Abstract
Objectives
To investigate the epidemiology of herpes simplex virus type 1 (HSV-1) in Latin America and the Caribbean.
Methods
Systematic review and meta-analytics guided by the Cochrane Collaboration Handbook and reported following the PRISMA guidelines.
Results
Thirty-three relevant reports were identified including 35 overall (and 95 stratified) seroprevalence measures, and five and nine proportions of virus isolation in genital ulcer disease (GUD) and in genital herpes, respectively. Pooled mean seroprevalence was 57.2% (95% CI: 49.7–64.6%) among children and 88.4% (95% CI: 85.2–91.2%) among adults. Pooled mean seroprevalence was lowest at 49.7% (95% CI: 42.8–56.6%) in those aged ≤10, followed by 77.8% (95% CI: 67.9–84.8%) in those aged 10–20, 82.8% (95% CI: 73.1–90.8%) in those aged 20–30, 92.5% (95% CI: 89.4–95.1%) in those aged 30–40, and 94.2% (95% CI: 92.7–95.5%) in those aged ≥40. Age was the strongest source of heterogeneity in seroprevalence, explaining 54% of variation. Evidence was found for seroprevalence decline over time. Pooled mean proportion of HSV-1 isolation was 0.9% (95% CI: 0.0–3.6%) in GUD and 10.9% (95% CI: 4.4–19.4%) in genital herpes.
Conclusions
HSV-1 is a widely prevalent infection in this region, but its epidemiology may be slowly transitioning, with still limited contribution for HSV-1 in genital herpes.
Introduction
Infection with herpes simplex virus type 1 (HSV-1) is prevalent globally [1]. HSV-1 is responsible for a range of mild to serious morbidities [2, 3], with its typical clinical manifestation being orolabial herpes lesions [2, 4]. The infection, lifelong and mostly asymptomatic, is usually acquired orally and in childhood [3]. However, mounting evidence suggests an HSV-1 epidemiological transition in Europe and North America [4–7] and in Asia [8], associated with decreasing oral acquisition in childhood and increasing sexual acquisition (through oral sex) in adulthood [4–6]. In multiple Western countries, HSV-1 is already the primary cause of first episode genital herpes, surpassing the role of that of HSV-2 [4, 5, 7, 9–11]. An epidemiological transition is defined here as a significant change in the occurrence of the infection and/or its mode of transmission patterns.
HSV-1 infection is of growing interest and a focus of an international multi-sectorial effort, guided by the World Health Organization, to develop a vaccine to control infection transmission [12, 13]. To inform these global health efforts, we aimed in the present study to provide a detailed investigation of the epidemiology of HSV-1 in Latin America and the Caribbean, by conducting a comprehensive systematic review and a range of meta-analytics. Importantly, we estimated HSV-1 antibody prevalence (seroprevalence), its associations and temporal trend, and assessed the role of HSV-1 as a cause of clinically-diagnosed genital ulcer disease (GUD) and clinically-diagnosed genital herpes.
Material and methods
The methodology of this study was adapted from that of a study investigating HSV-1 epidemiology in Asia [8].
Data sources and search strategy
The systematic review and meta-analyses were guided by the Cochrane collaboration Handbook [14], and were reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (checklist in S1 Table) [15]. PubMed, Embase, and LILACS databases were systematically searched up to September 12, 2018. The search strategies included MeSH/Emtree and broad terms with no language or year restrictions (S2 Table). The definition for the Latin America and the Caribbean region included 46 countries, as listed in S1 Box.
Study selection and inclusion and exclusion criteria
Search results were de-duplicated using a reference manager, Endnote (Thomson Reuters, USA). Titles and abstracts were screened for relevant and potentially relevant reports, and the full-texts of these relevant or potentially relevant reports were retrieved for further screening. Bibliographies of identified relevant reports and reviews were also screened for additional potentially relevant reports. Initial screening was conducted by LS and MA, and double screening was conducted by MH.
Reports met the inclusion criteria if they reported primary data on any of three outcome measures: 1) HSV-1 seroprevalence based on a valid diagnostic method (i.e. strictly type-specific glycoprotein-G based assays), 2) proportion of HSV-1 virus isolation in clinically-diagnosed GUD, or 3) proportion of HSV-1 virus isolation in clinically-diagnosed genital herpes.
Only measures with a sample size ≥10 were included. Case reports, editorials, letters to editors, commentaries, and reviews were excluded. HSV-1 seroprevalence measures among newborns <3 months of age were excluded, as they may reflect maternal antibodies as opposed to current infection.
In this systematic review, a “report” denotes a publication reporting a relevant outcome measure, while a “study” denotes the extracted details of an outcome measure.
Data extraction and synthesis
Relevant reports were extracted by LS and MA, and double-extracted by MH. Extracted data included publication details, population characteristics, study methodology characteristics, and outcome measures. The extracted variables are listed in S2 Box. Extracted overall outcome measures for the full sample were replaced by stratified measures (if available), based on a pre-defined protocol for the stratification hierarchy, provided that the sample size in each stratum was ≥10.
For HSV-1 seroprevalence measures, extracted strata were prioritized for population type (Fig 1), followed by age bracket (children (≤15 years of age) versus adults (>15 years of age)), and age group (≤10, 10–20, 20–30, 30–40, and ≥40 years of age). These age ranges were informed by the actually available age strata in extracted studies. For the proportions of HSV-1 virus isolation in GUD or in genital herpes, the stratification hierarchy included primary versus recurrent episode, followed by study site (hospital versus sexually transmitted infection clinic).
Fig 1. Population type definition and classification.
Abbreviation: HSV-1 = Herpes simplex virus type 1.
Quality assessment
Given the documented limitations in the sensitivity and specificity of HSV-1 serology diagnostic assays [16, 17], the validity of the type-specific diagnostic method of each study was investigated and determined in consultation with an expert advisor in HSV-1 serology, Professor Rhoda Ashley-Morrow, University of Washington, Seattle. Studies where the validity of the diagnostic method could not be confirmed, were excluded from the systematic review and meta-analytics.
Informed by the Cochrane approach [14], studies with valid assays were further classified into low versus high precision based on the number of individuals tested for HSV-1 in that study (<100 versus ≥100). Moreover, studies were classified into low versus high risk of bias (ROB) using two quality domains: sampling method (probability-based versus non-probability-based sampling) and response rate (≥80% versus <80%). Studies with no information on a quality domain were classified as having an “unclear” ROB for that domain.
Precision and ROB domains were included in the meta-regression analyses (as described below), to examine their associations with seroprevalence, that is the influence of the characteristics of the study methodology on observed HSV-1 seroprevalence.
Meta-analyses
Pooled means were estimated for HSV-1 seroprevalence and its relevant strata by population type, age bracket, age group, and year of publication category (<2000, 2000–2009, and 2010–2018), as well as for the proportions of HSV-1 virus isolation in GUD and in genital herpes, whenever ≥3 measures were available. The estimates were calculated in R version 3.4.1 [18] using a DerSimonian-Laird random-effects model [19], as applied in the meta package [20]. The Freeman-Tukey type arcsine square-root transformation [21] was utilized to stabilize the variance of each included measure. Forest plots were produced to visualise estimates and their 95% confidence intervals (CIs).
Heterogeneity was assessed using three complementary metrics: 1) Cochrane Q statistics to test for existence of heterogeneity [19, 22], 2) I2 to provide the magnitude of heterogeneity that is explained by true differences in the outcome measures across studies (as opposed to being due to sampling variation) [19, 23], and 3) prediction interval to provide the range of true effect sizes of the outcome measures around the pooled mean [19, 23].
Meta-regressions
Associations with HSV-1 seroprevalence and sources of between-study heterogeneity were investigated using univariable and multivariable random-effects meta-regression analyses. Independent variables with a p-value ≤0.1 in univariable analysis were included in the multivariable analyses. In the multivariable models, a p-value of ≤0.05 for any given independent variable indicated strong evidence for an association with HSV-1 seroprevalence.
The included independent variables were set a priori and consisted of: age bracket, age group, sex, population type, country’s income, assay type (Western blot, enzyme-linked immunosorbent assay, and others), sample size (<100 versus ≥100), sampling method (non-probability-based versus probability-based), response rate (≥80 versus otherwise), year of publication category, year of data collection, and year of publication.
The variable of country’s income (for countries with available data and per World Bank classification [24]) categorized the countries into upper-middle-income countries (Brazil, Colombia, Costa Rica, Jamaica, Mexico, and Peru), high-income countries (Barbados, Chile, and Argentina), and “mixed” for studies including different countries in the study sample.
Missing values for the year of data collection were imputed utilizing data for the year of publication as adjusted by the median difference between year of publication and year of data collection (for studies with non-missing data).
The meta-regressions were conducted on the log-transformed proportions (with inverse-variance weighting) in Stata/SE version 13 [25], using the metareg package [26].
Results
Search results and scope of evidence
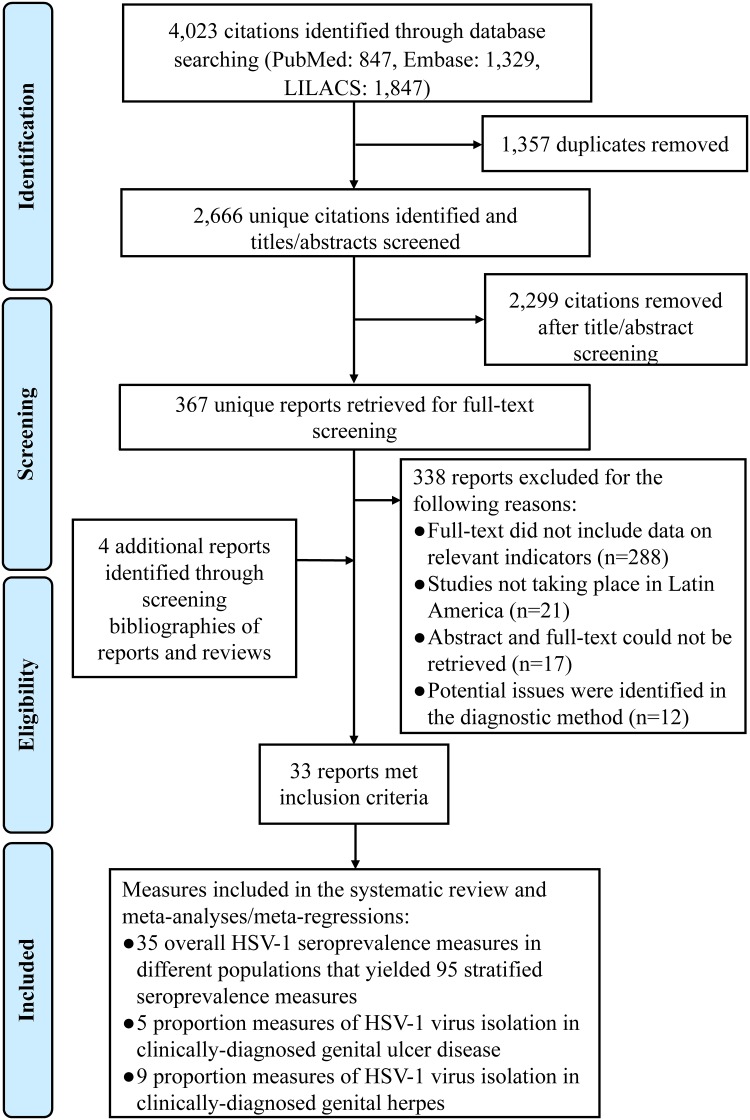
Fig 2 details the study selection process per PRISMA guidelines [15]. The search identified 4,023 citations (PubMed: 847, Embase: 1,329, and LILACS 1,847) of which duplicates were removed. Title and abstract screening yielded 367 relevant and potentially relevant reports. Full-text screening of these latter reports identified 29 reports that met the inclusion criteria. Four additional relevant reports [27–30] were identified through bibliography screening of reviews and relevant reports.
Fig 2. Flow chart of article selection for the systematic review of HSV-1 infection in Latin America and the Caribbean, per the PRISMA guidelines [15].
Abbreviation: HSV-1 = Herpes simplex virus type 1.
Extracted measures included: 35 overall HSV-1 seroprevalence measures yielding 95 stratified seroprevalence measures, five proportions of viral HSV-1 isolation in GUD, and nine proportions of viral HSV-1 isolation in genital herpes. No HSV-1 seroprevalence measure was identified among clinical children populations.
Overview of HSV-1 seroprevalence
Table 1 lists the extracted stratified HSV-1 seroprevalence measures and their characteristics (number of measures (n) = 95). Most measures were from studies conducted prior to 2010 (n = 76; 80.0%), and were based on convenience samples (n = 68; 71.0%). Seroprevalence across all measures ranged between 7.7–100% with a median of 86.0% (n = 95; Table 2).
Table 1. Studies reporting HSV-1 seroprevalence in Latin America and the Caribbean.
| Author, year | Year(s) of data collection | Country | Study site | Study design | Sampling method | Population | HSV-1 serological assay | Sample size | HSV-1 seroprevalence (%) |
|---|---|---|---|---|---|---|---|---|---|
| Healthy children populations (n = 19) | |||||||||
| Clemens, 2010 [43] | 1996–97 | Brazil | Community | CS | RS | 1–5 years old boys | ELISA | 52 | 44.2 |
| Clemens, 2010 [43] | 1996–97 | Brazil | Community | CS | RS | 6–10 years old boys | ELISA | 49 | 55.1 |
| Clemens, 2010 [43] | 1996–97 | Brazil | Community | CS | RS | 11–15 years old boys | ELISA | 125 | 65.6 |
| Clemens, 2010 [43] | 1996–97 | Brazil | Community | CS | RS | 1–5 years old girls | ELISA | 47 | 38.3 |
| Clemens, 2010 [43] | 1996–97 | Brazil | Community | CS | RS | 6–10 years old girls | ELISA | 50 | 58.0 |
| Clemens, 2010 [43] | 1996–97 | Brazil | Community | CS | RS | 11–15 years old girls | ELISA | 126 | 74.6 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | 1–9 years old girls | ELISA | 252 | 51.0 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | 1–9 years old boys | ELISA | 264 | 50.0 |
| Cowan, 2003 [45] | - | Brazil | Outpatient clinic | CS | Conv | 1–4 years old children | ELISA | 232a | 36.0 |
| Cowan, 2003 [45] | - | Brazil | Outpatient clinic | CS | Conv | 5–9 years old children | ELISA | 232a | 52.4 |
| Cowan, 2003 [45] | - | Brazil | Outpatient clinic | CS | Conv | 10–14 years old children | ELISA | 233a | 68.1 |
| De Salles-Gomes, 1981 [46] | 1980 | Brazil | Outpatient clinic | CS | Conv | 7–11 month babies | IF | 13 | 7.7 |
| De Salles-Gomes, 1981 [46] | 1980 | Brazil | Outpatient clinic | CS | Conv | 1–4 years old children | IF | 50 | 38.0 |
| De Salles-Gomes, 1981 [46] | 1980 | Brazil | Outpatient clinic | CS | Conv | 5–9 years old children | IF | 50 | 64.0 |
| De Salles-Gomes, 1981 [46] | 1980 | Brazil | Outpatient clinic | CS | Conv | 10–14 years old children | IF | 50 | 92.0 |
| Robinson, 2002 [47] | - | Multiple countries in South America | Community | CS | Conv | ≤3 years old children | WB | 23 | 29.0 |
| Robinson, 2002 [47] | - | Multiple countries in South America | Community | CS | Conv | 4–6 years old children | WB | 56 | 72.0 |
| Robinson, 2002 [47] | - | Multiple countries in South America | Community | CS | Conv | 7–9 years old children | WB | 68 | 76.0 |
| Robinson, 2002 [47] | - | Multiple countries in South America | Community | CS | Conv | 10–13 years old children | WB | 54 | 81.0 |
| Healthy adult populations (n = 51) | |||||||||
| Arriaga-Demeza, 2008 [48] | 2002–03 | Mexico | Community | CS | Conv | 18–20 years old females | WB | 195 | 50.3 |
| Arriaga-Demeza, 2008 [48] | 2002–03 | Mexico | Community | CS | Conv | 21–25 years old females | WB | 153 | 53.6 |
| Arriaga-Demeza, 2008 [48] | 2002–03 | Mexico | Community | CS | Conv | ≥26 years old females | WB | 31 | 74.2 |
| Arriaga-Demeza, 2008 [48] | 2002–03 | Mexico | Community | CS | Conv | 18–20 years old males | WB | 103 | 46.6 |
| Arriaga-Demeza, 2008 [48] | 2002–03 | Mexico | Community | CS | Conv | 21–25 years old males | WB | 102 | 61.8 |
| Arriaga-Demeza, 2008 [48] | 2002–03 | Mexico | Community | CS | Conv | ≥26 years old males | WB | 18 | 55.6 |
| Morrow, 2014 [16] | 2000–01 | Argentina | Community | CS | Conv | Argentinian women | WB | 99 | 98.9 |
| Morrow, 2014 [16] | 2000–01 | Costa Rica | Community | CS | Conv | Costa Rican women | WB | 98 | 92.9 |
| Morrow, 2014 [16] | 2000–01 | Mexico | Community | CS | Conv | Mexican women | WB | 100 | 98.0 |
| Clemens, 2010 [43] | 1996–97 | Brazil | Community | CS | RS | 16–20 years old males | ELISA | 119 | 69.8 |
| Clemens, 2010 [43] | 1996–97 | Brazil | Community | CS | RS | 21–30 years old males | ELISA | 107 | 76.6 |
| Clemens, 2010 [43] | 1996–97 | Brazil | Community | CS | RS | 31–40 years old males | ELISA | 78 | 85.9 |
| Clemens, 2010 [43] | 1996–97 | Brazil | Community | CS | RS | 16–20 years old females | ELISA | 128 | 75.8 |
| Clemens, 2010 [43] | 1996–97 | Brazil | Community | CS | RS | 21–30 years old females | ELISA | 126 | 81.0 |
| Clemens, 2010 [43] | 1996–97 | Brazil | Community | CS | RS | 31–40 years old females | ELISA | 82 | 81.7 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | 20–29 years old females | ELISA | 252a | 78.0 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | 30–39 years old females | ELISA | 252a | 96.0 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | 40–49 years old female | ELISA | 252a | 91.0 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | 50–59 years old females | ELISA | 252a | 98.0 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | ≥60 years old females | ELISA | 252a | 95.0 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | 20–29 years old males | ELISA | 264a | 91.0 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | 30–39 years old males | ELISA | 264a | 91.0 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | 40–49 years old male | ELISA | 264a | 93.0 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | 50–59 years old males | ELISA | 264a | 95.0 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | ≥60 years old males | ELISA | 264a | 94.0 |
| Corona, 2010 [28] | 2002–05 | Mexico | Community | CS | Conv | ≥ 26 years old students | ELISA | 59 | 72.9 |
| Corona, 2010 [28] | 2002–05 | Mexico | Community | CS | Conv | 21–25 years old students | ELISA | 412 | 59.7 |
| Corona, 2010 [28] | 2002–05 | Mexico | Community | CS | Conv | 18–20 years old students | ELISA | 335 | 50.1 |
| Cowan, 2003 [45] | - | Brazil | Outpatient clinic | CS | Conv | 15–19 years old adults | ELISA | 146 | 83.3 |
| Cowan, 2003 [45] | - | Brazil | Outpatient clinic | CS | Conv | 20–29 years old adults | ELISA | 147a | 83.6 |
| Cowan, 2003 [45] | - | Brazil | Outpatient clinic | CS | Conv | 30–34 years old adults | ELISA | 147a | 95.2 |
| Cowan, 2003 [45] | - | Brazil | Outpatient clinic | CS | Conv | 35–39 years old adults | ELISA | 147a | 92.9 |
| Cowan, 2003 [45] | - | Brazil | Outpatient clinic | CS | Conv | 40–44 years old adults | ELISA | 147a | 96.0 |
| Cowan, 2003 [45] | - | Brazil | Outpatient clinic | CS | Conv | ≥45 years old adults | ELISA | 147a | 94.6 |
| De Salles-Gomes, 1981 [46] | 1980 | Brazil | Outpatient clinic | CS | Conv | 15–19 years old adults | IF | 50 | 90.0 |
| De Salles-Gomes, 1981 [46] | 1980 | Brazil | Outpatient clinic | CS | Conv | 20–24 years old adults | IF | 50 | 84.0 |
| De Salles-Gomes, 1981 [46] | 1980 | Brazil | Outpatient clinic | CS | Conv | 25–29 years old adults | IF | 50 | 86.0 |
| De Salles-Gomes, 1981 [46] | 1980 | Brazil | Outpatient clinic | CS | Conv | 30–34 years old adults | IF | 60 | 98.3 |
| De Salles-Gomes, 1981 [46] | 1980 | Brazil | Outpatient clinic | CS | Conv | 35–39 years old adults | IF | 50 | 90.0 |
| De Salles-Gomes, 1981 [46] | 1980 | Brazil | Outpatient clinic | CS | Conv | ≥40 years old adults | IF | 50 | 96.0 |
| Evans, 1974 [49] | - | Brazil | Outpatient clinic | CCC | Conv | Healthy adults | IF | 26 | 87.5 |
| Jimemez, 1979 [50] | - | Costa Rica | Outpatient clinic | CS | Conv | ≥18 years old students | NAb | 16 | 50.0 |
| Levett, 2005 [51] | - | Barbados | Outpatient clinic | CS | Conv | Blood donors | ELISA | 184 | 81.0 |
| Levett, 2005 [51] | - | Barbados | Outpatient clinic | CS | Conv | Ante-natal clinic attendees | ELISA | 122 | 83.6 |
| Lupi, 2011 [52] | 1996–97 | Brazil | Outpatient clinic | Cohortb | Conv | Blood donors | ELISA | 155 | 68.0 |
| Oberle, 1989 [53] | 1984–85 | Costa Rica | Community | CS | MCS | ≥25 years old females | MAb | 766 | 97.1 |
| Patnaik, 2007 [54] | 1985–97 | Peru | Community | CS | Conv | Peruvian women | WB | 171 | 91.8 |
| Patnaik, 2007 [54] | 1985–97 | Colombia | Community | CS | Conv | Colombian women | WB | 65 | 89.2 |
| Prabhakar, 1984 [55] | - | Jamaica | Hospital | CCC | Conv | Healthy Jamaican women | NAb | 60 | 38.3 |
| Smith, 2002 [29] | 1996–97 | Peru | Hospital | CCC | Conv | Healthy Peruvian women | WB | 171 | 91.8 |
| Smith, 2002 [29] | 1985–88 | Colombia | Community | CCC | Conv | Healthy Colombian women | WB | 65 | 89.2 |
| Healthy age-mixed populations (n = 2) | |||||||||
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | 10–19 years old females | ELISA | 252 | 70.0 |
| Conde-Glez, 2013 [44] | 2005–06 | Mexico | Community | CS | RS | 10–19 years old males | ELISA | 264 | 71.0 |
| Clinical adult populations (n = 7) | |||||||||
| Calderon, 2018 [56] | 2014–15 | Peru | Outpatient clinic | CS | Conv | Women with breast cancer | ELISA | 44 | 88.6 |
| Evans, 1974 [49] | - | Brazil | Outpatient clinic | CCC | Conv | Patients with Hodgkin’s disease | IF | 26 | 84.4 |
| Moreira, 2018 [57] | 2015–16 | Brazil | Outpatient clinic | CCC | Conv | Women from a highly ZIKV-affected region | WB | 32 | 93.8 |
| Moreira, 2018 [57] | 2015–16 | Brazil | Outpatient clinic | CCC | Conv | Women from a highly ZIKV-affected region | WB | 160 | 95.0 |
| Smith, 2002 [29] | 1996–97 | Peru | Hospital | CCC | Conv | Women with squamous-cell carcinoma | WB | 166 | 91.5 |
| Smith, 2002 [29] | 1996–97 | Peru | Hospital | CCC | Conv | Women with adeno-squamous carcinoma | WB | 24 | 100 |
| Smith, 2002 [29] | 1985–88 | Colombia | Hospital | CCC | Conv | Women with squamous-cell carcinoma | WB | 78 | 74.4 |
| Other populations (n = 16) | |||||||||
| Levett, 2005 [51] | - | Barbados | Outpatient clinic | CS | Conv | HIV-positive adults | ELISA | 120 | 89.2 |
| Luchsinger, 2010 [58] | 2005–06 | Chile | Outpatient clinic | CS | Conv | HIV-positive adults | ELISA | 400 | 92.2 |
| Boulos, 1992 [59] | - | Haiti | Outpatient clinic | CS | Conv | Healthy/clinical women | ELISA | 228 | 96.9 |
| Conde-Glez, 1999 [60] | 1992 | Mexico | Outpatient clinic | CS | Conv | 16–22 years old FSWs | WB | 302 | 92.7 |
| Conde-Glez, 1999 [60] | 1992 | Mexico | Outpatient clinic | CS | Conv | 23–27 years old FSWs | WB | 330 | 93.1 |
| Conde-Glez, 1999 [60] | 1992 | Mexico | Outpatient clinic | CS | Conv | 28–32 years old FSWs | WB | 187 | 94.7 |
| Conde-Glez, 1999 [60] | 1992 | Mexico | Outpatient clinic | CS | Conv | 33–37 years old FSWs | WB | 101 | 94.1 |
| Conde-Glez, 1999 [60] | 1992 | Mexico | Outpatient clinic | CS | Conv | >37 years old FSWs | WB | 77 | 100 |
| Duenas, 1972 [61] | - | Colombia | Outpatient clinic | CS | Conv | 14–15 years old FSWs | NAb | 15 | 100 |
| Duenas, 1972 [61] | - | Colombia | Outpatient clinic | CS | Conv | 16–17 years old FSWs | NAb | 56 | 100 |
| Duenas, 1972 [61] | - | Colombia | Outpatient clinic | CS | Conv | 18–19 years old FSWs | NAb | 43 | 100 |
| Duenas, 1972 [61] | - | Colombia | Outpatient clinic | CS | Conv | 20–21 years old FSWs | NAb | 34 | 100 |
| Duenas, 1972 [61] | - | Colombia | Outpatient clinic | CS | Conv | 22–25 years old FSWs | NAb | 46 | 100 |
| Duenas, 1972 [61] | - | Colombia | Outpatient clinic | CS | Conv | 26–35 years old FSWs | NAb | 95 | 100 |
| Duenas, 1972 [61] | - | Colombia | Outpatient clinic | CS | Conv | ≥36 years old FSWs | NAb | 54 | 100 |
| Lupi, 2011 [52] | 1996–97 | Brazil | Hospital | Cohortb | Conv | Men who have sex with men | ELISA | 170 | 85.0 |
a Study included sample size only for the total sample, but not for the strata. Each stratum sample size was set at total sample size divided by the number of strata.
b The original study design of the study is prospective cohort. The included seroprevalence measures are those for the baseline measures at the onset of the study, before start of follow-up.
c The original study design of the study is case-control. The included seroprevalence measures are those for each of cases and controls, separately. The population type classification was assigned based on the actual population type for each of cases and controls, separately.
Abbreviations: Conv = Convenience, CS = Cross-sectional, CC = Case-control, ELISA = Enzyme-linked immunosorbent type-specific assay, FSWs = Female sex workers, HIV = Human immunodeficiency virus, HSV-1 = Herpes simplex virus type 1, IF = Indirect immunofluorescence, MAb = Monoclonal antibody, MCS = Multistage cluster sampling, NAb = Neutralizing antibody, RS = Random sampling, WB = Western blot, ZIKV = Zika virus.
Table 2. Pooled mean estimates for HSV-1 seroprevalence in Latin America and the Caribbean.
| Population type | Outcome measures | Samples | HSV-1 seroprevalence | Pooled mean HSV-1 seroprevalence | Heterogeneity measures | |||
|---|---|---|---|---|---|---|---|---|
| Total n |
Total N |
Range | Median | Mean (95% CI) |
Qa (p-value) |
I2b (%) (95% CI) |
Predictionc Interval (%) | |
| Healthy general populations | ||||||||
| Children | 19 | 2,026 | 7.7–92.0 | 55.1 | 57.2 (49.7–64.6) | 190.8 (p<0.001) | 90.6 (86.8–93.3) | 24.7–86.7 |
| Adults | 51 | 7,917 | 38.3–98.9 | 87.6 | 84.5 (79.9–88.5) | 1,323.5 (p<0.001) | 96.2 (95.7–96.8) | 46.1–100 |
| Age-mixed | 2 | 516 | 70.0–71.0 | 70.5 | 70.3 (66.2–74.2) d | - | - | - |
| All healthy general populations | 72 | 10,459 | 7.7–98.9 | 81.0 | 77.7 (72.9–82.2) | 2,269.1 (p<0.001) | 96.9 (96.5–97.3) | 32.6–100 |
| Clinical populations | ||||||||
| Adults | 7 | 530 | 74.4–100 | 91.5 | 90.9 (84.2–95.9) | 25.9 (p<0.001) | 76.8 (51.5–88.9) | 65.5–100 |
| All clinical populations | 7 | 530 | 74.4–100 | 91.5 | 90.9 (84.2–95.9) | 25.9 (p<0.001) | 76.8 (51.5–88.9 | 65.5–100 |
| Other populations | ||||||||
| HIV positive patients | 2 | 520 | 89.2–92.2 | 90.7 | 91.5 (88.8–93.7)d | - | - | - |
| Female sex workers | 12 | 1,340 | 93.1–100 | 100 | 98.5 (96.4–99.8) | 46.3 (p<0.001) | 76.2 (58.4–96.4) | 88.4–100 |
| Men who have sex with men | 1 | 170 | - | - | 85.3 (79.1–90.2)d | - | - | - |
| Mixed healthy/clinical adults populations | 1 | 228 | - | - | 96.9 (93.8–98.7)d | - | - | - |
| Age group | ||||||||
| ≤10 years | 14 | 1,438 | 7.7–76.0 | 50.5 | 49.7 (42.8–56.6) | 76.4 (p<0.001) | 83.0 (72.7–89.4) | 24.8–74.7 |
| 10–20 years | 17 | 2,294 | 46.6–100 | 74.6 | 77.8 (67.9–84.8) | 280.8 (p<0.001) | 94.3 (92.2–95.8) | 40.0–99.5 |
| 20–30 years | 12 | 1,926 | 53.6–100 | 82.5 | 82.8 (73.1–90.8) | 276.9 (p<0.001) | 96.0 (94.5–97.2) | 39.1–100 |
| 30–40 years | 9 | 1,181 | 81.7–98.3 | 92.9 | 92.5 (89.4–95.1) | 24.6 (p = 0.002) | 67.4 (34.3–83.8) | 81.4–99.0 |
| ≥40 years | 11 | 2,128 | 89.2–98.0 | 94.6 | 94.2 (92.7–95.5) | 17.5 (p = 0.064) | 42.9 (0.0–71.8) | 89.9–97.4 |
| Mixed | 32 | 4,280 | 38.3–100 | 90.3 | 89.6 (85.7–93.9) | 405.7 (p<0.001) | 92.4 (90.2–94.0) | 62.9–100 |
| Age bracket | ||||||||
| All children | 19 | 2,026 | 7.7–92.0 | 55.1 | 57.2 (49.7–64.6) | 190.8 (p<0.001) | 90.6 (86.8–93.3) | 24.7–86.7 |
| All adults | 73 | 10,690 | 38.3–100 | 91.0 | 88.4 (85.2–91.2) | 1,588.2 (p<0.001) | 95.5 (94.8–96.0) | 54.8–100 |
| All age-mixed | 3 | 531 | 70.0–100 | 71.0 | 77.5 (65.8–87.5) | 12.3 (p = 0.002) | 83.7 (50.8–94.6) | 0.0–100 |
| Year of publication category | ||||||||
| <2000 | 28 | 2,935 | 7.7–100 | 93.6 | 90.8 (85.8–94.9) | 394.7 (p<0.001) | 93.2 (91.2–94.7) | 57.1–100 |
| 2000–2009 | 32 | 3,844 | 29.0–100 | 83.0 | 80.7 (73.6–87.0) | 847.7 (p<0.001) | 96.3 (95.6–97.0) | 34.0–100 |
| 2010–2018 | 35 | 6,468 | 38.3–98.0 | 78.0 | 78.8 (72.7–84.3) | 1,113.4 (p<0.001) | 96.9 (96.4–97.4) | 37.4–100 |
| All studies | 95 | 13,335 | 7.7–100 | 86.0 | 83.1 (79.3–86.5) | 2,772.4 (p<0.001) | 96.6 (96.2–97.0) | 40.2–100 |
a Q: The Cochran’s Q statistic is a measure assessing the existence of heterogeneity in pooled outcome measures, here HSV-1 seroprevalence.
b I2: A measure assessing the magnitude of between-study variation that is due to true differences in HSV-1 seroprevalence across studies rather than sampling variation.
c Prediction interval: A measure quantifying the distribution 95% interval of true HSV-1 seroprevalence around the estimated pooled mean.
d No meta-analysis was done as number of studies was <3. If there was only one study, the reported 95% CI is the 95% CI of this study. If there were two studies, both samples were merged to yield one sample size, for which the 95% CI was calculated.
Abbreviations: CI = Confidence interval, HIV = Human immunodeficiency virus, HSV-1 = Herpes simplex virus type 1.
HSV-1 seroprevalence ranged between 7.7–92.0% with a median of 55.1% among healthy children populations (n = 19), between 38.3–98.9% with a median of 87.6% among healthy adult populations (n = 51), and between 74.4–100% with a median of 91.5% among clinical adult populations (n = 7). Table 2 lists summaries for other population categories.
Pooled mean estimates for HSV-1 seroprevalence
Table 2 displays the results of the meta-analyses. The overall pooled mean HSV-1 seroprevalence (n = 95) was 83.1% (95% CI: 79.2–86.5%).
The pooled mean HSV-1 seroprevalence was 57.2% (95% CI: 49.7–64.6%) among healthy children populations, 84.5% (95% CI: 79.9–88.5%) among healthy adult populations, and 90.9% (95% CI: 84.2–95.9%) among clinical adult populations.
The pooled mean seroprevalence increased with age. It was lowest at 49.7% (n = 14; 95% CI: 42.8–56.6%) in those aged ≤10, followed by 77.8% (n = 17; 95% CI: 67.9–84.8%) in those aged 10–20, 82.8% (n = 12; 95% CI: 73.1–90.8%) in those aged 20–30, 92.5% (n = 9; 95% CI: 89.4–95.1%) in those aged 30–40, and 94.2% (n = 11; 95% CI: 92.7–95.5%) in those aged ≥40.
The pooled mean seroprevalence decreased with time. It was highest at 90.8 (95% CI: 85.8–94.9%) before the year 2000, followed by 80.7% (95% CI: 73.6–87.0%) in 2000–2009, and 78.8% (95% CI: 72.7–84.3) in 2010–2018.
Forest plots for all adult populations and all children populations can be found in S1 Fig. All meta-analyses showed evidence of heterogeneity (Table 2). Heterogeneity was attributed to true variability in seroprevalence across studies rather than chance (Table 2). The heterogeneity was affirmed by the wide prediction intervals (Table 2).
Predictors of HSV-1 seroprevalence
Table 3 and S3 Table display the results of the univariable and multivariable analyses. In the univariable analyses, age bracket, age group, sex, population type, year of publication category, year of data collection, and year of publication qualified to be included in the multivariable analysis (p<0.1). Country’s income, assay type, response rate, sample size, and sampling method all had a p-value >0.1, and hence, were not included in the multivariable analyses.
Table 3. Univariable and multivariable meta-regression models for HSV-1 seroprevalence in Latin America and the Caribbean.
| Outcome measures | Samples | Univariable analysis | Multivariable analysisa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1a | Model 2b | ||||||||||
| Total n | Total N | RR (95%CI) | p-value | Adjusted R2 (%) | ARR (95%CI) | p-value | ARR (95%CI) | p-value | |||
| Population Characteristics | Age bracket | Children | 19 | 2,026 | 1.00 | - | 1.00 | - | - | - | |
| Adults | 73 | 10,690 | 1.45 (1.29–1.64) | <0.001 | 1.39 (1.24–1.57) | <0.001 | - | - | |||
| Age-mixed | 3 | 531 | 1.35 (1.04–1.75) | 0.022 | 35.37 | 1.30 (1.00–1.67) | 0.042 | - | - | ||
| Age group | ≤10 | 14 | 1,438 | 1.00 | - | - | - | 1.00 | - | ||
| 10–20 | 17 | 2,294 | 1.44 (1.24–1.67) | <0.001 | - | - | 1.36 (1.19–1.56) | <0.001 | |||
| 20–30 | 12 | 1,926 | 1.53 (1.31–1.79) | <0.001 | - | - | 1.44 (1.25–1.65) | <0.001 | |||
| 30–40 | 9 | 1,181 | 1.76 (1.49–2.08) | <0.001 | - | - | 1.70 (1.47–1.97) | <0.001 | |||
| ≥40 | 11 | 2,128 | 1.81 (1.54–2.11) | <0.001 | - | - | 1.81 (1.58–2.08) | <0.001 | |||
| Mixed | 32 | 4,280 | 1.68 (1.47–1.93) | <0.001 | 53.99 | - | - | 1.54 (1.35–1.75) | <0.001 | ||
| Sex | Female | 46 | 6,723 | 1.00 | - | 1.00 | - | 1.00 | - | ||
| Male | 17 | 2,771 | 0.86 (0.75–1.00) | 0.053 | 0.96 (0.85–1.09) | 0.572 | 0.97 (0.88–1.07) | 0.557 | |||
| Mixed | 32 | 3,751 | 0.93 (0.82–1.05) | 0.277 | 3.62 | 1.03 (0.92–1.14) | 0.618 | 1.00 (0.92–1.09) | 0.956 | ||
| Population type | Healthy | 72 | 10,456 | 1.00 | - | 1.00 | - | 1.00 | - | ||
| Clinical | 7 | 530 | 1.19 (0.99–1.43) | 0.062 | 1.10 (0.93–1.29) | 0.249 | 1.12 (0.97–1.28) | 0.116 | |||
| Other | 16 | 2,258 | 1.28 (1.12–1.45) | <0.001 | 17.13 | 1.15 (1.01–1.31) | 0.035 | 1.16 (1.04–1.29) | 0.006 | ||
| Country’s income | UMIC | 85 | 11,891 | 1.00 | - | - | - | - | - | ||
| HIC | 5 | 925 | 1.12 (0.88–1.42) | 0.324 | - | - | - | - | |||
| Otherc | 5 | 429 | 0.95 (0.73–1.22) | 0.665 | 0.00 | - | - | - | - | ||
| Study methodology characteristics | Assay type | Western blot | 27 | 3,029 | 1.00 | - | - | - | - | - | |
| ELISA | 46 | 8,508 | 0.93 (0.82–1.05) | 0.277 | - | - | - | - | |||
| Others | 22 | 1,710 | 1.05 (0.90–1.22) | 0.496 | 4.83 | - | - | - | - | ||
| Sample sized | <100 | 13 | 791 | 1.00 | - | - | - | - | - | ||
| ≥100 | 82 | 12,454 | 0.93 (0.75–1.08) | 0.364 | 0.26 | - | - | - | - | ||
| Sampling method | Non-probability-based | 69 | 8,536 | 1.00 | - | - | - | - | - | ||
| Probability-based | 26 | 4,701 | 0.93 (0.82–1.45) | 0.210 | 1.41 | - | - | - | - | ||
| Response rate | ≥80 | 22 | 5,155 | 1.00 | - | - | - | - | - | ||
| Otherwisee | 73 | 8,091 | 0.91 (0.80–1.03) | 0.164 | 0.93 | - | - | - | - | ||
| Temporal measures | Year of publication category | <2000 | 28 | 2,935 | 1.00 | - | - | - | - | - | |
| 2000–2009 | 32 | 3,844 | 0.87 (0.76–0.91) | 0.053 | - | - | - | - | |||
| 2010–2018 | 35 | 6,468 | 0.86 (0.75–0.70) | 0.023 | 8.67 | - | - | - | - | ||
| Year of data collection | 95 | 13,335 | 0.99 (0.99–1.00) | 0.047 | 6.86 | - | - | - | - | ||
| Year of publication | 95 | 13,335 | 0.99 (0.99–0.99) | 0.035 | 7.66 | 0.99 (0.99–1.00) | 0.389 | 0.99 (0.99–0.99) | 0.043 | ||
a Variance explained by the final multivariable model 1 (adjusted R2) = 42.82%.
b Variance explained by the final multivariable model 2 (adjusted R2) = 69.57%.
c Other includes one measure of a low income country (Haiti) and the measures extracted from studies including different countries.
d Sample size denotes the sample size of the study population found in the original publication.
e Otherwise indicates either response rate was <80% or response rate not included in the report.
Abbreviations: ARR = Adjusted risk ratio, CI = Confidence interval, ELISA = Enzyme-linked immunosorbent type-specific assay, HIC = High-income country, HSV-1 = Herpes simplex virus type 1, RR = Risk ratio, UMIC = Upper-middle-income country.
Since age bracket and age group are variables that are not independent of each other, two multivariable models were analyzed, each using one of these variables. For a similar consideration, the year of publication was included in the multivariable analyses, instead of year of data collection, given its more complete data. As for the multivariable analyses including the year of publication category, instead of the linear year of publication term, the results can be found in S3 Table.
The first model included age bracket, sex, population type, and year of publication. It explained 42.82% of the seroprevalence variation. In adults, seroprevalence was 1.39-fold (95% CI: 1.24–1.57) higher than that in children.
The second model included age group, sex, population type, and year of publication. It explained 69.57% of the seroprevalence variation. Compared to those aged ≤10, seroprevalence was 1.36-fold (95% CI: 1.19–1.56) higher in those aged 10–20, 1.44-fold (95% CI: 1.25–1.65) higher in those aged 20–30, 1.70-fold (95% CI: 1.47–1.97) higher in those aged 30–40, and 1.81-fold (95% CI: 1.58–2.08) higher in those aged ≥40. There was evidence here for a statistically-significant declining seroprevalence over time by 0.99-fold (95% CI: 0.99–0.99) per year, in contrast to the first model analysis (Table 3) and the analyses including the year of publication as a category (S3 Table), where the evidence for the decline in sero-prevalence did not reach statistical significance.
HSV-1 virus isolation in genital ulcer disease and in genital herpes
Tables 4 and 5 summarize the extracted proportions of HSV-1 virus isolation in GUD (n = 5) and in genital herpes (n = 9), as well as their pooled mean estimates.
Table 4. Studies reporting proportions of HSV-1 virus isolation in clinically-diagnosed GUD and in clinically-diagnosed genital herpes in Latin America and the Caribbean.
| Author, year | Year(s) of data collection | Country | Study site | Study design | Sampling method | Population | HSV-1 biological assay | Sample size | Proportion of HSV-1 isolation (%) |
|---|---|---|---|---|---|---|---|---|---|
| HSV-1 virus isolation in clinically-diagnosed GUD (n = 5) | |||||||||
| Gomes Naveca, 2013 [62] | 2008 | Brazil | Outpatient clinic | CS | Conv | Patients with GUD | PCR | 15 | 6.6 |
| Gomes Naveca, 2013 [62] | 2008 | Brazil | Outpatient clinic | CS | Conv | Patients with primary GUD | PCR | 324 | 4.0 |
| Gomes Naveca, 2013 [62] | 2008 | Brazil | Outpatient clinic | CS | Conv | Patients with recurrent GUD | PCR | 95 | 1.1 |
| Noda, 2016 [63] | 2012 | Cuba | Outpatient clinic | CS | Conv | Men with GUD | PCR | 113 | 0.0 |
| Valdespino-Gomez, 1995 [64] | 1990 | Mexico | Community | CS | Conv | FSWs with genital ulcers | IFA | 71 | 0.0 |
| HSV-1 virus isolation in clinically-diagnosed genital herpes (n = 9) | |||||||||
| Balachandran, 1982 [27] | - | Puerto Ricco | Outpatient clinic | CS | Conv | STI clinic attendees | IFA | 12 | 8.3 |
| Belli, 1990 [65] | 1982–83 | Argentina | Outpatient clinic | CS | Conv | Patients with genital herpes | IFA | 25 | 20.0 |
| Do Nascimento, 1998 [30] | 1995 | Brazil | Outpatient clinic | CS | Conv | HIV patients with genital herpes | PCR | 36 | 5.0 |
| Hun,1987 [66] | - | Costa Rica | Outpatient clinic | CS | Conv | STI clinic attendees | Culture | 12 | 25.0 |
| Prabhakar, 1987 [67] | 1982 | Jamaica | Outpatient clinic | CS | Conv | STI clinic attendees | IFA | 40 | 0.0 |
| Schultz, 1994 [68] | 1988 | Chile | Outpatient clinic | CS | Conv | Pregnant women with genital herpes | DFA | 20 | 10.0 |
| Suarez, 1988 [69] | 1985 | Chile | Outpatient clinic | CS | Conv | Patients with primary genital herpes | IFA | 14 | 28.5 |
| Suarez, 1988 [69] | 1985 | Chile | Outpatient clinic | CS | Conv | Patients with recurrent genital herpes | IFA | 61 | 9.8 |
| Suarez, 1989 [70] | 1984 | Chile | Outpatient clinic | CS | Conv | Women with genital herpes | DFA | 13 | 23.1 |
Abbreviations: Conv = Convenience, CS = Cross sectional, DFA = Direct fluorescent assay, FSWs = Female sex workers, GUD = Genital ulcer disease, HSV-1 = Herpes simplex virus type 1, IFA = Indirect immunofluorescence assay, PCR = Polymerase chain reaction, RS = Random Sampling, STI = Sexually transmitted infection.
Table 5. Pooled proportions of HSV-1 virus isolation in clinically-diagnosed GUD and in clinically-diagnosed genital herpes in Latin America and the Caribbean.
| Population type | Outcome measures | Samples | Proportion of HSV-1 isolation (%) | Pooled proportion of HSV-1 isolation (%) | Heterogeneity measures | |||
|---|---|---|---|---|---|---|---|---|
| Total n |
Total N |
Range | Median | Mean (95% CI) |
Qa (p-value) |
I2b (%) (95% CI) |
Prediction Intervalc (%) | |
| Patients with clinically-diagnosed GUD | 5 | 618 | 0.0–6.6 | 1.1 | 0.9 (0.0–3.6) | 12.9 (p = 0.0116) | 69.1 (20.7–88.0) | 0.0–14.6 |
| Patients with clinically-diagnosed genital herpes | 9 | 233 | 0.0–28.5 | 10.0 | 10.9 (4.4–19.4) | 21.1 (p = 0.0069) | 62.1 (21.7–81.6) | 0.0–40.4 |
a Q: The Cochran’s Q statistic is a measure assessing the existence of heterogeneity in pooled outcome measures, here proportions of HSV-1 virus isolation.
b I2: A measure assessing the magnitude of between-study variation that is due to true differences in proportions of HSV-1 virus isolation across studies rather than sampling variation.
c Prediction interval: A measure quantifying the distribution 95% interval of true proportions of HSV-1 virus isolation around the estimated pooled mean.
Abbreviations: CI = Confidence interval, GUD = Genital ulcer disease, HSV-1 = Herpes simplex virus type 1.
In GUD cases, the virus isolation proportion ranged between 0.0–6.6%, with a median of 1.1% and a pooled mean of 0.9% (95% CI: 0.0–3.6%). In genital herpes cases, the proportion ranged between 0.0–28.5%, with a median of 10.0% and a pooled mean of 10.9% (95% CI: 4.4–19.4%). Both meta-analyses of proportions showed strong evidence of heterogeneity (Table 5). Forest plots can be found in S2 Fig.
Quality assessment
A total of 31 reports were included in the systematic review, while an additional 12 reports were excluded due to potential issues in their diagnostic method (Fig 2).
Summary of the precision and ROB assessments are in S4 Table. High precision was found in the majority of studies (62.9%). High ROB in the sampling method domain was found in the vast majority of studies (94.3%). Low ROB in the response rate domain was found in 25.7% of studies, while the remaining studies had a high ROB (2.9%), or an unclear ROB (71.4%).
Since none of the study characteristics of sample size, sampling method, and response rate were found associated with HSV-1 seroprevalence (Table 3), it is not likely that precision nor ROB have affected the results of the present study.
Discussion
The systematic review and meta-analytics reported here indicate that HSV-1 infection is widely prevalent in Latin America and the Caribbean, at a seroprevalence level that is higher than that of the global population at 67% [1]. Nearly 60% of children and 90% of adults are infected, a higher seroprevalence than that in Western Countries [31] and Asia [8], though lower than that in Africa [32] and the Middle East and North Africa (MENA) [33]. Seroprevalence increased steadily with age, but most HSV-1 acquisitions still occurred in childhood (Tables 2 and 3).
Age was by far the strongest predictor of infection, explaining alone >50% of the seroprevalence variation (Table 3). Meanwhile, sex, clinical condition, and country’s income did not affect HSV-1 seroprevalence (Table 3), in broad agreement with the results of similar studies for Africa [32], Asia [8], and MENA [33]. These findings affirm the notion that HSV-1 is a truly general population infection, with largely homogenous exposure risk in the population.
There was evidence for a declining seroprevalence over the last three decades, but the exact effect size of the decline and nature of the decline (linear or not) are not yet certain with currently available data (Tables 2 and 3 and S3 Table). While seroprevalence declines have been also observed in North America and Europe [31, 34–41], no evidence for such declines was found in Africa [32], Asia [8], and MENA [33]. The large gap in HSV-1 seroprevalence between children and adults (Tables 2 and 3), supports also the interpretation of recent declines in seroprevalence, with the currently older cohorts experiencing higher infection risk in earlier times. As observed in North America [31], improvements in hygiene and standard of living may have driven the seroprevalence declines.
With this evidence for a possible slow transition in HSV-1 epidemiology in Latin America and the Caribbean, there is a cause for concern for genital herpes, as increasingly a larger fraction of adolescents may initiate sexual activity with no antibodies to protect them against acquiring HSV-1 sexually, and thus at risk of genital herpes. Indeed, we found evidence supporting a role for HSV-1 as the etiological cause of genital herpes (Tables 4 and 5), though at rates much lower than those observed in Western countries [4, 5, 7, 9–11] and Asia [8].
This study has limitations. Data were available only for 14 mostly populous countries (Tables 1 and 4), with no data found for the remaining 32 smaller countries. Studies varied in methods and quality and used different diagnostic assays, with potentially different sensitivity and specificity profiles [16, 17]. However, no effect was found on seroprevalence for assay type, sample size, sampling method, and response rate (Table 3), indicating that the variability in study methods may not have impacted the results and findings of the present study.
Conclusions
As in North America, Europe, and Asia [5, 7–11, 31, 35, 42], there is evidence for a possible transitioning HSV-1 epidemiology in Latin America and the Caribbean, though at a slower rate and with still limited contribution for HSV-1 in genital herpes and as a sexually transmitted infection. HSV-1 seroprevalence appears to be declining, with the younger cohorts experiencing lower infection risk than those experienced by the younger cohorts in earlier times. Yet, HSV-1 persists as a widely prevalent infection in this region, with 60% of children and 90% of adults being infected. These findings support the need for surveillance to monitor trends in seroprevalence and genital herpes etiology, and highlight the need for a vaccine to prevent infection and associated disease burden.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This publication was made possible by NPRP grant number 9-040-3-008 from the Qatar National Research Fund (a member of Qatar Foundation) to LJA-R. The statements made herein are solely the responsibility of the authors. The authors are also grateful for pilot funding provided by the Biomedical Research Program and infrastructure support provided by the Biostatistics, Epidemiology, and Biomathematics Research Core, both at Weill Cornell Medicine in Qatar to LJA-R.
References
- 1.Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PloS one. 2015;10(10):e0140765 10.1371/journal.pone.0140765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady RC, Bernstein DI. Treatment of herpes simplex virus infections. Antiviral Res. 2004;61(2):73–81. . [DOI] [PubMed] [Google Scholar]
- 3.Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57(5):737–63; quiz 64–6. 10.1016/j.jaad.2007.06.027 . [DOI] [PubMed] [Google Scholar]
- 4.Bernstein DI, Bellamy AR, Hook EW 3rd, Levin MJ, Wald A, Ewell MG, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56(3):344–51. Epub 2012/10/23. 10.1093/cid/cis891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30(10):797–800. 10.1097/01.OLQ.0000092387.58746.C7 . [DOI] [PubMed] [Google Scholar]
- 6.Ayoub HH, Chemaitelly H, Abu-Raddad LJ. Characterizing the Transitioning Epidemiology of Herpes Simplex Virus Type 1 in the United States: Model-Based Predictions. under review. 2018. [DOI] [PMC free article] [PubMed]
- 7.Lowhagen GB, Tunback P, Andersson K, Bergstrom T, Johannisson G. First episodes of genital herpes in a Swedish STD population: a study of epidemiology and transmission by the use of herpes simplex virus (HSV) typing and specific serology. Sex Transm Infect. 2000;76(3):179–82. 10.1136/sti.76.3.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khadr L, Harfouche M, Omori R, Schwarzer G, Chemaitelly H, Abu-Raddad LJ. The epidemiology of herpes simplex virus type 1 in Asia: systematic review, meta-analyses, and meta-regressions. Clin Infect Dis. 2018. Epub 2018/07/19. 10.1093/cid/ciy562 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilsen A, Myrmel H. Changing trends in genital herpes simplex virus infection in Bergen, Norway. Acta Obstet Gynecol Scand. 2000;79(8):693–6. . [PubMed] [Google Scholar]
- 10.Samra Z, Scherf E, Dan M. Herpes simplex virus type 1 is the prevailing cause of genital herpes in the Tel Aviv area, Israel. Sex Transm Dis. 2003;30(10):794–6. 10.1097/01.OLQ.0000079517.04451.79 . [DOI] [PubMed] [Google Scholar]
- 11.Gilbert M, Li X, Petric M, Krajden M, Isaac-Renton JL, Ogilvie G, et al. Using centralized laboratory data to monitor trends in herpes simplex virus type 1 and 2 infection in British Columbia and the changing etiology of genital herpes. Can J Public Health. 2011;102(3):225–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlieb SL, Giersing B, Boily MC, Chesson H, Looker KJ, Schiffer J, et al. Modelling efforts needed to advance herpes simplex virus (HSV) vaccine development: Key findings from the World Health Organization Consultation on HSV Vaccine Impact Modelling. Vaccine. 2017. 10.1016/j.vaccine.2017.03.074 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottlieb SL, Deal CD, Giersing B, Rees H, Bolan G, Johnston C, et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: Update and next steps. Vaccine. 2016;34(26):2939–47. 10.1016/j.vaccine.2016.03.111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons; 2011. [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS med. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashley-Morrow R, Nollkamper J, Robinson NJ, Bishop N, Smith J. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2004;10(6):530–6. Epub 2004/06/12. 10.1111/j.1469-0691.2004.00836.x . [DOI] [PubMed] [Google Scholar]
- 17.Ashley RL. Performance and use of HSV type-specific serology test kits. Herpes. 2002;9(2):38–45. . [PubMed] [Google Scholar]
- 18.RStudio Team. RStudio: Integrated Development for R. RStudio, Inc, Boston, MA: URL http://www.rstudio.com/. 2015. [Google Scholar]
- 19.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons; 2011. [Google Scholar]
- 20.Schwarzer G. Meta: An R package for meta-analysis. R news. 2007;7(3):40–5. [Google Scholar]
- 21.Freeman MF, Tukey JW. Transformations Related to the Angular and the Square Root. Ann Math Statist 1950;21:607–11. [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327(7414):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins J, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society). 2009;172(1):137–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Bank. World Bank country and lending groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed in August 2017.
- 25.StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 26.Harbord RM, Higgins J. Meta-regression in Stata. Meta. 2008;8(4):493–519. [Google Scholar]
- 27.Balachandran N, Frame B, Chernesky M, Kraiselburd E, Kouri Y, Garcia D, et al. Identification and typing of herpes simplex viruses with monoclonal antibodies. Journal of clinical microbiology. 1982;16(1):205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corona-Oregón E, Sánchez-Alemán MÁ, Barrón BL, Conde-González CJ. Virus del herpes simplex tipo 1: un posible agente de transmisión sexual en población universitaria. Gac Med Mex. 2010;146(2):98–102. [PubMed] [Google Scholar]
- 29.Smith JS, Herrero R, Bosetti C, Munoz N, Bosch FX, Eluf-Neto J, et al. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. Journal of the National Cancer Institute. 2002;94(21):1604–13. [DOI] [PubMed] [Google Scholar]
- 30.do Nascimento MC, Sumita LM, de Souza VA, Pannuti CS. Detection and direct typing of herpes simplex virus in perianal ulcers of patients with AIDS by PCR. Journal of clinical microbiology. 1998;36(3):848–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu F, Lee FK, Morrow RA, Sternberg MR, Luther KE, Dubin G, et al. Seroprevalence of herpes simplex virus type 1 in children in the United States. The Journal of pediatrics. 2007;151(4):374–7. Epub 2007/09/25. 10.1016/j.jpeds.2007.04.065 . [DOI] [PubMed] [Google Scholar]
- 32.Harfouche M, Chemaitelly H, Abu-Raddad LJ. Herpes simplex virus type 1 epidemiology in Africa: Systematic review, meta-analyses, and meta-regressions (under review). [DOI] [PubMed]
- 33.Chaabane S, Harfouche M, Chemaitelly H, Schwarzer G, Abu-Raddad LJ. Herpes simplex virus type 1 epidemiology in the Middle East and North Africa: systematic review, meta-analyses, and meta-regressions. Sci Rep. 2019;9(1):1136 Epub 2019/02/06. 10.1038/s41598-018-37833-8. 10.1038/s41598-018-37833-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith J, Robinson N. Age-SPecific Prevalence of Infection with Herpes Simplex Virus Types 2 and 1: A Global Review. The Journal of Infectious Diseases. 2002;186(Suppl 1):S3–S28. [DOI] [PubMed] [Google Scholar]
- 35.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964–73. 10.1001/jama.296.8.964 . [DOI] [PubMed] [Google Scholar]
- 36.Kramer MA, Uitenbroek DG, Ujcic-Voortman JK, Pfrommer C, Spaargaren J, Coutinho RA, et al. Ethnic differences in HSV1 and HSV2 seroprevalence in Amsterdam, the Netherlands. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2008;13(24). Epub 2008/09/03. . [PubMed] [Google Scholar]
- 37.Wutzler P, Doerr HW, Farber I, Eichhorn U, Helbig B, Sauerbrei A, et al. Seroprevalence of herpes simplex virus type 1 and type 2 in selected German populations-relevance for the incidence of genital herpes. J Med Virol. 2000;61(2):201–7. . [DOI] [PubMed] [Google Scholar]
- 38.Sauerbrei A, Schmitt S, Scheper T, Brandstadt A, Saschenbrecker S, Motz M, et al. Seroprevalence of herpes simplex virus type 1 and type 2 in Thuringia, Germany, 1999 to 2006. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2011;16(44). . [PubMed] [Google Scholar]
- 39.Pebody RG, Andrews N, Brown D, Gopal R, De Melker H, Francois G, et al. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex Transm Infect. 2004;80(3):185–91. 10.1136/sti.2003.005850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aarnisalo J, Ilonen J, Vainionpaa R, Volanen I, Kaitosaari T, Simell O. Development of antibodies against cytomegalovirus, varicella-zoster virus and herpes simplex virus in Finland during the first eight years of life: a prospective study. Scand J Infect Dis. 2003;35(10):750–3. . [DOI] [PubMed] [Google Scholar]
- 41.Vyse AJ, Gay NJ, Slomka MJ, Gopal R, Gibbs T, Morgan-Capner P, et al. The burden of infection with HSV-1 and HSV-2 in England and Wales: implications for the changing epidemiology of genital herpes. Sex Transm Infect. 2000;76(3):183–7. 10.1136/sti.76.3.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernstein DI, Bellamy AR, Hook EW III, Levin MJ, Wald A, Ewell MG, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clinical Infectious Diseases. 2012;56(3):344–51. 10.1093/cid/cis891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clemens SA, Farhat CK. Seroprevalence of herpes simplex 1–2 antibodies in Brazil. Revista de saude publica. 2010;44(4):726–34. Epub 2010/08/03. [DOI] [PubMed] [Google Scholar]
- 44.Conde-Glez C, Lazcano-Ponce E, Rojas R, DeAntonio R, Romano-Mazzotti L, Cervantes Y, et al. Seroprevalences of varicella-zoster virus, herpes simplex virus and cytomegalovirus in a cross-sectional study in Mexico. Vaccine. 2013;31(44):5067–74. Epub 2013/09/12. 10.1016/j.vaccine.2013.08.077 . [DOI] [PubMed] [Google Scholar]
- 45.Cowan FM, French RS, Mayaud P, Gopal R, Robinson NJ, de Oliveira SA, et al. Seroepidemiological study of herpes simplex virus types 1 and 2 in Brazil, Estonia, India, Morocco, and Sri Lanka. Sex Transm Infect. 2003;79(4):286–90. Epub 2003/08/07. 10.1136/sti.79.4.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Salles-Gomes LF, Sakuma ME, Curti SP. Present status of the frequency of antibodies against Herpesvirus hominis in inhabitants of Sao Paulo City, Brazil. Revista do Instituto Adolfo Lutz. 1981;41(2):107–14. [Google Scholar]
- 47.Robinson LG, Black FL, Lee FK, Sousa AO, Owens M, Danielsson D, et al. Helicobacter pylori prevalence among indigenous peoples of South America. J Infect Dis. 2002;186(8):1131–7. Epub 2002/10/02. 10.1086/343803 . [DOI] [PubMed] [Google Scholar]
- 48.Arriaga-Demeza RC, Conde-Glez CJ, Uribe-Salas FJ, Eguiza-Fano S, Garcia-Cisneros S, Sanchez-Aleman MA. Different patterns of herpes simplex virus type 1 infection among college students from Cuernavaca, Mexico. Sexual health. 2008;5(4):365–7. Epub 2008/12/09. . [DOI] [PubMed] [Google Scholar]
- 49.Evans AS, Carvalho RP, Frost P, Jamra M, Pozzi DH. Epstein-Barr virus infections in Brazil. II. Hodgkin’s disease. J Natl Cancer Inst. 1978;61(1):19–26. [DOI] [PubMed] [Google Scholar]
- 50.Jimemez JM, Fuentes LG, Cordero C, Alfaro G. [Seroepidemiological study of Herpes simplex virus type 2 in adult women of Costa Rica]. Revista de biologia tropical. 1979;27(2):207–16. Epub 1979/12/01. . [PubMed] [Google Scholar]
- 51.Levett PN. Seroprevalence of HSV-1 and HSV-2 in Barbados. Medical microbiology and immunology. 2005;194(1–2):105–7. Epub 2004/05/25. 10.1007/s00430-004-0222-5 . [DOI] [PubMed] [Google Scholar]
- 52.Lupi O. Prevalence and risk factors for herpes simplex infection among patients at high risk for HIV infection in Brazil. International journal of dermatology. 2011;50(6):709–13. Epub 2011/05/21. 10.1111/j.1365-4632.2010.04863.x . [DOI] [PubMed] [Google Scholar]
- 53.Oberle MW, Rosero-Bixby L, Lee FK, Sanchez-Braverman M, Nahmias AJ, Guinan ME. Herpes simplex virus type 2 antibodies: high prevalence in monogamous women in Costa Rica. The American journal of tropical medicine and hygiene. 1989;41(2):224–9. Epub 1989/08/01. . [PubMed] [Google Scholar]
- 54.Patnaik P, Herrero R, Morrow RA, Munoz N, Bosch FX, Bayo S, et al. Type-specific seroprevalence of herpes simplex virus type 2 and associated risk factors in middle-aged women from 6 countries: the IARC multicentric study. Sex Transm Dis. 2007;34(12):1019–24. Epub 2007/12/15. . [PubMed] [Google Scholar]
- 55.Prabhakar P, Rao BN, Hosang R, Narla VR, Persaud V. Antibodies to herpes simplex virus (types 1 and 2) in Jamaican patients with cervical dysplasia and neoplasia. The West Indian medical journal. 1984;33(2):63–7. Epub 1984/06/01. . [PubMed] [Google Scholar]
- 56.Calderon G, Pinto M, Pizarro R, De la Torre Juan Carlos G, Guerra H. Viral infections associated in breast cancer patients in a Latin American cancer institute. Annals of Surgical Oncology. 2018;25(2 Supplement 1):148–9. http://0-dx.doi.org.elibrary.qatar-weill.cornell.edu/10.1245/s10434-018-6534-2.29063297 [Google Scholar]
- 57.Moreira-Soto A, Cabral R, Pedroso C, Eschbach-Bludau M, Rockstroh A, Vargas LA, et al. Exhaustive TORCH Pathogen Diagnostics Corroborate Zika Virus Etiology of Congenital Malformations in Northeastern Brazil. 2018;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luchsinger V, Luzoro A, Martinez MJ. [High seroprevalence of cytomegalovirus, herpes simplex type 1 virus and Epstein Barr virus infection among human immunodeficiency virus-infected adults]. Revista medica de Chile. 2010;138(7):809–14. Epub 2010/11/04. . [DOI] [PubMed] [Google Scholar]
- 59.Boulos R, Ruff AJ, Nahmias A, Holt E, Harrison L, Magder L, et al. Herpes simplex virus type 2 infection, syphilis, and hepatitis B virus infection in Haitian women with human immunodeficiency virus type 1 and human T lymphotropic virus type I infections. The Johns Hopkins University (JHU)/Centre pour le Developpement et la Sante (CDS) HIV Study Group. J Infect Dis. 1992;166(2):418–20. Epub 1992/08/01. . [DOI] [PubMed] [Google Scholar]
- 60.Conde-Glez CJ, Juarez-Figueroa L, Uribe-Salas F, Hernandez-Nevarez P, Schmid DS, Calderon E, et al. Analysis of herpes simplex virus 1 and 2 infection in women with high risk sexual behaviour in Mexico. International journal of epidemiology. 1999;28(3):571–6. Epub 1999/07/16. . [DOI] [PubMed] [Google Scholar]
- 61.Duenas A, Adam E, Melnick JL, Rawls WE. Herpesvirus type 2 in a prostitute population. American journal of epidemiology. 1972;95(5):483–9. Epub 1972/05/01. . [DOI] [PubMed] [Google Scholar]
- 62.Gomes Naveca F, Sabido M, Amaral Pires de Almeida T, Araujo Veras E, Contreras Mejia Mdel C, Galban E, et al. Etiology of genital ulcer disease in a sexually transmitted infection reference center in Manaus, Brazilian Amazon. PLoS One. 2013;8(5):e63953 Epub 2013/05/25. 10.1371/journal.pone.0063953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noda AA, Blanco O, Correa C, Perez L, Kouri V, Rodriguez I. Etiology of Genital Ulcer Disease in Male Patients Attending a Sexually Transmitted Diseases Clinic: First Assessment in Cuba. Sex Transm Dis. 2016;43(8):494–7. Epub 2016/07/16. 10.1097/OLQ.0000000000000470 . [DOI] [PubMed] [Google Scholar]
- 64.Valdespino-Gomez JL, Garcia-Garcia Mde L, del Rio-Chiriboga C, Cruz-Palacios C, Loo-Mendez E, Lopez-Sotelo A. [Sexually transmitted diseases and the HIV/AIDS epidemic]. Salud publica de Mexico. 1995;37(6):549–55. Epub 1995/11/01. . [PubMed] [Google Scholar]
- 65.Belli L, Irigoyen MH, Casco RH, Castronovo S, Torres RA. Pautas para el manejo de la infección herpética genital en la experiencia de un centro de atención de ETS en Buenos Aires (Argentina). Med Cutan Ibero Lat Am. 1990;18(1):44–8. [PubMed] [Google Scholar]
- 66.Hun L, Fuentes LG. Diagnóstico del laboratorio de virus herpes simplex en Costa Rica. Rev Costarric Cienc Med. 1987;8(3):143–8. [Google Scholar]
- 67.Prabhakar P, Allam MG, Prabhu PS, Bailey A, Brathwaite AF. Genital herpes in Jamaica. A clinical and pathological study (1982–1984). The West Indian medical journal. 1987;36(3):154–8. Epub 1987/09/01. . [PubMed] [Google Scholar]
- 68.Schultz R, Suárez M, Saavedra T. Seguimiento de embarazadas en alto riesgo de infección por virus del herpes simple. 1994.
- 69.Suarez M, Labbe V, Saavedra T, Ojeda JM. Tipos viricos del herpes simple asociados a infecciones genitales primarias y recurrentes en Chile. Bol Oficina Sanit Panam. 1988;105(1):13–9. [PubMed] [Google Scholar]
- 70.Suarez M, Briones H, Dubinovsky S, Aliaga P, Alarcon G, de Diego S, et al. [Genital herpes infection in Chilean female university students]. Bol Oficina Sanit Panam. 1989;106(5):389–95. Epub 1989/05/01. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.