Abstract
Background
Because of the strong link between childhood obesity and adulthood obesity comorbidities, and the difficulty in decreasing body mass index (BMI) later in life, effective strategies are needed to address this condition in early childhood. The ability to predict obesity before age five could be a useful tool, allowing prevention strategies to focus on high risk children. The few existing prediction models for obesity in childhood have primarily employed data from longitudinal cohort studies, relying on difficult to collect data that are not readily available to all practitioners. Instead, we utilized real-world unaugmented electronic health record (EHR) data from the first two years of life to predict obesity status at age five, an approach not yet taken in pediatric obesity research.
Methods and findings
We trained a variety of machine learning algorithms to perform both binary classification and regression. Following previous studies demonstrating different obesity determinants for boys and girls, we similarly developed separate models for both groups. In each of the separate models for boys and girls we found that weight for length z-score, BMI between 19 and 24 months, and the last BMI measure recorded before age two were the most important features for prediction. The best performing models were able to predict obesity with an Area Under the Receiver Operator Characteristic Curve (AUC) of 81.7% for girls and 76.1% for boys.
Conclusions
We were able to predict obesity at age five using EHR data with an AUC comparable to cohort-based studies, reducing the need for investment in additional data collection. Our results suggest that machine learning approaches for predicting future childhood obesity using EHR data could improve the ability of clinicians and researchers to drive future policy, intervention design, and the decision-making process in a clinical setting.
Introduction
Childhood obesity has been increasing since the 1970s [1]. As of 2016, 18.5% of US children and adolescents aged 2–19 had obesity, with a significantly higher prevalence among boys than girls [2]. Although there has been recent cause to suspect obesity rates for adults and children might be leveling off [3, 4], more recent data question this conclusion [5]: data from 2015–2016 showed increases in obesity rates across children of all ages, including a large increase among children at the youngest ages, 2–5 years old [2]. Growth trajectory simulation models suggest that 57% of children today will have obesity at age 35 [6]. This upward trend is concerning as childhood obesity can lead to diabetes, hypertension, and other conditions in adulthood [7–9]. Because of the strong link between childhood obesity and adult comorbidities, and the difficulty in decreasing BMI later in life, effective strategies are needed to address the condition early in life. In fact, a growing number of early obesity prevention interventions are being developed to decrease obesity-promoting feeding and lifestyle practices beginning in pregnancy and infancy. Some are beginning to demonstrate promising impacts on both promoting healthy habits and decreasing early childhood obesity; however, they currently focus on universal interventions [10–18]. If we were instead able to predict the risk level of a child developing obesity, we would then be able to better target intervention resources through the measurement of the effect of an intervention relative to a child’s risk of developing obesity.
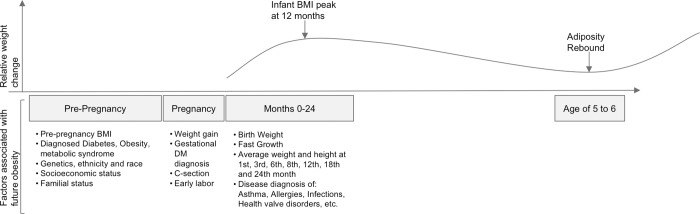
Two critical periods in the development of obesity include the prenatal and infancy period, and early childhood (Fig 1). The first 1,000 days [19, 20], from conception until the end of the second year of life, mark the first critical period in the development of obesity. The second period starts at age five, where the adiposity rebound marks a BMI minimum and a shift into childhood growth.
Fig 1. Factors at the prenatal and infancy periods associated with early childhood obesity by age five.
Adapted from González-Muniesa et al. [21].
Obesity during the early childhood critical period significantly increases the risk of obesity later in life [22, 23]. The ability to predict obesity before age five could be a useful tool, allowing prevention strategies to focus on children with a high risk of developing obesity. Primary care represents a promising platform for early childhood obesity prevention given the high frequency of visits during pregnancy and infancy, which provides access to infants and pregnant women. Additionally, a number of prenatal and infancy conditions are known risk factors for obesity at age five (Fig 1) [21].
Risk factors previously associated with childhood obesity range from 1) individual and parental biological factors, such as the infant’s birth weight [24–27], microbiome composition [28], maternal factors (including health diagnoses and weight gain), to 2) other family influences, such as race/ethnicity [29] and income [30]), and 3) neighborhood-level factors [31] (e.g., food availability, crime, and built environment). However, because of the complexity of the disease, this list likely still misses unknown key factors as well as the overall interdependence between the already identified determinants, making it challenging to predict with precision a child’s risk for developing obesity.
There are a few existing prediction models for obesity in infants, children, and adolescents that have primarily used data from prospective longitudinal cohort studies, and tend to employ traditional statistical methods, not machine learning approaches [32–37]. These studies demonstrated that it is possible to predict obesity during critical developmental periods, and offered quantitative insights on how different covariates correlated with key outcomes. However, these models are not generalizable to a broad clinical setting, given the high costs of data collection, and the fragility of those models in the cases of missing variables or small inaccuracies. This limitation significantly impacts the generalizability of predictions offered by such studies.
As of 2015, EHR systems were implemented in 84% of all US hospitals and approximately 87% of physicians’ offices [38–40]. Their widespread adoption also means that medical histories for each patient can be readily available for quantitative analysis at limited additional cost. In medicine, machine learning approaches have already seen successes, for example, in diagnostic medical imaging [41, 42], drug target discovery [43], early prediction of sepsis [44], type 2 diabetes [45], multiple families of diseases [46–50], and patient selection for clinical trials [51, 52]. There have been some machine learning approaches to predict later childhood obesity; however, research in this field is limited and there still exist a significant number of open issues. The most related research to our project is Dugan, et al. [53] which is explained in further detail below; other existing studies include some that proposed algorithms but without reporting results, leaving no point of comparison for future work [54–56]; another used data for children between ages 9 and 11, reducing its clinical utility to stop the development of obesity [57]; one that only utilizes 12 children, which gives insight into a small set of children but cannot be generalized to a broader population [58]; and two studies that compare a wide range of commonly used algorithms in machine learning, but report a static set of metrics, making it hard to compare performance across a set of metrics [53, 59]. The work of Dugan, et al. [53], however, demonstrated that it is possible to train machine learning models for obesity prediction using data from a custom clinical decision support system that incorporates both precise measurements and questionnaire data in a safety net hospital system in Indiana. Their work found race, the development of overweight between the ages of one and two, and accelerated weight gain to be important factors for prediction [53].
Similar to Dugan, et al. [53], our study used existing EHR data from the first critical period (pre-pregnancy through age two) from a safety net health system (ours in New York City) to predict future childhood obesity using machine learning. The substantive differences are that 1) we aimed to predict obesity at age five where adiposity is at a minimum during development, compared to obesity occurring at some point between the ages of two and ten, 2) we focused on reporting results across a sliding scale for the risk of developing obesity at age five, as opposed to all children who will become obese, as knowing the risk earlier may help to guide intervention studies, and 3) we used standard EHR data combined with census data, which requires no additional work from the clinician during a visit, rather than EHR data with supplementary, site-specific questionnaire data. Although EHR datasets are often noisy and incomplete due to numerous issues such as data entry errors and selective form fills, our model’s ability to make predictions using EHR data may allow for the approach to be more widely implemented, as it avoids the limitations of expensive cohort studies. Because machine learning models can be more effective than traditional statistical methods in handling missing, noisy, and asymmetric data (a common limitation of EHR data) we argue that our study can become more widely applicable in a clinical setting for guiding intervention efforts, compared to those that require the use of highly accurate and symmetrically collected cohort data, which is often not possible in a clinical setting due to resource limitations.
Methods
We conducted a retrospective cohort study using EHR data from patients in a safety net health system that serves a racially and ethnically diverse urban community in New York City: Family Health Centers at NYU Langone (formerly, Lutheran Family Health Centers)—one of the largest Federally Qualified Health Centers in the U.S.—which is composed of 8 primary care and specialty locations and over 40 school-based clinics in Brooklyn, New York. The EHR data employed by this study spanned from January 1, 2008 to August 31, 2016 and contained the records of 52,945 children of various ages, and 36,244 of their respective mothers for visits ranging from well-child visits to inpatient and outpatient services. Because not all mothers had given birth or received care in the study health system, there was not always a one-to-one match between mothers and their children. Additionally, some mothers had given birth to more than one child during the data collection period, also contributed to a lower number of mothers represented in the data set than children. The work was approved by the New York University School of Medicine’s Institutional Review Board and we were granted a waiver of informed consent as well as a waiver of authorization to use private health information for research.
The first set of criteria for a child to be considered in our study was to have at least one BMI measurement between the ages of 4.5 and 5.5 years (11,494 children) and be in the range of 10–40 kg/m2 (11,484 children), values outside of the CDC reference table minimums and maximums, to ensure there were no erroneous data points [60]. The second was that each child had to have at least one visit in the first two years of life (5,746 children). The third was to have the mother’s data available (3,451 children). When all three criteria are combined our study cohort shrunk to 3,449 children (1,751 boys, and 1,698 girls). Table 1 summarizes the effect of these three inclusion criteria used for this study on the full dataset. We included all children who passed our selection criteria for both modeling and prediction. As such, our selected cohort is not intended to be a random population sample.
Table 1. Number of children included at each selection criteria.
| Selection Criteria (in order) | N Boys | N Girls | N |
|---|---|---|---|
| 1) Full data set | 26,507 | 26,438 | 52,945 |
| 2a) BMI reading between 4.5 and 5.5 years | 5,775 | 5,719 | 11,494 |
| 2b) BMI reading is valid | 5,770 | 5,714 | 11,484 |
| 3) At least one data point prior to 2 years of age | 2,860 | 2,886 | 5,746 |
| 4) Maternal data available | 1,751 | 1,700 | 3,451 |
| Study Cohort Final | 1,751 | 1,698 | 3,449 |
Feature engineering
The EHR data used in this analysis—from both children and respective mothers—included the following features for each of their encounters or visit to a healthcare facility, for any purpose, in the study health system: demographic information (ethnicity, race, country of origin, nationality, and languages spoken), home address (allowing us to determine zip code and census tract), vital signs, medications, all laboratory test orders and results, diagnosis codes, and all medical procedures administered.
For maternal data we used vital signs, diagnosis codes, procedure, and laboratory results during six separate time periods: pre-pregnancy (prior to 40 weeks before birth), first trimester (0–14 weeks before birth), second trimester (14–27 weeks before birth), third trimester (27–40 weeks before birth), post-pregnancy, and during any other pregnancy. Taking these six time periods into account separately allowed us to understand the potential relationships between maternal health before, during, or after pregnancy and the child’s growth. For all other EHR data, such as delivery age or ethnicity, we only created one feature for each possibility as they do not change over time.
For the children’s data, we created features that group vital signs into averages over 11 time periods: at birth, 0–1 months, 1–3 months, 3–5 months, 5–7 months, 7–10 months, 10–13 months, 13–16 months, 16–19 months, 19–24 months, and latest measurements available (before 24 months), to capture the timeframes surrounding the standard well-child visits during the first two years of life [61]. Additionally, we calculated the change between each of these time periods as well as the change from birth to age two for all vital signs. For all other EHR data—diagnosis codes, demographic data, labs, or medications—we only created a single feature for each of the individual variables in the two-year time frame. For any data point that was not available, we filled in the corresponding matrix value with a zero.
Further, using the Clinical Classification Software categories, we collapsed all of the International Classification of Diseases 9th Revision diagnosis codes into 283 standard disease groupings to account for multiple related diagnosis codes. We then created binary encodings for all of the disease groups to indicate the presence of a diagnosis during each of the aforementioned time periods for mothers and for children at any point during the first two years of childhood. For lab results and vitals, we considered the average value for the maternal and childhood time periods. For features where we only considered whether or not they exist, i.e., medications, procedures, and demographic information, we created binary variables to indicate their presence.
Given the likely role of neighborhoods in the development of obesity [31], we also examined 17 continuous features at the census tract level derived from 2015 American Community Survey 5-year Estimates by geocoding each child’s address closest to birth and age two, using the NYCgbat Geosupport Desktop Edition [62]. These tract-level features included: percentage of population with a disability, education level, percentage of households participating in Supplemental Nutritional Assistance Program (SNAP, or food stamps), unemployment rate, and median household income. In addition, we created a binary variable for each of the 652 unique residential zip codes in the data to determine if there were any zip code-level influences not picked up by the census tract characteristics.
A total of 19,290 variables were created from all of the mentioned feature categories (e.g., diagnosis codes, labs, and ethnicity) for use in this analysis from all of the EHR data available combined with the census data. In Table 2, we show the number of features by category. The size of the feature space was a result of the sheer number of possible diagnosis codes, lab tests, and medications available. However, this did not necessarily translate to a positive impact on modeling because our feature space shrunk to 12% of the original 19,290 features when we look at variables that contain any information, and to 8% when we consider features with enough information to be useful (minimum of five children with information for a given variable). Many of these features are rare to begin with, such as most diagnosis codes or medications, however, for other features, there was likely genuinely missing information in our records. In addition, in the Jupyter Notebook in S1 File all of the generated features are included with number of occurrences and descriptive statistics for the overall data and valid cohort (combined and separated by gender).
Table 2. Number of features by category.
| Feature Category | Number of Features | Number of Features with at Least 1 Occurrence | Number of Features with at Least 5 Occurrences |
|---|---|---|---|
| Diagnosis | 566 | 160 | 107 |
| Lab | 549 | 73 | 57 |
| Medication | 2,968 | 78 | 14 |
| Gender | 2 | 2 | 2 |
| Ethnicity | 2 | 2 | 2 |
| Race | 11 | 9 | 8 |
| Vital | 475 | 255 | 255 |
| Number of visits | 1 | 1 | 1 |
| Zip code | 652 | 207 | 86 |
| Census | 34 | 34 | 34 |
| Maternal diagnosis | 3,962 | 473 | 257 |
| Newborn diagnosis | 566 | 52 | 22 |
| Maternal ethnicity | 4 | 3 | 3 |
| Primary insurance | 419 | 67 | 29 |
| Secondary insurance | 120 | 16 | 3 |
| Maternal race | 7 | 7 | 5 |
| Maternal language | 30 | 7 | 5 |
| Maternal nationality | 126 | 61 | 24 |
| Maternal marriage status | 7 | 5 | 5 |
| Maternal birthplace | 142 | 56 | 23 |
| Maternal delivery age | 1 | 1 | 1 |
| Maternal lab history | 5,700 | 573 | 477 |
| Maternal procedure history | 2,946 | 169 | 89 |
| Total | 19,290 | 2,311 | 1,509 |
Outcome definition
To predict obesity, we first calculated the BMI percentile by age, in months, and gender per the Center for Disease Control and Prevention (CDC) guidelines, for each BMI reading between the ages of 4.5 and 5.5 years [60]. If more than one record was available, we computed the median age, BMI, and BMI percentile as the final reading. We then determined obesity status by creating a binary variable to indicate whether or not a child is obese as defined by the CDC: BMI percentile being greater than or equal to the 95th percentile, according to the standard percentiles defined in [60].
Analysis methods
We used both regression and classification techniques for predicting childhood obesity. In the classification task, we used class probabilities to predict the binary outcome of obesity status: obese/not obese. In the regression task we normalized the median BMI value, as is standard practice for continuous variables. Using the predicted normalized BMI, we classified children as having obesity if they had a predicted value greater than the threshold for obesity.
For predicting our dichotomous measures of obese/not obese we used logistic regression with L1 loss, a random forest classifier, and gradient boosting classifier. For predicting our continuous BMI values we employed LASSO regression, random forest regression, and gradient boosting regression. These algorithms were the implemented versions in Python’s Scikit-learn package (version 0.19.1) [63]. LASSO regression and logistic regression were used as a baseline for machine learning performance. Random forest and gradient boosting were chosen because of their reported high performance across many tasks, especially those with a large feature space such as our own. As is standard practice, we normalized all of our continuous features before training each algorithm by subtracting the mean from each value and dividing by the standard deviation, respective to the values column mean and standard deviation.
To assess the performance of each of our models we randomly selected 20% of our data (350 boys and 339 girls) to be held out as a test set for all analyses for maintaining a consistent comparison of performance. Using the remaining data, we used bootstrap cross validation to validate our models by randomly sampling 90% of the data in each iteration without replacement, then performing a 70%/30% split for training and validation, and utilized our test data to assess final performance. Bootstrapping allowed us to compute the average AUC, along with a 95% confidence interval and represented a more real-world scenario for model implementation as opposed to a k-fold cross validation. For the comparison of classification and regression models 20 bootstraps were used. Final results on the best performing set of models were further refined by running 100 bootstraps.
For each of the regression and classification algorithms, we performed a series of feature selection techniques to further refine our methods and to test the effects that certain categories of features had on performance. In total, there were 13 variations of the data for each of the boys’ and girls’ cohorts that were used to train a model for each of the three regression and classification algorithms, making a total of 156 analyses. To create the 13 variations, we combined three category-based feature sets and three feature selection techniques. The three feature sets were: the full feature set (including variables with no information), only EHR features (which exclude census and zip code features), and non-weight or BMI features; the three feature selection methods consisted of no feature selection, features with at least five non-zero entries, and 10 bootstrap LASSO feature selection. In the LASSO feature selection, we selected all features whose average feature weight was non-zero in a 10 bootstrap LASSO regression process. We then created nine feature sets by considering all possible combinations of feature selection and feature category-based subset methods. The remaining four models used single features, and acted as a baseline of performance, given their importance to childhood obesity: the average weight for length (WFL) z-score between 19 and 24 months, the latest WFL available before 24 months, the average BMI between 19 and 24 months, and the latest BMI reading available before 24 months. Although WFL is more clinically meaningful for assessing childhood obesity, it has been suggested that BMI-z is more closely associated with later childhood obesity than WFL from a prediction standpoint [64], and thus we have incorporated both.
Results
The first column of Table 3 shows the demographic breakdown of our EHR population prior to applying our inclusion criteria. These results are comparable to our modeling cohorts with the exceptions of the “No Data Available” categories. Using all 3,449 children (1,751 boys and 1,698 girls) in the study cohort (Table 1) we assessed each variable’s association with the binary obesity outcome between the ages of 4.5 and 5.5. We compared these associations with obesity to the reference group (defined in each feature category section) and show a subset of those variables in Table 3. Overall, 18.6% of our cohort was obese at age five, which is less than the NYC estimate of children attending public schools in grades Kindergarten through eighth grade of 21% [31]. Only a single diagnoses category had a significant association (p<0.001) with obesity at age five: maternal diabetes mellitus, with no infant diagnoses determined to have had a significant association with obesity.
Table 3. Individual feature associations with obesity between ages 4.5 and 5.5.
| Variable | % of EHR Population | Girls | Boys | ||||
|---|---|---|---|---|---|---|---|
| % of Cohort | Odds Ratio (95% CI) | p-value for OR | % of Cohort | Odds Ratio (95% CI) | p-value for OR | ||
| Total Number | 52,945 | 1,698 | - | - | 1751 | - | - |
| Ethnicity | |||||||
| Not Hispanic/Latina | 24% | 17% | 0.587 (0.395, 0.874) | 0.009 | 21% | 0.714 (0.529, 0.963) | 0.027 |
| Hispanic/Latino | 49% | 82% | 1.546 (1.053, 2.269) | 0.026 | 79% | 1.399 (1.039, 1.884) | 0.027 |
| Other/Not Reported | 27% | 0% | - | - | 0% | - | - |
| Race | |||||||
| Caucasian/White | 15% | 5% | 1.151 (0.65, 2.038) | 0.630 | 4% | 0.827 (0.482, 1.421) | 0.492 |
| African Amer/Black | 13% | 5% | 1.913 (1.119, 3.27) | 0.018 | 5% | 0.907 (0.526, 1.565) | 0.725 |
| Asian | 10% | 9% | 0.204 (0.089, 0.466) | p<0.001 | 10% | 0.623 (0.419, 0.925) | 0.019 |
| Multiracial | 42% | 77% | 1.085 (0.786, 1.497) | 0.622 | 68% | 1.283 (0.979, 1.681) | 0.071 |
| Other | 14% | 3% | 1.828 (0.965, 3.462) | 0.064 | 1.321 (0.748, 2.332) | 0.337 | |
| Unknown/No Response | 6% | 0% | - | - | 0% | - | - |
| Maternal Marriage Status | |||||||
| Married | 7% | 36% | 0.702 (0.526, 0.938) | 0.017 | 36% | 0.875 (0.689, 1.111) | 0.272 |
| Divorced | 0% | 0% | 1.885 (0.378, 9.389) | 0.439 | 1% | 2.546 (0.804, 8.067) | 0.112 |
| Partnered | 4% | 32% | 1.135 (0.856, 1.504) | 0.379 | 30% | 1.067 (0.836, 1.363) | 0.601 |
| Single | 6% | 31% | 1.15 (0.867, 1.524) | 0.332 | 33% | 1.057 (0.832, 1.343) | 0.651 |
| Other/Unknown/No Response | 0% | 1% | 5.725 (1.645, 19.92) | 0.006 | 0.587 (0.131, 2.635) | 0.487 | |
| No Data Available | 83% | 0% | - | - | 0% | - | - |
| Maternal Birthplace | |||||||
| United States | 3% | 12% | 1.436 (0.992, 2.081) | 0.055 | 13% | 1.041 (0.749, 1.447) | 0.810 |
| China | 2% | 9% | 0.25 (0.115, 0.539) | p<0.001 | 11% | 0.64 (0.431, 0.951) | 0.027 |
| Dominican Republic | 1% | 3% | 1.681 (0.871, 3.243) | 0.122 | 4% | 2.369 (1.441, 3.896) | p<0.001 |
| Ecuador | 1% | 5% | 2.443 (1.475, 4.048) | p<0.001 | 5% | 1.011 (0.591, 1.729) | 0.968 |
| Mexico | 7% | 53% | 0.788 (0.604, 1.028) | 0.079 | 50% | 1.078 (0.86, 1.351) | 0.515 |
| El Salvador | 0% | 3% | 1.425 (0.703, 2.887) | 0.326 | 3% | 1.011 (0.496, 2.06) | 0.977 |
| Guatemala | 1% | 5% | 0.589 (0.292, 1.187) | 0.139 | 5% | 0.695 (0.401, 1.203) | 0.193 |
| Other | 2% | 10% | 1.418 (0.94, 2.139) | 0.096 | 0.905 (0.603, 1.358) | 0.629 | |
| No Data Available | 83% | 0% | - | - | 0% | - | - |
| Maternal Diagnosis | |||||||
| Diabetes Mellitus in pregnancy | - | 10% | 2.045 (1.396, 2.995) | p<0.001 | 11% | 1.605 (1.15, 2.24) | 0.005 |
| Diabetes Mellitus without complications | - | 5% | 2.093 (1.262, 3.47) | 0.004 | 5% | 1.935 (1.216, 3.08) | 0.005 |
| Hypertension in pregnancy | - | 9% | 1.745 (1.167, 2.61) | 0.007 | 12% | 1.377 (0.987, 1.92) | 0.060 |
| Complications at birth | - | 43% | 1.29 (0.988, 1.685) | 0.061 | 45% | 1.158 (0.923, 1.452) | 0.204 |
| OB-related perin trauma | - | 41% | 0.781 (0.592, 1.029) | 0.078 | 39% | 0.815 (0.645, 1.031) | 0.088 |
| Pelvic obstruction | - | 2% | 1.36 (0.552, 3.35) | 0.503 | 2% | 1.931 (1.02, 3.653) | 0.043 |
| Infant Diagnosis | |||||||
| Nutritional diagnosis | - | 0% | 0 (0, 0) | 0.083 | 0% | 0 (0, 0) | 0.000 |
| Epilepsy/convulsions | - | 1% | 2.483 (1.053, 5.853) | 0.766 | 1% | 2.483 (1.053, 5.853) | 0.038 |
| Liver Diseases | - | 10% | 0.743 (0.492, 1.122) | 0.153 | 10% | 0.743 (0.492, 1.122) | 0.158 |
| Skin Diseases | - | 11% | 1.022 (0.735, 1.419) | 0.252 | 14% | 1.022 (0.735, 1.419) | 0.899 |
| Kidney Diseases | - | 1% | 1.144 (0.556, 2.356) | 0.334 | 2% | 1.144 (0.556, 2.356) | 0.714 |
| Circulatory Diseases | - | 1% | 2.386 (0.968, 5.88) | 0.000 | 1% | 2.386 (0.968, 5.88) | 0.059 |
Additionally, we found that both BMI and weight for length z-score (at the last reading available and at the end of the second year) were strongly associated with obesity outcomes at age five. The characteristic tables for these features are summarized in Tables 4 and 5 for girls and boys, respectively. Our analysis validates previous findings that a number of variables during infancy have significant associations with obesity later in childhood, which falls in line with previous findings that weight early in life can predict weight later in life [33, 35, 37, 53].
Table 4. Individual feature associations for girls with obesity between ages 4.5 and 5.5.
| Variable | Total Number | Total Average (SD) | % Obese (N) | Obese Average (SD) | % Not Obese (N) | Not Obese Average (SD) | p-value |
|---|---|---|---|---|---|---|---|
| Weight for Length Z-score (average 19 to 24 months) | 1,347 | 1.042 (1.106) | 22.7% (316) | 1.899 (1.029) | 77.3% (1,076) | 0.79 (0.996) | p<0.001 |
| BMI (average 19 to 24 months) | 1,355 | 17.547 (1.786) | 22.7% (318) | 18.869 (1.818) | 77.3% (1,083) | 17.158 (1.578) | p<0.001 |
| Weight for Length Z-score (latest available reading) | 1,612 | 0.99 (1.166) | 22.1% (368) | 1.806 (1.135) | 77.9% (1,297) | 0.759 (1.066) | p<0.001 |
| BMI (latest available reading) | 1,624 | 17.509 (1.806) | 22.1% (371) | 18.734 (1.953) | 77.9% (1,304) | 17.161 (1.599) | p<0.001 |
Table 5. Individual feature associations for boys with obesity between ages 4.5 and 5.5.
| Variable | Total Number | Total Average (SD) | % Obese (N) | Obese Average (SD) | % Not Obese (N) | Not Obese Average (SD) | p-value |
|---|---|---|---|---|---|---|---|
| Weight for Length Z-score (average 19 to 24 months) | 1,392 | 1.042 (1.106) | 23.5% (316) | 1.899 (1.029) | 79.9% (1,076) | 0.79 (0.996) | p<0.001 |
| BMI (average 19 to 24 months) | 1,401 | 17.547 (1.786) | 23.5% (318) | 18.869 (1.818) | 79.9% (1,083) | 17.158 (1.578) | p<0.001 |
| Weight for Length Z-score (latest available reading) | 1,665 | 0.99 (1.166) | 22.8% (368) | 1.806 (1.135) | 80.5% (1,297) | 0.759 (1.066) | p<0.001 |
| BMI (latest available reading) | 1,675 | 17.509 (1.806) | 22.8% (371) | 18.734 (1.953) | 80.3% (1,304) | 17.161 (1.599) | p<0.001 |
Obesity prediction using EHR and machine learning
For our binary obesity classification and regression models, we were able to achieve performance comparable to, or better than, similar cohort-based studies [32–36]. However, we are not able to compare our results to directly to Dugan et al. because of the differences in reporting methods. We found that our regression models outperformed their classification counterparts for predicting obesity at age five with data from the first two years of life. On average, AUC on the test set with a 95% confidence interval was 0.042 [0.031, 0.052] higher for girls, and 0.033 [0.023, 0.043] higher for boys in the regression task than the classification task. The difference is significant because the confidence intervals do not overlap. An overview of performance assessment can be seen in the Jupyter Notebook in S2 File.
The best performing model with the highest mean AUC for girls was LASSO regression on the full feature set with LASSO feature selection. LASSO looks for a sparse solution, therefore the model only utilized 35 features. Details of these features can be found in S1 Table. Similarly, the best performing multivariate model by highest mean AUC in the regression analysis for boys was LASSO using only EHR data without feature selection with only 144 features being utilized. A summary of these 144 features can be found in S2 Table. However, the performance of this model was consistently lower than the best single feature model, average WFL z-score between 19 and 24 months, whereas the other three single feature models performed comparably to the best multivariate model. The details of these analyses can be seen in the Jupyter Notebook in S3 File.
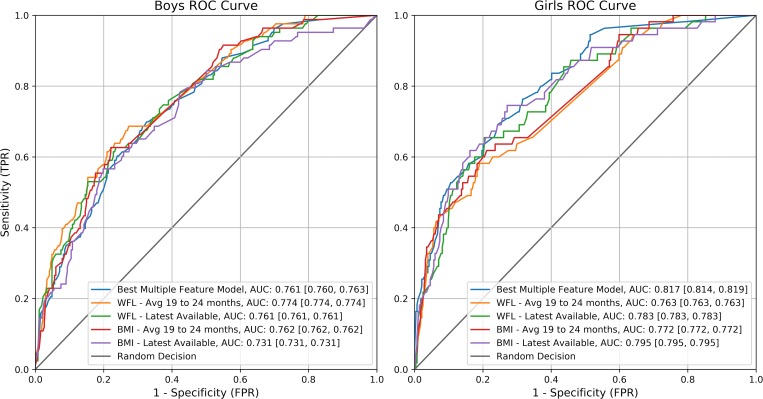
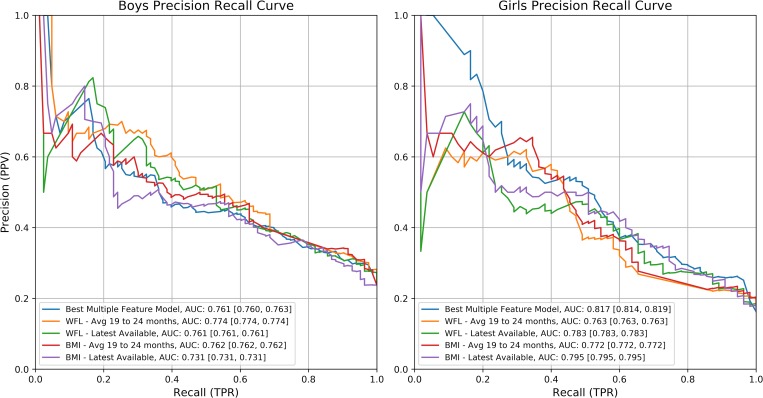
Using our best performing multivariate model we were able to predict obesity on the test set with a mean AUC of 81.7% [81.4%, 81.9%] and 76.1% [76.0%, 76.3%] for girls and boys, respectively. Using these models we found that 34.3% and 28.1% of the variance of BMI at age five being explained for girls and boys respectively. The results for each of the models are shown in S3 Table and S4 Table for girls and boys, respectively. In Figs 2 and 3, we present the ROC curves and precision recall curves, respectively, for each of these highest performing models against the each of our individual feature models.
Fig 2. ROC curves for the top performing model compared to individual feature predictions.
Fig 3. Precision recall curves for the top performing model compared to individual feature predictions.
Threshold values for these plots can be seen in Table 6 and Table 7, for girls and boys respectively. We found that we had modest performance if the goal is to reach a high sensitivity, but when focusing on predicting children most at risk of having obesity at age five, then we are able to achieve higher levels of accuracy. While it is important to consider predicting obesity outright, we are focused on a mechanism for targeted intervention for high risk children, so we focus on the results where a high PPV is achieved. It can be seen that where PPV is high (at least 70%) our model accuracy as well as the Matthews Correlation Coefficient (MCC) are maximized. This means that both our accuracy and the tradeoffs between error types in our model are performing best for this task. This tradeoff is ideal when attempting to craft a more tailored intervention study where resources should be focused on children who are at a higher risk of developing obesity and not all children who may become obese.
Table 6. Performance tradeoffs for the best performing model for girls.
| Sensitivity | PPV | Specificity | Accuracy | F1 | MCC | N Obese (TP + FP) | N Not Obese (TN + FN) |
|---|---|---|---|---|---|---|---|
| 0.145 | 0.889 | 0.996 | 0.858 | 0.250 | 0.352 | 9 | 330 |
| 0.200 | 0.786 | 0.989 | 0.861 | 0.319 | 0.126 | 14 | 325 |
| 0.291 | 0.571 | 0.958 | 0.850 | 0.386 | 0.030 | 28 | 311 |
| 0.418 | 0.535 | 0.930 | 0.847 | 0.469 | 0.021 | 43 | 296 |
| 0.491 | 0.519 | 0.912 | 0.844 | 0.505 | 0.018 | 52 | 287 |
| 0.600 | 0.371 | 0.803 | 0.770 | 0.458 | 0.007 | 89 | 250 |
| 0.691 | 0.355 | 0.757 | 0.746 | 0.469 | 0.006 | 107 | 232 |
| 0.800 | 0.293 | 0.627 | 0.655 | 0.429 | 0.004 | 150 | 189 |
| 0.891 | 0.261 | 0.511 | 0.572 | 0.403 | 0.003 | 188 | 151 |
Table 7. Performance tradeoffs for the best performing model for boys.
| Sensitivity | PPV | Specificity | Accuracy | F1 | MCC | N Obese (TP + FP) | N Not Obese (TN + FN) |
|---|---|---|---|---|---|---|---|
| 0.084 | 0.700 | 0.989 | 0.774 | 0.151 | 0.071 | 10 | 340 |
| 0.205 | 0.567 | 0.951 | 0.774 | 0.301 | 0.021 | 30 | 320 |
| 0.301 | 0.543 | 0.921 | 0.774 | 0.388 | 0.015 | 46 | 304 |
| 0.398 | 0.458 | 0.854 | 0.746 | 0.426 | 0.008 | 72 | 278 |
| 0.506 | 0.442 | 0.801 | 0.731 | 0.472 | 0.007 | 95 | 255 |
| 0.602 | 0.435 | 0.757 | 0.720 | 0.505 | 0.006 | 115 | 235 |
| 0.699 | 0.397 | 0.670 | 0.677 | 0.507 | 0.005 | 146 | 204 |
| 0.795 | 0.346 | 0.532 | 0.594 | 0.482 | 0.003 | 191 | 159 |
| 0.904 | 0.306 | 0.363 | 0.491 | 0.457 | 0.003 | 245 | 105 |
The factor that emerged as most predictive for girls was the average maternal post-pregnancy weight despite having a weak AUC as its own predictor; however, weight and height related features for the infant were all but seven of the model’s 35. For the best performing multivariate model for boys, weight and BMI features made up 122 of the 144 total features. However, only 85 of the 144 features had beta coefficients greater than or equal to 0.001, with 71 of those features also relating to weight and BMI.
Discussion
Since the Surgeon General's “Call to Action to Prevent and Decrease Overweight and Obesity" in 2001 [65], obesity and its causes has been the focus of numerous scientific studies [8, 66, 67]. Similarly, thousands of state-level policies have been enacted to encourage healthy lifestyles [68]. Despite the massive investments in money and effort so far, very few interventions have been effective at preventing obesity [69]. In this study, we used EHR and machine learning algorithms to identify young children with a high risk of developing obesity that could be specifically targeted for intervention. Using LASSO regression, we could predict obesity, between the ages of 4.5 and 5.5 years old on a held-out test set, achieving average AUC scores of 81.8% for girls and 76.1% for boys (Fig 1).
Some previous intervention studies have focused on known risk factors, such as maternal ethnicity [70, 71]. If we had used this broad cohort specification, such as that in Gross, et al. [18], as opposed to machine learning methods, our PPV would have been 18.3% for girls and 25.7% for boys (Jupyter Notebook in S2 File). This means that 81.7% of intervention targets, for girls, and 74.3% of the intervention targets, for boys, did not have much risk of becoming obese in the first place. Potentially, these broad inclusion criteria could be contributing to the small effects found in intervention studies, likely leading to the limited effectiveness of the interventions themselves. In contrast, with our full model, the achieved PPV (at 20% sensitivity) are 78% and 56% for girls and boys, respectively. This is significant because it allows for researchers to be able to set thresholds for inclusion in a study to measure the impact of an obesity intervention relative to the risk of developing obesity. High confidence predictions for future obesity (high PPV) capture less of the overall population that will develop obesity but those predictions will contain fewer false positives as opposed to predictions that lead to capturing a larger portion of the obesity developing population. The former approach would likely produce higher statistical power in a study because of the rebalanced distribution of false positives from previous studies, along with the added ability to measure effects relative to the risk level would allow for better understandings of where specific intervention methods are most effective.
We found significant differences in AUC performance between the best performing models and the most predictive factors for girls and boys. Other work has found similar differences [72] though it is not straightforward to determine the reason why this might be the case. These differences suggest boys and girls follow different growth trajectories and/or are subject to different obesity influencing factors, as can be seen in S1 Table and S2 Table. For instance, there was an environmental influence for predicting future obesity in girls, as can be seen with some census features existing in the selected features, as well as influence from maternal health variables. This suggests that there may be more external influences leading to childhood obesity in girls that can be tracked outside of growth measures. However, for boys, we found that nearly all of the selected features directly related to measures of obesity. Additionally, our study aligns with previous work that prior weight and obesity status can predict later in childhood obesity status [33, 35, 37, 53].
A limitation of our study is that our cohort is not demographically representative of NYC at large, coupled with a relatively small sample size. We expect that future studies incorporating bigger cohorts with more regionally representative demographics could further improve model performance. In addition, the size of our study sample through using a single health system was the likely the culprit for representation issues within the data set.
Another limitation, but also a feature of our study, was the noisy and incomplete nature of EHR datasets. Like most EHR data, we had many sparse records with low information content. For some features, such as newborn diagnoses, the rarity of a specified observation was inherent to the features themselves. For others, the sparsity of information within a feature came from not having complete patient history in the specific healthcare system. We underline that this is a feature of our approach, as we utilize the inherent redundancy of the EHR variables to become robust to certain level of data incompleteness.
A real-time, predictive health tracker, sitting on top of existing EHR systems (particularly those that were linked across systems), could be powered by models like ours, to alert clinicians of children at high risk of developing obesity with a goal of improving their decision-making process. To best achieve such a goal of real-time health tracking, denser datasets, summarizing a child and their mother’s entire medical history would enrich our feature space and potentially improve performance. The model presented here is a very promising step towards achieving this goal of using EHR for early identification of patients at-risk for developing childhood obesity.
In this study, we have shown that we are able to detect with reasonable accuracy which children will have obesity by age five with data from the first two years of life. While our available data, despite a large number of visits, is limited compared to traditional prospective studies with curated cohorts and expensive to collect data [33–35, 53, 73], our models perform just as well or better. We have been able to train accurate prediction models, demonstrating that real-life EHR data can be a useful tool in aiding childhood obesity intervention research, by allowing clinicians to select cohorts with higher future obesity prevalence, leading to more effective intervention studies and clinical trials, and, consequently, more targeted intervention programs and policies.
Supporting information
This file provides an overview of the features used in the paper’s analyses. The file can also be viewed in the following link on our GitHub through Jupyter’s NBViewer: https://nbviewer.jupyter.org/github/NYUMedML/ObesityPY/blob/master/src/Pediatric_Obesity_Prediction_Feature_Data.ipynb.
(IPYNB)
This file demonstrates the methods and results used to compare the performance of regression and classification techniques for prediction. The file can also be viewed in the following link on our GitHub through Jupyter’s NBViewer: https://nbviewer.jupyter.org/github/NYUMedML/ObesityPY/blob/master/src/Pediatric_Obesity_Prediction_Regression_Classification_Comparison.ipynb.
(IPYNB)
This file provides an overview of the final analyses performed. The file can also be viewed in the following link on our GitHub through Jupyter’s NBViewer: https://nbviewer.jupyter.org/github/NYUMedML/ObesityPY/blob/master/src/Pediatric_Obesity_Prediction_Regression_100_bootstraps.ipynb.
(IPYNB)
“*” indicates a feature whose unadjusted odds ratio is significantly greater than or less than 1.
(XLSX)
“*” indicates a feature whose unadjusted odds ratio is significantly greater than or less than 1.
(XLSX)
(XLSX)
(XLSX)
Data Availability
The data are electronic health records owned by NYU Langone Health and contain protected health information and personally identifiable information. They were not anonymized for this study. It is a restricted data set and public sharing of these data would violate the HIPPA security rule, however a deidentified data set will be available by request through https://www.icpsr.umich.edu/icpsrweb/. Our code and the subsequent analyses can be viewed on our GitHub page at https://github.com/NYUMedML/ObesityPY.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Alston JM, Okrent AM. The effects of farm and food policy on obesity in the United States New York, NY, U.S.A.: Palgrave Macmillan; 2017. xxii, 393 pages p. [Google Scholar]
- 2.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics. 2018. Epub 2018/02/28. 10.1542/peds.2017-3459 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–90. Epub 2012/01/19. 10.1001/jama.2012.40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. Epub 2012/01/19. 10.1001/jama.2012.39 . [DOI] [PubMed] [Google Scholar]
- 5.Ludwig DS. Epidemic Childhood Obesity: Not Yet the End of the Beginning. Pediatrics. 2018. Epub 2018/02/28. 10.1542/peds.2017-4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of Growth Trajectories of Childhood Obesity into Adulthood. N Engl J Med. 2017;377(22):2145–53. Epub 2017/11/25. 10.1056/NEJMoa1703860 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101(3 Pt 2):518–25. Epub 2002/09/13. . [PubMed] [Google Scholar]
- 8.Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. J Family Med Prim Care. 2015;4(2):187–92. Epub 2015/05/08. 10.4103/2249-4863.154628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and risk of the adult metabolic syndrome: a systematic review. Int J Obes (Lond). 2012;36(1):1–11. Epub 2011/11/02. 10.1038/ijo.2011.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniels LA, Mallan KM, Battistutta D, Nicholson JM, Perry R, Magarey A. Evaluation of an intervention to promote protective infant feeding practices to prevent childhood obesity: outcomes of the NOURISH RCT at 14 months of age and 6 months post the first of two intervention modules. Int J Obes (Lond). 2012;36(10):1292–8. Epub 2012/06/20. 10.1038/ijo.2012.96 . [DOI] [PubMed] [Google Scholar]
- 11.Wen LM, Baur LA, Simpson JM, Rissel C, Wardle K, Flood VM. Effectiveness of home based early intervention on children's BMI at age 2: randomised controlled trial. BMJ. 2012;344:e3732 Epub 2012/06/28. 10.1136/bmj.e3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell KJ, Lioret S, McNaughton SA, Crawford DA, Salmon J, Ball K, et al. A parent-focused intervention to reduce infant obesity risk behaviors: a randomized trial. Pediatrics. 2013;131(4):652–60. Epub 2013/03/06. 10.1542/peds.2012-2576 . [DOI] [PubMed] [Google Scholar]
- 13.Savage JS, Birch LL, Marini M, Anzman-Frasca S, Paul IM. Effect of the INSIGHT Responsive Parenting Intervention on Rapid Infant Weight Gain and Overweight Status at Age 1 Year: A Randomized Clinical Trial. JAMA Pediatr. 2016;170(8):742–9. Epub 2016/06/09. 10.1001/jamapediatrics.2016.0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor BJ, Gray AR, Galland BC, Heath AM, Lawrence J, Sayers RM, et al. Targeting Sleep, Food, and Activity in Infants for Obesity Prevention: An RCT. Pediatrics. 2017;139(3). Epub 2017/03/01. 10.1542/peds.2016-2037 . [DOI] [PubMed] [Google Scholar]
- 15.French GM, Nicholson L, Skybo T, Klein EG, Schwirian PM, Murray-Johnson L, et al. An evaluation of mother-centered anticipatory guidance to reduce obesogenic infant feeding behaviors. Pediatrics. 2012;130(3):e507–17. Epub 2012/08/15. 10.1542/peds.2011-3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machuca H, Arevalo S, Hackley B, Applebaum J, Mishkin A, Heo M, et al. Well Baby Group Care: Evaluation of a Promising Intervention for Primary Obesity Prevention in Toddlers. Child Obes. 2016;12(3):171–8. Epub 2016/04/02. 10.1089/chi.2015.0212 . [DOI] [PubMed] [Google Scholar]
- 17.Gross RS, Mendelsohn AL, Gross MB, Scheinmann R, Messito MJ. Randomized Controlled Trial of a Primary Care-Based Child Obesity Prevention Intervention on Infant Feeding Practices. J Pediatr. 2016;174:171–7 e2. Epub 2016/04/27. 10.1016/j.jpeds.2016.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross RS, Mendelsohn AL, Yin HS, Tomopoulos S, Gross MB, Scheinmann R, et al. Randomized controlled trial of an early child obesity prevention intervention: Impacts on infant tummy time. Obesity (Silver Spring). 2017;25(5):920–7. Epub 2017/03/24. 10.1002/oby.21779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo Baidal JA, Criss S, Goldman RE, Perkins M, Cunningham C, Taveras EM. Reducing Hispanic children's obesity risk factors in the first 1000 days of life: a qualitative analysis. J Obes. 2015;2015:945918 Epub 2015/04/16. 10.1155/2015/945918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lumeng JC, Taveras EM, Birch L, Yanovski SZ. Prevention of obesity in infancy and early childhood: a National Institutes of Health workshop. JAMA Pediatr. 2015;169(5):484–90. Epub 2015/03/17. 10.1001/jamapediatrics.2014.3554 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, Loos RJF, et al. Obesity. Nat Rev Dis Primers. 2017;3:17034 Epub 2017/06/16. 10.1038/nrdp.2017.34 . [DOI] [PubMed] [Google Scholar]
- 22.DiPietro L, Mossberg HO, Stunkard AJ. A 40-year history of overweight children in Stockholm: life-time overweight, morbidity, and mortality. Int J Obes Relat Metab Disord. 1994;18(9):585–90. Epub 1994/09/01. . [PubMed] [Google Scholar]
- 23.Freedman DS, Shear CL, Burke GL, Srinivasan SR, Webber LS, Harsha DW, et al. Persistence of juvenile-onset obesity over eight years: the Bogalusa Heart Study. Am J Public Health. 1987;77(5):588–92. Epub 1987/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danielzik S, Czerwinski-Mast M, Langnase K, Dilba B, Muller MJ. Parental overweight, socioeconomic status and high birth weight are the major determinants of overweight and obesity in 5–7 y-old children: baseline data of the Kiel Obesity Prevention Study (KOPS). Int J Obes Relat Metab Disord. 2004;28(11):1494–502. Epub 2004/08/25. 10.1038/sj.ijo.0802756 . [DOI] [PubMed] [Google Scholar]
- 25.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320(7240):967–71. Epub 2001/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labayen I, Moreno LA, Ruiz JR, Gonzalez-Gross M, Warnberg J, Breidenassel C, et al. Small birth weight and later body composition and fat distribution in adolescents: the Avena study. Obesity (Silver Spring). 2008;16(7):1680–6. Epub 2008/05/10. 10.1038/oby.2008.258 . [DOI] [PubMed] [Google Scholar]
- 27.Sorensen HT, Sabroe S, Rothman KJ, Gillman M, Fischer P, Sorensen TI. Relation between weight and length at birth and body mass index in young adulthood: cohort study. BMJ. 1997;315(7116):1137 Epub 1997/12/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig SJC, Blankenberg D, Parodi ACL, Paul IM, Birch LL, Savage JS, et al. Child Weight Gain Trajectories Linked To Oral Microbiota Composition. Sci Rep. 2018;8(1):14030 Epub 2018/09/21. 10.1038/s41598-018-31866-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caprio S, Daniels SR, Drewnowski A, Kaufman FR, Palinkas LA, Rosenbloom AL, et al. Influence of race, ethnicity, and culture on childhood obesity: implications for prevention and treatment. Obesity (Silver Spring). 2008;16(12):2566–77. Epub 2009/03/13. 10.1038/oby.2008.398 . [DOI] [PubMed] [Google Scholar]
- 30.Currie A, Shields MA, Price SW. The child health/family income gradient: Evidence from England. J Health Econ. 2007;26(2):213–32. Epub 2006/09/12. 10.1016/j.jhealeco.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 31.Elbel B, Corcoran SP, Schwartz AE. Neighborhoods, Schools and Obesity: The Potential for Place-Based Approaches to Reduce Childhood Obesity. PLoS ONE. 2016;11(6):e0157479 10.1371/journal.pone.0157479 PMC4910992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santorelli G, Petherick ES, Wright J, Wilson B, Samiei H, Cameron N, et al. Developing prediction equations and a mobile phone application to identify infants at risk of obesity. PLoS One. 2013;8(8):e71183 Epub 2013/08/14. 10.1371/journal.pone.0071183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graversen L, Sorensen TI, Gerds TA, Petersen L, Sovio U, Kaakinen M, et al. Prediction of adolescent and adult adiposity outcomes from early life anthropometrics. Obesity (Silver Spring). 2015;23(1):162–9. Epub 2014/10/31. 10.1002/oby.20921 . [DOI] [PubMed] [Google Scholar]
- 34.Weng SF, Redsell SA, Nathan D, Swift JA, Yang M, Glazebrook C. Estimating overweight risk in childhood from predictors during infancy. Pediatrics. 2013;132(2):e414–21. Epub 2013/07/17. 10.1542/peds.2012-3858 . [DOI] [PubMed] [Google Scholar]
- 35.Morandi A, Meyre D, Lobbens S, Kleinman K, Kaakinen M, Rifas-Shiman SL, et al. Estimation of newborn risk for child or adolescent obesity: lessons from longitudinal birth cohorts. PLoS One. 2012;7(11):e49919 Epub 2012/12/05. 10.1371/journal.pone.0049919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redsell SA, Weng S, Swift JA, Nathan D, Glazebrook C. Validation, Optimal Threshold Determination, and Clinical Utility of the Infant Risk of Overweight Checklist for Early Prevention of Child Overweight. Child Obes. 2016;12(3):202–9. Epub 2016/04/20. 10.1089/chi.2015.0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Druet C, Stettler N, Sharp S, Simmons RK, Cooper C, Smith GD, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012;26(1):19–26. Epub 2011/12/14. 10.1111/j.1365-3016.2011.01213.x . [DOI] [PubMed] [Google Scholar]
- 38.Prevention CfDCa, Statistics NCfH. Electronic Medical Records/Electronic Health Records (EMRs/EHRs). 2015.
- 39.Henry J, Pylypchuk Y, Searcy T, Patel V. Adoption of Electronic Health Record Systems among U.S. Non-Federal Acute Care Hospitals: 2008–2015. The Office of the National Coordinator for Health Information Technology, 2016.
- 40.Charles D, Gabriel, H., Searcy, T. Adoption of Electronic Health Record Systems among U.S. NonFederal Acute Care Hospitals: 2008–2014 The Office of the National Coordinator for Health Information Technology, 2015.
- 41.Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278(2):563–77. Epub 2015/11/19. 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenspan H, Ginneken B, Summers RM. Guest Editorial Deep Learning in Medical Imaging: Overview and Future Promise of an Exciting New Technique. IEEE Transactions on Medical Imaging. 2016;35(5). [Google Scholar]
- 43.Lavecchia A. Machine-learning approaches in drug discovery: methods and applications. Drug Discov Today. 2015;20(3):318–31. Epub 2014/12/03. 10.1016/j.drudis.2014.10.012 . [DOI] [PubMed] [Google Scholar]
- 44.Henry KE, Hager DN, Pronovost PJ, Saria S. A targeted real-time early warning score (TREWScore) for septic shock. Sci Transl Med. 2015;7(299):299ra122 Epub 2015/08/08. 10.1126/scitranslmed.aab3719 . [DOI] [PubMed] [Google Scholar]
- 45.Razavian N, Blecker S, Schmidt AM, Smith-McLallen A, Nigam S, Sontag D. Population-Level Prediction of Type 2 Diabetes From Claims Data and Analysis of Risk Factors. Big Data. 2015;3(4):277–87. Epub 2016/07/22. 10.1089/big.2015.0020 . [DOI] [PubMed] [Google Scholar]
- 46.Lipton ZC, Kale DC, Elkan C, Wetzell R. Learning to diagnose with LSTM recurrent neural networks. arXiv:151103677. 2016.
- 47.Razavian N, Marcus J, Sontag D. Multi-task Prediction of Disease Onsets from Longitudinal Lab Tests. arXiv.org2016.
- 48.Suresh H, Hunt N, Johnson A, Celi LA, Szolovits P, Ghassemi M. Clinical Intervention Prediction and Understanding using Deep Networks. arXiv:170508498. 2017.
- 49.Chapman WW, Bridewell W, Hanbury P, Cooper GF, Buchanan BG. A simple algorithm for identifying negated findings and diseases in discharge summaries. J Biomed Inform. 2001;34(5):301–10. Epub 2002/07/19. 10.1006/jbin.2001.1029 . [DOI] [PubMed] [Google Scholar]
- 50.Baumel T, Nassour-Kassis J, Cohen R, Elhadad M, Elhadad Ne. Multi-Label Classification of Patient Notes a Case Study on ICD Code Assignment. arXiv:170909587. 2017.
- 51.Guyon I, Weston J, Barnhill S, Vapnik V. Gene Selection for Cancer Classification using Support Vector Machines. Machine Learning. 2002;46(1–3):389–422. [Google Scholar]
- 52.Chekroud AM, Zotti RJ, Shehzad Z, Gueorguieva R, Johnson MK, Trivedi MH, et al. Cross-trial prediction of treatment outcome in depression: a machine learning approach. Lancet Psychiatry. 2016;3(3):243–50. Epub 2016/01/25. 10.1016/S2215-0366(15)00471-X . [DOI] [PubMed] [Google Scholar]
- 53.Dugan TM, Mukhopadhyay S, Carroll A, Downs S. Machine Learning Techniques for Prediction of Early Childhood Obesity. Appl Clin Inform. 2015;6(3):506–20. Epub 2015/10/09. 10.4338/ACI-2015-03-RA-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hariz M, Adnan B, Husain W, Damanhoori F. A Survey on Utilization of Data Mining for Childhood Obesity Prediction. 2011.
- 55.Novak B, Bigec M, editors. Childhood obesity prediction with artificial neural networks. Proceedings ninth IEEE symposium on computer-based medical systems; 1996: IEEE.
- 56.Novak B, Bigec M, editors. Application of artificial neural networks for childhood obesity prediction. Proceedings 1995 Second New Zealand International Two-Stream Conference on Artificial Neural Networks and Expert Systems; 1995: IEEE.
- 57.Muhamad Adnan M, Husain W, Rashid N, editors. Parameter identification and selection for childhood obesity prediction using data mining. 2nd International Conference on Management and Artificial Intelligence Singapore: IACSIT Press; 2012.
- 58.Adnan MHBM, Husain W, editors. A hybrid approach using Naïve Bayes and Genetic Algorithm for childhood obesity prediction. 2012 International Conference on Computer & Information Science (ICCIS); 2012: IEEE.
- 59.Zhang S, Tjortjis C, Zeng X, Qiao H, Buchan I, Keane JJISF. Comparing data mining methods with logistic regression in childhood obesity prediction. 2009;11(4):449–60. [Google Scholar]
- 60.Prevention CfDCa, Statistics NCfH. CDC growth charts: United States. 2000.
- 61.Hagan JF, Shaw JS, Duncan PM. Bright futures: guidelines for health supervision of infants, children, and adolescents 4th ed: American Academy of Pediatrics; 2017. [Google Scholar]
- 62.Geosupport Desktop Edition NDoCP. Available from: http://www1.nyc.gov/site/planning/data-maps/open-data/dwn-gde-home.page.
- 63.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: Machine Learning in Python. J Mach Learn Res. 2011;12:2825–30. [Google Scholar]
- 64.Roy SM, Spivack JG, Faith MS, Chesi A, Mitchell JA, Kelly A, et al. Infant BMI or Weight-for-Length and Obesity Risk in Early Childhood. Pediatrics. 2016;137(5). Epub 2016/06/01. 10.1542/peds.2015-3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.United States. Public Health Service. Office of the Surgeon General., United States. Office of Disease Prevention and Health Promotion., Centers for Disease Control and Prevention (U.S.), National Institutes of Health (U.S.). The Surgeon General's call to action to prevent and decrease overweight and obesity. Rockville, MD Washington, DC: U.S. Dept. of Health and Human Services, Public Health Service For sale by the Supt. of Docs., U.S. G.P.O.; 2001. xv, 60 p. p.
- 66.Roberson LL, Aneni EC, Maziak W, Agatston A, Feldman T, Rouseff M, et al. Beyond BMI: The "Metabolically healthy obese" phenotype & its association with clinical/subclinical cardiovascular disease and all-cause mortality—a systematic review. BMC Public Health. 2014;14:14 Epub 2014/01/10. 10.1186/1471-2458-14-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Albuquerque D, Stice E, Rodriguez-Lopez R, Manco L, Nobrega C. Current review of genetics of human obesity: from molecular mechanisms to an evolutionary perspective. Mol Genet Genomics. 2015;290(4):1191–221. Epub 2015/03/10. 10.1007/s00438-015-1015-9 . [DOI] [PubMed] [Google Scholar]
- 68.Obesity URCfFP. Legislation Database—Tracks Policies Related to Obesity. Available from: http://www.uconnruddcenter.org/legislation-database.
- 69.Stice E, Shaw H, Marti CN. A meta-analytic review of obesity prevention programs for children and adolescents: the skinny on interventions that work. Psychol Bull. 2006;132(5):667–91. Epub 2006/08/17. 10.1037/0033-2909.132.5.667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barlow MA. Preventing Early Childhood Obesity in American Indian Populations.: NIH; 2017.
- 71.Beck AL, Heyman M, Chao C, Wojcicki J. Full fat milk consumption protects against severe childhood obesity in Latinos. Prev Med Rep. 2017;8:1–5. Epub 2017/09/01. 10.1016/j.pmedr.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Govindan M, Gurm R, Mohan S, Kline-Rogers E, Corriveau N, Goldberg C, et al. Gender Differences in Physiologic Markers and Health Behaviors Associated With Childhood Obesity. Pediatrics. 2013. 10.1542/peds.2012-2994 [DOI] [PubMed] [Google Scholar]
- 73.Pediatrics AA. Recommendations for Preventive Pediatric Health Care. 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file provides an overview of the features used in the paper’s analyses. The file can also be viewed in the following link on our GitHub through Jupyter’s NBViewer: https://nbviewer.jupyter.org/github/NYUMedML/ObesityPY/blob/master/src/Pediatric_Obesity_Prediction_Feature_Data.ipynb.
(IPYNB)
This file demonstrates the methods and results used to compare the performance of regression and classification techniques for prediction. The file can also be viewed in the following link on our GitHub through Jupyter’s NBViewer: https://nbviewer.jupyter.org/github/NYUMedML/ObesityPY/blob/master/src/Pediatric_Obesity_Prediction_Regression_Classification_Comparison.ipynb.
(IPYNB)
This file provides an overview of the final analyses performed. The file can also be viewed in the following link on our GitHub through Jupyter’s NBViewer: https://nbviewer.jupyter.org/github/NYUMedML/ObesityPY/blob/master/src/Pediatric_Obesity_Prediction_Regression_100_bootstraps.ipynb.
(IPYNB)
“*” indicates a feature whose unadjusted odds ratio is significantly greater than or less than 1.
(XLSX)
“*” indicates a feature whose unadjusted odds ratio is significantly greater than or less than 1.
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The data are electronic health records owned by NYU Langone Health and contain protected health information and personally identifiable information. They were not anonymized for this study. It is a restricted data set and public sharing of these data would violate the HIPPA security rule, however a deidentified data set will be available by request through https://www.icpsr.umich.edu/icpsrweb/. Our code and the subsequent analyses can be viewed on our GitHub page at https://github.com/NYUMedML/ObesityPY.