG, et al. on behalf of the keynote-024 investigators. Pembrolizumab versus chemotherapy for PD-L1–positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]