Abstract
Reproduction is a process that is extremely sensitive to changes in nutritional status. The nutritional control of oogenesis via insulin signaling has been reported; however, the mechanism underlying its sensitivity and tissue specificity has not been elucidated. Here, we determined that Drosophila Makorin RING finger protein 1 gene (Mkrn1) functions in the metabolic regulation of oogenesis. Mkrn1 was endogenously expressed at high levels in ovaries and Mkrn1 knockout resulted in female sterility. Mkrn1-null egg chambers were previtellogenic without egg production. FLP-FRT mosaic analysis revealed that Mkrn1 is essential in germline cells, but not follicle cells, for ovarian function. As well, AKT phosphorylation via insulin signaling was greatly reduced in the germline cells, but not the follicle cells, of the mutant clones in the ovaries. Furthermore, protein-rich diet elevated Mkrn1 protein levels, without increased mRNA levels. The p-AKT and p-S6K levels, downstream targets of insulin/Tor signaling, were significantly increased by a nutrient-rich diet in wild-type ovaries whereas those were low in Mkrn1exS compared to wild-type ovaries. Taken together, our results suggest that nutrient availability upregulates the Mkrn1 protein, which acts as a positive regulator of insulin signaling to confer sensitivity and tissue specificity in the ovaries for proper oogenesis based on nutritional status.
Introduction
Reproduction is an energetically expensive process because it involves massive cell proliferation and growth that requires synthesis of various proteins. Because of this high-energy demand, reproduction is tightly regulated by nutrient availability to ensure that reproduction only happens when there is a surplus of nutrients. When nutrients are scarce, reproduction is compromised to preserve energy for survival. Reproductive pauses in response to starvation are common among species of the animal kingdom; this reproductive dormancy can be quickly reversed by nutritional status [1–3].
Drosophila ovaries are composed of ovarioles that consist of sequentially developing egg chambers [4]. At the anterior end of an ovariole, germline stem cells reside in a structure called the germarium and proliferate to produce 16 interconnected germline cells, one of which becomes an oocyte while the remaining 15 cells become nurse cells. Groups of interconnected germline cells are called as germline cysts and newly produced germline cysts are enclosed by follicle cells and develop separately. Oogenesis can be roughly divided into 14 stages that can each be characterized by size and morphology. While germline cells do not divide after 16 cells, follicle cells go through mitosis until stage 6 and switch to endocycle stage (ME switch), where only genome replications occur. Notch signaling controls this ME switch [5, 6]. The ME switch is nutrient dependent and requires the Foxo gene [7]. After the ME switch, vitellogenesis, the yolk formation process by oocyte uptake of precursor proteins, and oocyte maturation occur. Whether oogenesis should continue or not is determined at a mid-oogenesis check point, which occurs right after the ME switch and before the vitellogenesis. The chief determining factor for oogenesis progression is if there are enough nutrients for reproduction. Under starvation conditions, programmed cell death occurs at stage 8 egg chambers [8].
The hormonal system of organisms relays nutritional information to organs. Insulin and insulin-like growth factor signaling is one of the most important hormonal systems for nutritional regulation. Insulin is released upon nutrient uptake and subsequently regulates processes that require energy, such as cellular growth, metabolism, and reproduction [9, 10]. Female reproductive organs rely on the insulin signaling system for proper function and to ensure that oogenesis occurs only when there are enough nutrients available [10]. The insulin signaling pathway and its role in ovarian development is conserved among species [11]. In flies, insulin receptor (InR) and insulin substrate protein 1 (chico) genetic mutation cause female sterility [12, 13]. Also, ovarian cells require the insulin signaling pathway for nutrient-dependent growth and vitellogenesis [8]. Insulin signaling also affects mammalian oocyte growth and development. In humans, ovarian malfunctions have been associated with defects in insulin signaling, such as insulin resistance [14, 15]. However, the mechanisms by which insulin regulates oocyte development have not been well established in vivo, despite evidence that insulin is involved in androgen production, gonadotropin signaling, PCOS (Polycystic Ovary Syndrome), and obesity-induced infertility [16].
Nutrient availability has been shown to delay or accelerate reproductive capabilities [17, 18]. Therefore, it can be inferred that ovaries possess a mechanism to distinguish quantitative differences in nutrient levels. We wanted to know how this quantitative nutritional information is interpreted at the molecular level and postulated that the strength of the nutrition-dependent regulatory signaling, such as insulin signaling, is directly influenced by the amount of nutrients available. Moreover, systemic insulin signaling is variable and tissue-specific because vital organs are preferentially spared from starvation. Indeed, ovaries are the most sensitive organ to reduced insulin signaling. In flies, mutation of InRE19, lnk4Q3/6S2, and chico1 cause reduction, but not complete abolition, of insulin signaling without exhibiting defects in vital organs; however, oogenesis is greatly compromised [12, 13, 19]. Mutation of Irs-2 in mice also causes female sterility with other major defects [20]. Furthermore, it has been shown that mouse ovaries remain sensitive to insulin signaling while the pituitary gland becomes insulin resistant in a diet-induced obesity model [21]. However, the mechanism of tissue specificity and sensitivity of insulin signaling remains poorly understood.
The target of rapamycin (TOR) signaling pathway is another highly conserved nutrient sensing signal that is required for cell growth and proliferation [22, 23]. While insulin signaling senses and delivers systematic nutrient information, TOR signaling senses cellular amino acid levels and regulates anabolic processes. Insulin and TOR signaling interact to ensure cells integrate nutrient information from difference sources and perform appropriate cellular processes. In Drosophila, the ovaries require TOR activity for the proliferation and maintenance of germline cells [22]. Hence, interactions between the insulin and TOR signaling pathways are the most evident in ovaries. In Drosophila, PRAS40, the inhibitor of TOR signaling pathway, affects the interaction of the insulin and TOR signaling only in ovaries [23]
Steroid hormone signaling is also important for reproduction. In Drosophila, ecdysone, a steroid hormone that controls molting in insects, is required for reproductive processes. Developing egg chambers degenerate without ecdysone signaling [24, 25]. In general, steroid signaling is influenced by the nutritional and metabolic status of an organism. For example, nutrition affects ecdysone concentrations and InR mutation impairs ecdysone synthesis in Drosophila [26, 27]. In mammals, ovarian folliculogenesis is regulated by the interplay of hormones such as gonadotropins, steroids and insulin-like growth factors [28–30].
Mkrn1 is a Drosophila homologue of mammalian makorin family and encodes RING zinc finger proteins with ubiquitin ligase activity. In mammals, Mkrn1 reportedly destabilizes many substrates including p53, p21, PPARγ, hTERT, PTEN, APC, and AMPK [31–36], and is therefore implicated in various cellular events. In our previous study, we showed that Drosophila Mkrn1 is involved in the proper timing of the larval to pupal transition and affects final body size by regulating ecdysone synthesis in the prothoracic gland suggesting Mkrn1 plays a role in the transition from growth to maturation [37]. Here, we found that female Mkrn1 null mutant (Mkrn1exS) flies are sterile, and further demonstrated that Mkrn1 is required for Drosophila oogenesis. Furthermore, Mkrn1 was strongly expressed in ovaries and was upregulated by a protein-rich diet. Mkrn1 null female flies exhibited vitellogenesis failure, which also occurs when flies were starved or nutrient signaling was perturbed. Insulin/Tor signaling was greatly reduced in Mkrn1exS ovaries, implying a role as a positive regulator in the insulin/Tor signaling pathway. Collectively, our data support the notion that Mkrn1 functions as a tissue-specific regulator of the insulin/Tor signaling pathway to activate oogenesis in ovaries in a nutrient-sensitive manner.
Materials and methods
Drosophila strains and culture conditions
The Mkrn1 and Mkrn1exS mutant Drosophila strains were generated as previously described [37]. Two lines with Mkrn1 gene deletions—Df(3L)BSC418/TM6C (BDSC 24922) and Df(3L)BSC419/TM6C (BDSC 24923)—were used in this study. NRE-EGFP (BDSC 30727) was used to examine Notch signaling and PRAS40KO (BDSC 76339) was used to examine epistatic interactions with Mkrn1exS.
All Drosophila stocks were maintained on a standard cornmeal food at room temperature except for the experimental groups that required nutrient-poor and -rich diet conditions. The nutrient-poor diet contained 10% sucrose and 2% agar, whereas the nutrient-rich diet contained 10% sucrose, 2% agar, and yeast paste. Females were cultured for 1 day under different nutritional conditions less than 8 hours after eclosion.
Generation and analysis of mosaic clones
For FLP-FRT mosaic analysis, the FRT80B Mkrn1exS strain was generated by FRT80B and Mkrn1exS recombination followed by crossing with hsflp; FRT80B, ubi-GFP females. F1 generation, one-day-old, female flies were heat-shocked for 1 hr at 37°C twice a day for 3 consecutive days and kept in a 25°C incubator. Ovaries were dissected and subjected to immunostaining 10 days after the first shock.
Quantitative real-time PCR
Adult female heads, thoraxes, abdomens, and ovaries were dissected. From these tissues, total RNA was isolated using QIAzol lysis reagent (QIAGEN) and reverse-transcribed with oligo-dT primers using Prime Script reverse transcriptase (TAKARA). Quantitative real-time PCR was performed using a Rotor Gene 6000 (QIAGEN) with SYBR Premix Ex Taq (Tli RNase H Plus; TAKARA). The following primers were used: Mkrn1-forward, 5’-GACGTGCGGCATCTGCTTTG-3’; Mkrn1-reverse, 5’-TGTTTGGCCTGACGCCATGT-3’; Phm-forward, 5’-GCTTGCATTTCCGAGACGAT-3’; Phm-reverse, 5’-ACGATCATCGAACCACCCTT-3’; E74-forward, 5’-CAAACCGAAGCTGGAGATGG-3’; and E74-reverse, 5’-TCGTCCACTTGATGAAACGC-3’. Cbp20 or actin mRNA levels were used to normalize gene expression levels using the following primer sequence: cbp20-forward, 5’-GTATAAGAAGACGCCCTGC-3’; cbp20-reverse, 5’-TTCACAAATCTCATGGCCG-3’; actin-forward, 5’-CATGTTTGAGACCTTCAACACCCC-3’; actin-reverse, 5’-GCCATCTCCTGCTCGAAGTCTAG-3’. Data were analyzed and quantified using Rotor Gene 6000 software.
Immunoblot analysis
We used our previously generated antibody against Drosophila Mkrn1 [37]. Protein extracts from the head, thorax, abdomen, and ovaries of adult female Drosophila were prepared using lysis buffer (10 mM HEPES [pH 7.5]; 50 mM KCl; 10% glycerol; 5 mM Tris-HCl [pH 7.5]) with freshly added 5 mM EDTA, 1 mM DTT, 0.1% Triton X-100, protease inhibitor (Sigma), 1 mM Na3VO4, and 0.25 mM NaF (final concentration). For p-AKT and p-S6K analysis, phosphatase inhibitor cocktail 2 and 3 (Sigma) were used instead of Na3VO4 and NaF. The protein extracts were resolved by SDS-PAGE and the resulting blots were probed using the following primary antibodies: rabbit anti-Mkrn1, 1:3000 [37]; rabbit anti-phospho-AKT (Ser505), 1:1000 (Cell Signaling Technology, 4054); rabbit anti-AKT, 1:1000 (Cell signaling Technology, 9272); rabbit anti-phospho-S6K (Thr398), 1:1000 (Cell Signaling Technology, 9209); guinea pig anti-dS6K, 1:3000 [38]; mouse anti-Armadillo, 1:1000 (DSHB, N2 7A1); rabbit anti-p44/42 MAPK (Erk1/2), 1:2000 (Cell Signaling Technology, 9102). Band intensities were quantified using ImageJ software.
Immunostaining of ovaries and image analysis
The ovaries of female adult flies were dissected in PBS; and approximately twenty ovaries for each sample were analyzed by immunostaining. The ovaries were fixed in 4% paraformaldehyde and rinsed with 0.5% PBST solution (1× PBS containing 0.5% Triton X-100). The fixed ovaries were incubated for 1 hr in blocking solution comprised of 0.5% PBST and 10% horse serum. Primary antibodies were diluted in blocking solution and incubated with samples overnight at 4°C followed by washing with 0.5% PBST. The samples were subsequently incubated with secondary antibodies diluted in blocking solution overnight at 4°C. The ovaries were washed and stained with hoechst33342 (Sigma, 1:1000), mounted on slides, and incubated with Phalloidin-TRITC (Sigma, P1951) in PBS (2.5 ug/ml) for 30 min to stain the actin filaments. Confocal images were obtained with a LSM710 or LSM800 Microscope (Zeiss) and processed with Zen software (Zeiss). The following antibodies and final dilutions were used: rabbit anti-Mkrn1, 1:3000 [37]; goat anti-VASA, 1:500 (Santa Cruz, sc-26877); mouse anti-Cut, 1:100 (DSHB, 2B10); mouse anti-Hindsight, 1:100 (DSHB, 1G9); mouse anti-Broad Core, 1:100 (DSHB, 25E9.D7); rabbit anti-phospho-AKT (Ser473), 1:100 (Cell Signaling Technology, 4060), Alexa 555-conjugated goat, anti-mouse and goat, anti-rabbit IgG, 1:100 and 1:200, respectively (Sigma); Alexa 488-conjugated donkey, anti-goat IgG, 1:200 (Sigma).
Rapamycin treatment
Rapamycin (Sigma) was dissolved in ethanol and diluted to a final concentration of 1 μM in PBS. Ovaries were dissected from female files 6 to 8 hours after eclosion and incubated in PBS with or without rapamycin for 1 hour, or 2 hours. After incubation, the ovaries were immediately processed for western blot analysis.
Results
Mkrn1 expression was enriched in Drosophila ovaries and Mkrn1-null females were sterile
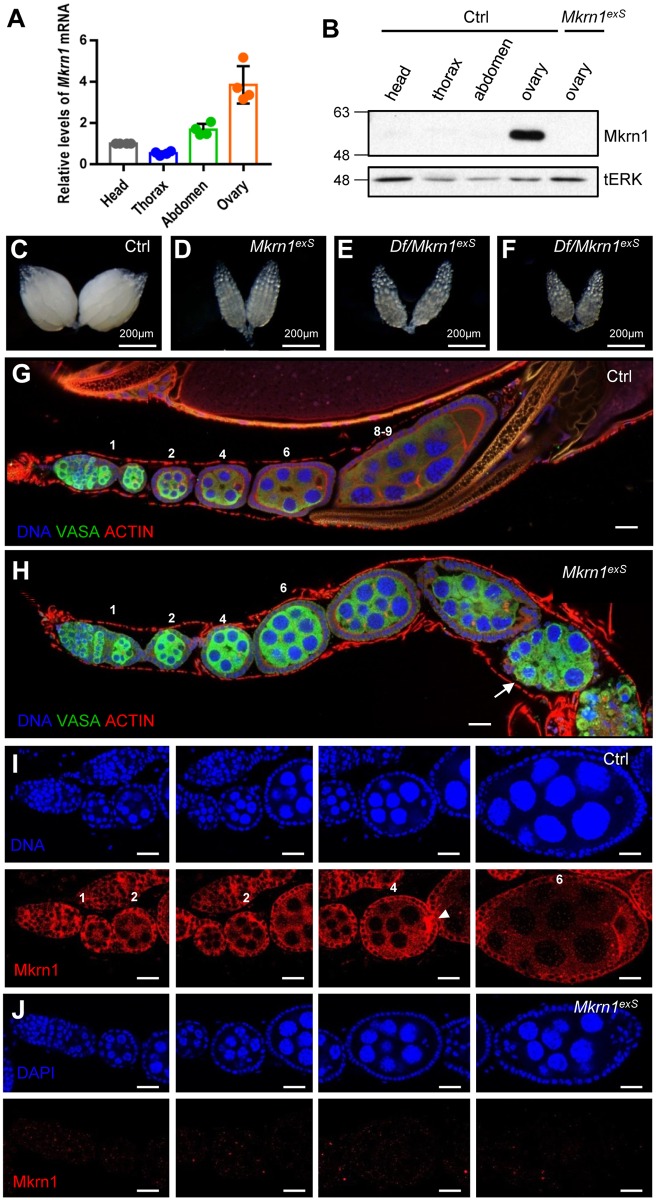
Mkrn1-null flies (Mkrn1exS,[37]) are completely viable with no obvious phenotype. However, we found that the female flies were sterile whereas the male flies were fertile. This was surprising because our previous study did not reveal sexual dimorphism of Mkrn1-mutant flies during pupariation and Mkrn1 is expressed in prothoracic endocrine glands [37]. To determine the role of Mkrn1 in female reproduction, we first examined Mkrn1 mRNA levels in ovaries. We found that Mkrn1 mRNA expression was highly enriched in ovaries compared to the head, thorax, and abdomen (Fig 1A), which is consistent with our observed phenotype of Mkrn1-mutant female sterility. Next, we analyzed Mkrn1 protein levels using anti-Mkrn1 antibody and further confirmed that it is highly enriched in ovaries compared to other tissues, suggesting an important role of Mkrn1 in Drosophila female fertility (Fig 1B).
Fig 1. Mkrn1 expression was highly enriched in control ovaries and Mkrn1-null ovaries exhibited a phenotype.
(A) One-day-old, control female flies from standard nutrient conditions were dissected for baseline analyses. Total mRNA was extracted from the heads, thoraxes, abdomens, and ovaries and analyzed by quantitative real-time PCR. Relative mRNA levels for Mkrn1 and reference gene Actin are shown. Error bars represent the SEM from four independent experiments. (B) Protein extracts from indicated tissues were analyzed by immunoblot using the anti-Mkrn1 antibody and tERK was used as a loading control. (C–F) Bright-field images of whole ovaries from control (C), Mkrn1exS (D), Df(3L) BSC418/Mkrn1exS (E), and Df(3L) BSC419/Mkrn1exS flies. (G, H) Confocal images of ovariole immunostaining from control (G) and Mkrn1exS (H) flies. Dissected ovaries were stained for Vasa (green), Actin (red, phalloidin), and the DNA (blue, hoechst). Please note that Mkrn1exS egg chambers did not progress to vitellogenic stages and eventually degenerated from stage 7 resulting in egg chambers with only germline cells (arrow). (I, J) Ovaries were immunostained for DNA (blue, top panel) and Mkrn1 (red, bottom panel) from control (I) and Mkrn1exS (J) flies. Mkrn1 was ubiquitously observed in cytoplasm of follicle cells, nurse cells, and oocytes throughout oogenesis, and Mkrn1 was enriched in the pole plasm (arrow head) in the control. Absence of Mkrn1 detection in Mkrn1exS ovaries validates the gene knockout and specificity of the Mkrn1 antibody. Numbers above each egg chamber indicate the developing stage of ovarioles. Scale bars = 20μm.
Vitellogenesis did not occur in Mkrn1-null ovaries
The ovaries of Mkrn1exS flies were much smaller than control ovaries and did not contain mature eggs (Fig 1C and 1D). To confirm that the female sterility phenotype was truly due to loss of function of the Mkrn1 gene, we crossed Mkrn1exS with two different deficiency lines uncovering this locus for the complementation testing. Female heterozygotes of both strains, Df(3L)BSC418/Mkrn1exS and Df(3L)BSC419/Mkrn1exS, were sterile. Moreover, ovarian phenotypes and size were indistinguishable among the Df(3L)BSC418/Mkrn1exS, Df(3L)BSC419/Mkrn1exS, and Mkrn1exS homozygotes, confirming that this allele is the true null allele of Mkrn1 (Fig 1E and 1F).
To examine the defects of oogenesis more closely, ovaries were stained with phalloidin and antibody against VASA protein. All egg chambers were previtellogenic and lacked the dramatic morphological defects apparent in egg chambers prior to the previtellogenic stage (Fig 1G and 1H). Oogenesis did not proceed after stage 7. After several strings of stage 7 egg chambers, the follicle cells degenerated, resulting in egg chambers with only germline cells. Because Mkrn1 deficiency displayed stage-dependent defect, we examined if Mkrn1 had a stage-specific expression pattern using the Mkrn1 antibody. We first confirmed that our antibody recognized the Mkrn1 protein by immunofluorescence staining of ovaries since Mkrn1 staining disappears in Mkrn1exS (Fig 1J). Mkrn1 was ubiquitously expressed in follicle cells, nurse cells, and oocytes throughout oogenesis (Fig 1I).
Cell-autonomous role of Mkrn1 was displayed in germline cells
Hormone signaling dysfunction, such as impaired insulin or ecdysone signaling pathways, can cause female sterility with vitellogenesis defects in flies [12, 24]. Although the previous study suggests a role of Mkrn1 in neuroendocrine cells in controlling the timing of pupariation [37], ovary-enriched expression of Mkrn1 and female specific sterility suggest that Mkrn1 function is autonomously required for normal ovarian function. To test this, we performed a mosaic clonal analysis using FLP-FRT mitotic recombination and examined mutant clones in the ovaries. This experiment also made it possible to further dissect the specific cell types that required Mkrn1 function in the ovaries.
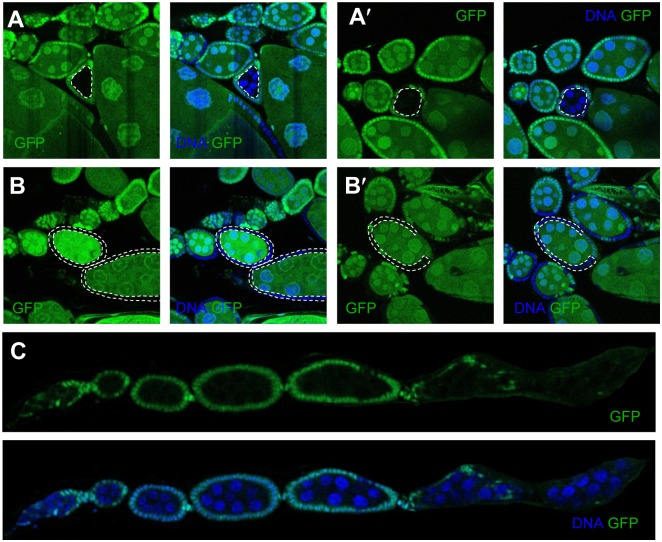
Interestingly, the egg chambers with Mkrn1-mutant germline cysts enclosed by wild-type follicle cells were smaller than its anterior cysts, which was abnormal because oogenesis proceeds serially, resulting in larger posterior than anterior egg chambers (Fig 2A and 2A′). The smaller size of Mkrn1exS germline cysts suggests that Mkrn1 autonomously regulates germline cyst growth. In contrast, the egg chambers with wild-type germline cells enclosed by Mkrn1exS follicle cells did not exhibit phenotypic differences from neighboring wild-type egg chambers. Even when all of the follicle cells of the egg chamber were Mkrn1 mutants, if germline cells were wild-type, the egg chambers developed normally and reached vitellogenic stages suggesting that the loss of Mkrn1 in follicle cells is not responsible for the oogenesis defect (Fig 2B and 2B′). We also found that ovarioles consisting of egg chambers with Mkrn1 mutant germline cysts enclosed by wild-type follicle cells exhibited exactly the same phenotype as Mkrn1exS ovarioles (Fig 2C). Mkrn1—null germline cyst development did not proceed past stage7 before the egg chamber degenerated. From these results, we concluded that the function of Mkrn1 is autonomous in ovaries, more specifically in germline cells.
Fig 2. Mkrn1 function was required in germline cells, but not follicle cells, for egg chamber growth.
(A, B) Mkrn1exS cells are marked by the absence of GFP (green). Posterior egg chambers consisting of Mkrn1exS germline cysts and wild-type follicle cells were abnormally smaller than the anterior egg chambers (A and A′). Egg chambers with wild-type germline cysts and mutant follicle cells grew normally and proceeded to vitellogenic stages (B and B′). (C) Ovarioles with all egg chambers had Mkrn1exS mutant germline cysts and wild-type follicle cells phenocopied the Mkrn1exS mutant. The dashed line indicates the Mkrn1-null clones.
Mkrn1-mutant ovaries did not display aberrant Notch signaling during mid-oogenesis
The ME switch occurs during mid-oogenesis. This transition is a prerequisite for egg chambers to enter vitellogenesis, and Notch signaling regulates this transition around stage 6 [5–7].
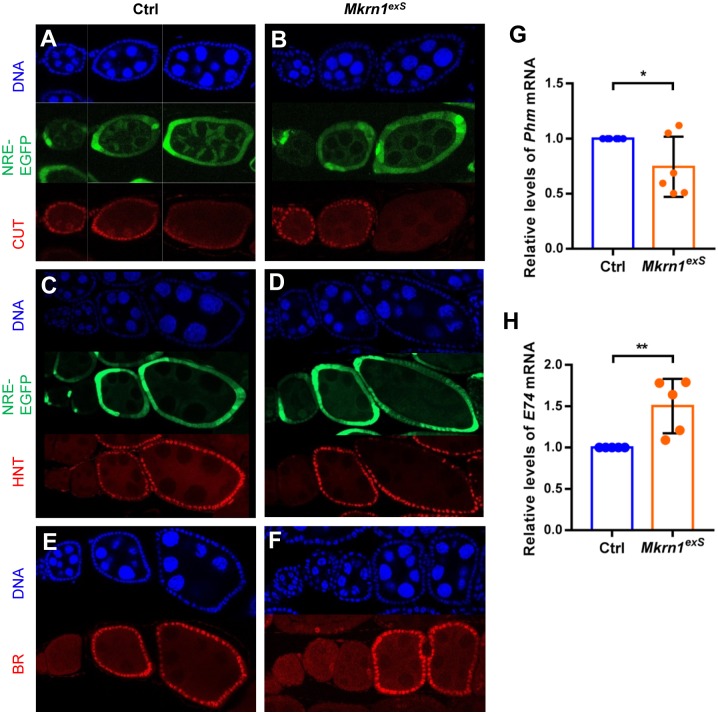
Because Mkrn1 mutants exhibit terminated development at the stages immediately after the ME switch, we examined if Notch signaling was affected in Mkrn1exS flies. Notch regulates the expression of transcription factors such as cut, hindsight (hnt), and broad (br) during the ME switch. We introduced Notch reporter NRE-EGFP, in which Notch response element drives expression of EGFP, into control and Mkrn1exS flies. NRE-EGFP expression was stronger around stage 6. The onset pattern of NRE-EGFP expression was indistinguishable in control and Mkrn1exS flies (Fig 3A–3D). We also examined three well-known Notch targets: cut, hnt, and br to determine Notch activity. When Notch signaling is activated around stage 6, cut expression is repressed and hnt and br expression is induced. In Mkrn1 mutants, all three Notch targets showed similar expression patterns compared to the control (Fig 3A–3F). Our results showed that Mkrn1 did not affect the onset of Notch signaling in follicle cells during the ME switch.
Fig 3. Expression of Notch targets and ecdysone-responsive genes were not affected in Mkrn1-null Drosophila ovaries.
(A–F) Egg chambers from control and Mkrn1exS flies were stained for Cut (A and B), Hnt (C and D), and Br (E and F) (shown in red). (A–D) NRE-EGFP (GFP) was used as a Notch signaling reporter and was activated around stage 6 in both the control and Mkrn1exS ovaries. Cut was expressed up to stage 6 and Hnt was activated around stage 6 in both control and Mkrn1exS ovaries. Br was also activated after stage 6 in both the control and Mkrn1exS ovaries. (G and H) Control and Mkrn1exS ovaries were dissected from one-day-old females. Quantitative real-time PCR was performed to measure mRNA levels of Phm (G) and E74 (H). Relative mRNA levels are shown and error bars represent SEM from five independent experiments. Asterisks indicate statistically significant differences (Student’s t-test: *P < 0.05; **P < 0.01).
Ecdysone signaling was not affected in Mkrn1-null Drosophila ovaries
In Drosophila ovaries, ecdysone signaling is cell-autonomously required in germline cells for proper development. Ovaries display previtellogenic egg chambers without appropriate ecdysone signaling, as shown in ecdysone receptor (EcR) and ecdysoneless cell cycle regulator (ecd) mutants, which are similar to the Mkrn1exS phenotype [24, 25]. We previously showed that ecdysone synthesis was down-regulated in prothoracic glands during larval development in Mkrn1exS larvae [37]. Thus, we tested if reduced ecdysone signaling was responsible for the oogenesis defects in Mkrn1exS ovaries. In adults, ovaries can also produce ecdysone [39], We measured mRNA levels of phantom (phm), an enzyme required for ecdysone synthesis, and found its transcript levels to be slightly reduced in Mkrn1exS ovaries compared to controls (Fig 3G). These data are consistent with what was observed in Mkrn1exS larvae, suggesting the mechanism by which Mkrn1 regulates ecdysone synthesis could be preserved in ovaries. Next, as a surrogate indicator for ecdysone signaling, the expression of the early ecdysone response gene, E74, was examined by quantitative real-time PCR. E74 transcript levels were reduced in Mkrn1exS larvae [37]; however, its expression levels were 1.5-fold higher in Mkrn1exS ovaries. Thus, the reduction of ecdysone synthesis did not appear to significantly affect ecdysone signaling transduction (Fig 3H), presumably due to the ecdysone synthesized by other tissues [39–41]. Moreover, br, another target of ecdysone signaling [5, 42], was not affected in Mkrn1exS ovaries, further supporting this postulation (Fig 3E and 3F). On the other hand, excessive ecdysone signaling can induce apoptosis of nurse cells at stage 8 and 9 [43]. Although Mkrn1exS ovaries showed a slight increase in ecdysone signaling, egg chambers remained intact following stages 8–9 and nurse cells did not undergo apoptosis, as shown by the absence of condensed nuclei; therefore, the increased ecdysone signaling was not the cause of the ovarian defects observed in Mkrn1exS flies.
Insulin signaling was significantly reduced in Mkrn1-mutant cysts
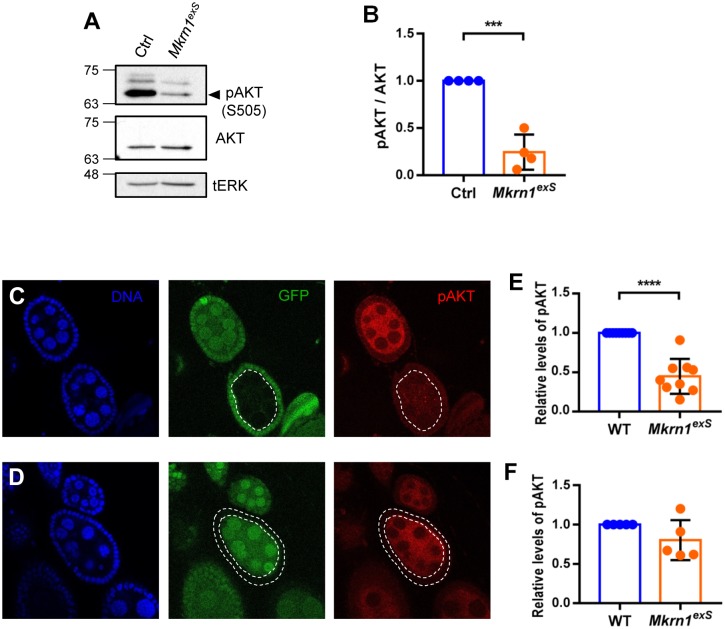
The mid-oogenesis check point is the stage right before the onset of vitellogenesis. In addition to Notch and ecdysone signaling pathways, insulin and TOR signaling pathways are also essential to ensure that there are enough nutrients for the egg production progression. InR hypomorphs show very similar phenotypes to Mkrn1exS ovaries in that they are both previtellogenic [12]. The similarity of ovarian phenotypes of Mkrn1exS and insulin signaling pathway mutants led us to examine the role of Mkrn1 in the insulin signaling pathway. We used phosphorylated AKT (p-AKT) as a readout of insulin signaling pathway activation. p-AKT levels were measured by western blot analysis of ovarian protein extracts from one-day-old female flies to ensure that both control and mutant flies had similar sized ovaries at the same stages of oogenesis for proper molecular comparison. We found that AKT phosphorylation was greatly reduced in Mkrn1exS ovaries compared to control, indicating that insulin signaling is reduced in Mkrn1exS ovaries (Fig 4A and 4B).
Fig 4. p-AKT levels were reduced in Mkrn1exS ovaries.
(A) Protein extracts were prepared from ovaries of one-day-old control and Mkrn1exS female flies and analyzed by western blot using anti-p-AKT and anti-AKT antibodies, and tERK as the loading control. (B) Relative p-AKT levels were quantified by measuring band intensities and normalized by total AKT protein levels. Error bars represent SEM from four independent experiments. Asterisks indicate statistically significant differences (Student’s t-test: ***P<0.001). (C–F) Confocal images of immunostaining for p-AKT (red) and DNA (blue) of ovaries from Mkrn1exSflies containing mutant germline and follicle cells. Mkrn1exS cells are distinguished by the absence of GFP (green). p-AKT level were significantly reduced in Mkrn1exS germline cells, but not in Mkrn1exS follicle cells. Fluorescence intensities of p-AKT were quantified and Mkrn1exS germline cysts were compared with similarly sized neighboring wild-type germline cysts. Mkrn1exS follicle cells were quantitated and compared to nearby wild-type follicle cells. Error bars represent SEM for 10 germline clones and five follicle clones. Asterisks indicate statistically significant differences (Student’s t-test: *P < 0.05; ****P < 0.0001).
As described earlier, Mkrn1 function is essential for germline cyst, but not follicle cell, growth. If this growth defect is due to decreased insulin signaling, we could expect different influences of Mkrn1 on various cell types. To determine if there are cell-type specific differences in insulin signaling, ovaries were immunostained for p-AKT. Levels of phosphorylated AKT were significantly reduced in Mkrn1exS germline cells and there was no difference in Mkrn1exS follicle cells (Fig 4C–4F). This result is consistent with the growth-limiting phenotypes of Mkrn1exS only manifested in germline cells but not in follicle cells. Reduction of insulin signaling in germline clones of Mkrn1exS implies that Mkrn1 function is required for insulin signaling and normal development of ovaries.
Mkrn1 levels depend on nutrient availability and TOR signaling
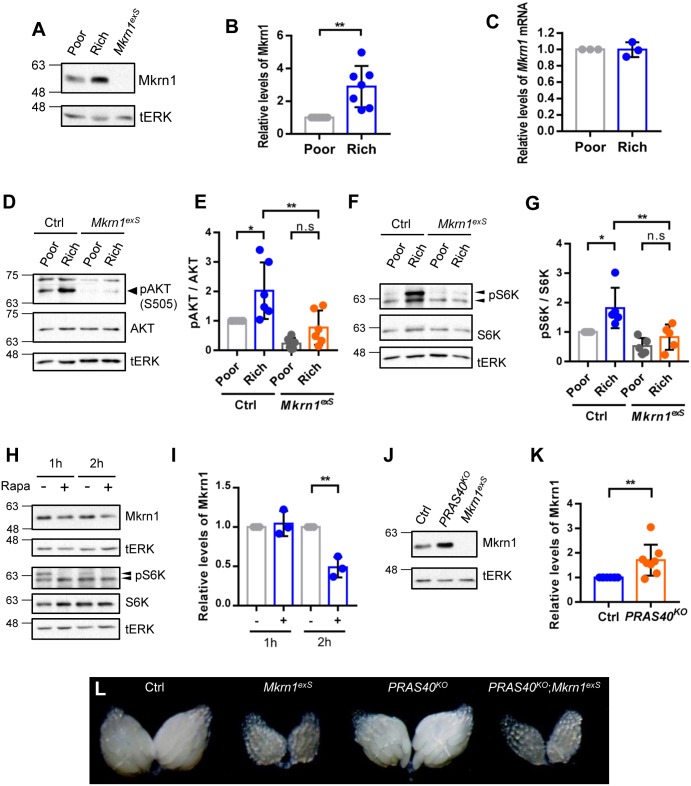
The ovarian phenotype of Mkrn1exS is very similar to those of nutrient signaling pathway hypomorphs as well as observations under starvation conditions. To test if Mkrn1 protein levels are regulated by nutrient availability, flies were subjected to nutrient-poor or -rich diet conditions for 1 day. Because we wanted to focus on signaling events in early stages of egg chambers, we used one-day-old females for the analysis. Protein extracts from ovaries of female flies, maintained in either nutrient-poor or -rich diet conditions, were examined by western blot analysis. We found that Mkrn1 protein levels were significantly reduced in ovaries of starved flies (Fig 5A and 5B). To determine if this reduction in Mkrn1 protein levels was due to reduced transcription, we performed quantitative real-time PCR for Mkrn1 mRNA transcript levels; Mkrn1 mRNA levels were not affected by nutritional status (Fig 5C). Thus, Mkrn1 is regulated by nutritional status at the post-translational level. To examine how Mkrn1 affects insulin and TOR signaling in ovaries depending on nutritional status, we examined the levels of p-AKT (Fig 5D and 5E) and p-S6K (Fig 5F and 5G), downstream effector of insulin and TOR signaling, in control and Mkrn1exS ovaries under nutrient-poor and -rich conditions. We found that both p-AKT and p-S6K signals were significantly increased by a nutrient-rich diet in control flies but not in Mkrn1exS. Moreover, the levels were very low compared to control flies under both nutrient conditions. This indicates that Mkrn1 is an important modulator of the insulin and TOR signaling pathway that holds the ability to respond to nutritional status signals.
Fig 5. Mkrn1 ovarian protein levels are regulated by nutritional status and TOR signaling in Drosophila.
(A) Newborn female flies were reared for 24 hours under the indicated nutritional conditions. Protein extracts from flies under nutrient-poor or -rich conditions were subjected to immunoblot analysis using anti-Mkrn1 antibody, and tERK as the loading control. (B) Mkrn1 protein levels were quantified by measuring band intensities and the relative levels are shown. Error bars represent SEM from seven independent experiments. Asterisks indicate statistically significant differences (Student’s t-test: **P<0.0.01). (C) Ovaries from flies under the same nutrient conditions were used for mRNA extraction. Quantitative real-time PCR was performed to measure Mkrn1 mRNA levels and the relative levels are shown. Error bars represent SEM from three independent experiments. (D-G) Control and Mkrn1exS females were reared under nutrient-poor or -rich conditions for 24 hours. Protein extracts were then obtained from the ovaries and subjected to immunoblot analysis using anti-p-AKT and anti-AKT antibodies (D), anti-pS6K and anti-S6K (F) and tERK as the loading control. Relative levels of p-AKT (E) and pS6K (G) were quantified by measuring band intensities and normalized to the total AKT protein levels (E) and S6K protein levels (G). (H and I) The ovaries were dissected and incubated in PBS with or without rapamycin (1μM) for the indicated time. Protein extracts from the ovaries were subjected to immunoblot analysis using anti-Mkrn1 antibody and tERK as the loading control. Relative levels of Mkrn1 were determined as described above. The error bars represent SEM from three independent experiments and the asterisks indicate statistically significant differences (Student’s t-test: **P<0.01). (J and K) Ovaries from control and PRAS40KO female flies were dissected and subjected to western blot analysis of the Mkrn1 levels using anti-Mkrn1 antibody and tERK as the loading control. Relative levels of Mkrn1 were determined as described above. The error bars represent SEM from eight independent experiments and the asterisks indicate statistically significant differences (Student’s t-test: *P<0.05, **P<0.01). (L) Bright filed images of ovaries from control, Mkrn1exS, PRAS40KO, and Mkrn1exS PRAS40KO flies are shown.
Because Mkrn1 level were increased by a nutrient- rich diet, we sought to determine if TOR signaling regulates Mkrn1 protein levels. First, we treated ovaries from one-day-old females with rapamycin, a TOR inhibitor. Compared to vehicle treated ovaries, Mkrn1 levels were reduced in rapamycin treated ovary (Fig 5H and 5I). Because PRAS40 functions as an ovary-specific negative regulator of TORC1 in Drosophila [23], we also examined MKRN1 levels in PRAS40 mutant ovaries, and found that Mkrn1 levels were increased in the ovaries from PRAS40KO, PRAS40 null mutants (Fig 5J and 5K). These data confirmed that Mkrn1 levels are regulated by TOR signaling. A prior report indicated that PRAS40 acts downstream of insulin signaling in the ovaries and rescued the sterile phenotype observed in chico1 mutants [23]. To test whether PRAS40 could also rescue the phenotype observed in Mkrn1exS, we generated double mutants for PRAS40 and Mkrn1. In contrast to the chico1 mutant, the introduction of the PRAS40 mutant to Mkrn1exS did not rescue the sterility observed in Mkrn1exS females. In addition, the ovaries from the double mutants were indistinguishable from the ovaries obtained from Mkrn1exS females, suggesting that Mkrn1 is epistatically downstream of PRAS40 (Fig 5L), which was consistent with the finding that MKRN1 levels were elevated in the absence of PRAS40. Taken together, the enrichment of Mkrn1 in the ovaries suggests that Mkrn1 acts as a tissue-specific factor that is regulated by a protein-rich diet through TOR signaling and that Mkrn1 regulates the insulin/Tor signaling pathway to drive oogenesis only in the presence of sufficient nutrients.
Discussion
In this study, we revealed a previously unknown role of Mkrn1 in oogenesis. Mkrn1 loss leads to previtellogenic ovaries, which are also observed in hypomorphic models of nutrient-dependent signaling pathway components. Nutrient-dependent control of signaling pathways is especially important for the onset of vitellogenesis because the following states require high amounts of energy for protein synthesis. Our data showing Mkrn1 as a positive regulator of the insulin signaling pathway is consistent with its mutant phenotype.
Clonal analysis of the Mkrn1 mutation revealed that Mkrn1 is required in germline cells, but not in the follicle cells in egg chambers. Reduction of p-Akt was only observed in germline cysts, but not in follicle cells. These results contrast with the ubiquitous expression of Mkrn1 in ovaries and the preferential degeneration of follicle cells in Mkrn1 null mutants. The degeneration of follicle cells also occurred in ovarioles consisting of wild-type follicle cells and Mkrn1 mutant germline cells (Fig 2C), indicating a non-autonomous role of Mkrn1 in the degeneration of neighboring follicle cells. A Similar phenotype was described as ‘peas without pods’ (Pwop) in egg chambers harboring insulin and TOR signaling pathway mutant germline clones, such as InR hypomorph, chico1, and S6K mutants [44]. The similarity between InR, chico, S6K, and Mkrn1 mutant germline clones suggests that Mkrn1 has a regulatory role in insulin signaling in germline cells. Collectively, we think that germline cells might be protected from degeneration induced by a reduction in insulin signaling caused either by the Mkrn1 mutation or by other components of the insulin signaling pathway, but not the follicle cells.
Unlike key components of the insulin signaling pathway, Mkrn1 expression is highly enriched in ovaries. This explains why Mkrn1exS flies do not exhibit alterations in body size while chico, lnk, and InR hypormorphs exhibit a reduction in body size in addition to the female-sterile phenotype. This implies that Mkrn1 is a tissue-specific modulator of the insulin signaling pathway. However, the significance of Mkrn1 enrichment in ovaries remains unclear. According to our current study, as well as others, we can infer that tissue-specific differences in the dynamics and strength of insulin signaling is required based on the organ for proper homeostasis. For example, in developing flies, brain growth is spared during nutrient restriction. This tissue-specific effect is achieved by strong Alk expression in the brain, which regulates the TOR and insulin signaling pathways [45]. In adult male flies, the genitals are also resistant to starvation. This is made possible by decreasing the expression FOXO in this tissue to ensure the insulin signal is not inhibited by nutrient restriction [46]. Conversely, ovaries are immediately wasted during starvation to preserve energy for survival and are particularly sensitive to the availability of amino acids [8].
Ovary-specific factors or mechanisms are required to trigger events that are restricted to the ovaries by ubiquitous nutritional changes or by the genetic manipulation of insulin signaling. A prior study of Drosophila PRAS40 supports the tissue specific regulation of the nutrient signaling pathway [23]. The authors showed that an ovary specific connection existed between the insulin and TOR signaling pathways. Although PRAS40 is ubiquitously expressed, the loss of PRAS40 function only resulted in enlarged ovaries with increased p-S6K levels, but a normal body size. PRAS40 acts downstream of insulin signaling and specifically regulates TORC1 activities in the ovaries. Our data showed that PRAS40 negatively regulates Mkrn1 levels and that Mkrn1, in turn, positively regulates insulin/Tor signaling. Hence, because it is highly expressed in the ovaries, regulated by nutritional status and TOR signaling, and positively regulates the insulin/Tor signaling pathway, we propose that Mkrn1 is a nutrient-dependent, tissue-specific regulator of insulin/Tor signaling in the ovaries. Tissue-specific modulators of insulin signaling account for the different responses of organs in mammals as well. Prior studies have shown reduced insulin sensitivity in the ovaries of lean mice, and increased insulin sensitivity in the ovaries of obese mice compared to peripheral metabolic tissues. This could be due to enhanced regulation of insulin signaling by Irs-1 and Irs-2 in the ovaries compared to only Irs-1 regulation in the periphery [21]. To this end, a prior study demonstrated that the deletion of Irs-2 caused female infertility in mice [20].
The question of how Mkrn1 regulates insulin signaling in response to nutritional status thus remains. The potential molecular function of Mkrn1 as a ubiquitin E3 ligase has been studied. The Mkrn1 substrates identified include p53, p21, PPARγ, hTERT, Pten, APC, and AMPK [31–36]. Among these substrates, AMPK and Pten are regulators of energy-dependent signaling pathways. AMPK was recently identified as a Mkrn1 target [33]. Because AMPK is activated by low cellular-energy levels, it may play a critical role in ovaries by suppressing ovarian growth and development in a dietary-restricted environment. It could be hypothesized that AMPK levels become constitutively high and inhibit anabolic processes and vitellogenesis in Mkrn1exS flies. However, an energy-dependent role of AMPK would only be critical in follicle cells and dispensable in the germline cells of Drosophila ovaries [47]. This would not be consistent with our results that Mkrn1 is essential in germline cells, but not follicle cells, for proper oogenesis. Thus, AMPK does not seem to be responsible for the previtellogenic ovarian phenotype of Mkrn1exS flies. Pten is a key regulator of the insulin signaling pathway and s destabilized by Mkrn1. Interestingly, Mkrn1 functions as a positive-feedback regulator of the PI3K/AKT signals in cervical cancer progression because it is stabilized by AKT-mediated phosphorylation and it destabilizes Pten, which leads to further activation of the insulin signaling pathway [34]. Hence, we speculated that Mkrn1 destabilizes Pten in the ovaries and that the increased PTEN levels in Mkrn1exS flies could account for the decreased level of AKT phosphorylation. However, the absence of a reliable Drosophila Pten antibody and the lethal Pten mutation phenotype made it difficult for us to test this idea. Thus, whether or not Mkrn1 regulates the insulin signaling pathway through Pten or other target(s) during oogenesis remains to be determined.
Although our data suggests an important role of Mkrn1 in insulin/TOR signaling during oogenesis, it is still possible that other known targets of Mkrn1 may account for the sterile phenotype observed in mkrn1 null mutant. For example, APC was recently identified as an Mkrn1 substrate and functions in WNT signaling [36]. In Drosophila, WNT signaling is required for stem cell maintenence [48] but its role in mid-oogenesis is unknown. If Mkrn1 destabilizes APC in Drosophila ovaries, the increase in APC in Mkrn1exS would theoretically lead to the degradation of Armadillo, a Drosophila homologue of mammalian β-catenin. However, this was not the case in Mkrn1exS. Armadillo levels were increased in Mkrn1exS compared to controls (S1 Fig) suggesting that APC is not responsible for the Mkrn1exS female sterility phenotype.
Intriguingly, we observed that Mkrn1 protein is highly enriched in pole plasm in this study (Fig 1I). Pole plasm contains osk and staufen mRNAs, which are important regulators of germ cell development [49, 50]. It has been previously reported that Mkrn1 is an RNA-binding protein [51–53]. Mkrn1 was suggested to control protein translation in mammalian neuronal cells [53]. Collectively, our findings along with others raise the possibility that Mkrn1 may be actively involved in translational control of mRNAs in the pole plasm, thereby regulating the development of germ cell. Although this idea requires further investigation, it does not conflict with the E3 ligase role of Mkrn1 in the insulin signaling pathway. Translational control is ultimately a downstream event of nutrient-dependent signaling, such as the insulin and mTOR pathways, and therefore, should be precisely regulated. Mkrn1 could perform dual functions as a translational activator and a E3 ligase to better coordinate energy-dependent signaling and subsequent protein translation.
Insulin signaling for the regulation of oocyte development is highly conserved in mammals [16]. Although the hypothalamic-pituitary-gonadal axis of the hormonal system is well established in mammalian reproduction, insulin signaling is also heavily implicated in ovarian development. Challenging situations, such as obesity-induced infertility, can be reversed by modulating insulin signaling [54]. However, precise regulation of insulin signaling is poorly understood. For example, the ovary-specific deletion of Pten or Pdk1 causes premature ovarian failure despite opposite regulatory roles in the insulin signaling pathway, implying the complexity of the mechanism [55, 56]. We suggest that additional positive regulators such as Mkrn1 and the tissue specific interaction of insulin and TOR signaling may introduce additional complexity and dynamics to the system. Moreover, our study provides new insights into the mechanism underlying the tissue specificity of the insulin signaling pathway and the mechanisms leading to diet-related female fertility.
Supporting information
(PDF)
Acknowledgments
We are very grateful to Aurelio A. Teleman (German Cancer Research Center (DKFZ), Germany) for generously providing anti-S6K antibody and Jongkyeong Chung (Seoul National University, Republic of Korea) for kindly providing hsflp; FRT80B, ubi-GFP flies.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was supported by the Korea Health Industry Development Institute (KHIDI) grant funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HI16C2061) and the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT, Republic of Korea (grant No. 2012R1A5A048183) to Eun Young Kim. Eunjoo Cho was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT, Republic of Korea (grant No. 2017R1D1A1B03033549).
References
- 1.Group ECW. Nutrition and reproduction in women. Hum Reprod Update. 2006;12(3):193–207. Epub 2006/02/02. 10.1093/humupd/dmk003 . [DOI] [PubMed] [Google Scholar]
- 2.Hahn DA, Denlinger DL. Energetics of insect diapause. Annu Rev Entomol. 2011;56:103–21. Epub 2010/08/10. 10.1146/annurev-ento-112408-085436 . [DOI] [PubMed] [Google Scholar]
- 3.Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab. 2010;21(2):96–103. Epub 2009/11/17. 10.1016/j.tem.2009.10.001 . [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin JM, Bratu DP. Drosophila melanogaster Oogenesis: An Overview. Methods Mol Biol. 2015;1328:1–20. Epub 2015/09/02. 10.1007/978-1-4939-2851-4_1 . [DOI] [PubMed] [Google Scholar]
- 5.Jia D, Tamori Y, Pyrowolakis G, Deng WM. Regulation of broad by the Notch pathway affects timing of follicle cell development. Dev Biol. 2014;392(1):52–61. 10.1016/j.ydbio.2014.04.024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Deng WM. Notch-dependent downregulation of the homeodomain gene cut is required for the mitotic cycle/endocycle switch and cell differentiation in Drosophila follicle cells. Development. 2005;132(19):4299–308. 10.1242/dev.02015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jouandin P, Ghiglione C, Noselli S. Starvation induces FoxO-dependent mitotic-to-endocycle switch pausing during Drosophila oogenesis. Development. 2014;141(15):3013–21. 10.1242/dev.108399 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231(1):265–78. 10.1006/dbio.2000.0135 . [DOI] [PubMed] [Google Scholar]
- 9.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. Epub 2001/12/14. 10.1038/414799a . [DOI] [PubMed] [Google Scholar]
- 10.Sliwowska JH, Fergani C, Gawalek M, Skowronska B, Fichna P, Lehman MN. Insulin: its role in the central control of reproduction. Physiol Behav. 2014;133:197–206. Epub 2014/05/31. 10.1016/j.physbeh.2014.05.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das D, Pal S, Maitra S. Releasing prophase arrest in zebrafish oocyte: synergism between maturational steroid and Igf1. Reproduction. 2016;151(1):59–72. 10.1530/REP-15-0389 . [DOI] [PubMed] [Google Scholar]
- 12.Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292(5514):107–10. 10.1126/science.1057987 . [DOI] [PubMed] [Google Scholar]
- 13.Richard DS, Rybczynski R, Wilson TG, Wang Y, Wayne ML, Zhou Y, et al. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J Insect Physiol. 2005;51(4):455–64. 10.1016/j.jinsphys.2004.12.013 . [DOI] [PubMed] [Google Scholar]
- 14.Wu XK, Zhou SY, Liu JX, Pollanen P, Sallinen K, Makinen M, et al. Selective ovary resistance to insulin signaling in women with polycystic ovary syndrome. Fertil Steril. 2003;80(4):954–65. . [DOI] [PubMed] [Google Scholar]
- 15.Dunaif A, Wu X, Lee A, Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS). Am J Physiol Endocrinol Metab. 2001;281(2):E392–9. 10.1152/ajpendo.2001.281.2.E392 . [DOI] [PubMed] [Google Scholar]
- 16.Das D, Arur S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol Reprod Dev. 2017;84(6):444–59. Epub 2017/04/06. 10.1002/mrd.22806 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boland MP, Lonergan P, O’Callaghan D. Effect of nutrition on endocrine parameters, ovarian physiology, and oocyte and embryo development. Theriogenology. 2001;55(6):1323–40. Epub 2001/05/01. . [DOI] [PubMed] [Google Scholar]
- 18.Soliman A, De Sanctis V, Elalaily R. Nutrition and pubertal development. Indian J Endocrinol Metab. 2014;18(Suppl 1):S39–47. Epub 2014/12/30. 10.4103/2230-8210.145073 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werz C, Kohler K, Hafen E, Stocker H. The Drosophila SH2B family adaptor Lnk acts in parallel to chico in the insulin signaling pathway. PLoS Genet. 2009;5(8):e1000596 10.1371/journal.pgen.1000596 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burks DJ, Font de Mora J, Schubert M, Withers DJ, Myers MG, Towery HH, et al. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000;407(6802):377–82. Epub 2000/10/03. 10.1038/35030105 . [DOI] [PubMed] [Google Scholar]
- 21.Wu S, Divall S, Wondisford F, Wolfe A. Reproductive tissues maintain insulin sensitivity in diet-induced obesity. Diabetes. 2012;61(1):114–23. Epub 2011/11/15. 10.2337/db11-0956 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaFever L, Feoktistov A, Hsu HJ, Drummond-Barbosa D. Specific roles of Target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development. 2010;137(13):2117–26. Epub 2010/05/28. 10.1242/dev.050351 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pallares-Cartes C, Cakan-Akdogan G, Teleman AA. Tissue-specific coupling between insulin/IGF and TORC1 signaling via PRAS40 in Drosophila. Dev Cell. 2012;22(1):172–82. Epub 2012/01/24. 10.1016/j.devcel.2011.10.029 . [DOI] [PubMed] [Google Scholar]
- 24.Carney GE, Bender M. The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics. 2000;154(3):1203–11. Epub 2000/04/11. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaziova I, Bonnette PC, Henrich VC, Jindra M. Cell-autonomous roles of the ecdysoneless gene in Drosophila development and oogenesis. Development. 2004;131(11):2715–25. Epub 2004/05/07. 10.1242/dev.01143 . [DOI] [PubMed] [Google Scholar]
- 26.Terashima J, Takaki K, Sakurai S, Bownes M. Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster. J Endocrinol. 2005;187(1):69–79. 10.1677/joe.1.06220 . [DOI] [PubMed] [Google Scholar]
- 27.Tu MP, Yin CM, Tatar M. Impaired ovarian ecdysone synthesis of Drosophila melanogaster insulin receptor mutants. Aging Cell. 2002;1(2):158–60. . [DOI] [PubMed] [Google Scholar]
- 28.Mazerbourg S, Monget P. Insulin-Like Growth Factor Binding Proteins and IGFBP Proteases: A Dynamic System Regulating the Ovarian Folliculogenesis. Front Endocrinol (Lausanne). 2018;9:134 Epub 2018/04/13. 10.3389/fendo.2018.00134 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update. 2012;18(1):73–91. Epub 2011/11/10. 10.1093/humupd/dmr039 . [DOI] [PubMed] [Google Scholar]
- 30.Chou CH, Chen MJ. The Effect of Steroid Hormones on Ovarian Follicle Development. Vitam Horm. 2018;107:155–75. Epub 2018/03/17. 10.1016/bs.vh.2018.01.013 . [DOI] [PubMed] [Google Scholar]
- 31.Salvatico J, Kim JH, Chung IK, Muller MT. Differentiation linked regulation of telomerase activity by Makorin-1. Mol Cell Biochem. 2010;342(1–2):241–50. Epub 2010/05/18. 10.1007/s11010-010-0490-x . [DOI] [PubMed] [Google Scholar]
- 32.Lee EW, Lee MS, Camus S, Ghim J, Yang MR, Oh W, et al. Differential regulation of p53 and p21 by MKRN1 E3 ligase controls cell cycle arrest and apoptosis. EMBO J. 2009;28(14):2100–13. Epub 2009/06/19. 10.1038/emboj.2009.164 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MS, Han HJ, Han SY, Kim IY, Chae S, Lee CS, et al. Loss of the E3 ubiquitin ligase MKRN1 represses diet-induced metabolic syndrome through AMPK activation. Nat Commun. 2018;9(1):3404 Epub 2018/08/26. 10.1038/s41467-018-05721-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MS, Jeong MH, Lee HW, Han HJ, Ko A, Hewitt SM, et al. PI3K/AKT activation induces PTEN ubiquitination and destabilization accelerating tumourigenesis. Nat Commun. 2015;6:7769 Epub 2015/07/18. 10.1038/ncomms8769 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JH, Park KW, Lee EW, Jang WS, Seo J, Shin S, et al. Suppression of PPARgamma through MKRN1-mediated ubiquitination and degradation prevents adipocyte differentiation. Cell Death Differ. 2014;21(4):594–603. Epub 2013/12/18. 10.1038/cdd.2013.181 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HK, Lee EW, Seo J, Jeong M, Lee SH, Kim SY, et al. Ubiquitylation and degradation of adenomatous polyposis coli by MKRN1 enhances Wnt/beta-catenin signaling. Oncogene. 2018;37(31):4273–86. Epub 2018/05/02. 10.1038/s41388-018-0267-3 . [DOI] [PubMed] [Google Scholar]
- 37.Tran HT, Cho E, Jeong S, Jeong EB, Lee HS, Jeong SY, et al. Makorin 1 Regulates Developmental Timing in Drosophila. Mol Cells. 2018;41(12):1024–32. Epub 2018/11/07. 10.14348/molcells.2018.0367 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahn K, Miranda M, Francis VA, Vendrell J, Zorzano A, Teleman AA. PP2A regulatory subunit PP2A-B’ counteracts S6K phosphorylation. Cell Metab. 2010;11(5):438–44. Epub 2010/05/07. 10.1016/j.cmet.2010.03.015 . [DOI] [PubMed] [Google Scholar]
- 39.Schwedes CC, Carney GE. Ecdysone signaling in adult Drosophila melanogaster. J Insect Physiol. 2012;58(3):293–302. 10.1016/j.jinsphys.2012.01.013 . [DOI] [PubMed] [Google Scholar]
- 40.Handler AM. Ecdysteroid titers during pupal and adult development in Drosophila melanogaster. Dev Biol. 1982;93(1):73–82. Epub 1982/09/01. . [DOI] [PubMed] [Google Scholar]
- 41.Petryk A, Warren JT, Marques G, Jarcho MP, Gilbert LI, Kahler J, et al. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci U S A. 2003;100(24):13773–8. Epub 2003/11/12. 10.1073/pnas.2336088100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buszczak M, Freeman MR, Carlson JR, Bender M, Cooley L, Segraves WA. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development. 1999;126(20):4581–9. . [DOI] [PubMed] [Google Scholar]
- 43.Terashima J, Bownes M. E75A and E75B have opposite effects on the apoptosis/development choice of the Drosophila egg chamber. Cell Death Differ. 2006;13(3):454–64. Epub 2005/10/08. 10.1038/sj.cdd.4401745 . [DOI] [PubMed] [Google Scholar]
- 44.Pritchett TL, McCall K. Role of the insulin/Tor signaling network in starvation-induced programmed cell death in Drosophila oogenesis. Cell Death Differ. 2012;19(6):1069–79. Epub 2012/01/14. 10.1038/cdd.2011.200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng LY, Bailey AP, Leevers SJ, Ragan TJ, Driscoll PC, Gould AP. Anaplastic lymphoma kinase spares organ growth during nutrient restriction in Drosophila. Cell. 2011;146(3):435–47. Epub 2011/08/06. 10.1016/j.cell.2011.06.040 . [DOI] [PubMed] [Google Scholar]
- 46.Koyama T, Mendes CC, Mirth CK. Mechanisms regulating nutrition-dependent developmental plasticity through organ-specific effects in insects. Front Physiol. 2013;4:263 Epub 2013/10/18. 10.3389/fphys.2013.00263 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laws KM, Drummond-Barbosa D. AMP-activated protein kinase has diet-dependent and -independent roles in Drosophila oogenesis. Dev Biol. 2016;420(1):90–9. Epub 2016/10/21. 10.1016/j.ydbio.2016.10.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song X, Xie T. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development. 2003;130(14):3259–68. Epub 2003/06/05. . [DOI] [PubMed] [Google Scholar]
- 49.Lasko P. mRNA localization and translational control in Drosophila oogenesis. Cold Spring Harb Perspect Biol. 2012;4(10). Epub 2012/08/07. 10.1101/cshperspect.a012294 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lehmann R. Germ Plasm Biogenesis—An Oskar-Centric Perspective. Curr Top Dev Biol. 2016;116:679–707. Epub 2016/03/13. 10.1016/bs.ctdb.2015.11.024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carpenedo RL, Cassar PA, Stanford WL. MKRN1: Uncovering function by an unbiased systems approach. Cell Cycle. 2016;15(3):303–4. Epub 2015/12/15. 10.1080/15384101.2015.1124698 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cassar PA, Carpenedo RL, Samavarchi-Tehrani P, Olsen JB, Park CJ, Chang WY, et al. Integrative genomics positions MKRN1 as a novel ribonucleoprotein within the embryonic stem cell gene regulatory network. EMBO Rep. 2015;16(10):1334–57. Epub 2015/08/13. 10.15252/embr.201540974 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miroci H, Schob C, Kindler S, Olschlager-Schutt J, Fehr S, Jungenitz T, et al. Makorin ring zinc finger protein 1 (MKRN1), a novel poly(A)-binding protein-interacting protein, stimulates translation in nerve cells. J Biol Chem. 2012;287(2):1322–34. Epub 2011/12/01. 10.1074/jbc.M111.315291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu S, Divall S, Nwaopara A, Radovick S, Wondisford F, Ko C, et al. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes. 2014;63(4):1270–82. Epub 2014/01/01. 10.2337/db13-1514 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, Tohonen V, et al. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18(15):2813–24. Epub 2009/05/09. 10.1093/hmg/ddp217 . [DOI] [PubMed] [Google Scholar]
- 56.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319(5863):611–3. Epub 2008/02/02. 10.1126/science.1152257 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the manuscript.