Introduction
Bipolar Disorder (BD) is a debilitating psychiatric disorder characterized by recurrent, episodic disturbances in mood, sleep, behavior, perception, and cognition, rendering it a leading cause of disability, morbidity, and mortality worldwide(Mahon et al., 2010). BD affects 1–3% of the adult population and has a heritability of 59–87%, placing first-degree relatives of individuals with BD at a 10-fold increased risk of the disorder versus relatives of unaffected controls(Merikangas et al., 2007; Phillips and Swartz, 2014; Singh and Chang, 2013; Smoller and Finn, 2003). Yet, the absence of objective biomarkers of BD makes it difficult to identify young individuals who are likely to develop BD in the future.
Neuroimaging studies can identify such biomarkers by detecting abnormal structure and activity in neural circuitries important for processes aberrant in individuals with BD, such as emotion processing(Phillips and Swartz, 2014). Neural regions implicated in emotion processing include the amygdala, ventrolateral prefrontal cortex (vlPFC), and anterior cingulate cortex (ACC)(Dolcos et al., 2011; Phillips et al., 2003; Phillips et al., 2008). Studies have reported elevated amygdala activity(Blumberg et al., 2005; Lawrence et al., 2004), lower vlPFC activity(Hafeman et al., 2014; Phillips et al., 2003; Phillips et al., 2008), and lower ACC activity(Blumberg et al., 2005) during emotion processing tasks in youth and adults with BD versus healthy controls.
Given that structural integrity of white matter is key for ensuring intact functioning of a given neural circuitry, studying relationships between white matter tract (WMT) structure and activity may provide a more comprehensive understanding of BD. Abnormal WMT structure in youth and adults with BD is observed in several WMTs important for emotion processing, including the cingulum(Benedetti et al., 2011b; Linke et al., 2013; Versace et al., 2014), forceps minor(Benedetti et al., 2011b; Chaddock et al., 2009; Haller et al., 2011; Versace et al., 2014; Wang et al., 2008b), uncinate fasciculus(Benedetti et al., 2011a; Linke et al., 2013; Versace et al., 2008; Versace et al., 2014), and superior longitudinal fasciculus(Benedetti et al., 2014; Benedetti et al., 2011b; Chaddock et al., 2009; Raichle et al., 2001; van der Schot et al., 2010; Versace et al., 2008; Versace et al., 2010a). Specific abnormalities include the following in frontal WMTs(Emsell et al., 2013; Mahon et al., 2010; Versace et al., 2008; Versace et al., 2014; Wang et al., 2008a; Wang et al., 2008b) and WMTs connecting prefrontal cortical to anterior limbic(Benedetti et al., 2011a; Benedetti et al., 2011b) and temporal regions(Ashtari, 2012; Bruno et al., 2008; Mahon et al., 2013; Saricicek et al., 2016; Versace et al., 2014): lower fractional anisotropy (FA), likely reflecting lower collinearity of longitudinally-aligned fibers(Versace et al., 2008); greater radial diffusivity (RD), reflecting abnormal myelination, more obliquely oriented fibers, and/or local inflammation(Mahon et al., 2010; Song et al., 2005); and reduced tract length, likely reflecting altered axonal myelination or myelin loss(Atmaca et al., 2007; Barnea-Goraly et al., 2009; Brambilla et al., 2003; Hong et al., 2011; Torgerson et al., 2013; Wang et al., 2008b).
There are several gaps in the literature that hinder progress in understanding the underlying pathophysiology of BD. First, while most neuroimaging studies examined individuals diagnosed with BD, few examined youth at genetic risk for the disorder(Ladouceur et al., 2013; Olsavsky et al., 2012; Phillips et al., 2008; Singh and Chang, 2013; Singh et al., 2014; Tseng et al., 2015; Versace et al., 2010b). Focusing on BD at-risk youth unaffected by the disorder may identify biomarkers of BD before illness onset. The few studies of activity in BD at-risk youth reported abnormally elevated amygdala and lower ACC activity during facial emotion processing(Chan et al., 2016; Olsavsky et al., 2012; Phillips et al., 2008; Tseng et al., 2015) and abnormally elevated vlPFC activity during reward processing(Singh et al., 2014). Studies of WMTs in BD at-risk youth reported lower FA widespread, in tracts connecting prefrontal cortical and limbic regions, and in the anterior limb of the internal capsule(Ganzola et al., 2018; Ganzola et al., 2017; McIntosh et al., 2005; Versace et al., 2010b).
Second, while several WMT and activity abnormalities have been identified in youth with, and at risk for, BD, few studies have examined the relationships between them in this population. Combining diffusion imaging and functional magnetic resonance imaging (fMRI) techniques has become increasingly important in fields of cognitive and clinical neuroscience(Zhu et al., 2014). Such studies have examined relationships between WMT structure and either blood-oxygen-level dependent (BOLD) activity(Baird et al., 2005; Conturo et al., 1999; Madden et al., 2007; O’Donnell et al., 2012; Olesen et al., 2003; Toosy et al., 2004; Werring et al., 1999; Ystad et al., 2011) or functional connectivity(Calamante et al., 2013; Greicius et al., 2009; Guye et al., 2003; Koch et al., 2002; Supekar et al., 2010; van den Heuvel et al., 2008). Both types of structure-function relationships have the potential to contribute to our understanding of mechanisms underlying psychiatric disorders; however, such studies have yet to be performed in youth with, or at risk for, BD.
Third, relating WMT-activity measures and symptoms is very important in OBP, as youth at genetic risk for BD with greater symptom severity are likely to be more at risk for developing BD in the future. Specifically, symptoms of depression, mania, affective lability, and anxiety have been shown to be precursors of BD in OBP(Hafeman et al., 2016). Yet, no studies to date have combined structural and functional imaging to study WMT-activity relationships and their relationships with symptoms in BD at-risk youth.
Additionally, of the studies that examined BD at-risk youth, few compared youth at genetic risk for BD to those at risk for other disorders(Manelis et al., 2016; Manelis et al., 2015; Soehner et al., 2016). It thus remains difficult to determine the extent to which neuroimaging findings represent biomarkers of specific risk for BD. The Bipolar Offspring Study (BIOS) examines emotion processing neural circuitries in offspring of bipolar parents (OBP) and offspring of comparison parents (OCP) who have non-BD disorders, including Major Depressive Disorder, Attention-Deficit/Hyperactivity Disorder, and/or an Anxiety Disorder(Birmaher et al., 2009). While OBP and OCP are heterogeneous on a risk continuum, putting the sample at risk for factors that may contribute to sample skew or group differences, studies have shown that OBP are more likely to develop a bipolar spectrum disorder by age 21 (23%) than OCP (3.2%)(Axelson et al., 2015), placing OBP at greater risk for developing BD than OCP. OCP thus serve as a control group both for genetic risk for non-BD disorders, since OBP are also at higher risk for these disorders than the general population(Birmaher et al., 2009), and for the presence of non-BD disorders in parents, since parents with BD have high rates of non-BD comorbidity(Merikangas et al., 2007). The few neuroimaging studies comparing OBP and OCP found patterns of activity and functional connectivity in the amygdala and vlPFC that distinguish OBP from OCP(Manelis et al., 2016; Manelis et al., 2015; Soehner et al., 2016). No studies of OBP and OCP to date, however, employed multimodal neuroimaging techniques to identify biomarkers of specific risk for BD. Studies are needed to determine whether neuroimaging techniques can identify biomarkers that confer specific risk for BD in OBP.
Furthermore, while non-BD disorders may confound neuroimaging findings, these disorders are common in BD at-risk youth. Including at-risk youth with, and without, these disorders in neuroimaging studies can help determine the extent to which findings are confounded, or not, by present psychopathology. Indeed, we previously reported that neuroimaging findings distinguishing OBP from OCP remained even after excluding youth with non-BD disorders(Manelis et al., 2016; Manelis et al., 2015). However, the effects of non-BD disorders on WMT-activity relationships have yet to be studied. Further examination of the effects of these disorders in at-risk youth may also enhance our understanding of how WMT-activity relationships confer risk for BD.
The goal of the present study was thus to explore relationships between WMT structure and activity in emotion processing neural circuitry that distinguish youth at genetic risk for BD from youth at risk for non-BD disorders. We examined the effects of GROUP(OBP,OCP)xWMT interactions on activity in emotion processing circuitry to identify whether WMT-activity relationships distinguished OBP from OCP, and how non-BD disorders impacted these relationships. We hypothesized that:
OBP would show relationships between lower prefrontal WMT (cingulum, forceps minor, uncinate fasciculus, superior longitudinal fasciculus) fiber collinearity and greater amygdala and/or lower prefrontal (vlPFC, ACC) cortical activity.
These WMT-activity relationships would distinguish OBP from OCP.
These relationships would remain when excluding youth with non-BD disorders.
Additional analyses examined: how these relationships compared to a reference group of healthy offspring of healthy parents (OHP); the relationships between WMT-activity and symptoms; correlations between WMT measures and FA; and whether or not main findings were affected by psychotropic medications or age.
Methods
Participants
OBP and OCP, ages 8–17 years, were recruited from BIOS. OBP had at least one parent with BD, while OCP had at least one parent with a non-BD disorder, including Major Depressive Disorder, Attention-Deficit/Hyperactivity Disorder, and/or an Anxiety Disorder. A third group of OHP, ages 8–17 years, were recruited from the healthy comparison youth group of the Longitudinal Assessment of Manic Symptoms (LAMS) study(Findling et al., 2010; Horwitz et al., 2010). OHP had parents with no psychiatric diagnoses.
Exclusion criteria included: history of serious medical illness, head injury, or neurological disorder; IQ <70, as assessed by the Wechsler Abbreviate Scale of Intelligence(Wechsler, 1999); diagnosis of BD, autism, or schizophrenia; MRI contraindication (e.g., pregnancy, metal in the body); and substance abuse on the day of the scan or substance abuse disorder in the last three months. For OHP, additional exclusion criteria included history of DSM-5 disorder.
Thirty-two OBP (mean age=13.81(2.45), 15 female), thirty OCP (mean age=13.98(2.30), 12 female), and twenty-four OHP (mean age=13.80(1.72), 10 female), matched for age, sex, IQ, and socioeconomic status (SES), were included in this study (Table 1). Fourteen OBP and fifteen OCP had non-BD disorders. Prior to study participation, parents and guardians provided written informed consent, and youth provided written informed assent. Participants received monetary compensation.
Table 1.
Comparison of OBP (n=32), OCP (n=30), and OHP (n=24).
| OBP n=32 | OCP n=30 | OHP n=24 | Statistic | p | |
|---|---|---|---|---|---|
| Demographic Information | |||||
| Age | 13.81(2.45) | 13.98(2.30) | 13.80(1.72) | F = 0.061 | .941 |
| Sex (females) | 15 | 12 | 10 | χ2 = 0.324 | .851 |
| IQ | 99.97(15.80) | 102.97(14.22) | 104.00(13.60) | F = 0.591 | .556 |
| SES (primary caregiver education) | χ2 = 7.037 | .134 | |||
| No/some HS or HS Diploma | 16 | 7 | 7 | ||
| Some post HS | 6 | 5 | 3 | ||
| Associate’s Degree or Higher | 10 | 18 | 14 | ||
| Clinical Measures | |||||
| Diagnosis | 14 | 15 | N/A | t = −0.486 | .629 |
| Major Depressive Disorder | 4 | 4 | N/A | t = −0.096 | .924 |
| Anxiety Disorder | 4 | 6 | N/A | t = −0.793 | .431 |
| Attention Deficit/Hyperactivity Disorder | 7 | 7 | N/A | t = −0.135 | .893 |
| Oppositional Defiant or Conduct Disorder | 2 | 3 | N/A | t = −0.534 | .595 |
| Obsessive Compulsive Disorder | 0 | 2 | N/A | t = −1.439 | .161 |
| Eating Disorder | 2 | 0 | N/A | t = 1.438 | .161 |
| Psychotropic Medication Use | 3 | 0 | N/A | t = 1.791 | .083 |
| Scan day assessments | |||||
| SCARED-P | 9.10(5.67) | 10.27(11.61) | 4.75(4.59) | F = 3.084 | .051 |
| SCARED-C | 12.52(15.20) | 8.62(13.40) | 10.23(11.66) | F = 0.651 | .524 |
| CALS-P | 7.93(9.57) | 3.73(4.54) | 1.67(2.55) | F = 7.494 | .001 |
| CALS-C | 9.97(12.67) | 6.42(10.57) | 6.00(13.08) | F = 0.889 | .415 |
| MFQ-P | 6.52(9.38) | 3.81(3.56) | 1.63(2.06) | F = 4.211 | .018 |
| MFQ-C | 8.07(10.89) | 8.62(11.20) | 5.83(10.66) | F = 0.506 | .605 |
| Assessment closest to scan | |||||
| KMRS | 1.86(2.80) | 0.27(0.60) | 0.08(0.28) | F = 10.524 | .000 |
P indicates parent version and C indicates child version. Data are mean (SD) for age, IQ, and clinical assessments. For all other variables, data are total n.
Abbreviations: Offspring of Bipolar Parents (OBP); Offspring of Comparison Parents (OCP); Offspring of Healthy Parents (OHP); Screen for Child Anxiety Related Emotional Disorders (SCARED); Children’s Affective Lability Sale (CALS); Mood and Feelings Questionnaire (MFQ); Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale (KMRS); Socioeconomic Status (SES); High School (HS).
Psychiatric diagnoses were confirmed by a licensed psychiatrist or psychologist prior to scanning using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS)-Present and Lifetime Version(Kaufman et al., 1997) for offspring and the Structural Clinical Interview for DSM-IV(First, 1996) and Family History Screen(Weissman et al., 2000) for parents. Symptom assessments included the Screen for Child Anxiety Related Disorders (SCARED)(Birmaher et al., 1999; Birmaher et al., 1997), Children’s Affective Lability Sale (CALS)(Gerson et al., 1996), Mood and Feelings Questionnaire (MFQ)(Sund et al., 2001), and K-SADS Mania Rating Scale (KMRS)(Axelson et al., 2003). Parent- and child-reported SCARED, CALS, and MFQ were administered on the scan day; KMRS interviews, based on both parent and child information, were administered, on average, two months after the scan. Three OBP were taking psychotropic medications for non-BD diagnoses.
Neuroimaging Data Acquisition and Analyses
Participants completed an emotional face processing task during fMRI (Supplementary Figure 1)(Almeida et al., 2011; Perlman et al., 2012; Phillips et al., 2008; Tottenham et al., 2009). Functional images were preprocessed using Statistical Parametric Mapping (SPM8), including realignment and unwarping steps. Task stimulus contrasts of interest included positive (happy) and negative (sad, angry, and fearful, averaged together) emotional faces versus shapes. Regions of interest (ROIs) were anatomically defined using Center for Morphometric Analysis standard labels proposed in FreeSurfer. Individual-level averaged BOLD waveforms to the onset of each stimulus type were extracted in native space from anatomic ROIs to main stimulus contrasts per task. Global probabilistic tractography determined the distributions of 18 WMTs, and FA, RD, Axial Diffusivity (AD), volume, and length were extracted for each tract.
Statistical Analyses
Elastic net regression analysis was used for variable selection and reduction through GLMNET(Friedman, 2014; Zou and Hastie, 2005). We used a k=10-fold cross-validation approach. 6 outcome variables included activity in the bilateral amygdala, bilateral vlPFC, and caudal (cACC) and rostral (rACC) ACC to positive and negative emotional faces, in two separate models, in OBP and OCP. 163 predictor variables included: demographics (age, gender, IQ, SES (assessed by the Hollingshead Four Factor Index of Social Status(Hollingshead, 1975)), handedness, and highest parental education); diagnoses; WMT measures (RD, AD, volume, and length of the forceps major/minor and the left/right anterior thalamic radiation, cingulum-angular bundle and -cingulate gyrus, corticospinal tract, inferior longitudinal fasciculus, superior longitudinal fasciculus-parietal and -temporal, and uncinate fasciculus); and GROUP(OBP,OCP)xWMT measure interactions to examine between-group differences in WMT-activity relationships.
Elastic net is particularly useful when the number of predictor variables is much larger than the number of observations, or subjects(Zou and Hastie, 2005). Thus, to maximize the usefulness of our model, we increased the number of predictors by including all WMT measures for all tracts identifiable through TRActs Constrained by UnderLying Anatomy (TRACULA)(Yendiki et al., 2011). While FA is the most widely used invariant measure of anisotropy used in diffusion tensor imaging, it is calculated from the same eigenvectors (λ1, λ2, and λ3) that are used to calculate AD (λ1) and RD ((λ2+ λ3)/2)(Alexander et al., 2007). This strong correlation between FA and both AD and RD rendered us unable to put all three measures in a single model. In keeping with our aim to maximize our model’s usefulness, we included twice as many variables (AD and RD) in the model, in lieu of FA, and instead examined FA in additional analyses.
This was followed with post-hoc analyses to examine the contribution of non-zero variables observed with elastic net to the dependent variables, as well as the proportion of variance in dependent variables explained by the models. A test statistic or p-value for elastic net that has a simple and exact asymptotic null distribution is still under development(Lockhart et al., 2014); however, significance was determined in all other analyses.
The goal of the present study was to identify WMT-activity relationships that differed between OBP and OCP. Thus, only GROUPxWMT interactions were examined further. For all non-zero predictors of GROUPxWMT interactions on activity measures, post-hoc analyses determined the nature of between-group differences in the slopes of WMT-activity relationships, using(Paternoster et al., 1998): Z=(SlopeOBP – SlopeOCP)/√(SE2OBP + SE2OCP). To control for multiple parallel tests of between-group differences in slopes of the above relationships, sequential goodness of fit (SGoF) metatests were used(Carvajal-Rodriguez et al., 2009). This method was chosen because it is a multitest adjustment methodology that increases its statistical power when the number of tests increases(Carvajal-Rodríguez et al., 2009). Under favorable conditions, this test can show a statistical power up to two orders of magnitude higher than Benjamini and Hochberg and Bonferroni methods without appreciably increasing the false discovery rate(Carvajal-Rodríguez et al., 2009). Thus, it is an important tool for multitest adjustment when working with high-dimensional biological data(Carvajal-Rodríguez et al., 2009), rendering it well-suited for the large number of multiple comparison adjustments performed in this study.
Additional Analyses
Additional analyses focused on WMT-activity relationships that significantly differentiated OBP from OCP. We repeated the above analyses separating youth into those with and without non-BD disorders. We also conducted the above analyses in OHP as a comparison group for OBP and OCP. We determined how WMT measures correlated with FA and age. We examined between-group differences in WMT and activity measures and determined whether main findings remained after excluding youth taking psychotropic medications. Finally, we examined between-group differences in symptom severity (using SCARED, CALS, MFQ, and KMRS) and determined whether symptoms that differed between groups impacted significant between-group differences in WMT-activity relationships. Here, we examined how symptom severity measures moderated WMT-activity relationships by determining whether there were significant interactions between symptom severity and WMT measures on neural activity.
See Supplementary Material for more information regarding participant diagnoses and medications, power analyses, neuroimaging data acquisition and analyses, and elastic net.
Results
Analyses Testing Hypotheses 1–2
When examining responses to negative emotional faces in all ROIs, no predictors optimized model fit, indicating that there was no significant relationship between any of the predictors and activity to negative emotions. Thus, we will focus on findings pertaining to positive (i.e. happy) emotional faces.
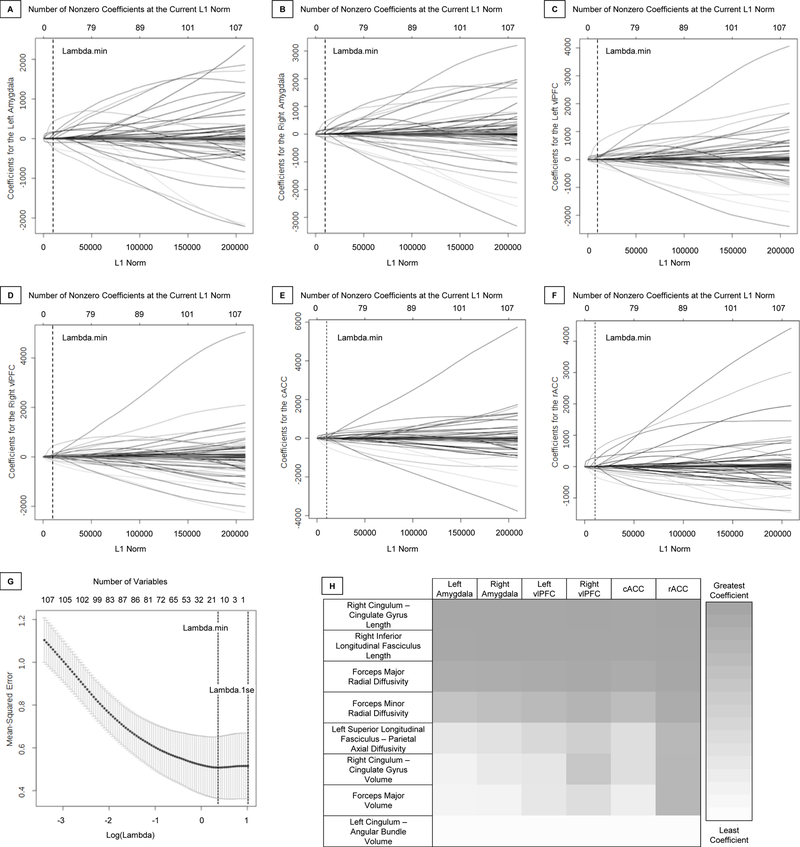
When examining responses to happy faces in all ROIs, 14 non-zero predictors together optimized model fit using the minimum λ (λ=1.436) identified by cross-validation (Figure 1A–G). Eight GROUPxWMT interactions showed relationships with activity in all ROIs (inverse for OBP, positive for OCP): forceps minor RD, right cingulum-cingulate gyrus volume and length, right inferior longitudinal fasciculus length, left cingulum-angular bundle volume, forceps major volume and RD, and left superior longitudinal fasciculus-parietal AD. Four variables showed positive relationships with activity in all ROIs for all youth: left cingulum-cingulate gyrus volume, left superior longitudinal fasciculus-temporal volume and length, and left handedness. Two variables showed inverse relationships with activity in all ROIs for all youth: right handedness and medium SES.
Figure 1. Elastic Net Plots Generated in GLMNET and Heat Map of Select Exponentiated Coefficients.
A–F. Plots of variable fit for activity in response to happy faces in the left amygdala (A), right amygdala (B), left vlPFC (C), right vlPFC (D), cACC (E), and rACC (F). Each curve corresponds to an independent variable in the full model prior to optimization. Curves indicate the path of each variable coefficient as λ varies. Lambda.min (λ=1.436) corresponds to the λ which minimizes mean squared error in the model and was used for the selection of the 14 predictor variables. G. Plot of non-zero variable fit after cross validation. Representation of the 10-fold cross validation performed for the elastic net regression that chooses the optimal λ. Lambda.min corresponds to the λ which minimizes mean squared error and was used for variable selection. Lambda.1se (λ=2.754) corresponds to the λ that is one standard error from the lambda.min. H. Heat map representing color-coded exponentiated coefficients for group x white matter interaction variables in the elastic net model. Each row represents a variable with group interaction between OBP and OCP that was found to be a significant predictor variable in the model. Each column represents one of the six regions for which the predictor variables predicted activity in response to happy faces. Exponentiated coefficients, representing the degree to which the predictor variables are associated with activity, are depicted with increased coefficients ranging from white to gray, representing the least and greatest coefficient observed of these variables in this model, respectively.
Abbreviations: Ventrolateral Prefrontal Cortex (vlPFC); Caudal Anterior Cingulate Cortex (cACC); Rostral Anterior Cingulate Cortex (rACC); Offspring of Bipolar Parents (OBP); Offspring of Comparison Parents (OCP).
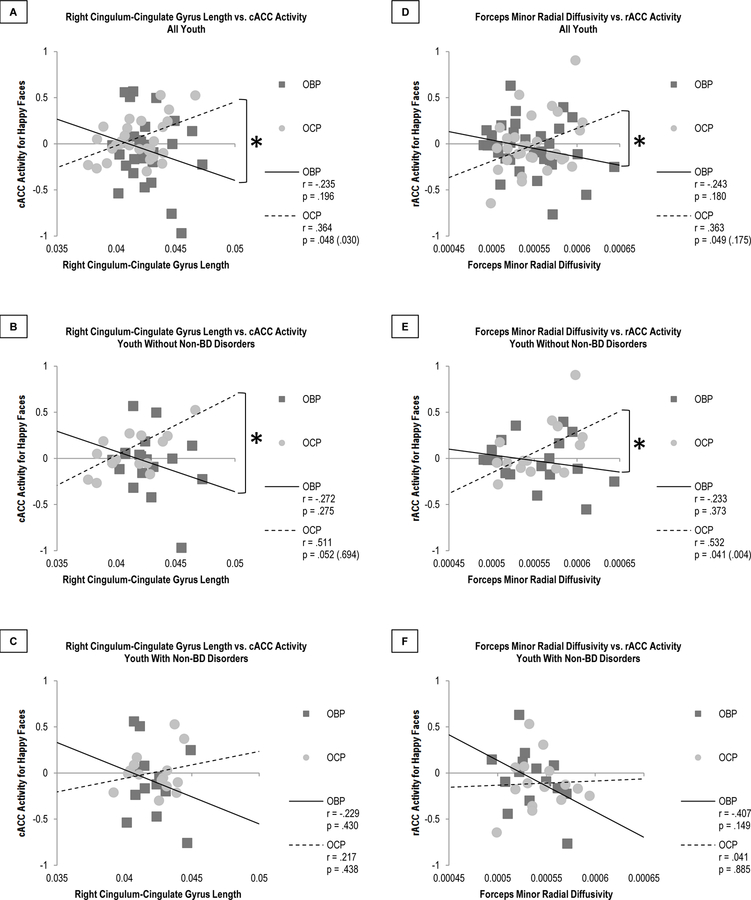
A pseudo r-squared of .165 was calculated containing 14 predictors from the model versus an intercept only model, indicating that 16.5% of the variance in activity to happy faces in all ROIs was explained by the 14 predictors. Eight of these predictors were GROUPxWMT interaction variables (Figure 1H). Of these interactions, the slopes of 2 WMT-activity relationships significantly differed between OBP and OCP after correcting for multiple comparisons (Table 2): right cingulum-cingulate gyrus length and cACC activity (p=0.033), and forceps minor RD and rACC activity (p<0.001) (Figures 2A, 2D). In OBP, longer right cingulum-cingulate gyrus length and greater forceps minor RD were associated with lower cACC and rACC activity to happy faces, respectively. Conversely, in OCP, longer right cingulum-cingulate gyrus length and greater forceps minor RD were associated with greater cACC and rACC activity, respectively.
Table 2.
Slope comparisons between OBP (n=32) and OCP (n=30) for the eight white matter-activity relationships for which there were non-zero group x white matter interaction predictors of ipsilateral neural activity variables.
| Predictor Variable | Outcome Variable | Z | p = |
|---|---|---|---|
| Right CCG Length | Right Amygdala | −2.17 | .030 (.451) |
| Right vlPFC | −1.38 | .168 | |
| cACC | −2.26 | .024 (.054) | |
| rACC | −0.72 | .472 | |
| Right ILF Length | Right Amygdala | −0.95 | .342 |
| Right vlPFC | −0.71 | .478 | |
| cACC | −1.28 | .201 | |
| rACC | −1.01 | .312 | |
| FMAJOR Radial Diffusivity | Left Amygdala | −1.68 | .093 |
| Right Amygdala | −1.80 | .072 | |
| Left vlPFC | −0.29 | .772 | |
| Right vlPFC | −0.86 | .390 | |
| cACC | −0.45 | .653 | |
| rACC | −0.17 | .865 | |
| FMAJOR Radial Diffusivity | Left Amygdala | −2.07 | .038 (.941) |
| Right Amygdala | −1.89 | .059 | |
| Left vlPFC | −1.56 | .119 | |
| Right vlPFC | −1.37 | .171 | |
| cACC | −1.80 | .072 | |
| rACC | −2.47 | .014 (.014) | |
| Left SLFP xial Diffusivity | Left Amygdala | −1.62 | .105 |
| Left vlPFC | −1.59 | .112 | |
| cACC | −2.07 | .038 (1.00) | |
| rACC | −2.20 | .028 (.173) | |
| Right CCG Volume | Right Amygdala | −0.27 | .787 |
| Right vlPFC | 0.65 | .516 | |
| cACC | −0.30 | .764 | |
| rACC | 0.08 | .936 | |
| FMAJOR Volume | Left Amygdala | −0.55 | .582 |
| Right Amygdala | −1.08 | .280 | |
| Left vlPFC | 0.41 | .682 | |
| Right vlPFC | −0.47 | .638 | |
| cACC | −0.73 | .465 | |
| rACC | 0.00 | 1.00 | |
| Left CAB Volume | Left Amygdala | 0.91 | .363 |
| Left vlPFC | 0.90 | .368 | |
| cACC | 0.49 | .624 | |
| rACC | 1.45 | .147 | |
p-values are uncorrected, with SGoF-corrected values in parentheses. Bolded p-values are those that are significant after SGoF correction.
Abbreviations: Offspring of Bipolar Parents (OBP); Offspring of Control Parents (OCP); Ventrolateral Prefrontal Cortex (vlPFC); Caudal Anterior Cingulate Cortex (cACC); Rostral Anterior Cingulate Cortex (rACC); Cingulum – Cingulate Gyrus (CCG); Inferior Longitudinal Fasciculus (ILF); Forceps Major (FMAJOR); Superior Longitudinal Fasciculus (SLFP); Cingulum – Angular Bundle (CAB).
Figure 2. Comparison of Significant White Matter Tract-Activity Relationships in OBP and OCP.
A–C. Comparison of relationships between right cingulum-cingulate gyrus length and cACC activity for happy faces. This relationship significantly differed in all youth (p = .024 (.033), A) and in those without non-BD disorders (p = 0.023 (0.002), B) but not in youth with non-BD disorders (p = 0.276, C). D–F. Comparison of relationships between forceps minor radial diffusivity and rACC activity for happy faces. This relationship significantly differed in all youth (p = 0.014 (<0.001), D) and in those without non-BD disorders (p = 0.017 (<0.001), E) but not in youth with non-BD disorders (p = 0.204, F).
p-values are uncorrected, with SGoF-corrected values in parentheses. Bolded p-values are those that are significant after SGoF correction.
Abbreviations: Offspring of Bipolar Parents (OBP); Offspring of Comparison Parents (OCP); Caudal Anterior Cingulate Cortex (cACC); Rostral Anterior Cingulate Cortex (rACC); Bipolar Disorder (BD); Sequential Goodness of Fit (SGoF).
Additional Analyses
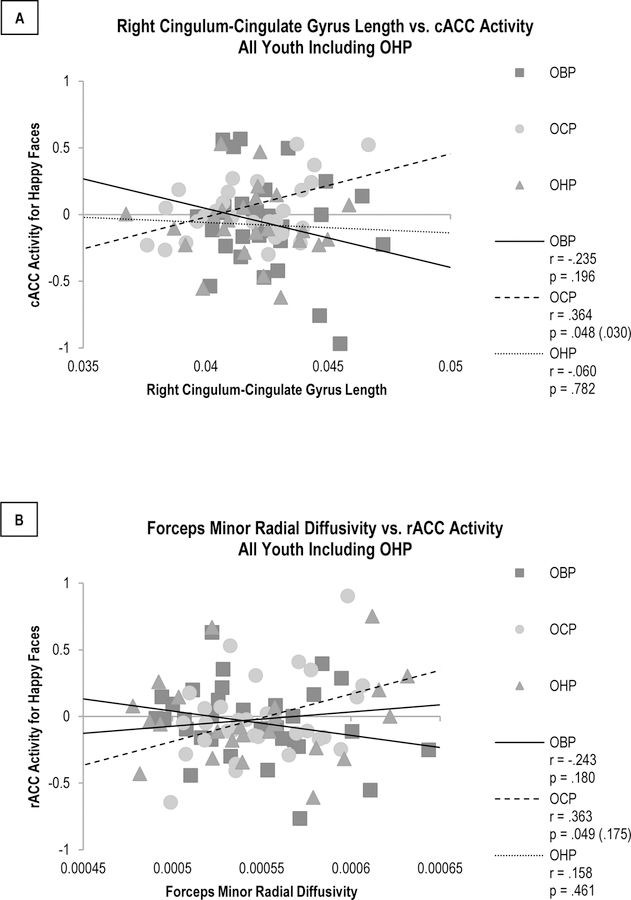
These WMT-activity relationships remained significantly different between OBP and OCP only in youth without non-BD disorders (Figures 2B–C, 2E–F). The relationships for OHP were in between those of OBP and OCP, but differences in these relationships were not statistically significant (Figure 3, Supplementary Table 1). Removing youth taking psychotropic medications caused the significance of the right cingulum-cingulate gyrus length-cACC activity relationship to be substituted by trend-level significance (p=0.069), but not the significance of the forceps minor RD-rACC activity relationship (p=0.017)(Supplementary Figure 2). Additional analyses thus focused on the forceps minor RD-rACC activity relationship, which was not susceptible to medication effects.
Figure 3. Comparison of Significant White Matter Tract-Activity Relationships in OHP Compared to OBP and OCP.
A. Comparison of relationships between right cingulum-cingulate gyrus length and cACC activity for happy faces. This relationship did not significantly differ between OHP and either BOP (p = .401) or OCP (p = .126). B. Comparison of relationships between forceps minor radial diffusivity and rACC activity for happy faces. This relationship did not significantly differ between OHP and either OBP (p = .258) or OCP (p = .107).
p-values are uncorrected, with SGoF-corrected values in parentheses.
Abbreviations: Offspring of Bipolar Parents (OBP); Offspring of Comparison Parents (OCP); Offspring of Healthy Parents (OHP); Caudal Anterior Cingulate Cortex (cACC); Rostral Anterior Cingulate Cortex (rACC); Sequential Goodness of Fit (SGoF).
Greater forceps minor RD was significantly associated with lower forceps minor FA (p<0.001). Age was not significantly associated with forceps minor RD or rACC activity (Supplementary Figure 3). When comparing individual WMT and activity measures in all OBP and OCP, no group differences were found.
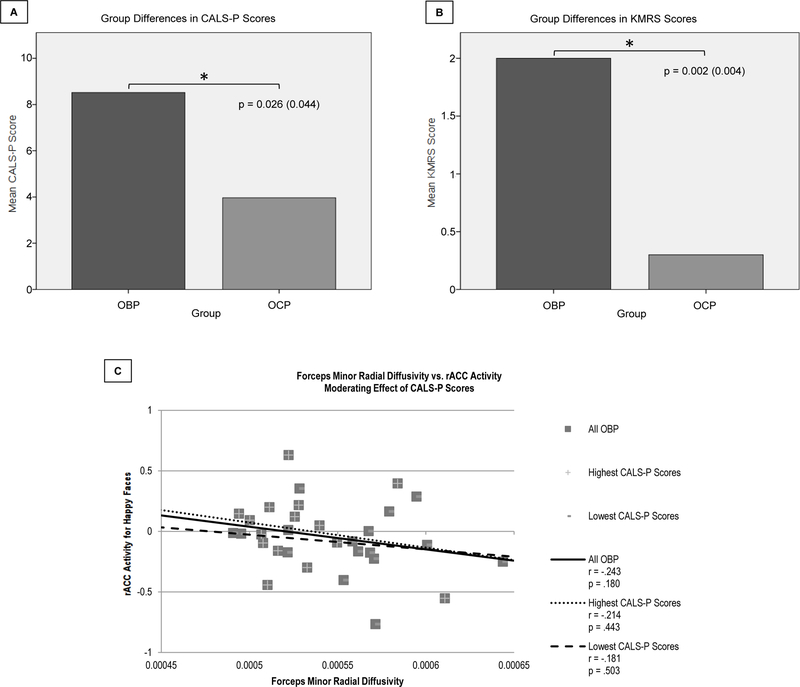
OBP had significantly greater CALS-P and KMRS scores versus OCP (p=0.044 and p=0.004, respectively) (Figure 4A–B). Regression analyses showed a significant interaction between CALS-P score and forceps minor RD on rACC activity in OBP (F(1,29)=5.566, p=.036), but not in OCP. Separating OBP into those with higher and lower CALS-P scores, based on a median split, revealed that those with higher CALS-P scores (M(SD)=15.33(10.52)) had greater inverse WMT-activity relationships (r=−.214, p=.443) than those with lower scores (M(SD)=2.13(2.00)) (r=−.181, p=.503) (Figure 4C).
Figure 4. Group Comparisons in Clinical Scores, and Effects on White Matter Tract-Activity Relationships.
A–B. Comparison of CALS-P and KMRS scores for OBP versus OCP. OBP had significantly greater CALS-P (p=0.026(0.044), A) and KMRS (p=0.002(0.004), B) scores versus OCP. C. Moderating effect of CALS-P scores on the relationship between forceps minor radial diffusivity and rACC activity for happy faces. CALS-P scores had a significant moderating effect on the relationship between forceps minor radial diffusivity and rACC activity in OBP (F(1,29)=5.566, p=.036). OBP with higher CALS-P scores (M=15.33; SD=10.52) had more negative white matter tract-activity relationships (r=−.214, p=.443) than OBP with lower CALS-P scores (M=2.13; SD=2.00) (r=−.181, p=.503).
p-values are uncorrected, with SGoF-corrected values in parentheses. Bolded p-values are those that are significant after SGoF correction.
Abbreviations: Children’s Affective Lability Scale – Parent (CALS-P); Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale (KMRS); Offspring of Bipolar Parents (OBP); Offspring of Comparison Parents (OCP); Rostral Anterior Cingulate Cortex (rACC); Sequential Goodness of Fit (SGoF).
Discussion
To our knowledge, this is the first study to use multimodal neuroimaging techniques to identify WMT-activity relationships that distinguish youth at genetic risk for BD from youth at risk for non-BD psychiatric disorders. Our goal was to explore WMT-activity relationships in emotion processing circuitry that distinguish OBP from OCP which may lead to the identification of potential biomarkers of BD that precede illness onset. An elastic net regression model indicated that 16.5% of the variance in activity to happy faces in the amygdala, vlPFC, and ACC was predicted by 14 GROUPxWMT interaction, WMT, and demographic variables. This admittedly small amount of variance may be partly explained by the fact that six outcome regions were included in a single elastic net regression model. In other words, the 14 predictor variables, together, explained 16.5% of the variance in all 6 outcome regions at the same time. This also points toward the complex nature of the interaction between WMT measures and activity in the amygdala and prefrontal cortical regions and suggests that many additional factors are likely contributing to activity in these regions.
The primary aim of the elastic net regression analysis was to determine which WMT variables had significant relationships with activity in the amygdala, vlPFC, and/or ACC that distinguished OBP from OCP. Of the 8 GROUPxWMT interaction variables that resulted from the model, only 2 relationships significantly differed between OBP and OCP: right cingulum-cingulate gyrus length-cACC activity, and forceps minor RD-rACC activity. However, neither relationship significantly differentiated either at-risk group from OHP. Removing youth taking psychotropic medications caused the significance of the right cingulum-cingulate gyrus length-cACC activity relationship to be substituted by trend-level significance; however, medications did not affect the forceps minor RD-rACC activity relationship. The latter finding was thus the main focus of our additional analyses.
Greater forceps minor RD was associated with lower rACC activity to happy faces in OBP but greater activity in OCP. Additional analyses revealed that greater forceps minor RD was associated with lower FA and, thus, lower fiber collinearity. This indicates that, in OBP alone, lower fiber collinearity in the forceps minor was significantly associated with lower activity in the rACC. When comparing this WMT-activity relationship in youth with and without non-BD disorders, the between-group differences in this relationship remained significant only in OBP and OCP without disorders. Together, these findings indicate that the key WMT-activity relationship differentiating OBP from OCP was the forceps minor RD-rACC activity relationship to happy faces, which remained evident when excluding youth with non-BD psychiatric disorders.
Previous neuroimaging studies of youth and adults with BD provide similar findings regarding WMT and activity abnormalities during emotion processing. Studies reported that individuals with, and at risk for, BD have abnormally low ACC activity(Blumberg et al., 2005; Chan et al., 2016; Dolcos et al., 2011; Phillips et al., 2003; Phillips et al., 2008) and fiber collinearity in the forceps minor(Benedetti et al., 2011b; Chaddock et al., 2009; Emsell et al., 2013; Haller et al., 2011; Jenkins et al., 2016; Ji et al., 2017; Nortje et al., 2013; Versace et al., 2014; Versace et al., 2010b; Wang et al., 2008b). Our findings add to this literature by showing that, in OBP, lower fiber collinearity in the forceps minor was associated with lower activity to happy faces in the rACC. The forceps minor is the major interhemispheric WMT that anteriorly connects the cerebral hemispheres, integrates emotion, language, attention, arousal, memory, and sensory-motor functions, and is vulnerable to repeated stresses such as psychosis and impulsivity(Lavagnino et al., 2015; Sarrazin et al., 2015). The rACC has extensive connections to the amygdala and is involved in conditioned emotional learning, modulating internal emotional responses, and assigning emotional valence to internal and external stimuli(Devinsky et al., 1995; Zimmerman et al., 2006). Thus, the inverse relationship between forceps minor RD and rACC activity in OBP may indicate that, in this at-risk group, abnormal myelination and/or more obliquely oriented fibers in the forceps minor may contribute to lower activity in the rACC by reducing the integrity of connections between regions that are important to positive emotional processing(Morgan et al., 2009).
Conversely, the relationship between forceps minor RD and rACC activity was positive in OCP such that lower fiber collinearity in forceps minor contributed to greater rACC activity in this group. Additionally, while not statistically significant, the forceps minor RD-rACC relationship for OHP was intermediate between those of OBP and OCP. Thus, because the at-risk groups did not differ from the healthy controls, it is not possible at this point to determine whether this WMT-activity relationship is a biomarker preceding BD illness. Given the significantly greater genetic risk for BD in OBP versus OCP, however, we may speculate that the forceps minor RD-rACC relationship suggests diverging pathophysiological mechanisms in OBP versus OCP. Further study, including larger sample sizes and longitudinal analyses, is needed to understand the implications of this main finding to BD risk and development.
Despite the lack of differences with OHP, additional analyses showed that OBP had significantly greater affective lability and manic symptom severity than OCP. Furthermore, there was a significant interaction between parent-reported affective lability severity and forceps minor RD on rACC activity to happy faces in OBP: greater affective lability was associated with a greater inverse WMT-activity relationship. Given that affective lability is a precursor of BD in OBP(Hafeman et al., 2016), the forceps minor RD-rACC activity relationship to happy faces may represent a neural basis for this clinical risk marker in OBP.
Six other variables (right cingulum-cingulate gyrus volume, right inferior longitudinal fasciculus length, left cingulum-angular bundle volume, forceps major volume and RD, and left superior longitudinal fasciculus-parietal AD) showed GROUPxWMT interactions (inverse for OBP, positive for OCP). These measures, except left superior longitudinal fasciculus-parietal AD, were inversely associated with FA, indicating that lower right cingulum-cingulate gyrus, right inferior longitudinal fasciculus, left cingulum-angular bundle, and forceps major fiber collinearity were associated with lower activity in OBP, but greater activity in OCP; the opposite was true for the left superior longitudinal fasciculus-parietal AD relationship. None of these relationships significantly differed between groups after SGoF corrections, however. Three WMT variables (left cingulum-cingulate gyrus volume and left superior longitudinal fasciculus-temporal volume and length) showed positive relationships with activity to happy faces in all ROIs for all youth. These measures were inversely associated with FA, indicating that lower left cingulum-cingulate gyrus and left superior longitudinal fasciculus-temporal fiber collinearity were associated with greater activity in all ROIs in OBP and OCP. Handedness also showed relationships with activity in all ROIs for all youth (inverse for right, positive for left); however, very few youth were left- (n=4) or mixed-handed (n=3), suggesting that handedness did not have a significant effect on the model. Similarly, youth with medium SES were relatively few (n=11), and neither very low, low, high, nor very high SES had any predictive value in the elastic net model, suggesting that SES also did not have a significant effect on the model. In summary, none of these relationships significantly differed between OBP and OCP. Thus, while these variables showed relationships with activity in emotion processing neural circuitry to happy faces, they are unlikely to be markers that either distinguish OBP from OCP or indicate specific risk for BD.
All findings were specific to happy faces, reflecting the importance of positive emotion processing abnormalities in the development of BD. A common theme that has emerged from neuroimaging studies of BD is that of abnormal activity in emotion processing circuity to positive emotional stimuli(Phillips and Swartz, 2014). Specifically, an emerging pattern is that of abnormally elevated amygdala, striatal, and medial prefrontal cortical activity in response to positive emotional stimuli in individuals affected with BD(Blumberg et al., 2005; Lawrence et al., 2004). Several studies have shown that adults with BD have abnormally increased amygdala and medial prefrontal cortex activity(Keener et al., 2012; Surguladze et al., 2010), as well as abnormally decreased positive bilateral orbitofrontal cortex-amygdala effective connectivity(Almeida et al., 2009), to emotional faces, particularly happy faces. These results suggest that individuals with BD have a dysregulated amygdala response to positive emotional stimuli(Phillips and Swartz, 2014). Overall, our findings suggest that abnormal perception of happy faces may reflect an underlying attentional bias to positive emotional stimuli, which may predispose to deficits in social processing, heightened perception of social reward, and, ultimately, hypo/mania.
There were limitations to this study. The primary limitations were the exploratory nature of our analyses and limited sample size, particularly when comparing subsamples of high-risk youth who had a non-BD disorder to those who did not have any disorders. Small sample size may have contributed to the reason why no significant differences were found between OHP and either OBP or OCP. Future studies should aim to replicate our findings with more targeted hypotheses and larger sample sizes. As this was the first study to examine structure-function relationships in BD at-risk youth, we chose to initially examine relationships between WMT structure and BOLD activity; future studies can examine relationships among functional connectivity, gray matter volume, and cortical thickness measures to additionally enhance our understanding of relationships between brain structure and function in BD at-risk youth. WMT length may, in part, be influenced by the tractography propagation mask and definition of end regions; future studies can employ different approaches to examine WMT length in OBP and OCP. We assumed a linear model between WMT structure and activity; nonlinear models may be considered in future studies. While other clinical assessments could have been included, our primary aim was to determine measures of specific symptoms that, at subthreshold levels, may confer risk for BD(Hafeman et al., 2016). Additionally, while we showed that age did not significantly affect either structural or functional measure, pubertal development, as well as other environmental effects such as childhood adversity, cannot be entirely ruled out as contributing factors in our results. Furthermore, while most parents with non-BD psychiatric disorders were beyond the most common age of onset for BD, it is possible that some of these individuals may have been misdiagnosed or may still develop BD later in life. Every effort was made, however, to ensure the correct diagnoses for each offspring and parent involved in this study, including follow-up evaluations that were conducted at the time of the scan. Finally, recent studies have debated the possible inflation of predictions in neuroimaging studies in individuals with psychiatric disorders(Whelan and Garavan, 2014). We used a well-validated approach that penalizes complex models using regularization, cross-validation, and sparsity enforcement in model fit. Future studies can aim to replicate our findings and employ longitudinal follow-up designs to determine how the WMT-activity relationships identified in this study predict future BD in OBP.
In this study, we showed that the relationship between forceps minor RD and rACC activity significantly differentiated youth at genetic risk for BD from youth at risk for non-BD psychiatric disorders, was evident in youth unaffected by psychiatric disorders, and was moderated by symptoms of affective lability. Given these findings, it is possible that this WMT-activity relationship reflects underlying neuropathological processes that contribute to affectively labile youth at risk for BD and may help differentiate them from youth at risk for other psychiatric disorders. This is an important step toward identifying neurobiological measures in BD risk to improve accuracy in identifying youth most at risk for future BD.
Supplementary Material
Abbreviations
- BD
Bipolar Disorder
- WMT
White Matter Tract
- OBP
Offspring of Bipolar Parents
- OCP
Offspring of Comparison Parents
- vlPFC
Ventrolateral Prefrontal Cortex
- ACC
Anterior Cingulate Cortex
- FA
Fractional Anisotropy
- RD
Radial Diffusivity
- BIOS
Bipolar Offspring Study
- OHP
Offspring of Healthy Parents
- LAMS
Longitudinal Assessment of Manic Symptoms
- MRI
Magnetic Resonance Imaging
- SES
Socioeconomic Status
- K-SADS
Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children
- SCARED
Screen for Child Anxiety Related Disorders
- CALS
Children’s Affective Lability Sale
- MFQ
Mood and Feelings Questionnaire
- KMRS
K-SADS Mania Rating Scale
- SPM
Statistical Parametric Mapping
- ROIs
Regions of Interest
- AD
Axial Diffusivity
- cACC
Caudal ACC
- rACC
Rostral ACC
- SGoF
Sequential Goodness of Fit
References
- Alexander AL, Lee JE, Lazar M, Field AS, 2007. Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JR, Kronhaus DM, Sibille EL, Langenecker SA, Versace A, Labarbara EJ, Phillips ML, 2011. Abnormal left-sided orbitomedial prefrontal cortical-amygdala connectivity during happy and fear face processing: a potential neural mechanism of female MDD. Front Psychiatry 2, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML, 2009. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry 66, 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, 2012. Anatomy and functional role of the inferior longitudinal fasciculus: a search that has just begun. Dev Med Child Neurol 54, 6–7. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Ozdemir H, Yildirim H, 2007. Corpus callosum areas in first-episode patients with bipolar disorder. Psychol Med 37, 699–704. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N, 2003. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol 13, 463–470. [DOI] [PubMed] [Google Scholar]
- Axelson D, Goldstein B, Goldstein T, Monk K, Yu H, Hickey MB, Sakolsky D, Diler R, Hafeman D, Merranko J, 2015. Diagnostic precursors to bipolar disorder in offspring of parents with bipolar disorder: a longitudinal study. American Journal of Psychiatry 172, 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird AA, Colvin MK, Vanhorn JD, Inati S, Gazzaniga MS, 2005. Functional connectivity: integrating behavioral, diffusion tensor imaging, and functional magnetic resonance imaging data sets. Journal of cognitive neuroscience 17, 687–693. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Chang KD, Karchemskiy A, Howe ME, Reiss AL, 2009. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol Psychiatry 66, 238–244. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Absinta M, Rocca MA, Radaelli D, Poletti S, Bernasconi A, Dallaspezia S, Pagani E, Falini A, Copetti M, Colombo C, Comi G, Smeraldi E, Filippi M, 2011a. Tract-specific white matter structural disruption in patients with bipolar disorder. Bipolar Disord 13, 414–424. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Bollettini I, Radaelli D, Poletti S, Locatelli C, Falini A, Smeraldi E, Colombo C, 2014. Adverse childhood experiences influence white matter microstructure in patients with bipolar disorder. Psychol Med 44, 3069–3082. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Yeh PH, Bellani M, Radaelli D, Nicoletti MA, Poletti S, Falini A, Dallaspezia S, Colombo C, Scotti G, Smeraldi E, Soares JC, Brambilla P, 2011b. Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biol Psychiatry 69, 309–317. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, Obreja M, Ehmann M, Iyengar S, Shamseddeen W, Kupfer D, Brent D, 2009. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry 66, 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M, 1999. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry 38, 1230–1236. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM, 1997. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry 36, 545–553. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, Gueorguieva R, Fulbright RK, McGlashan TH, Gore JC, Krystal JH, 2005. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 183, 308–313. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Nicoletti MA, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC, 2003. Magnetic resonance imaging study of corpus callosum abnormalities in patients with bipolar disorder. Biol Psychiatry 54, 1294–1297. [DOI] [PubMed] [Google Scholar]
- Bruno S, Cercignani M, Ron MA, 2008. White matter abnormalities in bipolar disorder: a voxel-based diffusion tensor imaging study. Bipolar Disord 10, 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamante F, Masterton RA, Tournier JD, Smith RE, Willats L, Raffelt D, Connelly A, 2013. Track-weighted functional connectivity (TW-FC): a tool for characterizing the structural-functional connections in the brain. Neuroimage 70, 199–210. [DOI] [PubMed] [Google Scholar]
- Carvajal-Rodriguez A, de Una-Alvarez J, Rolan-Alvarez E, 2009. A new multitest correction (SGoF) that increases its statistical power when increasing the number of tests. BMC Bioinformatics 10, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal-Rodríguez A, de Uña-Alvarez J, Rolán-Alvarez E, 2009. A new multitest correction (SGoF) that increases its statistical power when increasing the number of tests. BMC bioinformatics 10, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock CA, Barker GJ, Marshall N, Schulze K, Hall MH, Fern A, Walshe M, Bramon E, Chitnis XA, Murray R, McDonald C, 2009. White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry 194, 527–534. [DOI] [PubMed] [Google Scholar]
- Chan SW, Sussmann JE, Romaniuk L, Stewart T, Lawrie SM, Hall J, McIntosh AM, Whalley HC, 2016. Deactivation in anterior cingulate cortex during facial processing in young individuals with high familial risk and early development of depression: fMRI findings from the Scottish Bipolar Family Study. Journal of Child Psychology and Psychiatry 57, 1277–1286. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, McKinstry RC, Burton H, Raichle ME, 1999. Tracking neuronal fiber pathways in the living human brain. Proceedings of the National Academy of Sciences of the United States of America 96, 10422–10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA, 1995. Contributions of anterior cingulate cortex to behaviour. Brain 118 ( Pt 1), 279–306. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Iordan AD, Dolcos S, 2011. Neural correlates of emotion-cognition interactions: A review of evidence from brain imaging investigations. J Cogn Psychol (Hove) 23, 669–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsell L, Leemans A, Langan C, Van Hecke W, Barker GJ, McCarthy P, Jeurissen B, Sijbers J, Sunaert S, Cannon DM, McDonald C, 2013. Limbic and callosal white matter changes in euthymic bipolar I disorder: an advanced diffusion magnetic resonance imaging tractography study. Biol Psychiatry 73, 194–201. [DOI] [PubMed] [Google Scholar]
- Findling RL, Youngstrom EA, Fristad MA, Birmaher B, Kowatch RA, Arnold LE, Frazier TW, Axelson D, Ryan N, Demeter CA, Gill MK, Fields B, Depew J, Kennedy SM, Marsh L, Rowles BM, Horwitz SM, 2010. Characteristics of children with elevated symptoms of mania: the Longitudinal Assessment of Manic Symptoms (LAMS) study. J Clin Psychiatry 71, 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, SPitzer RL, Gibbon M, Williams J, 1996. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) American Psychiatric Press, Washington, DC. [Google Scholar]
- Friedman J, Hastie T, Simon N Tibshirani R, 2014. GLMNET
- Ganzola R, McIntosh AM, Nickson T, Sprooten E, Bastin ME, Giles S, Macdonald A, Sussmann J, Duchesne S, Whalley HC, 2018. Diffusion tensor imaging correlates of early markers of depression in youth at high-familial risk for bipolar disorder. Journal of child psychology and psychiatry, and allied disciplines [DOI] [PubMed]
- Ganzola R, Nickson T, Bastin ME, Giles S, Macdonald A, Sussmann J, McIntosh AM, Whalley HC, Duchesne S, 2017. Longitudinal differences in white matter integrity in youth at high familial risk for bipolar disorder. Bipolar disorders 19, 158–167. [DOI] [PubMed] [Google Scholar]
- Gerson AC, Gerring JP, Freund L, Joshi PT, Capozzoli J, Brady K, Denckla MB, 1996. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res 65, 189–198. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF, 2009. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guye M, Parker GJ, Symms M, Boulby P, Wheeler-Kingshott CA, Salek-Haddadi A, Barker GJ, Duncan JS, 2003. Combined functional MRI and tractography to demonstrate the connectivity of the human primary motor cortex in vivo. Neuroimage 19, 1349–1360. [DOI] [PubMed] [Google Scholar]
- Hafeman DM, Bebko G, Bertocci MA, Fournier JC, Bonar L, Perlman SB, Travis M, Gill MK, Diwadkar VA, Sunshine JL, Holland SK, Kowatch RA, Birmaher B, Axelson D, Horwitz SM, Arnold LE, Fristad MA, Frazier TW, Youngstrom EA, Findling RL, Drevets W, Phillips ML, 2014. Abnormal deactivation of the inferior frontal gyrus during implicit emotion processing in youth with bipolar disorder: attenuated by medication. J Psychiatr Res 58, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman DM, Merranko J, Axelson D, Goldstein BI, Goldstein T, Monk K, Hickey MB, Sakolsky D, Diler R, Iyengar S, Brent D, Kupfer D, Birmaher B, 2016. Toward the Definition of a Bipolar Prodrome: Dimensional Predictors of Bipolar Spectrum Disorders in At-Risk Youths. The American journal of psychiatry 173, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Xekardaki A, Delaloye C, Canuto A, Lovblad KO, Gold G, Giannakopoulos P, 2011. Combined analysis of grey matter voxel-based morphometry and white matter tract-based spatial statistics in late-life bipolar disorder. J Psychiatry Neurosci 36, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB, 1975. Four factor index of social status
- Hong S, Ke X, Tang T, Hang Y, Chu K, Huang H, Ruan Z, Lu Z, Tao G, Liu Y, 2011. Detecting abnormalities of corpus callosum connectivity in autism using magnetic resonance imaging and diffusion tensor tractography. Psychiatry Res 194, 333–339. [DOI] [PubMed] [Google Scholar]
- Horwitz SM, Demeter CA, Pagano ME, Youngstrom EA, Fristad MA, Arnold LE, Birmaher B, Gill MK, Axelson D, Kowatch RA, Frazier TW, Findling RL, 2010. Longitudinal Assessment of Manic Symptoms (LAMS) study: background, design, and initial screening results. J Clin Psychiatry 71, 1511–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LM, Barba A, Campbell M, Lamar M, Shankman SA, Leow AD, Ajilore O, Langenecker SA, 2016. Shared white matter alterations across emotional disorders: A voxel-based meta-analysis of fractional anisotropy. Neuroimage Clin 12, 1022–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji A, Godwin D, Rutlin J, Kandala S, Shimony JS, Mamah D, 2017. Tract-based analysis of white matter integrity in psychotic and nonpsychotic bipolar disorder. J Affect Disord 209, 124–134. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Keener MT, Fournier JC, Mullin BC, Kronhaus D, Perlman SB, LaBarbara E, Almeida JC, Phillips ML, 2012. Dissociable patterns of medial prefrontal and amygdala activity to face identity versus emotion in bipolar disorder. Psychol Med 42, 1913–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Norris DG, Hund-Georgiadis M, 2002. An investigation of functional and anatomical connectivity using magnetic resonance imaging. Neuroimage 16, 241–250. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Diwadkar VA, White R, Bass J, Birmaher B, Axelson DA, Phillips ML, 2013. Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm. Dev Cogn Neurosci 5, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavagnino L, Cao B, Mwangi B, Wu MJ, Sanches M, Zunta-Soares GB, Kapczinski F, Soares J, 2015. Changes in the corpus callosum in women with late-stage bipolar disorder. Acta Psychiatr Scand 131, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML, 2004. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 55, 578–587. [DOI] [PubMed] [Google Scholar]
- Linke J, King AV, Poupon C, Hennerici MG, Gass A, Wessa M, 2013. Impaired anatomical connectivity and related executive functions: differentiating vulnerability and disease marker in bipolar disorder. Biol Psychiatry 74, 908–916. [DOI] [PubMed] [Google Scholar]
- Lockhart R, Taylor J, Tibshirani RJ, Tibshirani R, 2014. A SIGNIFICANCE TEST FOR THE LASSO. Ann Stat 42, 413–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA, 2007. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiology of aging 28, 459–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon K, Burdick KE, Ikuta T, Braga RJ, Gruner P, Malhotra AK, Szeszko PR, 2013. Abnormal temporal lobe white matter as a biomarker for genetic risk of bipolar disorder. Biol Psychiatry 73, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon K, Burdick KE, Szeszko PR, 2010. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev 34, 533–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelis A, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, Dwojak AC, Axelson D, Goldstein BI, Goldstein TR, Bebko G, Bertocci MA, Gill MK, Birmaher B, Phillips ML, 2016. Altered functioning of reward circuitry in youth offspring of parents with bipolar disorder. Psychol Med 46, 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelis A, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, Dwojak AC, Axelson D, Goldstein BI, Goldstein TR, Bebko G, Bertocci MA, Hafeman DM, Gill MK, Birmaher B, Phillips ML, 2015. Altered amygdala-prefrontal response to facial emotion in offspring of parents with bipolar disorder. Brain : a journal of neurology 138, 2777–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead TWJ, Harrison LK, Lawrie SM, Johnstone EC, 2005. White matter density in patients with schizophrenia, bipolar disorder and their unaffected relatives. Biological psychiatry 58, 254–257. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC, 2007. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 64, 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan VL, Mishra A, Newton AT, Gore JC, Ding Z, 2009. Integrating functional and diffusion magnetic resonance imaging for analysis of structure-function relationship in the human language network. PLoS One 4, e6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nortje G, Stein DJ, Radua J, Mataix-Cols D, Horn N, 2013. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J Affect Disord 150, 192–200. [DOI] [PubMed] [Google Scholar]
- O’Donnell LJ, Rigolo L, Norton I, Wells WM 3rd, Westin CF, Golby AJ, 2012. fMRI-DTI modeling via landmark distance atlases for prediction and detection of fiber tracts. Neuroimage 60, 456–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T, 2003. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Brain research. Cognitive brain research 18, 48–57. [DOI] [PubMed] [Google Scholar]
- Olsavsky AK, Brotman MA, Rutenberg JG, Muhrer EJ, Deveney CM, Fromm SJ, Towbin K, Pine DS, Leibenluft E, 2012. Amygdala hyperactivation during face emotion processing in unaffected youth at risk for bipolar disorder. J Am Acad Child Adolesc Psychiatry 51, 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternoster R, Brame R, Mazerolle P, Piquero A, 1998. USING THE CORRECT STATISTICAL TEST FOR THE EQUALITY OF REGRESSION COEFFICIENTS. Criminology 36, 859–866. [Google Scholar]
- Perlman SB, Almeida JR, Kronhaus DM, Versace A, Labarbara EJ, Klein CR, Phillips ML, 2012. Amygdala activity and prefrontal cortex-amygdala effective connectivity to emerging emotional faces distinguish remitted and depressed mood states in bipolar disorder. Bipolar disorders 14, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R, 2003. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry 54, 515–528. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC, 2008. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 13, 829, 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA, 2014. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. The American journal of psychiatry 171, 829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL, 2001. A default mode of brain function. Proc Natl Acad Sci U S A 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saricicek A, Zorlu N, Yalin N, Hidiroglu C, Cavusoglu B, Ceylan D, Ada E, Tunca Z, Ozerdem A, 2016. Abnormal white matter integrity as a structural endophenotype for bipolar disorder. Psychol Med 46, 1547–1558. [DOI] [PubMed] [Google Scholar]
- Sarrazin S, d’Albis MA, McDonald C, Linke J, Wessa M, Phillips M, Delavest M, Emsell L, Versace A, Almeida J, Mangin JF, Poupon C, Le Dudal K, Daban C, Hamdani N, Leboyer M, Houenou J, 2015. Corpus callosum area in patients with bipolar disorder with and without psychotic features: an international multicentre study. J Psychiatry Neurosci 40, 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Chang KD, 2013. Brain structural response in individuals at familial risk for bipolar disorder: a tale of two outcomes. Biol Psychiatry 73, 109–110. [DOI] [PubMed] [Google Scholar]
- Singh MK, Kelley RG, Howe ME, Reiss AL, Gotlib IH, Chang KD, 2014. Reward processing in healthy offspring of parents with bipolar disorder. JAMA Psychiatry 71, 1148–1156. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Finn CT, 2003. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet 123C, 48–58. [DOI] [PubMed] [Google Scholar]
- Soehner AM, Bertocci MA, Manelis A, Bebko G, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, Axelson D, Goldstein BI, Goldstein TR, Birmaher B, Phillips ML, 2016. Preliminary investigation of the relationships between sleep duration, reward circuitry function, and mood dysregulation in youth offspring of parents with bipolar disorder. J Affect Disord 205, 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC, 2005. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26, 132–140. [DOI] [PubMed] [Google Scholar]
- Sund AM, Larsson B, Wichstrom L, 2001. Depressive symptoms among young Norwegian adolescents as measured by the Mood and Feelings Questionnaire (MFQ). Eur Child Adolesc Psychiatry 10, 222–229. [DOI] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V, 2010. Development of functional and structural connectivity within the default mode network in young children. Neuroimage 52, 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, Marshall N, Schulze K, Hall MH, Walshe M, Bramon E, Phillips ML, Murray RM, McDonald C, 2010. Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. Neuroimage 53, 58–64. [DOI] [PubMed] [Google Scholar]
- Toosy AT, Ciccarelli O, Parker GJ, Wheeler-Kingshott CA, Miller DH, Thompson AJ, 2004. Characterizing function-structure relationships in the human visual system with functional MRI and diffusion tensor imaging. Neuroimage 21, 1452–1463. [DOI] [PubMed] [Google Scholar]
- Torgerson CM, Irimia A, Leow AD, Bartzokis G, Moody TD, Jennings RG, Alger JR, Van Horn JD, Altshuler LL, 2013. DTI tractography and white matter fiber tract characteristics in euthymic bipolar I patients and healthy control subjects. Brain imaging and behavior 7, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C, 2009. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 168, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng WL, Bones BL, Kayser RR, Olsavsky AK, Fromm SJ, Pine DS, Leibenluft E, Brotman MA, 2015. An fMRI study of emotional face encoding in youth at risk for bipolar disorder. Eur Psychiatry 30, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Mandl R, Luigjes J, Hulshoff Pol H, 2008. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci 28, 10844–10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schot AC, Vonk R, Brouwer RM, van Baal GC, Brans RG, van Haren NE, Schnack HG, Boomsma DI, Nolen WA, Hulshoff Pol HE, Kahn RS, 2010. Genetic and environmental influences on focal brain density in bipolar disorder. Brain 133, 3080–3092. [DOI] [PubMed] [Google Scholar]
- Versace A, Almeida JR, Hassel S, Walsh ND, Novelli M, Klein CR, Kupfer DJ, Phillips ML, 2008. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry 65, 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Almeida JR, Quevedo K, Thompson WK, Terwilliger RA, Hassel S, Kupfer DJ, Phillips ML, 2010a. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry 68, 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Andreazza AC, Young LT, Fournier JC, Almeida JR, Stiffler RS, Lockovich JC, Aslam HA, Pollock MH, Park H, Nimgaonkar VL, Kupfer DJ, Phillips ML, 2014. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Mol Psychiatry 19, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Ladouceur CD, Romero S, Birmaher B, Axelson DA, Kupfer DJ, Phillips ML, 2010b. Altered development of white matter in youth at high familial risk for bipolar disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry 49, 1249–1259, 1259 e1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Jackowski M, Kalmar JH, Chepenik LG, Tie K, Qiu M, Gong G, Pittman BP, Jones MM, Shah MP, Spencer L, Papademetris X, Constable RT, Blumberg HP, 2008a. Abnormal anterior cingulum integrity in bipolar disorder determined through diffusion tensor imaging. Br J Psychiatry 193, 126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, Edmiston E, Chepenik LG, Bhagwagar Z, Spencer L, Pittman B, Jackowski M, Papademetris X, Constable RT, Blumberg HP, 2008b. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biol Psychiatry 64, 730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1999. Wechsler Abbreviated Scale of Intelligence The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M, 2000. Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry 57, 675–682. [DOI] [PubMed] [Google Scholar]
- Werring DJ, Clark CA, Parker GJ, Miller DH, Thompson AJ, Barker GJ, 1999. A direct demonstration of both structure and function in the visual system: combining diffusion tensor imaging with functional magnetic resonance imaging. Neuroimage 9, 352–361. [DOI] [PubMed] [Google Scholar]
- Whelan R, Garavan H, 2014. When optimism hurts: inflated predictions in psychiatric neuroimaging. Biol Psychiatry 75, 746–748. [DOI] [PubMed] [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, Stevens A, Zöllei L, Augustinack J, Wang R, Salat D, Ehrlich S, Behrens T, 2011. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in neuroinformatics 5, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M, Hodneland E, Adolfsdottir S, Haasz J, Lundervold AJ, Eichele T, Lundervold A, 2011. Cortico-striatal connectivity and cognition in normal aging: a combined DTI and resting state fMRI study. Neuroimage 55, 24–31. [DOI] [PubMed] [Google Scholar]
- Zhu D, Zhang T, Jiang X, Hu X, Chen H, Yang N, Lv J, Han J, Guo L, Liu T, 2014. Fusing DTI and fMRI data: a survey of methods and applications. NeuroImage 102, 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman ME, DelBello MP, Getz GE, Shear PK, Strakowski SM, 2006. Anterior cingulate subregion volumes and executive function in bipolar disorder. Bipolar Disord 8, 281–288. [DOI] [PubMed] [Google Scholar]
- Zou H, Hastie T, 2005. Regularization and variable selection via the elastic net. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 67, 301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.